Abstract
Background
Threshold values for 13C‐urea breath test (13C‐UBT) positivity may be affected by various sociodemographic, host, bacterial, and laboratory factors. Manufacturer recommended cutoffs for 13C‐UBT assays may not be applicable in all settings. Optimizing 13C‐UBT cutoffs may have profound public health ramifications. We aimed to determine the optimal threshold for 13C‐UBT positivity in our population.
Methods
Consecutive test samples collected at our central laboratory from patients undergoing a first‐time 13C‐UBT between 1 January 2010 and 31 December 2015 were included. The difference between values at 30 minutes and at baseline (T30–T0) was expressed as delta over baseline (DOB). Cluster analysis was performed on the 13C‐UBT test results to determine the optimal cutoff point with minimal interclass variance.
Results
Two lakhs thirty four thousand eight hundred thirty one patients (87 291 (37.2%) male, age 39.9 ± 19.9) underwent a first‐time 13C‐UBT, including 124 701 (53.1%) negative and 110 130 (46.9%) positive tests, using the manufacturer‐recommended cutoff of 3.5 DOB. Cluster analysis determined an optimized cutoff of 2.74 DOB, representing an additional 2180 (0.93%) positive subjects who had been previously categorized as negative according to the manufacturer‐specified cutoff of 3.5 DOB. Mean positive and negative DOB values were 19.54 ± 14.95 and 0.66 ± 0.51, respectively. The cutoffs for male and female subjects were 2.23 and 3.05 DOB, respectively. Threshold values for <45‐year‐olds, 45‐60‐year‐olds and >60‐year‐olds were 2.67, 2.55, and 2.93 DOB, respectively. Of the 2180 (0.93%) patients with DOB 2.73‐3.49, 289 (13.3%) performed a subsequent 13C‐UBT and 140 (48.4%) remained positive when tested at 20.3 ± 14.4 months.
Conclusions
Major referral laboratories should optimize threshold values for 13C‐UBT positivity for their geographical location. Different cutoff values should be applied for male and female subjects.
Keywords: 13C‐urea breath test, cluster, cutoff, Helicobacter pylori, threshold
1. INTRODUCTION
Helicobacter pylori (H. pylori) infection affects up to 50% of the world's population and is the leading cause of peptic ulcer disease, gastric cancer, and MALT lymphoma.1 The most widely available and accurate noninvasive test for the diagnosis of H. pylori is the 13C‐urea breath test (13C‐UBT).2 This test exploits the urease producing characteristic of the organism, and involves measuring expired 13CO2 following oral ingestion of 13C‐urea. When H. pylori infection is present, 13C‐urea is cleaved by bacterial urease to 2NH3 and 13CO2. Absorbed 13CO2 is excreted via the lungs and measured in the expired air at baseline and 30 minutes following ingestion of the tracer. A positive result is indicated by an increase in expired 13C over baseline, or delta over baseline (DOB).3, 4, 5
A cutoff value of 5 DOB has been widely used to discriminate between positive and negative test and was originally recommended in the standard European protocol.6 Later, by using receiver operator characteristic (ROC) curves, Johnston, et al validated a cutoff of 3.5 DOB.7 Others have used cluster analysis to identify a cutoff of 3.0 DOB.8 Similarly, it had been suggested that different cutoff values be applied to different populations. In children, for example, diagnostic accuracy is improved by employing a cutoff of 7.0 DOB in children younger than 6 years and 4.0 DOB in children over 6 years.9
The magnitude of DOB value is affected by multiple patient‐related factors including gender, nationality, fasting, medications, and posture.10, 11, 12, 13 Furthermore, test characteristics such as 13C‐urea dosage, test meal, test duration, and laboratory equipment, are directly related to 13C‐UBT accuracy.14, 15, 16, 17, 18, 19
It follows that the 13C‐UBT cutoff value should be adjusted in different populations and laboratory settings.20, 21, 22 Nevertheless, most laboratories use manufacturer‐specified cutoff values without regard for these variables. In the present study, we aimed to identify the optimal cutoff value for 13C‐UBTs analyzed at our referral laboratory and optimize the cutoff according to age and gender.
2. METHODS
2.1. Patients
Patients over the age of 18 years who had undergone a 13C‐urea breath test (13C‐UBT) at Clalit Health Service (CHS) during a capture period of 1 January 2010‐31 December 2015 were identified. Only one 13C‐UBT result per patient was included in the final data set (the index test). Repeat tests were not included. Patients with any 13C‐UBT performed during the 2 years before inclusion (1 January 2008 to 31 December 2009) were excluded. Therefore, our cohort included patients undergoing 13C‐UBT for the initial diagnosis of H. pylori infection, rather than for the confirmation of eradication following treatment.
2.2. Sample acquisition and analysis
We collected breath samples from all CHS affiliated facilities, including 1300 primary care and referral clinics and 14 hospitals spread geographically across Israel. The test meal consisted of 75 mg of C13‐urea dissolved in 200 mL 0.1 mol/L citric acid solution or 200 mL orange juice. Samples were processed at the Gastroenterology Laboratory at Rabin Medical Center (Petah Tikva, Israel) using an AP 2003 Isotope Ratio Mass Spectrometer (IRMS) (Analytical Precision, Phoenix, AZ, USA) and a Gilson XL 222 Automatic Breath Sampler (Gilson, Middleton, WI, USA). At baseline and 30 minutes after intake of the test meal, we assayed expired 13C:12C (parts per thousand), and the difference between these scores (delta over baseline, DOB) was the test result. According to the manufacturer, DOB above 3.5 was indicative of H. pylori infection.
2.3. Data extraction
We retrieved demographic data and 13C‐UBT test results from the CHS database. Approval was obtained from the Rabin Medical Center ethics committee, and the study was performed in accordance with the principles of Good Clinical Practice.
2.4. Statistical analysis
The Kolmogorov‐Smirnov test (P < 0.01) was used to analyze continuous variables (mean ± SD). Cluster analysis was performed on the 13C‐UBT test results to determine the optimal cutoff point with minimal interclass variance. SPSS version 24.0 software (IBM, Armonk, NY) was used.
3. RESULTS
Two lakhs thirty four thousand eight hundred thirty one patients (87 291 (37.2%) male, age 39.9 ± 19.9) underwent a first‐time 13C‐UBT, including 124 701 (53.1%) negative and 110 130 (46.9%) positive tests, using the manufacturer‐recommended cutoff of 3.5 DOB. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Total, N(%) | 234831 (100) |
| Male, N(%) | 87291 (37.2) |
| Age, mean (SD) | 39.9 (19.9) |
SD, standard deviation.
Cluster analysis determined an optimized cutoff of 2.74 DOB for the entire data set (Figure 1). Mean positive and negative DOB values were 19.54 ± 14.95 and 0.66 ± 0.51, respectively (Table 2).
Figure 1.
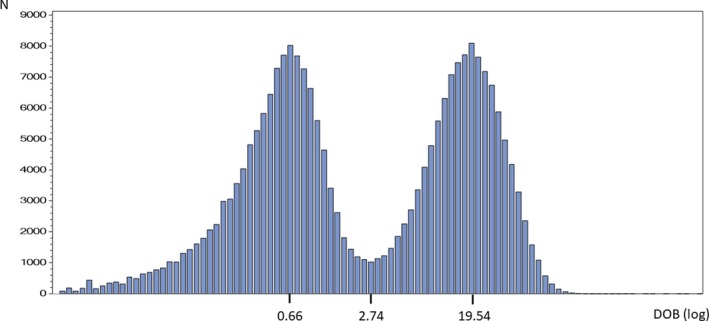
Cluster analysis in the whole study population. The histogram of the logarithmically transformed 13C‐urea breath test values shows two distinct populations: Helicobacter pylori ‐negative and H pylori‐positive. DOB, delta over baseline
Table 2.
Optimized 13C‐urea breath test threshold level in the tested population
| N (%) | Cutoff (DOB) | H. pylori‐positive | H. pylori‐negative | |||
|---|---|---|---|---|---|---|
| N (%) | Mean DOB (SD) | N (%) | Mean DOB (SD) | |||
| All | 234831 (100) | 2.74 | 112310 (47.8) | 19.54 (14.95) | 122521 (52.2) | 0.66 (0.51) |
| Male | 87291 (37.2) | 2.23 | 42604 (48.8) | 15.29 (13.26) | 44687 (51.2) | 0.56 (0.44) |
| Female | 147540 (62.8) | 3.05 | 69979 (47.4) | 22.06 (15.34) | 77561 (52.6) | 0.71 (0.53) |
DOB, delta over baseline; SD, standard deviation.
The cutoffs for male and female subjects were 2.23 and 3.05 DOB, respectively. Threshold values for <45‐year‐olds, 45‐60‐year‐olds and >60‐year‐olds were 2.67, 2.55, and 2.93 DOB, respectively (Table 3).
Table 3.
Optimized 13C‐urea breath test thresholds in different age groups (DOB)
| <45 y | 45‐60 y | >60 y | |
|---|---|---|---|
| All | 2.67 | 2.55 | 2.93 |
| Male | 2.16 | 2.06 | 2.53 |
| Female | 3.00 | 2.83 | 3.12 |
DOB, delta over baseline.
Adjustment of the cutoff identified an additional 2180 (0.93%) positive subjects who had been previously categorized as negative according to the manufacturer‐specified cutoff of 3.5 DOB. Of the 2180 (0.93%) patients with 2.73‐3.49 DOB, 289 (13.3%) performed a subsequent 13C‐UBT and 140 (48.4%) remained positive when tested at 20.3 ± 14.4 months.
4. DISCUSSION
The 13C‐UBT is the preferred noninvasive test for the diagnosis of H. pylori infection.1 In our large cohort, we determined an optimal cutoff of DOB 2.74 for subjects undergoing 13C‐UBT. The optimized cutoff was higher among women (3.05 DOB) and among subjects over the age of 60 years (2.93 DOB).
Minor adjustments in 13C‐UBT threshold levels can have major public health ramifications. Upwards adjustment of the cutoff has the potential to considerably decrease exposure to antibiotics with a concomitant decrease in antibiotic‐associated morbidity and microbial resistance. Downwards adjustment of the cutoff, as performed in our study, will lead to an increased detection of H. pylori. This has the potential to decrease the burden of gastric cancer in our population.23 Furthermore, a high detection rate of H. pylori infection among subjects with uninvestigated dyspepsia may lead to timely treatment and fewer referrals for endoscopy.24 It follows that adjustment of the cutoff value either upwards or downwards may lead to decreased health‐related costs.
In our study, we reclassified 2180 subjects as positive after having received a negative result using the manufacturer‐specified cutoff of 3.5 DOB. Among these subjects, 13.3% performed a subsequent 13C‐UBT. This proportion is higher than expected, since the likelihood of a positive 13C‐UBT following a negative 13C‐UBT is less than 1%, and primary care physicians would likely to be hesitant to request a test with such a low pretest probability.25 The fact that so many subjects were referred for repeat testing despite this, might indicate that patients had ongoing symptoms due to undiagnosed H. pylori infection. Over half of subjects were negative (<2.74 DOB) upon repeat testing; however, we cannot exclude a situation in which subjects who were initially incorrectly classified as negative (2.74‐3.49 DOB) subsequently underwent a endoscopy‐based test for H. pylori, received treatment, and performed the second 13C‐UBT following treatment. Among the 140 subjects who were initially incorrectly classified as negative (2.74‐3.49 DOB) and performed a second 13C‐UBT which was positive (>2.74 DOB), 128 (85.7%) would have tested positive even using the manufacturer‐specified cutoff of 3.5 DOB.
Our findings are consistent with those of other laboratories who have demonstrated that manufacturer‐specified threshold levels may be too high. Mauro, et al identified a cutoff of 3.09 DOB in a cohort of 2232 Canadian subjects, with highest threshold levels observed in subjects over age 60 years. Among subjects undergoing 13C‐UBT for confirmation of eradication following treatment, threshold levels were lower (2.88 DOB).26 Others have identified cutoff values of 2.5‐3.0 DOB.8, 19
Age is a major factor which may affect the optimal threshold value for the 13C‐UBT. Yang, et al performed a validation study among children and recommended applying a cutoff value of 7.0 DOB in children below age 6 years and a cutoff value of 4.0 DOB in children older than 6 years.9 In addition, a large meta‐analysis showed that the 13C‐UBT test is less accurate for the diagnosis of H. pylori infection in children younger than 6 years, but using a cutoff value of at least 6.0 DOB may improve test accuracy.27
The need for a higher cutoff in children may be due to urease‐producing microorganisms, including Streptococcus salivarius, Proteus mirabilis, and Klebsiella pneumoniae, which are present in the oral cavity and cause false positive results when lower thresholds are used.28, 29 Although these organisms are often present in adults, children tend to retain 13C‐urea in the mouth leading to false positive results and the need for a higher cutoff value.30, 31 Another reason for higher cutoff values in children is related to the use of a standardized 13C‐urea dose (often >75 mg) regardless of age or body weight. Relatively higher doses of 13C‐urea in children may influence the DOB values and lead to false positive results.9 Finally, children produce relatively abundant endogenous 12CO2.32 This may increase the isotopic ratio of 13CO2/12CO2 as compared with adults thereby necessitating higher threshold values for 13C‐UBT. Similar microbiological and physiological studies have not been performed in the elderly; however, it is conceivable that some of the same mechanisms may account for the higher 13C‐UBT threshold values required among women and in subjects over the age of 60 (Table 3). Another possible cause for higher threshold values among women and the elderly may be an exaggerated effect of the citric acid test meal on gastric emptying and gastric acidity, compared to younger men. A standard dose of citric acid was administered without consideration of a lower mean body weight or a possible baseline impairment in gastric emptying.
In addition to patient factors which may influence optimal threshold values, various test characteristics should also be considered. Leodolter, et al identified a cutoff of 4.0 DOB using a test meal of 200 mL 0.1 mol/L citric acid and a test duration of 30 minutes.33 Malaty, et al found that a cutoff value of 2.4 DOB is 96% sensitive and 100% specific when shortening the test duration to 20 minutes, omitting the test meal and using 125 mg of 13C‐urea.17 Wang, et al suggest a cutoff value of 2.8 DOB when shortening the duration to 15 minutes, using fresh milk as the test meal and using 100 mg of 13C‐urea (99% sensitivity, 93% specificity).18 Tepes, et al used a cutoff value of 2.5 DOB and a novel test meal combining citric, malic, and tartaric acid, to achieve 92% sensitivity and 96% specificity despite treatment with a proton pump inhibitor.34 Ohara, et al suggest using a cutoff value of 2.5 DOB to achieve 98.1% sensitivity and 97.9% specificity with a test duration of 20 minutes, omitting the test meal and using 100 mg of 13C‐urea.19 As a result of this study, a cutoff of 2.5 DOB has been widely accepted in Japan. Nevertheless, among Japanese subjects performing the test following treatment, a cutoff of 3.5 DOB has been suggested.14
The main strength of our study is the large sample size. Limitations include the lack of a gold standard such as histology. This precluded us from being able to determine 13C‐UBT sensitivity and specificity using the optimized threshold. However, using a gold standard is clearly not feasible with such a large data set. Furthermore, previous studies have confirmed the validity of threshold values derived from cluster analysis.8, 14, 19, 26
In conclusion, our study demonstrates that manufacturer‐recommended 13C‐UBT threshold values may not be applicable in every setting. Different cutoff values should be applied for male and female subjects. Major referral laboratories should optimize threshold values for 13C‐UBT positivity in their geographical location.
DISCLOSURES
None.
Perets TT, Gingold‐Belfer R, Leibovitzh H, et al. Optimization of 13C‐urea breath test threshold levels for the detection of Helicobacter pylori infection in a national referral laboratory. J Clin Lab Anal. 2019;33:e22674 10.1002/jcla.22674
REFERENCES
- 1. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence consensus report. Gut. 2017;66:6‐30. [DOI] [PubMed] [Google Scholar]
- 2. Best LM, Takwoingi Y, Siddique S, et al. Non‐invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lotterer E, Ramaker J, Lüdtke FE, Tegeler R, Geletneky JV, Bauer FE. The simplified 13C‐urea breath test–one point analysis for detection of Helicobacter pylori infection. Z Gastroenterol. 1991;29:590‐594. [PubMed] [Google Scholar]
- 4. Atherton JC, Spiller RC. The urea breath test for Helicobacter pylori . Gut. 1994;35:723‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goddard AF, Logan RP. Review article: urea breath tests for detecting Helicobacter pylori . Aliment Pharmacol Ther. 1997;11:641‐649. [DOI] [PubMed] [Google Scholar]
- 6. Eggers RH, Kulp A, Tegeler R. A methodological analysis of the 13C‐urea breath test for detection of Helicobacter pylori infections: high sensitivity and specificity within 30 min using 75 mg of 13C‐urea. Eur J Gastro Hepatol. 1990;2:437‐444. [Google Scholar]
- 7. Johnston BJ, Levi S, Johnson PG. Cut‐off point for 13C‐urea breath test. Gut. 1996;39:A122. [Google Scholar]
- 8. Mion F, Rosner G, Rousseau M, Minaire Y. 13C‐urea breath test for Helicobacter pylori: Cut‐off point determination by cluster analysis. Clin Sci. 1997;93:3‐6. [DOI] [PubMed] [Google Scholar]
- 9. Yang HR, Seo JK. Diagnostic accuracy of the C‐urea breath test in children: adjustment of the cut‐off value according to age. J Gastroenterol Hepatol. 2005;20:264‐269. [DOI] [PubMed] [Google Scholar]
- 10. Gatta L, Vakil N, Ricci C, et al. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol. 2004;99:823‐829. [DOI] [PubMed] [Google Scholar]
- 11. Johnston BJ, Gruer N, Johnson P. Effect of subject position on the performance of the 13C‐urea breath test for the detection of Helicobacter pylori . Gut. 1998;43(2S):57A. [Google Scholar]
- 12. Eisdorfer I, Shalev V, Goren S, Chodick G, Muhsen K. Sex differences in urea breath test results for the diagnosis of Helicobacter pylori infection: a large cross‐sectional study. Biol Sex Differ. 2018;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bode G, Rothenbacher D, Brenner H, Adler G. Variation in the 13C‐urea breath test value by nationality in Helicobacter pylori ‐infected children. Scand J Gastroenterol. 1998;33:468‐472. [DOI] [PubMed] [Google Scholar]
- 14. Kato C, Sugiyama T, Sato K, et al. Appropriate cut‐off value of 13C‐urea breath test after eradication of Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2003;18:1379‐1383. [DOI] [PubMed] [Google Scholar]
- 15. Gatta L, Vakil N, Ricci C, et al. A rapid, low‐dose, 13C‐urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment Pharmacol Ther. 2003;17:793‐798. [DOI] [PubMed] [Google Scholar]
- 16. Domínguez‐Muñoz JE, Leodolter A, Sauerbruch T, Malfertheiner P. A citric acid solution is an optimal test drink in the 13C‐urea breath test for the diagnosis of Helicobacter pylori infection. Gut. 1997;40:459‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malaty HM, el‐Zimaity HM, Genta RM, Klein PD, Graham DY. Twenty‐minute fasting version of the US 13C‐urea breath test for the diagnosis of H. pylori infection. Helicobacter. 1996;1:165‐167. [DOI] [PubMed] [Google Scholar]
- 18. Wang WM, Lee SC, Ding HJ, et al. Quantification of Helicobacter pylori infection: simple and rapid 13C‐urea breath test in Taiwan. J Gastroenterol. 1998;33:330‐335. [DOI] [PubMed] [Google Scholar]
- 19. Ohara S, Kato M, Asaka M, Toyota T. Studies of 13C‐urea breath test for diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol. 1998;33:6‐13. [DOI] [PubMed] [Google Scholar]
- 20. Labenz J, Bärsch G, Peitz U, et al. Validity of a novel biopsy urease test (HUT) and a simplified 13C‐urea breath test for diagnosis of Helicobacter pylori infection and estimation of the severity of gastritis. Digestion. 1996;57:391‐397. [DOI] [PubMed] [Google Scholar]
- 21. Li Z‐X, Huang L‐L, Liu C, et al. Cut‐off optimization for 13C‐urea breath test in a community‐based trial by mathematic, histology and serology approach. Sci Rep. 2017;7:2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanahashi T, Kodama T, Yamaoka Y, et al. Analysis of the 13 C‐urea breath test for detection of Helicobacter pylori infection based on the kinetics of Δ‐ 13 CO 2 using laser spectroscopy. J Gastroenterol Hepatol. 1998;13:732‐737. [DOI] [PubMed] [Google Scholar]
- 23. Wong BC‐Y, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: a randomized controlled trial. JAMA 2004;291:187‐194. [DOI] [PubMed] [Google Scholar]
- 24. Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988‐1013. [DOI] [PubMed] [Google Scholar]
- 25. Niv Y, Hazazi R, Waked A, Lederfein T, Achiel K. Helicobacter pylori recurrence and infection rate in Israeli adults. Dig Dis Sci. 2008;53:1211‐1214. [DOI] [PubMed] [Google Scholar]
- 26. Mauro M, Radovic V, Zhou P, et al. 13C urea breath test for (Helicobacter pylori): determination of the optimal cut‐off point in a Canadian community population. Can J Gastroenterol. 2006;20:770‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leal YA, Flores LL, Fuentes‐Pananá EM, Cedillo‐Rivera R, Torres J. 13C‐urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta‐analysis. Helicobacter. 2011;16:327‐337. [DOI] [PubMed] [Google Scholar]
- 28. Jones NL, Sherman PM. Approaching Helicobacter pylori infection in children: level I evidence at last and a word of caution. J Pediatr. 2001;139:622‐623. [DOI] [PubMed] [Google Scholar]
- 29. Rowland M, Lambert I, Gormally S, et al. Carbon 13‐labeled urea breath test for the diagnosis of Helicobacter pylori infection in children. J Pediatr. 1997;131:815‐820. [DOI] [PubMed] [Google Scholar]
- 30. Peng NJ, Lai KH, Liu RS, et al. Clinical significance of oral urease in diagnosis of Helicobacter pylori infection by 13Curea breath test. Dig Dis Sci. 2001;46:1772‐1778. [DOI] [PubMed] [Google Scholar]
- 31. Imrie C, Rowland M, Bourke B, Drumm B. Limitations to carbon 13‐labeled urea breath testing for Helicobacter pylori in infants. J Pediatr. 2001;139:734‐737. [DOI] [PubMed] [Google Scholar]
- 32. Klein PD, Malaty HM, Czinn SJ, Emmons SC, Martin RF, Graham DY. Normalizing results of 13C‐urea breath testing for CO2 production rates in children. J Pediatr Gastroenterol Nutr. 1999;29:297‐301. [DOI] [PubMed] [Google Scholar]
- 33. Leodolter A, Domínguez‐Muñoz JE, von Arnim U, Kahl S, Peitz U, Malfertheiner P. Validity of a modified 13C‐urea breath test for pre‐ and posttreatment diagnosis of Helicobacter pylori infection in the routine clinical setting. Am J Gastroenterol. 1999;94:2100‐2104. [DOI] [PubMed] [Google Scholar]
- 34. Tepeš B, Malfertheiner P, Labenz J, Aygen S. Modified Helicobacter test using a new test meal and a 13C‐urea breath test in Helicobacter pylori positive and negative dyspepsia patients on proton pump inhibitors. World J Gastroenterol. 2017;23:5954‐5961. [DOI] [PMC free article] [PubMed] [Google Scholar]


