Abstract
Background
We aimed to assess the predictive value of long noncoding RNAs antisense noncoding RNA in the INK4 locus (lncRNAs ANRIL) for acute exacerbation of chronic obstructive pulmonary disease (COPD) and evaluate its correlation with inflammatory cytokines as well as the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage in COPD patients.
Methods
A total of 136 acute exacerbations of COPD (AECOPD) patients, 138 stable COPD patients, and 140 healthy controls (HCs) were consecutively recruited, and plasma samples were collected. Real‐time polymerase chain reaction was used to detect lncRNA ANRIL expression. Enzyme‐linked immunosorbent assay was performed to detect inflammatory cytokines expressions.
Results
LncRNA ANRIL expression was lower in AECOPD patients compared with stable COPD patients and HCs (Both P < 0.001). Receiver operating characteristic curves revealed lncRNA ANRIL could distinguish AECOPD patients from HCs (area under curve (AUC):0.700, 95% CI: 0.638‐0.762) and stable COPD patients (AUC: 0.659, 95% CI: 0.594‐0.724). For inflammatory cytokines, lncRNA ANRIL expression was negatively correlated with TNF‐α (P < 0.001), IL‐1β (P = 0.015), IL‐8 (P = 0.008), IL‐17A (P = 0.002), and LTB‐4 (P = 0.004) in AECOPD patients, while it was negatively correlated with TNF‐α (P = 0.049), IL‐1β (P = 0.005), IL‐17A (P = 0.030), and LTB‐4 (P = 0.011) in stable COPD patients. Furthermore, lncRNA ANRIL expression negatively correlated with GOLD stage in AECOPD patients (P = 0.001), but not in stable COPD patients (P = 0.131).
Conclusion
LncRNA ANRIL associates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in COPD patients.
Keywords: chronic obstructive pulmonary disease, disease conditions, inflammatory cytokines, LncRNA ANRIL
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD), one of the common chronic inflammatory diseases, is characterized by airflow limitation with incomplete reversibility, which devotes to abnormal inflammatory responses to noxious particles or gases in the lung.1 Most COPD patients are classified as stable COPD or acute exacerbations COPD (AECOPD) because the chronic and stable course of COPD could be aggravated during short periods due to viral and bacterial infection.2 According to World Health Organization (WHO), COPD is a major contributor to global morbidity and mortality, affecting estimated 251 million population and causing nearly 3 million deaths worldwide each year, and there is a strikingly raised tendency in its incidence especially in developing countries probably due to increased smoking rates and air pollution.3 Even though great improvements have been achieved in early diagnosis and disease management, COPD is still the fourth leading cause of death, and it is predicted to become the third one in 2030, suggesting that COPD is a huge challenge threatening the global health.3 In addition, AECOPD results in significant morbidity and has been reported to be among the major causes of death worldwide.4 Hence, exploring additional and convincing biomarkers are necessary for supervision of disease progress and improvement of treatments outcomes in COPD patients.
Long noncoding RNAs (lncRNAs), as non‐protein‐coding RNA transcripts longer than 200 nt, locate within intergenic stretches or overlapping antisense transcripts of protein‐coding genes, which closely correlate with chromosome modification, transcriptional activation or mRNA degradation to not only participate in normal physiological cellular activities, but also contribute to pathological mechanism, disease progression as well as disease prognosis.5, 6, 7, 8 LncRNA antisense noncoding RNA in the INK4 locus (ANRIL), antisense of the cyclin‐dependent kinase inhibitor 2B (CDKN2B) gene on chromosome 9p21, has been reported to involve into the processes of development and progression of chronic inflammatory diseases and contributes to inflammatory responses via the interaction with several genes or pathways including promoting caspase recruitment domain family member (CARD) 8 to repress nuclear factor kappa (NF‐κB) pathway in ischemic stroke, or decreasing proinflammatory factor TGF‐β1 expression to inducing thyroid cancer cells invasion and metastasis.9, 10, 11, 12, 13, 14 Although lncRNA ANRIL has been reported to be a potential regulator through directly or indirectly mediating inflammatory cytokines in some chronic inflammatory diseases (such as atherosclerosis or periodontitis), little is known about the role of lncRNA ANRIL in COPD.15, 16 Considering that COPD is one of typical inflammatory diseases, particularly in acute exacerbations of COPD that presents with more serious inflammatory responses as well as advanced disease conditions, and lncRNA ANRIL is a potential regulator related to inflammatory disorder, we conducted this study and the purpose of this study was to assess the predictive value of lncRNA ANRIL for acute exacerbations of COPD and to evaluate its correlation with inflammatory cytokines as well as Global initiative for chronic obstructive lung disease (GOLD) stage in COPD patients.
2. METHODS
2.1. Participants
A total of 136 AECOPD patients and 138 stable COPD patients in Shanghai East Hospital between January 2016 and December 2017 were consecutively recruited in the present study.
The inclusion criteria of AECOPD patients were as follows: (a) diagnosed as COPD according to GOLD criteria, (b) presentation of an acute exacerbation with at least two of the major symptoms or one major symptom and one minor symptom for at least two consecutive days. Major symptoms included increased dyspnea, increased sputum purulence, or increased sputum volume. Minor symptoms included nasal discharge/congestion, wheeze, sore throat, or cough, and (c) age ≥40 years. The exclusion criteria were as follows: (a) complicated with asthma, lung cancer, or other relevant lung diseases and (b) history of severe infection, malignant tumors, autoimmune diseases, or malignant hematologic diseases.
The inclusion criteria of stable COPD patients consisted of (a) diagnosed as COPD according to GOLD criteria, (b) without acute exacerbation at least 6 months, and (c) age ≥40 years. The exclusion criteria stable COPD included the following: (a) complicated with asthma, lung cancer, or other relevant lung diseases and (b) history of severe infection, malignant tumors, autoimmune diseases, or malignant hematologic diseases.
After the completion of the COPD patient's recruitment, we reviewed medical examination records of healthy subjects who underwent physical examinations in the Shanghai East Hospital from January 2016 to December 2017. As for eligible health subjects whose age and gender were matched to total COPD patients, they were invited to participate in this study. Finally, 140 healthy volunteers were recruited as healthy controls (HCs), and volunteers who had infection, lung diseases, renal or hepatic dysfunction, and a history of severe infection, malignant tumors, autoimmune, or malignant hematologic diseases were excluded.
2.2. Ethics
The Ethics Committee of Shanghai East Hospital approved the study protocol, and all participants or their family members provided written informed consents before enrollment.
2.3. Date collection
Demographic and clinical data were collected from all participants including age, gender, body mass index (BMI), and lung function test results (forced expiratory volume in one‐second (FEV1), FEV1 (% predicted), FEV1/forced vital capacity (FVC)). Moreover, GOLD stages of AECOPD and stable COPD patients were also evaluated by physicians.
2.4. Sample collection
Peripheral blood samples of AECOPD patients (within 24 hours after admission) and stable COPD patients were collected in tubes containing anticoagulant (ethylenediaminetetraacetic acid, EDTA). Separation of the plasma was performed within 2 hours by centrifugation at 1900 g for 10 minutes, followed by 10‐minutes high‐speed centrifugation at 16 000 g of blood samples. Then, the supernatant sera were obtained and stored at −80°C for further detection. In addition, peripheral blood samples of HCs were obtained from physical examinations, and the plasma was separated correspondingly.
2.5. Real‐time polymerase chain reaction
Total RNA was extracted from plasma using TRIzol Reagent (TaKaRa, Kusatsu, Japan) according to manufacturer's protocol and then converted to cDNA using the Universal cDNA Synthesis kit (Exiqon, Denmark). After that, quantitative RT‐PCR (qRT‐PCR) was carried out using SYBR Premix Ex Taq kit (Takara, China). All reactions were incubated on a 96‐well plate initial heat activation at 95°C for 5 minutes, followed by 40 cycles of amplification, each cycle included denaturation at 95°C for 5 seconds, annealing at 61°C for 15 seconds, and extension at 72°C for 30 seconds. Each sample was assayed in triplicate for the analysis. The relative expression of lncRNA ANRIL was calculated by the 2−△△t method with U6 as the internal reference. The primer of lncRNA ANRIL was forward primer: 5′‐GCCTAACGCACTATGGTATGGA‐3′, reverse primer:5′‐ATCTAAGTCACTGGTCTGAGTTCTT‐3′.
2.6. Enzyme‐linked immunosorbent assay
According to the manufacturer instructions, tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐1β (IL‐1β), IL‐6, IL‐8, IL‐17A, and leukotriene B4 (LTB4) expressions in plasma from AECOPD and stable COPD patients were determined using commercial ELISA kit (R&D, Minneapolis, Minnesota, USA).
2.7. Statistics
Statistical analysis was performed by SPSS 22.0 software (SPSS Inc, NewYork City, NY, USA) and GraphPad Prism 6.0 (GraphPad Software, NewYork City, NY, USA). Data were presented as mean value ± standard deviation, mean value (25th‐75th quantiles), or count (percentage). Comparison among three or above groups was determined by one‐way ANOVA test, chi‐square test or Kruskal‐Wallis H rank sum test. Comparison between two groups was determined by Wilcoxon rank sum test or chi‐square test. Receiver operating characteristic (ROC) curves were carried out to distinguish AECOPD patients and stable COPD patients from HCs, as well as AECOPD patients from stable COPD patients. The correlation was determined by Spearman test. P < 0.05 was considered significant.
3. RESULTS
3.1. Baseline characteristics
As presented in Table 1, no difference was found in age (P = 0.469), gender (P = 0.311), or BMI (P = 0.171) among AECOPD patients, stable COPD patients, and HCs, while FEV1 (P < 0.001), FEV1(%) (P < 0.001), and FEV1/FVC (P < 0.001) was decreased in AECOPD patients and stable COPD patients compared with HCs. For AECOPD patients and stable COPD patients, no difference in GOLD stage (P = 0.970) was observed, whereas inflammatory cytokines levels were all increased in AECOPD patients, including TNF‐α, IL‐1β, IL‐6, IL‐8, IL‐17, and LTB‐4 (All P < 0.001) compared with stable COPD patients.
Table 1.
Characteristics of participants
| Items | AECOPD patients (N = 136) | Stable COPD patients (N = 138) | HCs (N = 140) | P value |
|---|---|---|---|---|
| Age (y) | 67.7 ± 7.9 | 66.9 ± 8.2 | 66.4 ± 10.2 | 0.469 |
| Gender (Male/Female) | 98/38 | 104/34 | 94/46 | 0.311 |
| BMI (kg/m2) | 22.5 ± 2.9 | 22.2 ± 3.1 | 22.9 ± 3.3 | 0.171 |
| FEV1 (L) | 1.01 ± 0.44a | 0.99 ± 0.48 | 1.97 ± 0.39 | <0.001 |
| FEV1 (% predicted) | 45.5 ± 12.9a | 44.3 ± 13.7 | 92.8 ± 6.7 | <0.001 |
| FEV1/FVC (%) | 48.7 ± 10.8a | 47.9 ± 12.4 | 78.1 ± 3.3 | <0.001 |
| GOLD stage (n/%) | ||||
| I | 3 (2.2) | 3 (2.2) | ‐ | 0.970b |
| II | 44 (32.4) | 42 (30.4) | ‐ | |
| III | 80 (58.8) | 85 (61.6) | ||
| IV | 9 (6.6) | 8 (5.8) | ||
| Inflammatory cytokines | ||||
| TNF‐α (pg/mL) | 53.815 (22.606‐87.113) | 15.067 (7.020‐22.967) | ‐ | <0.001 b |
| IL‐1β (pg/mL) | 4.641 (2.990‐6.705) | 2.519 (1.342‐3.707) | ‐ | <0.001 b |
| IL‐6 (pg/mL) | 21.068 (13.586‐27.801) | 9.476 (6.807‐13.001) | ‐ | <0.001 b |
| IL‐8 (pg/mL) | 36.520 (17.147‐48.587) | 21.862 (9.781‐26.981) | ‐ | <0.001 b |
| IL‐17 (pg/mL) | 49.741 (17.190‐71.818) | 11.058 (5.062‐19.578) | ‐ | <0.001 b |
| LTB‐4 (pg/mL) | 49.996 (32.641‐79.249) | 11.669 (8.043‐17.366) | ‐ | <0.001 b |
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; HCs, healthy controls; BMI, body mass index; FEV1, forced expiratory volume in one‐second; FVC, forced vital capacity; GOLD, global initiative for chronic obstructive lung disease; TNF‐α, tumor necrosis factor‐α; IL‐1β, interleukin‐1β; IL‐6, interleukin‐6; IL‐8, interleukin‐8; IL‐17, interleukin‐17; LTB‐4, leukotriene B4.
Data were presented as mean value ± standard deviation, mean value (25th‐75th quantiles) or count (percentage). Comparison among 3 groups was determined by one‐way ANOVA test or chi‐square test. Comparison between 2 groups was determined by Wilcoxon rank sum test or chi‐square test. P < 0.05 was considered significant.
Notice: FEV1, FEV1 predicted, and FEV1/FVC in AECOPD patients were determined postbronchodilator.
Comparison was determined between AECOPD patients and stable COPD patients.
The bold values was considered significant.
3.2. Expression of lncRNA ANRIL in HCs, stable COPD patients, and AECOPD patients
Median values of lncRNA ANRIL expression in HCs, stable COPD patients, and AECOPD patients were 2.024 (0.866‐2.828), 1.718 (0.686‐2.881), and 1.022 (0.474‐1.632), respectively (Figure 1). LncRNA ANRIL expression was lower in AECOPD patients compared with stable COPD patients (P < 0.001) and HCs (P < 0.001), while no difference was observed in lncRNA ANRIL expression between stable COPD patients and HCs (P = 0.214).
Figure 1.
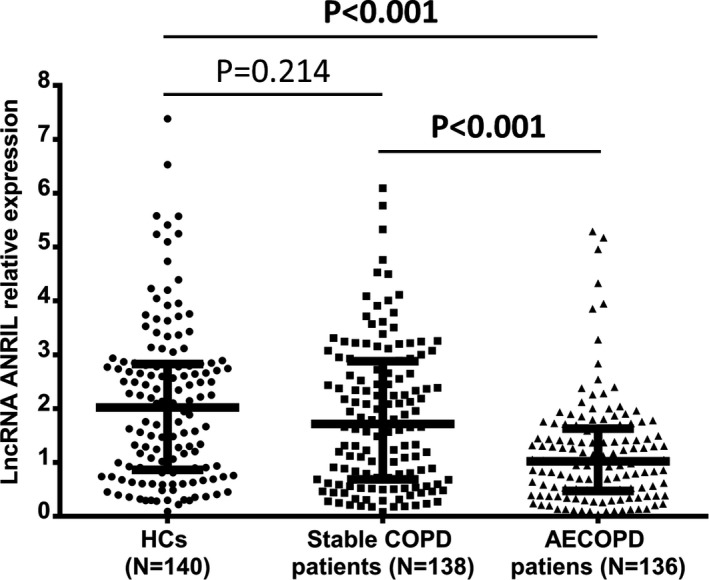
LncRNA ANRIL was lowly expressed in AECOPD patients compared with stable COPD patients and HCs. Downregulated lncRNA ANRIL expression was observed in AECOPD patients compared with stable COPD patients and HCs, while there was no difference in lncRNA ANRIL expression between stable COPD and HCs. The comparison was determined by Wilcoxon rank sum test. P < 0.05 was considered significant. LncRNA ANRIL, long noncoding RNAs antisense noncoding RNA in the INK4 locus; HCs, healthy controls; COPD, chronic obstructive pulmonary disease; AECOPD: acute exacerbations of chronic obstructive pulmonary disease
3.3. Predictive value of lncRNA ANRIL for acute exacerbation of COPD
Receiver operating characteristic curves revealed that lncRNA ANRIL could distinguish total COPD from HCs with area under curve (AUC) of 0.621 (95% CI: 0.564‐0.678); its sensitivity and specificity were 73.4% and 50.0%, respectively, at the best cutoff point where the largest sum of sensitivity and specificity occurred (Figure 2A). In addition, lncRNA ANRIL could not distinguish stable COPD patients from HCs (AUC: 0.543 (95% CI: 0.475‐0.611) with sensitivity and specificity being 69.6% and 40.0%, respectively, at the best cutoff point (Figure 2B), while lncRNA ANRIL could distinguish AECOPD patients from HCs (AUC:0.700, 95% CI: 0.638‐0.762) (Figure 2C) as well as stable COPD patients (AUC: 0.659, 95% CI: 0.594‐0.724) (Figure 2D); the sensitivity and specificity at the best cutoff point were 89.7% and 50.0%, as well as 89.7% and 44.2%, respectively. Thus, these results indicated that lncRNA ANRIL could predict acute exacerbation risk of COPD.
Figure 2.
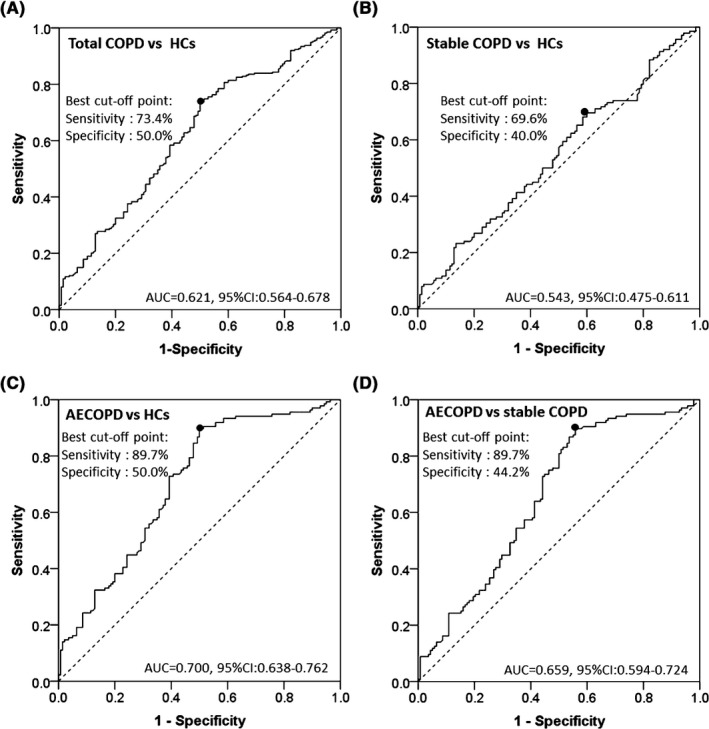
LncRNA ANRIL could distinguish AECOPD patients from stable COPD patients and HCs. LncRNA ANRIL could distinguish total COPD from HCs (A); lncRNA ANRIL could not distinguish stable COPD patients from HCs (B); lncRNA ANRIL could distinguish AECOPD patients from HCs (C); lncRNA ANRIL could distinguish AECOPD patients from stable COPD patients (D). Receiver operating characteristic curves were used to distinguish AECOPD patients and stable COPD patients from HCs, as well as distinguish AECOPD patients from stable COPD patients. LncRNA ANRIL, long noncoding RNAs antisense noncoding RNA in the INK4 locus; HCs, healthy controls; COPD, chronic obstructive pulmonary disease; AECOPD: acute exacerbations of chronic obstructive pulmonary disease
3.4. Correlation of lncRNA ANRIL with inflammatory cytokines in AECOPD patients
For AECOPD patients, lncRNA ANRIL expression was negatively correlated with TNF‐α (r = −0.340, P < 0.001) (Figure 3A), IL‐1β (r = −0.208, P = 0.015) (Figure 3B), IL‐8 (r = −0.225, P = 0.008) (Figure 3D), IL‐17A (r = −0.267, P = 0.002) (Figure 3E), and LTB‐4 (r = −0.246, P = 0.004) (Figure 3F). In addition, lncRNA ANRIL expression was numerically negatively associated with IL‐6 (r = −0.158, P = 0.067) (Figure 3C), but without statistical significance.
Figure 3.
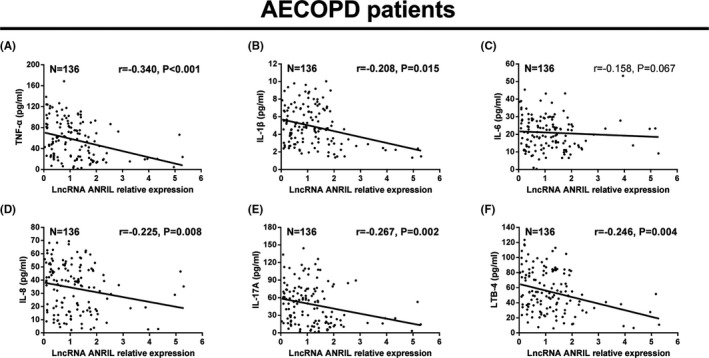
LncRNA ANRIL negatively correlated with inflammatory cytokines in AECOPD patients. LncRNA ANRIL expression was negatively associated with TNF‐α (A), IL‐1β (B), IL‐8 (D), IL‐17A (E), and LTB‐4 (F), while it numerically negatively correlated with IL‐6 (C), but without statistical significance. The correlation was determined by Spearman test. P < 0.05 was considered significant. LncRNA ANRIL, long noncoding RNAs antisense noncoding RNA in the INK4 locus; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; TNF‐α, tumor necrosis factor‐α; IL‐1β, interleukin (IL)‐1β; LTB4, leukotriene B4
3.5. Correlation of lncRNA ANRIL with Inflammatory cytokines in stable COPD patients
For stable COPD patients, lncRNA ANRIL expression was negatively associated with TNF‐α (r = −0.168, P = 0.049) (Figure 4A), IL‐1β (r = −0.237, P = 0.005) (Figure 4B), IL‐17A (r = −0.185, P = 0.030) (Figure 4E), and LTB‐4 (r = −0.215, P = 0.011) (Figure 4F). Moreover, lncRNA ANRIL expression was numerically negatively correlated with IL‐8 (r = −0.146, P = 0.087) (Figure 4D), but without statistical significance, while no correlation was found between lncRNA ANRIL expression and IL‐6 (r = −0.116, P = 0.176) (Figure 4C) in stable COPD patients.
Figure 4.
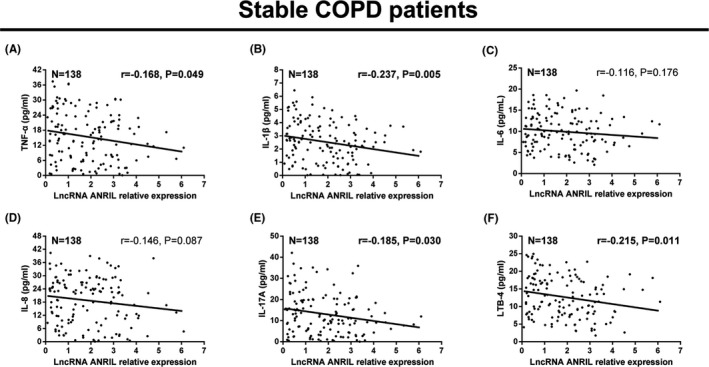
LncRNA ANRIL negatively correlated with inflammatory cytokines in stable COPD patients. Negative correlation was observed of lncRNA ANRIL expression with TNF‐α (A), IL‐1β (B), IL‐17A (E), and LTB‐4 (F), while it was numerically negatively correlated with IL‐8 (D), but without statistical significance. There was no correlation between lncRNA ANRIL expression and IL‐6 (C) in stable COPD patients. The correlation was determined by Spearman test. P < 0.05 was considered significant. LncRNA ANRIL, long noncoding RNAs antisense noncoding RNA in the INK4 locus; COPD, chronic obstructive pulmonary disease; TNF‐α, tumor necrosis factor‐α; IL‐1β, interleukin (IL)‐1β; LTB4, leukotriene B4
3.6. Correlation of lncRNA ANRIL with GOLD stage in AECOPD patients and stable COPD patients
LncRNA ANRIL expression was negatively associated with GOLD stage in AECOPD patients (P = 0.001) (Figure 5A), while there was no correlation of lncRNA ANRIL expression with GOLD stage in stable COPD patients (P = 0.131) (Figure 5B).
Figure 5.
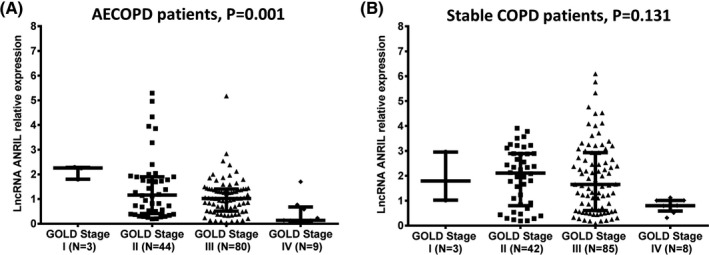
LncRNA ANRIL negatively correlated with GOLD stage in AECOPD patients but not in stable COPD patients. LncRNA ANRIL expression was negatively associated with GOLD stage in AECOPD patients (A), whereas no correlation was found in stable COPD patients (B). The comparison was determined by Kruskal‐Wallis H rank sum test. P < 0.05 was considered significant. LncRNA ANRIL, long noncoding RNAs antisense noncoding RNA in the INK4 locus; GOLD, global initiative for chronic obstructive lung disease; AECOPD: acute exacerbations of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease
4. DISCUSSION
In the current study, we observed that (a) lncRNA ANRIL expression was lower in AECOPD patients compared with stable COPD patients as well as HCs, and it could distinguish AECOPD patients from stable COPD patients and HCs, suggesting that it could predict acute exacerbation risk of COPD. (b) LncRNA ANRIL expression was negatively correlated with proinflammatory cytokines in AECOPD patients as well as stable COPD patients, and it was negatively associated with GOLD stage in AECOPD patients, but not in stable COPD patients.
Chronic obstructive pulmonary disease is a progressive inflammatory disease of airways, alveoli as well as microvasculature, and is featured by mucosal and glandular inflammation, epithelial cell hyperplasia as well as altered tissue repair in small conducting airways.17 LncRNAs, a large class of non‐protein‐coding transcripts, are considered as critical regulators in physiological and pathological processes of chronic diseases, particularly in inflammatory diseases. For instance, lncRNA taurine‐upregulated gene 1 (TUG1) inhibits miR‐29b and suppresses nuclear factor kappa (NF‐κB), Janus kinase‐signal transducer as well as activator of transcription (JAK‐STAT) pathways to reduce concentrations of proapoptotic factors and inflammatory cytokines, thereby repressing inflammatory responses in lipopolysaccharide (LPS)‐injured H9c2 cells.18 Also, lncRNA Mirt2, interacting with tumor necrosis factor (TNF) receptor‐associated factor (TRAF6), decreases activation of NF‐kB signaling and mitogen‐activated protein kinase (MAPK) pathway, thereby inhibiting inflammatory responses in primary cultured peritoneal macrophages obtained from C57BL/6 mice.19 Furthermore, lncRNA TUG1 has been reported to be involved in the processes of COPD onset and progression.20, 21 Therefore, these previous researches indicate that lncRNAs serve as potential regulators in inflammatory responses of chronic inflammatory diseases, including COPD.
LncRNA ANRIL is one of the well‐known functional lncRNAs, which is related to the development and prognosis of chronic inflammatory diseases. According to previous experiments, lncRNA ANRIL could induce caspase recruitment domain family member (CARD) 8 to repress NF‐κB pathway, thereby inhibiting inflammatory responses in ischemic stroke.11, 12 In addition, lncRNA ANRIL could promote thyroid cancer cells invasion and metastasis through downregulating TGF‐β1 expression (one of the proinflammatory factors).13, 14 These previous data indicate that lncRNA ANRIL might inhibit the progression of inflammatory diseases. In clinical trials, lncRNA ANRIL has been reported to be downregulated in some inflammatory diseases, including Crohn's disease (CD) and ulcerative colitis (UC), while few studies have been carried out about the expression of lncRNA ANRIL in COPD patients and its diagnostic value for AECOPD.22, 23 In the present study, we observed that lncRNA ANRIL was lowly expressed in AECOPD patients, and it could distinguish AECOPD patients from stable COPD patients and HCs. This might be due to that lncRNA ANRIL regulated genes or pathways (including suppressed NF‐kB pathway or downregulated TGF‐β1) to decrease inflammatory responses, which contributed to lower risk of acute exacerbation in COPD patients.
The correlation of lncRNA ANRIL with disease severity in chronic inflammatory diseases or respiratory system diseases is rarely known, and limited study discloses that lncRNA ANRIL overexpression correlates with higher TNM stage and advanced lymph node metastasis in lung cancer.24 Although little is known about the role of lncRNA ANRIL in COPD, lncRNA ANRIL is considered as an inflammatory suppresser, which might downregulate inflammatory cytokines to decrease inflammatory responses in COPD. Therefore, we assessed the relationship of lncRNA ANRIL with inflammatory cytokines in COPD patients and found that lncRNA ANRIL expression was negatively associated with inflammatory cytokines levels (including TNF‐α, IL‐1β, IL‐17A, and LTB‐4) in AECOPD patients and stable COPD patients. The possible reason was that lncRNA ANRIL interacted with some genes or signaling pathways (such as NF‐kB signaling pathway or TGF‐β1) to downregulate levels of inflammatory cytokines, thereby decreasing inflammatory responses in COPD patients.
Considering that GOLD stage acts as one of the standard indexes directly reflecting disease conditions of COPD, we further evaluated the association of lncRNA ANRIL with GOLD stage in COPD patients and observed that lncRNA ANRIL negatively correlated with GOLD stage in AECOPD patients but not in stable COPD patients.2 The possible explanation was that lncRNA ANRIL could decrease levels of inflammatory cytokines through downregulating multiple pathways or genes including NF‐kB or TGF‐β1, thereby repressing inflammatory responses, improving disease severity, and reducing GOLD stage in COPD patients. In addition, compared to stable COPD patients, AECOPD patients present with more serious inflammatory responses and advanced disease conditions; thus, the closer correlation of lncRNA ANRIL with GOLD stage might be observed in AECOPD patients. However, the detailed mechanism of lncRNA ANRIL on COPD is still unclear; thus, further study is greatly needed.
Even though some interesting results were found in this study, there were still some limitations. Firstly, the sample size was relatively small in this study (a total of 136 AECOPD patients and 138 stable COPD patients); this might result in poor statistic power. Secondly, the underlying mechanism of lncRNA ANRIL in COPD was not investigated. Thirdly, all the patients were recruited from our hospital. Therefore, further study with more COPD patients from multicenter is greatly needed.
In conclusion, lncRNA ANRIL associates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in COPD patients.
Ge J, Geng S, Jiang H. Long noncoding RNAs antisense noncoding RNA in the INK4 locus (ANRIL) correlates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in patients with chronic obstructive pulmonary disease. J Clin Lab Anal. 2019;33:e22678 10.1002/jcla.22678
REFERENCES
- 1. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347‐365. [DOI] [PubMed] [Google Scholar]
- 3. Chronic obstructive pulmonary disease(COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed Apr 20, 2018.
- 4. Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management of chronic obstructive pulmonary disease. 2006; http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed Oct 20, 2011.
- 5. Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non‐coding RNAs in mammals. Nature. 2009;458(7235):223‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagano T, Fraser P. No‐nonsense functions for long noncoding RNAs. Cell. 2011;145(2):178‐181. [DOI] [PubMed] [Google Scholar]
- 8. Sun M, Kraus WL. From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev. 2015;36(1):25‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song CL, Wang JP, Xue X, et al. Effect of Circular ANRIL on the Inflammatory Response of Vascular Endothelial Cells in a Rat Model of Coronary Atherosclerosis. Cell Physiol Biochem. 2017;42:1202‐1212. [DOI] [PubMed] [Google Scholar]
- 10. Bochenek G, Hasler R, El Mokhtari NE, et al. The large non‐coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 2013;22(22):4516‐4527. [DOI] [PubMed] [Google Scholar]
- 11. Bao MH, Szeto V, Yang BB, et al. Long non‐coding RNAs in ischemic stroke. Cell Death Dis. 2018;9(3):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai Y, Nie S, Jiang G, et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p. C10X) with ischemic stroke. Stroke. 2014;45(2):383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao JJ, Hao S, Wang LL, et al. Long non‐coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF‐beta/Smad signaling pathway. Oncotarget. 2016;7(36):57903‐57918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Rustenhoven J, Aalderink M, Scotter EL, et al. TGF‐beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaefer AS, Bochenek G, Jochens A, et al. Genetic evidence for PLASMINOGEN as a shared genetic risk factor of coronary artery disease and periodontitis. Circ Cardiovasc Genet. 2015;8(1):159‐167. [DOI] [PubMed] [Google Scholar]
- 16. Holdt LM, Beutner F, Scholz M, et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30(3):620‐627. [DOI] [PubMed] [Google Scholar]
- 17. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931‐1940. [DOI] [PubMed] [Google Scholar]
- 18. Zhang H, Li H, Ge A, et al. Long non‐coding RNA TUG1 inhibits apoptosis and inflammatory response in LPS‐treated H9c2 cells by down‐regulation of miR‐29b. Biomed Pharmacother. 2018;101:663‐669. [DOI] [PubMed] [Google Scholar]
- 19. Du M, Yuan L, Tan X, et al. The LPS‐inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun. 2017;8(1):2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bi H, Zhou J, Wu D, et al. Microarray analysis of long non‐coding RNAs in COPD lung tissue. Inflamm Res. 2015;64(2):119‐126. [DOI] [PubMed] [Google Scholar]
- 21. Tang W, Shen Z, Guo J, et al. Screening of long non‐coding RNA and TUG1 inhibits proliferation with TGF‐beta induction in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2951‐2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zacharopoulou E, Gazouli M, Tzouvala M, et al. The contribution of long non‐coding RNAs in Inflammatory Bowel Diseases. Dig Liver Dis. 2017;49(10):1067‐1072. [DOI] [PubMed] [Google Scholar]
- 23. Mirza AH, Berthelsen CH, Seemann SE, et al. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome Med. 2015;7(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin L, Gu ZT, Chen WH, et al. Increased expression of the long non‐coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]


