Abstract
Background
The objective of this study was to assess the effect of parathyroidectomy (PTX) treatment on prolonging overall survival (OS) as well as decreasing levels of intact parathyroid hormone (iPTH), calcium (Ca), and phosphorus (P) in elderly hemodialysis patients with severe secondary hyperparathyroidism (SHPT).
Methods
A total of 304 elderly hemodialysis patients with severe SHPT were consecutively enrolled in this cohort study. According to whether PTX operations were applied, patients were classified into PTX group (N = 112) and Control group (N = 192) and were followed up for 3 years. Mortality rate and OS were evaluated, and iPTH, Ca, and P levels were recorded.
Results
Compared to control group, increased iPTH (P < 0.001), higher Ca (P = 0.003), elevated AST (P = 0.022), and lower Hb (P = 0.049) concentrations were observed in the PTX group at baseline. The 1‐year mortality (P < 0.001), 2‐year mortality (P < 0.001), and 3‐year mortality (P < 0.001) was reduced in PTX group compared to Control group, and PTX was correlated with prolonged OS (P < 0.001). Multivariate Cox's regression analysis further revealed that PTX treatment (P < 0.001, HR = 0.177) was an independent factor for better OS. Moreover, patients in PTX group had decreased iPTH (P < 0.05) and Ca (P < 0.05) levels compared to Control group at M1‐M36, while no difference was found in serum P level between the two groups at M1‐M36.
Conclusion
Parathyroidectomy decreases iPTH and Ca levels, and it associates with favorable survival in elderly hemodialysis patients with severe SHPT.
Keywords: elderly, hemodialysis, overall survival, Parathyroidectomy, secondary hyperparathyroidism
1. INTRODUCTION
Chronic kidney disease (CKD) is one of the most common urinary system diseases and current international guidelines define the condition of CKD as decreased kidney function (glomerular filtration rate [GFR] <60 mL/min per 1.73 m2 or markers of kidney damage, or both) for at least 3 months duration.1 For CKD patients who undergo hemodialysis, most of them have many inevitable complications such as hypocalcemia, hyperphosphatemia, and decrease in 1,25‐dihydroxy vitamin D, which would further result in hypersecretion of parathyroid hormone (PTH) and secondary hyperparathyroidism (SHPT).2, 3 According to national statistical report, approximately 1.3% males and 2.2% females in China are diagnosed with CKD stages 3‐5 and need to receive hemodialysis therapy, leading to high prevalence of SHPT within hemodialysis populations.4 As the main complication in CKD patients receiving hemodialysis, SHPT is a tricky problem that impairs quality of life and induces a higher mortality rate, especially in elderly population due to poor tolerance and senile function.5 Thus, it is necessary to explore appropriate therapeutic approaches for SHPT to increase survival and improve prognosis in CKD patients receiving hemodialysis.
Although various conventional therapies (modification of hemodialysis characteristics) and new drugs (such as vitamin D, sevelamer hydrochloride, and calcimimetics) are frequently used for SHPT treatment, the efficacy of these treatments is still far from satisfaction due to the uncontrolled intact PTH (iPTH) levels and drugs toxicity in severe SHPT patients.6 Surgical treatment parathyroidectomy (PTX), consisting of subtotal, total PTX with the implant and total PTX, is recommended for SHPT patients refractory to medical therapy, and it has been demonstrated to have a great effect on decreasing mortality rate and improving prognosis in patients with SHPT or primary hyperparathyroidism, while for hemodialysis patients with severe SHPT, particularly in these elderly patients, the advantage of PTX has not been investigated.7, 8, 9 Therefore, the objective of this study was to assess the effect of PTX treatment on prolonging overall survival (OS) as well as decreasing levels of iPTH, Ca, and P in elderly hemodialysis patients with severe SHPT.
2. MATERIALS AND METHODS
2.1. Patients
A total of 304 elderly hemodialysis patients with severe SHPT admitted to The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology from 2010/1/1 to 2013/12/13 were consecutively enrolled in this cohort study. The inclusion criteria were: (a) Sustained iPTH level above 700 pg/mL associated with hypercalcemia and hyperphosphatemia; (b) Age above 60 years; (c) Duration of hemodialysis above 12 months; (d) Able to be regularly followed up. Patients with a history of kidney transplantation or other kidney surgery, history of percutaneous transluminal coronary angioplasty or bypass surgery, history of solid tumors or hematological malignancies were excluded from this study.
2.2. Ethics statement and approval
This study was conducted according to the Declaration of Helsinki. And Ethics Review Board of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology had approved the protocol of this study. All patients provided written informed consents.
2.3. Baseline data collection
Comprehensive baseline information of patients was collected including: age, gender, body mass index (BMI), medical history of hypertension, diabetes mellitus, hyperlipidemia, hyperuricemia and heart failure, duration of hemodialysis, Kt/V rate, ultrafiltration rate, iPTH, calcium (Ca), phosphorus (P), fibroblast growth factor 23 (FGF‐23), hemoglobin (Hb), C‐reactive protein (CRP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and serum uric acid (SUA).
2.4. Treatments
As a cohort study, treatments were not intervened in this study, and the treatments were determined according to clinical need, patients’ condition, and willingness. Corresponding to whether PTX operations were applied, patients were classified into PTX group and control group. The relevant data related to drugs used in glucose‐lowering therapy and antihypertensive therapy was shown in Table S1.
2.5. Assessments
Patients were followed up for 3 years according to the protocol, and 1‐, 2‐ and 3‐year mortalities were evaluated; meanwhile, overall survival (OS) was calculated from the data of enrollment to the date of death from any cause. In addition, levels of iPTH, Ca, P were measured at M0, M1, M3, M6, M12, M18, M24, M30, and M36. The detailed information about causes of postoperative deaths within 3 years was shown in Table S2.
2.6. Statistical analysis
Statistical analysis was performed using the SSPS 21.0 software (IBM, New York, NY, USA) and GraphPad Prism 5.01 (Graph Int, San Diego, CA, USA). Data were presented as mean ± SD, median (25th‐75th quantiles) or count (percentage). Comparison was determined by t test, Wilcoxon rank sum test or Chi‐square test. Analysis of OS was performed by Kaplan‐Meier (K‐M) curves and log‐rank test. The factors affecting OS was determined by univariate and multivariate Cox's proportional hazards regression. P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Study flow
A total of 579 patients were invited to participate in this study. Among them, 51 patients who declined to participate in this study were excluded. And then 528 patients were screened for eligibility, while 101 patients were excluded (including 37 patients were not eligible, 52 patients refused to sign the informed consents, 12 patients were excluded for other reasons). Subsequently, 427 patients were enrolled in this study, whereas 123 patients were excluded, among which, 65 patients lost follow‐up or did not have completed 3‐year survival data (including 43 patients in PTX group and 22 patients in control group), 31 non‐PTX patients initiated with PTX treatment during the study and 27 patients withdrew informed consents. At last, the remaining 304 patients were included in the final analysis including 192 patients in Control group and 112 patients in PTX group, as shown in Figure 1.
Figure 1.
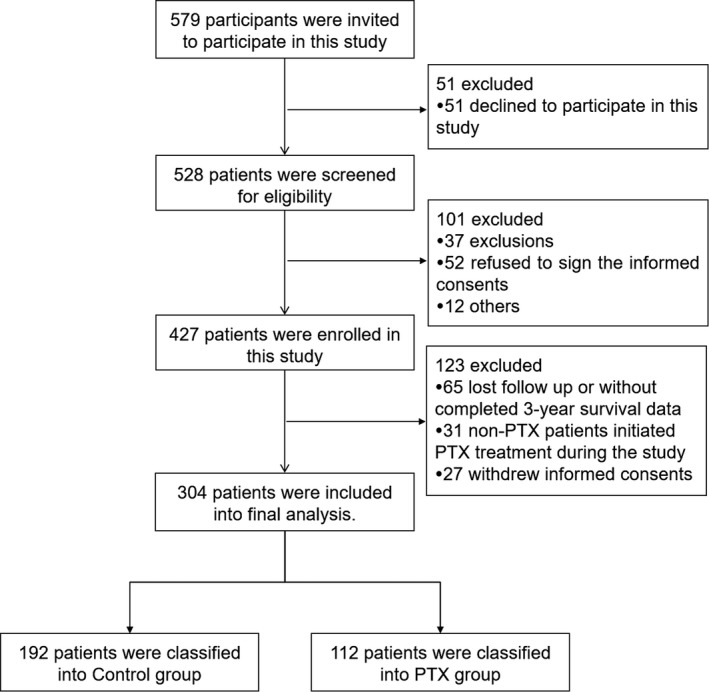
Study flow
3.2. Patients’ characteristics
The mean age of patients in the Control group and PTX group were 65.5 ± 7.1 and 64.7 ± 7.3 years, respectively. There were 113 males and 79 females in the Control group, as well as 78 males and 34 females in PTX group, respectively. No difference was found in age and gender between the two groups. In addition, patients in the PTX group had longer duration of hemodialysis (P = 0.008), increased iPTH level (P < 0.001), elevated Ca level (P = 0.003), lower Hb value (P = 0.049), and higher AST value (P = 0.022) compared to Control group. No difference was observed in other baseline characteristics between the two groups (Table 1).
Table 1.
Patients’ characteristics
| Parameters | Control group (n = 192) | PTX group (n = 112) | P value |
|---|---|---|---|
| Age (years) | 65.5 ± 7.1 | 64.7 ± 7.3 | 0.353 |
| Gender (Male/Female) | 113/79 | 78/34 | 0.060 |
| BMI (kg/m2) | 22.3 ± 3.3 | 22.9 ± 3.0 | 0.106 |
| Hypertension (n/%) | 133 (69.3) | 87 (77.7) | 0.114 |
| Diabetes mellitus (n/%) | 44 (22.9) | 20 (17.9) | 0.297 |
| Hyperlipidemia (n/%) | 69 (55.9) | 44 (39.3) | 0.560 |
| Hyperuricemia (n/%) | 57 (29.7) | 39 (34.8) | 0.353 |
| Heart failure (n/%) | 18 (9.4) | 16 (14.3) | 0.190 |
| Duration of dialysis (months) | 35 (28‐51) | 44 (34‐65) | 0.008 |
| Kt/V | 1.6 ± 0.5 | 1.7 ± 0.5 | 0.193 |
| Ultrafiltration rate (mL/min) | 6.6 ± 2.2 | 7.0 ± 2.1 | 0.117 |
| iPTH (pg/mL) | 1085 ± 533 | 1636 ± 724 | <0.001 |
| Ca (mg/dL) | 9.40 ± 1.21 | 9.89 ± 1.42 | 0.003 |
| P (mg/dL) | 6.95 ± 1.38 | 7.17 ± 1.26 | 0.158 |
| FGF‐23 (pg/mL) | 589 ± 190 | 635 ± 181 | 0.093 |
| Hb (g/dL) | 11.8 ± 2.6 | 11.2 ± 2.1 | 0.049 |
| CRP (mg/L) | 10.6 (4.1‐17.2) | 12.2 (5.2‐20.9) | 0.142 |
| ALB (g/L) | 36.7 ± 6.0 | 35.9 ± 5.5 | 0.238 |
| ALT (u/L) | 49.2 ± 27.5 | 53.8 ± 21.4 | 0.106 |
| AST (u/L) | 60.5 ± 18.3 | 66.3 ± 22.7 | 0.022 |
| ALP (u/L) | 267.8 ± 85.2 | 289.4 ± 116.3 | 0.087 |
| SUA (mg/dL) | 7.3 ± 2.2 | 7.7 ± 2.1 | 0.117 |
Data were presented as mean ± SD, median (25th‐75th quantiles) or count (percentage). Comparison was determined by t test, Wilcoxon rank sum test or Chi‐square test. P value < 0.05 (presented with bold font) was considered statistically significant. ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; Ca, calcium; CRP, C‐reactive protein; FGF‐23, fibroblast growth factor 23; Hb, hemoglobin; iPTH, intact parathyroid hormone; P, phosphorus; PTX, parathyroidectomy; SUA, serum uric acid.
3.3. Comparison of mortality rate and OS between control group and PTX group
Compared to Control group, patients in PTX group presented with lower 1‐year mortality (P < 0.001), 2‐year mortality (P < 0.001), and 3‐year mortality (P < 0.001; Figure 2A). In addition, OS in PTX group was better than that in Control group (P < 0.001; Figure 2B). Considering the effect of irregular hemodialysis on mortality, we exclude those patients with irregular hemodialysis and did further comparison of mortality between two groups, which were shown in Table S3.
Figure 2.
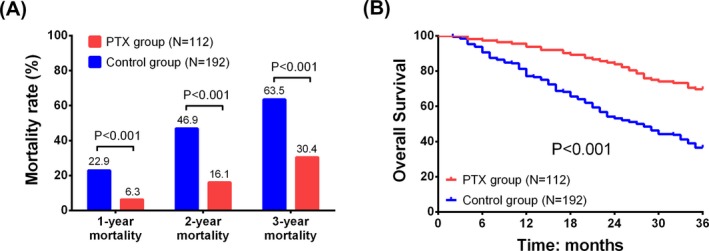
Comparison of mortality rate and OS between Control group and PTX group. A, patients in PTX group had lower 1‐y mortality, decreased 2‐y mortality, and reduced 3‐y mortality compared to Control group; B, prolonged OS was found in PTX group compared to Control group. Chi‐square test was used to analyze the difference in mortality between two groups. Kaplan‐Meier curve and the log‐rank test were used to compare the OS between two groups. P value < 0.05 was considered statistically significant. OS, overall survival; PTX, parathyroidectomy
3.4. Cox's proportional hazards regression analysis of factors affecting OS
Univariate Cox's proportional hazards regression analysis indicated that PTX treatment was correlated with prolonged OS (P < 0.001, HR = 0.353), whereas hypertension (P = 0.001, HR = 2.036), duration of hemodialysis (high; P < 0.001, HR = 2.315), FGF‐23 level (high; P < 0.001, HR = 2.288), CRP level (high; P < 0.001, HR = 3.484), and ALT level (high; P = 0.045, HR = 1.383) were associated with poor OS in elderly hemodialysis patients with severe SHPT. Moreover, multivariate Cox's regression analysis revealed that PTX treatment (P < 0.001, HR = 0.177) was an independent factor for better OS, while hypertension (P < 0.001, HR = 2.647), hyperuricemia (P < 0.004, HR = 1.795), duration of hemodialysis (high; P < 0.001, HR = 2.245), FGF‐23 (high; P < 0.001, HR = 2.881), and CRP (high; P < 0.001, HR = 4.046) could predict worse OS in elderly hemodialysis patients with severe SHPT (Table 2).
Table 2.
Cox's proportional hazards regression analysis of factors affecting OS
| Parameters | Univariate Cox's regression | Multivariate Cox's regression | ||||||
|---|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| PTX treatment (vs control) | <0.001 | 0.353 | 0.241 | 0.517 | <0.001 | 0.177 | 0.113 | 0.277 |
| Age (high) | 0.731 | 1.057 | 0.772 | 1.447 | 0.180 | 0.789 | 0.559 | 1.116 |
| Gender (male) | 0.174 | 0.801 | 0.582 | 1.103 | 0.587 | 1.113 | 0.757 | 1.638 |
| BMI (high) | 0.964 | 0.993 | 0.725 | 1.359 | 0.523 | 1.123 | 0.787 | 1.600 |
| Hypertension | 0.001 | 2.036 | 1.359 | 3.050 | <0.001 | 2.647 | 1.706 | 4.108 |
| Diabetes mellitus | 0.526 | 1.128 | 0.777 | 1.638 | 0.886 | 0.965 | 0.598 | 1.559 |
| Hyperlipidemia | 0.146 | 1.267 | 0.921 | 1.742 | 0.883 | 0.971 | 0.657 | 1.435 |
| Hyperuricemia | 0.257 | 1.213 | 0.869 | 1.692 | 0.004 | 1.795 | 1.209 | 2.666 |
| Heart failure | 0.323 | 1.091 | 0.918 | 1.297 | 0.612 | 1.048 | 0.874 | 1.257 |
| Duration of dialysis (high) | <0.001 | 2.315 | 1.660 | 3.228 | <0.001 | 2.245 | 1.548 | 3.256 |
| Kt/V (high) | 0.518 | 0.902 | 0.659 | 1.234 | 0.359 | 1.176 | 0.831 | 1.664 |
| Ultrafiltration rate (high) | 0.311 | 0.850 | 0.621 | 1.164 | 0.261 | 0.824 | 0.588 | 1.155 |
| iPTH (high) | 0.056 | 1.361 | 0.993 | 1.865 | 0.183 | 1.260 | 0.897 | 1.770 |
| Ca (high) | 0.343 | 1.165 | 0.850 | 1.596 | 0.649 | 0.919 | 0.640 | 1.321 |
| P (high) | 0.663 | 1.072 | 0.783 | 1.468 | 0.383 | 1.171 | 0.821 | 1.671 |
| FGF‐23 (high) | <0.001 | 2.288 | 1.648 | 3.178 | <0.001 | 2.881 | 1.979 | 4.194 |
| Hb (high) | 0.323 | 0.853 | 0.623 | 1.169 | 0.463 | 0.876 | 0.616 | 1.247 |
| CRP (high) | <0.001 | 3.484 | 2.453 | 4.949 | <0.001 | 4.046 | 2.770 | 5.912 |
| ALB (high) | 0.417 | 1.139 | 0.832 | 1.560 | 0.936 | 0.979 | 0.586 | 1.637 |
| ALT (high) | 0.045 | 1.383 | 1.008 | 1.899 | 0.734 | 1.093 | 0.656 | 1.820 |
| AST (high) | 0.266 | 1.195 | 0.873 | 1.637 | 0.806 | 1.046 | 0.728 | 1.505 |
| ALP (high) | 0.854 | 1.030 | 0.752 | 1.410 | 0.784 | 0.948 | 0.649 | 1.385 |
| SUA (high) | 0.192 | 1.233 | 0.900 | 1.688 | 0.093 | 1.364 | 0.949 | 1.962 |
Data were presented as P value, HR (hazard ratio) and 95% CI (confidence interval). Univariate and multivariate Cox's proportional hazards regression analyses were performed to determine the factors affecting OS. P value < 0.05 (presented with bold font) was considered statistically significant. ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; Ca, calcium; CRP, C‐reactive protein; FGF‐23, fibroblast growth factor 23; Hb, hemoglobin; iPTH, intact parathyroid hormone; OS, overall survival; P, phosphorus; PTX, parathyroidectomy; SUA, serum uric acid.
3.5. Comparison of serum iPTH, Ca, and P levels between control group and PTX group
At M0, serum iPTH (P < 0.05; Figure 3A) and Ca (P < 0.05) levels (Figure 3B) were higher in PTX group compared to Control group, while patients in PTX group had reduced iPTH (P < 0.05; Figure 3A) and Ca (P < 0.05) levels (Figure 3B) compared to Control group at M1, M3, M6, M12, M18, M24, M30, and M36. No difference was found in serum P level between the two groups at M0, M1, M3, M6, M12, M18, M24, M30, and M36 (Figure 3C).
Figure 3.
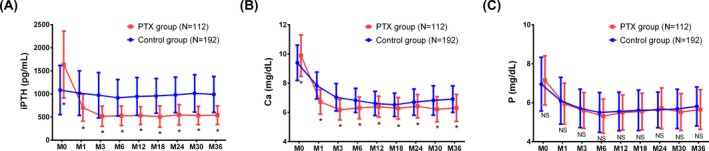
Comparison of patients’ serum levels of iPTH, Ca, and P within 3 y in the Control group and PTX group. A, Patients in PTX group had higher iPTH level at M0 and lower iPTH level compared to the Control group at M1‐M36. B, Patients in PTX group had higher Ca level at M0 and lower Ca level compared to the Control group at M1‐M36. C, No difference was found in serum P at M0‐M36 between the two groups. To compare the difference of iPTH, Ca, and P between the two groups, t test was used. P value < 0.05 was considered statistically significant. Ca, calcium; iPTH, intact parathyroid hormone; P, phosphorus; PTX, parathyroidectomy
4. DISCUSSION
In this prospective study, the prominent results manifested that: (a) patients in PTX group disclosed advanced disease conditions at baseline compared to Control group, (b) patients exhibited favorable OS as well as decreased levels of iPTH and Ca in PTX group compared to Control group, and PTX treatment was an independent factor for prolonged OS in elderly hemodialysis patients with severe SHPT.
SHPT is one of the most serious complications in hemodialysis patients, particularly in the elderly patients, which contributes to high‐turnover bone diseases, cardiovascular diseases, or even death.10, 11 Severe SHPT is defined by high intact PTH (iPTH) level, whereas two different guidelines (the Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines and the Japanese guideline)provide distinct levels of iPTH for defining severe SHPT (the iPTH level persistently exceeding 800 pg/mL or 500 pg/mL). Due to the lack of support of randomized clinical trial data in these two previous guidelines, the cutoff point of iPTH value exceeding 800 pg/mL or 500 pg/mL might be arbitrary for the definition of severe SHPT. According to a previous study that uses iPTH value exceeding 700 pg/mL to be the cutoff point defining severe SHPT, higher levels of iPTH and Ca are observed in PTX‐treated group compared to control group at baseline assessment. Considering the sample size which is relatively small in the previous study of 50 severe SHPT patients, the statistical power might be poor. In the present study, we enrolled 304 elderly hemodialysis patients with severe SHPT (iPTH value exceeding 700 pg/mL) and also discovered that patients in PTX group had elevated levels of iPTH and Ca at baseline assessment. This could be explained by that: CKD patients receiving hemodialysis underwent mineral disorders (hypocalcemia and hyperphosphatemia), subsequently leading to hypersecretion of parathyroid hormone (PTH), which contributed to worse disease conditions, thereby increasing the need for PTX treatment. Thus, patients in PTX group had enhanced disease conditions compared to control group.
In clinical trials, few studies have been performed to investigate the treatment efficacy of PTX in hemodialysis patients with SHPT. An interesting study from the United States Renal Database System (USRDS) discloses that PTX is correlated with lower long‐term mortality rates in SHPT patients receiving chronic dialysis.9 As to severe SHPT, a prospective cohort study indicates that PTX is associated with the decreased mortality rate in severe SHPT patients undergoing maintenance hemodialysis.12 Additionally, large‐scale and observational cohort studies from the Japanese Society for Hemodialysis Therapy Renal Data Registry (JRDR) also displays that PTX may improve survival in hemodialysis patients with severe SHPT.8 Although these above‐mentioned studies have verified the favorable role of PTX for SHPT treatment, its therapeutic efficacy is rarely known in the elderly hemodialysis patients with severe SHPT.8, 9, 12 In the current study, we discovered that PTX was associated with improved OS independently in elderly hemodialysis patients with severe SHPT, besides, we observed the obvious downtrend of iPTH and Ca levels in PTX group at M1‐M36 compared to Control group. The possible explanation might be that the removal of parathyroid by PTX effectively reduced the secretion of iPTH as well as the subsequent Ca and P levels, decreasing severity of SHPT, thereby leading to longer OS in elderly hemodialysis patients with severe SHPT.
There were some limitations in the present study. Firstly, as a cohort study, this study possibly has some confounding factors, such as financial status, compliance of HD and family support, which might influence comparison of mortality between two groups. Secondly, the follow‐up duration (3 years) was relatively short; the long‐term role of PTX in elderly hemodialysis patients with severe SHPT is unclear. Thirdly, factors affecting the quality of life (such as anemia, pruritus, bone pain, and cognition) were not evaluated in this study.
In conclusion, PTX decreases iPTH and Ca levels, and it associates with favorable survival in elderly hemodialysis patients with severe SHPT.
Supporting information
Wan J, Li W, Zhong Y. Parathyroidectomy decreases serum intact parathyroid hormone and calcium levels and prolongs overall survival in elderly hemodialysis patients with severe secondary hyperparathyroidism. J Clin Lab Anal. 2019;33:e22696 10.1002/jcla.22696
J. Wan and W. Li contributed equally to this work.
REFERENCES
- 1. Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet. 2017;389:1238‐1252. [DOI] [PubMed] [Google Scholar]
- 2. Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945‐1953. [DOI] [PubMed] [Google Scholar]
- 3. Reiss E, Canterbury JM, Kanter A. Circulating parathyroid hormone concentration in chronic renal insufficiency. Arch Intern Med. 1969;124:417‐422. [PubMed] [Google Scholar]
- 4. Hedgeman E, Lipworth L, Lowe K, et al. International burden of chronic kidney disease and secondary hyperparathyroidism: a systematic review of the literature and available data. Int J Nephrol. 2015;2015:184321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Block GA, Hulbert‐Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607‐617. [DOI] [PubMed] [Google Scholar]
- 6. Al Aly Z, Gonzalez EA, Martin KJ, et al. Achieving K/DOQI laboratory target values for bone and mineral metabolism: an uphill battle. Am J Nephrol. 2004;24:422‐426. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Parkerson S, Udelsman R. Parathyroidectomy in the elderly: do the benefits outweigh the risks? World J Surg. 1998;22:531‐536. discussion 535‐536. [DOI] [PubMed] [Google Scholar]
- 8. Komaba H, Taniguchi M, Wada A, et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;88:350‐359. [DOI] [PubMed] [Google Scholar]
- 9. Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010‐2016. [DOI] [PubMed] [Google Scholar]
- 10. Amann K, Ritz E, Wiest G, et al. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol. 1994;4:1814‐1819. [DOI] [PubMed] [Google Scholar]
- 11. Rashid G, Bernheim J, Green J, et al. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol. 2007;292:F1215–F1218. [DOI] [PubMed] [Google Scholar]
- 12. Moldovan D, Racasan S, Kacso IM, et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. Int Urol Nephrol. 2015;47:1871‐1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


