Abstract
Background
Small sample volumes may artificially elevate plasma osmolality (Posm) measured by freezing point depression. The purpose of this study was to compare two widely different sample volumes of measured Posm (mmol/kg) to each other, and to calculated osmolarity (mmol/L), across a physiological Posm range (~50 mmol/kg).
Methods
Posm was measured using freezing point depression and osmolarity calculated from measures of sodium, glucose, and blood urea nitrogen. The influence of sample volume was investigated by comparing 20 and 250 μL Posm samples (n = 126 pairs). Thirty‐two volunteers were tested multiple times while EUH (n = 115) or DEH (n = 11) by −4.0% body mass. Protinol™ (240, 280, and 320 mmol/kg) and Clinitrol™ (290 mmol/kg) reference solutions were compared similarly (n = 282 pairs).
Results
The 20 μL samples of plasma showed a 7 mmol/kg positive bias compared to 250 μL samples and displayed a nearly constant proportional error across the range tested (slope = 0.929). Calculated osmolarity was lower than 20 μL Posm by the same negative bias (−6.9 mmol/kg) but not different from 250 μL Posm (0.1 mmol/kg). The differences between 20 and 250 μL samples of Protinol™ were significantly higher than Clinitrol™.
Conclusions
These results demonstrate that Posm measured by freezing point depression will be ~7 mmol/kg higher when using 20 μL vs 250 μL sample volumes. Approximately half of this effect may be due to plasma proteins. Posm sample volume should be carefully considered when calculating the osmole gap or assessing hydration status.
Keywords: calculated osmolarity, freezing point depression, measured osmolality, osmole gap
1. INTRODUCTION
The measurement of plasma osmolality (Posm) is used in clinical settings to assess body fluid balance disturbance.1, 2, 3, 4, 5, 6, 7, 8 Moreover, Posm is used for the calculation of the osmole gap (measured − calculated) to help elucidate the presence of undiagnosed substances or metabolic disorders within patient populations.9, 10 In the field of sports medicine, Posm is routinely used for hydration assessment and has demonstrated superiority over other measures when specifically assessing exercise‐induced dehydration (ie, hypertonic‐hypovolemia).11, 12 Important details regarding Posm measurement techniques have been reported,13, 14, 15 and specifically, investigations have observed that timing of fluid,16 method of blood draw,17 and processing of blood (ie, whole blood vs plasma)18 can alter the values obtained and consequently the diagnostic interpretation.19
Previous work from our laboratory demonstrated that sample volume may impact Posm values.18 Our previous work and other investigations examining the impact of sample volume on Posm are not without limitations, as studies were comprised of limited data sets and/or narrow Posm ranges.17, 20 Using freezing point depression osmometry, Posm sample volumes range in the literature from 10 to 250 µL.17, 18 Posm measurement via micro‐osmometers has improved the use of osmolality measures in situations where limited biological sample volumes can be obtained.21, 22
To our knowledge, no prior investigations have systematically examined the independent impact of sample volume, through a significant sample volume and Posm range. Given the widespread use of Posm in research and clinical settings,23 the potential impact of sample volume on Posm measurement could be an important consideration. Small changes in Posm values can lead to differing diagnoses in both clinical and sports medicine settings.19, 24, 25, 26 Thus, knowledge of how sample volume may impact Posm interpretations is clearly warranted. Therefore, the purpose of the investigation was to determine whether sample volume independently impacts Posm values across a range representing euhydrated and dehydrated values.19 Furthermore, we sought to examine the role of biological fluid complexity in explaining our observations. Our hypothesis, based on previous work,18 was that osmometry using small sample volumes would result in meaningfully higher Posm measurements (>4 mmol/kg)19 and that sample fluid complexity would help explain the phenomenon.
2. MATERIALS AND METHODS
Thirty‐two physically active soldier and civilian volunteers took part in this study (25 males, 7 females; mean ± SD age, 24 ± 8 years; body mass (BM), 81.85 ± 17.66 kg; height, 177 ± 9 cm). All volunteers had passed the Army Physical Fitness Test (or equivalent fitness) within the previous 6 months and received a general medical clearance prior to participation; thus, all volunteers were considered physically fit and healthy. The use of alcohol, dietary supplements, and any medication other than an oral contraceptive was prohibited. Volunteers were provided informational briefings and gave voluntary, informed written consent to participate. Investigators adhered to AR 70‐25 and US Army Medical Research and Materiel Command Regulation 70‐25 on the use of volunteers in research. The US Army Research Institute of Environmental Medicine Human Use Review Committee approved this study.
Volunteer participation ranged between 1 and 8 days of experimental testing (1 day, n = 2; 3 days, n = 4; 4 days, n = 24; 8 days, n = 2) and provided a total of 126 independent blood samples for analysis. Volunteers arrived to the laboratory at 06:30 after ≥8 hour overnight fast and having consumed ≥3 L of water in the previous 24 hours. All volunteers were considered normally hydrated (euhydrated). After 20 minutes of controlled posture (sitting) in the laboratory, a venous blood sample was drawn without stasis from an antecubital vein. A subset of 11 volunteers underwent ≤3 hours of exercise‐heat stress with fluid restriction19 until they lost ~3.0 L of body water, which resulted in dehydration by 3.9 ± 0.6% of body mass. A second blood sample was then taken as described above using controlled posture, etc. The goal was to increase Posm by ~10 mmol/kg.26
Blood samples were collected into 2.7 mL lithium‐heparin tubes (Sarstedt Monovette®, Newton, NC). Samples were centrifuged at 1500 × g for 15 minutes at 5°C to acquire plasma and immediately transferred to sample cuvettes for osmolality determination. Note, plasma samples were vortexed prior to analysis. Using the same subjects’ plasma, osmometry was performed on two separate freezing point depression devices allowing for direct comparison of sample volume. A 20 µL aliquot sample of plasma was performed in triplicate via freezing point depression using a micro‐osmometer (Fiske® Micro‐osmometer, Model 210; Norwood, MA). Concurrently, samples were measured in triplicate on a larger 250 µL single sample osmometer (Advanced® Model 3250; Norwood, MA). When the triplicate intrasample measures differed by ≤3 mmol/kg (~1%), the median value was used. If the triplicate intrasample measures differed by >3 mmol/kg, two additional samples were measured and the median value was used.14 Both osmometers were calibrated before any plasma samples were run using ClinitrolTM 290 and Protinol™ 240, 280, and 320 reference solutions (Advanced Instruments, Norwood, MA).
Plasma samples (150 µL) were also analyzed for sodium and blood urea nitrogen by direct ion selective electrode (ISE), and glucose by enzymatic determination, all being done using a Stat Profile Critical Care Xpress (Nova Biomedical, Waltham, MA). In an effort to minimize user complexity wherever possible, plasma osmolarity was calculated from the simplest of the most accurate equations available: sodium, glucose, and blood urea nitrogen (BUN) (2× sodium [mmol/L] + glucose [mmol/L] + BUN [mmol/L]).27, 28
Sample volume comparisons were made using ordinary least squares regression (OLS), whereby proportional (slope), constant (y‐intercept), and random (SEE) errors were assessed in accordance with Westgard and Hunt.29 Bias and mean absolute error were also compared using conventional calculations. Lin’s concordance correlation coefficient30 was also computed by hand to uniquely examine agreement between sample volumes with respect to the line of identity (concordance line, perfect agreement), rather than the line of best fit (OLS). The difference between sample volumes using reference standards was compared using analysis of variance and Tukey’s post hoc procedure with a conventional P < 0.05 threshold. Differences of magnitude >4 mmol/kg were considered of practical importance, a priori.18, 19 Smaller differences that were statistically significant were considered for additional explanatory power of the hypotheses tested. All statistical analyses were completed with the use of computerized statistical software packages (GraphPad Prism® version 7 for Windows; Microsoft Excel, Microsoft Corporation, Redmond, WA).
3. RESULTS
A total of 126 paired plasma samples were compared. Figure 1A compares 20 and 250 µL samples. The 20 μL samples of plasma showed a 7 mmol/kg positive bias compared to 250 μL samples and displayed a nearly constant proportional error across the range tested (slope = 0.929). Bias and the MAE were nearly identical because errors were uniformly positive. Calculated osmolarity, when compared to 20 μL Posm, displayed negative bias (−6.9 mmol/kg; Figure 1B; Table 1) that was also uniform (MAE = 6.9 mmol/kg). However, calculated values were not different from 250 μL Posm (0.1 mmol/kg; Figure 1C; Table 1), and the MAE was also small (1.9 mmol/kg). The random error about the best fit line was small in all comparisons (2.4‐2.7 mmol/kg), but the concordance correlation coefficient (Table 1) showed substantial agreement31 with respect to the line of identity for the comparison in Figure 1C only.
Figure 1.
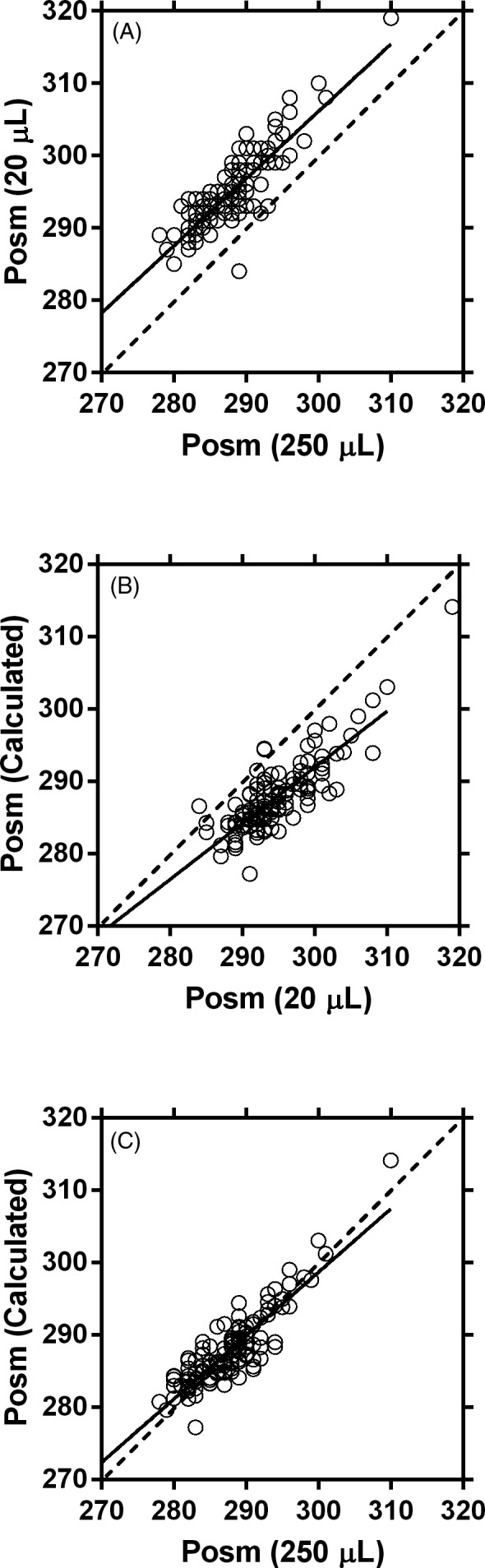
Relationship between plasma osmolality (Posm; mmol/kg) samples when using a 250 μL sample (X‐axis) and a 20 μL sample (Y‐axis) (A), 20 μL sample (X‐axis) and the calculated plasma osmolarity (mmol/L; Y‐axis) (B), and 250 μL sample (X‐axis) and the calculated plasma osmolarity (mmol/L; Y‐axis) (C). The dashed line represents the line of identity where Y = X. The solid line represents the line of best fit
Table 1.
Linear regression parameters and statistical results
| y | x | m | b | Sy | Bias | MAE | CCC |
|---|---|---|---|---|---|---|---|
| Posm 20 µL | Posm 200 µL | 0.929 | 27.29 | 2.74 | −7.0 | 7.1 | 0.439 |
| Posm calc | Posm 20 µL | 0.775 | 59.49 | 2.72 | 6.9 | 6.9 | 0.436 |
| Posm calc | Posm 200 µL | 0.877 | 35.57 | 2.42 | 0.1 | 1.9 | 0.876 |
n = 126 paired comparisons: m = slope, b = y‐intercept, Sy = standard error of the estimate, bias = difference (x – y), MAE = mean absolute error, CCC = concordance correlation coefficient.
A total of 282 pairs of standard reference solution comparisons were made. The differences between 20 and 250 μL samples of Protinol™ (240, 280, and 320 mmol) were significantly higher than Clinitrol™ 290 (Figure 2). In addition, Protinol™ 320 was significantly higher than Protinol™ 240. Mean differences for all Protinol™ standards were higher than the typical analytical variation for Clinitrol™ standards. While the mean difference fell below the typical day‐to‐day biological variation for Posm,19 the trend was for differences to progressively increase as the complexity of dissolved substances increased (Figure 2). For example, 59% of the Clinitrol™ samples showed a positive bias when examining 20‐250 µL volumes. For Protinol™, positive bias was 88% (240), 90% (280), and 96% (320).
Figure 2.
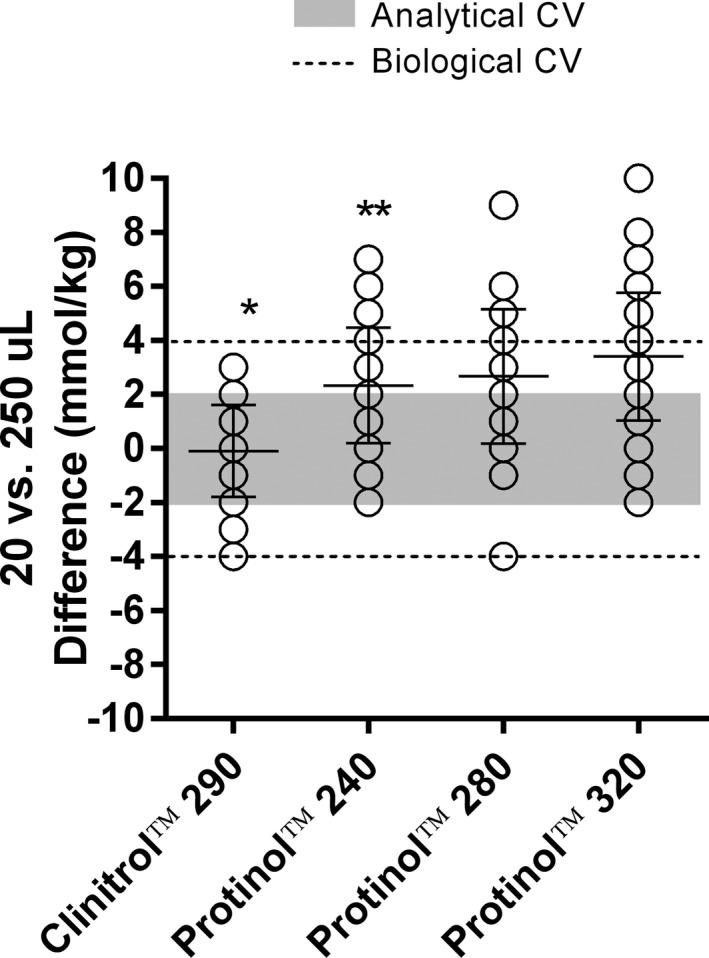
Differences in osmolality (mmol/kg) between 20 and 250 µL standards of Clinitrol™ 290 (n = 138 pairs) and Protinol™ 240 (n = 48 pairs), 280 (42 pairs), and 320 (54 pairs). Shaded region represents the typical analytical variation; dashed lines represent the typical day‐to‐day biological variation.19 Lines with error bars represent mean ± SD. Identical differences appear as single overlapping values within a column. *Significantly different from all Protinol™ trials (P < 0.05); **significantly different from Protinol™ 320
4. DISCUSSION
The primary purpose of this study was to examine the potential impact of sample volume on Posm measures. Furthermore, we sought to examine whether sample volume would influence calculated osmolarity and the corresponding osmole gap assessment. Our primary conclusion is that 20 μL Posm measures are both significant and importantly different from 250 μL Posm measures. Approximately half of this effect may be due to plasma proteins. Furthermore, our results suggest that large sample volumes (ie, 250 μL) improve Posm measures as reflected by the proximity to calculated Posm values (ie, reduced osmole gap).
In our previous investigation, we conducted preliminary work to assess whether sample volume (20 μL vs 250 μL) independently impacts the Posm values obtained.18 In a limited sample size (n = 10), those results demonstrated that the difference in Posm between sample volumes was 4 ± 2 mmol/kg (P < 0.01; 20 μL samples being higher), but differences ≤4 mmol/kg were considered marginal.19 Other investigations have similarly compared the impact of sample volume, but have also been limited in sample size, comparing a single Posm measure in 30 emergency department patients using 100 μL vs 200 μL (2‐fold) sample volumes20 or examining the difference between 10 μL vs 50 μL (5‐fold) sample volumes in 24 euhydrated subjects.17
The current investigation sought to further expand our previous results by investigating a larger number of observations (>100 measurements) through an increased range of Posm values tested (ie, euhydrated through dehydrated values) and using two common sample volumes that differ in range by more than 10‐fold. The results of the present investigation unequivocally demonstrate that the sample volume size, 20 μL volumes compared to 250 μL, independently produces a significant and meaningful ~7 mmol/kg higher value.
The sports medicine field uses Posm to assess hydration status with a commonly cited dehydration threshold value of 290 mmol/kg.11, 25 The results of our current investigation demonstrate that sample volume can dramatically alter hydration status classification. For example, a value of 290 mmol/kg (euhydrated) when obtained using a 250 μL volume would concurrently read 297 mmol/kg (dehydrated) from a 20 μL. Therefore, if investigators are using 20 μL samples, our results suggest that Posm values would have to be <283 mmol/kg to be considered euhydrated. Importantly, if dehydration is being monitored over time (ie, dynamic state measurement) while using reference change values (RCVs),26, 32 smaller sample volumes would still be appropriate since our results demonstrate that the error is constant and linear from the euhydrated through dehydrated Posm range.
In clinical settings, Posm measurements are used for the calculation of the osmole gap.33 This assessment allows clinicians the ability to detect various toxic substance ingestion or abnormal metabolic states within a patient.28, 34 Typically, a gap of >10 mmol warrants concern.9, 10 Previously, we determined that only 5 of 36 commonly used equations, when tested on a healthy population, are most accurate due to the small calculated osmole gap (range: 0.7‐4.5 mmol).27 Our current results demonstrate that the 20 μL sample volume results in a osmole gap of ~7 mmol/kg compared to the 250 μL osmole gap of <1 mmol/kg when using one of the most accurate (closest to measured) equations.28 Thus, if a user does not choose an accurate equation and measures osmolality using a small sample volume, the potential for compounded measurement and calculation errors could result in a significantly inflated osmole gap (>10 mmol), potentially altering a clinical diagnosis. Our results demonstrate that sample volume is an important consideration when assessing the osmole gap, with larger volumes (ie, 250 μL) being more advantageous. Importantly, when replicates of larger sample volumes are desired (eg, research), planned blood volume sampling requirements must be adjusted accordingly.
We observed no significant osmolality differences between 20 and 250 μL samples of Clinitrol. However, 20 μL Protinol samples were 2‐4 mmol/kg higher than 250 μL samples and contributed proportionally more with increasing protein content (Figure 2). This suggests a role for plasma proteins in contributing to approximately half the ~7 mmol/kg differences observed between 20 and 250 μL samples of plasma (Figure 1A). Blood plasma is a complex biological fluid containing various osmotically active components.35 When the components of plasma are not taken into account when measuring osmolality, results can be easily confounded.36 Previously, we demonstrated that whole blood osmolality was significantly different from plasma osmolality when using small sample volumes.18 Specifically, when large sample volumes were used (250 μL vs 20 μL), the differences between whole blood and plasma were dramatically reduced leading us to hypothesize that a volume‐dependent physical phenomenon was taking place. Our current work further validates this hypothesis. Furthermore, we used incrementally different calibration standards of dissimilar compositions to demonstrate that solutions with higher concentrations and increased complexity (ie, the presence of proteins) produce larger discrepancies between small and large sample volumes (see Figure 2), thus validating our presumption that when using freezing point osmometry, small sample volumes are influenced more when complex fluids—especially those with proteins—are analyzed. It is important to note that these conclusions are valid for the freezing point depression methodology only and may not directly apply to vapor pressure osmometry. For example, vapor pressure osmometry may not be affected by sample viscosity or suspended particles such as freezing point depression osmometry. However, vapor pressure osmometry is impacted by the presence of volatile compounds in the sample (eg, alcohols), which are of interest when assessing the osmole gap; thus, freezing point depression osmometry was used in this study because it is a historically preferred clinical methodology.36
5. CONCLUSION
In conclusion, this investigation provides strong evidence that when Posm is measured using freezing point depression osmometry, larger sample volumes will enhance the accuracy of the measurement; however, the ultimate use of the Posm measure will dictate the sample volume needed. Our results demonstrate that whether clinicians or researchers are using Posm to assess static hydration state, larger volumes would be recommended. If a dynamic dehydration assessment is desired, and RCVs are utilized, sample volume has little impact. However, in clinical situations where the osmole gap is of concern, larger sample volumes should be utilized since small sample volumes consistently and meaningfully create artificially higher osmole gaps. Clinicians and researchers alike should carefully consider sample volume when making Posm measures since sample volume can independently impact the outcome measure.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Jeffery S. Staab, Irene Potter, Adam J. Luippold, Katherine M. Mitchell, Karleigh E. Bradbury, Alex M. Posch, and Myra Jones for their expert assistance. The views, opinions, and/or findings contained in the chapter are those of the authors and should not be construed as an official Department of the Army position, or decision, unless so designated by other official documentation. Approved for public release; distribution unlimited.
Sollanek KJ, Kenefick RW, Cheuvront SN. Importance of sample volume to the measurement and interpretation of plasma osmolality. J Clin Lab Anal. 2019;33:e22727 10.1002/jcla.22727
Funding information
This work was supported by the United States Army Medical Research and Materiel Command (USAMRMC).
REFERENCES
- 1. Boyd DR, Baker RJ. Osmometry: a new bedside laboratory aid for the management of surgical patients. Surg Clin North Am. 1971;51(1):241‐250. [DOI] [PubMed] [Google Scholar]
- 2. Cheuvront SN, Kenefick RW, Sollanek KJ, Ely BR, Sawka MN. Water‐deficit equation: systematic analysis and improvement. Am J Clin Nutr. 2013;97(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 3. Davis JA, Harvey DR, Stevens JF. Osmolality as a measure of dehydration in the neonatal period. Arch Dis Child. 1966;41(218):448‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Current G. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310(2):102‐105. [DOI] [PubMed] [Google Scholar]
- 5. Haraway AW Jr, Becker EL. Clinical application of cryoscopy. JAMA. 1968;205(7):506‐512. [PubMed] [Google Scholar]
- 6. Holtfreter B, Bandt C, Kuhn SO, et al. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol Scand. 2006;50(8):970‐977. [DOI] [PubMed] [Google Scholar]
- 7. Langhoff E, Ladefoged J. Sodium activity, sodium concentration, and osmolality in plasma in acute and chronic renal failure. Clin Chem. 1985;31(11):1811‐1814. [PubMed] [Google Scholar]
- 8. Mattar JA, Weil MH, Shubin H, Stein L. Cardiac arrest in the critically ill. II. Hyperosmolal states following cardiac arrest. Am J Med. 1974;56(2):162‐168. [DOI] [PubMed] [Google Scholar]
- 9. Kruse JA, Cadnapaphornchai P. The serum osmole gap. J Crit Care. 1994;9(3):185‐197. [DOI] [PubMed] [Google Scholar]
- 10. Erstad BL. Osmolality and osmolarity: narrowing the terminology gap. Pharmacotherapy. 2003;23(9):1085‐1086. [DOI] [PubMed] [Google Scholar]
- 11. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377‐390. [DOI] [PubMed] [Google Scholar]
- 12. Cheuvront SN, Kenefick RW, Charkoudian N, Sawka MN. Physiologic basis for understanding quantitative dehydration assessment. Am J Clin Nutr. 2013;97(3):455‐462. [DOI] [PubMed] [Google Scholar]
- 13. Seifarth CC, Miertschischk J, Hahn EG, Hensen J. Measurement of serum and plasma osmolality in healthy young humans–influence of time and storage conditions. Clin Chem Lab Med. 2004;42(8):927‐932. [DOI] [PubMed] [Google Scholar]
- 14. Bohnen N, Terwel D, Markerink M, Ten Haaf JA, Jolles J. Pitfalls in the measurement of plasma osmolality pertinent to research in vasopressin and water metabolism. Clin Chem. 1992;38(11):2278‐2280. [PubMed] [Google Scholar]
- 15. Redetzki HM, Hughes JR, Redetzki JE. Differences between serum and plasma osmolalities and their relationship to lactic acid values. Proc Soc Exp Biol Med. 1972;139(1):315‐318. [DOI] [PubMed] [Google Scholar]
- 16. Sollanek KJ, Kenefick RW, Cheuvront SN, Axtell RS. Potential impact of a 500‐mL water bolus and body mass on plasma osmolality dilution. Eur J Appl Physiol. 2011;111(9):1999‐2004. [DOI] [PubMed] [Google Scholar]
- 17. Wittbrodt MT, Espinoza S, Millard‐Stafford ML. Biological variation of plasma osmolality obtained with capillary versus venous blood. Clin Chem Lab Med. 2015;53(10):1613‐1619. [DOI] [PubMed] [Google Scholar]
- 18. Cheuvront SN, Kenefick RW, Heavens KR, Spitz MG. A comparison of whole blood and plasma osmolality and osmolarity. J Clin Lab Anal. 2014;28(5):368‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr. 2010;92(3):565‐573. [DOI] [PubMed] [Google Scholar]
- 20. Er TK. Effect of sample volume for the measurement of osmolality by using the Advanced 3250 osmometer. Am J Emerg Med. 2008;26(9):1060‐1061. [DOI] [PubMed] [Google Scholar]
- 21. Assadi FK. Validity of whole blood osmolality measurement in sick neonates. Pediatr Nephrol. 1988;2(1):27‐28. [DOI] [PubMed] [Google Scholar]
- 22. Yang KC, Su BH, Tsai FJ, Peng CT. The comparison between capillary blood sampling and arterial blood sampling in an NICU. Acta Paediatr Taiwan. 2002;43(3):124‐126. [PubMed] [Google Scholar]
- 23. Stookey JD. High prevalence of plasma hypertonicity among community‐dwelling older adults: results from NHANES III. J Am Diet Assoc. 2005;105(8):1231‐1239. [DOI] [PubMed] [Google Scholar]
- 24. Fischbach FT. A Manual of Laboratory and Diagnostic Tests, 4th edn Philadelphia, PA: J. B. Lippincott Company; 1992. [Google Scholar]
- 25. IOM . Dietary Reference Intakes for Water, Potassium, Sodium Chloride, and Sulfate. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 26. Cheuvront SN, Fraser CG, Kenefick RW, Ely BR, Sawka MN. Reference change values for monitoring dehydration. Clin Chem Lab Med. 2011;49(6):1033‐1037. [DOI] [PubMed] [Google Scholar]
- 27. Heavens KR, Kenefick RW, Caruso EM, Spitz MG, Cheuvront SN. Validation of equations used to predict plasma osmolality in a healthy adult cohort. Am J Clin Nutr. 2014;100(5):1252‐1256. [DOI] [PubMed] [Google Scholar]
- 28. Mahon WA, Holland J, Urowitz MB. Hyperosmolar, non‐ketotic diabetic coma. Can Med Assoc J. 1968;99(22):1090‐1092. [PMC free article] [PubMed] [Google Scholar]
- 29. Westgard JO, Hunt MR. Use and interpretation of common statistical tests in method‐comparison studies. Clin Chem. 1973;19(1):49‐57. [PubMed] [Google Scholar]
- 30. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255‐268. [PubMed] [Google Scholar]
- 31. McBride GB.A proposal for strength‐of‐agreement criteria for lin's concordance correlation coefficient. NIWA Client Report (Ham2005‐062). Hamilton, New Zealand: National Institute of Water & Atmospheric Research Ltd; 2005.
- 32. Fraser CG. Biological Variation: from Principles to Practice. Washington, DC: American Association of Clinical Chemistry Press; 2001. [Google Scholar]
- 33. Khajuria A, Krahn J. Osmolality revisited–deriving and validating the best formula for calculated osmolality. Clin Biochem. 2005;38(6):514‐519. [DOI] [PubMed] [Google Scholar]
- 34. Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol. 1993;31(1):81‐93. [DOI] [PubMed] [Google Scholar]
- 35. Rasouli M. Basic concepts and practical equations on osmolality: Biochemical approach. Clin Biochem. 2016;49(12):936‐941. [DOI] [PubMed] [Google Scholar]
- 36. Sweeney TE, Beuchat CA. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am J Physiol. 1993;264(3 Pt 2):R469‐R480. [DOI] [PubMed] [Google Scholar]


