Abstract
Background
Heterophilic antibodies are still an important source of interference in immunoassays, but reports of interference with D‐dimers are rare. Are D‐dimer level abnormalities, found in the clinic, caused by heterophilic antibodies as well, or are other mechanisms involved? We will elaborate on this issue through two different examples in this article.
Methods
Serum from two patients with significantly elevated levels of D‐dimers were measured and compared by different methods, diluted, and dealt with heterophilic antibody blockers. At the same time, to retrieve the interference, we focused on the cause of D‐dimer false positives and made a systematic review of the literature.
Results
The D‐dimer values were normal (0.49 and 0.15 μg/mL) detected with different testing method and decreased after addition of heterophilic antibody blocking reagent. According to literature data, there were 66.7% (4/6) references showed the interference were heterophilic antibody.
Conclusions
The influence of heterophilic antibodies on the measurement of D‐dimers remains a big challenge. Different measuring instruments and methods may have significant differences in the measurement of D‐dimers. By using a combination of instrumental methods for measuring, incorporating heterophilic antibody blockers, and combining with clinical performance and imaging data, most of the interference can be eliminated.
Keywords: D‐dimer, D‐dimer assays, Heterophilic antibody, Immunoassay, Interference
1. INTRODUCTION
D‐dimers are fibrin degradation products formed by the action of 3 enzymes: thrombin, factor XIIIa, and plasmin (Figure 1).1, 2 During the conversion of fibrinogen to fibrin monomers, thrombin first cleaves two small fragments (ie, fibrinopeptides A and B) from fibrinogen. The negative charge of fibrinogen E (Figure 1, blue) is converted into a positive charge, allowing the fibrin monomer to spontaneously form a polymer through hydrogen bonds (black dotted lines). Thrombin also activates circulating transglutaminase factor XIII, which stabilizes the initial fibrin polymer by catalyzing the formation of covalent bonds to cross‐link adjacent D domains (Figure 1, solid red lines). Next, plasmin cleaves fibrin into various small fragments, referred to as fibrin degradation products (FDPs), among which are D‐dimer fragments, the smallest products of fibrin degradation (MW 180 kDa), which are composed of two adjacent cross‐linked fibrin monomers.2 It is this unique target epitope that is recognized by most reagent antibodies for the laboratory evaluation of thrombosis. The D‐dimer is a unique metabolite of secondary fibrinolysis that increases as a result of hypercoagulability and hyperfibrinolysis. The D‐dimer assay is a sensitive, but non‐specific indicator that has been widely used by clinicians to detect deep vein thrombosis (DVT) or pulmonary embolism (PE), and to diagnose or monitor disseminated intravascular coagulation (DIC) or serve as an effective indicator of thrombolytic or anticoagulant therapy.3 Many different D‐dimer analyses have been developed, which differ in the D‐dimer epitope targeted by the antibody, method of capture and detection, instrumentation required, and calibration standard. It has been reported that there are 20 different monoclonal antibodies used by 30 different D‐dimer assays.4 In general, these assays use homogeneous sandwiches of monoclonal antibodies to detect specific epitopes on cross‐linked D‐dimer fragments, including methods based on hemagglutination, fluorescence, chemiluminescence, or other techniques.5 In clinical laboratories, automated multi‐function instruments generally rely on various test methods, such as enzyme immunoassays, immunofiltration, and immunochromatography. However, immunoassays are inherently susceptible to heterophilic antibodies, that is, interference with endogenous antibodies that measure the antibody, and the consequences of this interference can be devastating (Figure 2).6 There have been many reports of heterophilic antibodies affecting assay results in various tests, such as human chorionic gonadotropin, cardiac troponin I, and prostate‐specific antigen.7, 8, 9 Various types of interfering antibodies affect about 0.05%‐0.5% of immunoassays10 and in 8 automated tumor marker immunoassays, the prevalence of heterophilic antibodies was 0.2%‐3.7%.11 In daily clinical work, acquisition of D‐dimer results inconsistent with clinical symptoms and imaging data is not unique, and we have encountered two such patients whose FDP values were normal, but D‐dimer levels were confusingly high. As the D‐dimer is a fibrinogen degradation product (Figure 1), D‐dimer levels are normally lower than FDP levels. Are such results credible? What are the causes of such abnormalities? Will there be heterophilic antibodies? Articles in this area are relatively infrequent. Therefore, we will elaborate on this issue through the two different examples in this article.
Figure 1.

Formation of FDPs and D‐dimers from fibrinogen
Figure 2.

Effects of heterophilic antibodies in the sandwich immunoassay
2. MATERIALS AND METHODS
2.1. Two experimental serum samples
Serum 1 was from Patient A, an 86‐year‐old woman who was admitted to the neurology ward for repeated dizziness and limb weakness in our hospital on March 27, 2018. She suffered necrosis of the right femoral head due to trauma 20 years ago and limited mobility for years. She has cataracts in both eyes before and made a right eye cataract surgery 10 years ago. Furthermore, the patient had a 5‐year history of hypertension and diabetes and took nifedipine and perindopril to control blood pressure. We performed coagulation functions, including D‐dimer examination to rule out DVT. However, all indicators of hemostatic function (including fibrin degradation products and FDP) were normal (3.7 μg/mL), excepting for a markedly elevated value of D‐dimers (336360 μg/L FEU; local reference range: <550 μg/L FEU), as measured by a commercial latex‐enhanced immunoturbidimetric assay (Siemens AG SYSMEX CS‐5100).
Serum 2 was from Patient B, a 70‐year‐old woman who was hospitalized in the respiratory department of our hospital because of paroxysmal shortness of breath. The main diagnosis was bronchial asthma exacerbation. She had undergone lung cancer surgery 5 years ago and received regular postoperative examinations. In addition, she had a history of hypertension and took antihypertensive drugs regularly. All of these are risk factors for DVT and PE, she recommended to undergo a coagulation test and D‐dimer examination. It showed that an increase in D‐dimers (4580 μg/L FEU; local reference range: <550 μg/L FEU), using the same instrument and method as Patient A, while no other indicators were significantly abnormal with FDP was within the normal range (1.5 μg/mL). In these two cases, there was no imaging data or clinical symptoms were abnormal and they both had no history of allergies and no family history of bleeding or thrombosis. D‐dimer measurements in both cases were performed under normal quality control conditions and excluded sample quality issues. According to national and institutional guidelines, informed consent was obtained for both serum samples. This study was approved by the Privacy Office of the First Affiliated Hospital of Shantou University Medical College.
2.2. Comparative test
Serum samples were tested by another instrument and testing method (HemosIL D‐dimer HS for ACL TOP700, latex‐enhanced immunoturbidimetric immunoassay) for D‐dimer values, under normal quality control conditions, and the operation strictly in accordance with the instructions.
2.3. Dilution test
Serum samples were serially diluted by twofold multiples (2, 4, 8, 16, and 32 times) and measured using the same methods and instruments as the initial measurements (commercial latex‐enhanced immunoturbidimetric assay, Siemens AG). Then, the dilution ratio was used as the abscissa, and the D‐dimer result as the ordinate to draw a linear plot.
2.4. Heterophilic antibody blocking reagent (HBR)
Samples were treated with a heterophilic blocking reagent (HBR, Scantibodies Laboratory Inc, USA) following the instructions. Briefly, the HBR was immediately thawed in a water bath and gently shaken upright then added directly to the experimental sample. After incubation at the temperature required by the instructions, a D‐dimer assay is performed. The control group was identical to the sample except that no HBR was added.
2.5. Systematic review and analysis
We systematically searched PubMed, Web of Science, and Scopus for articles about cases on heterophilic antibodies or other factors influencing D‐dimers and D‐dimer assays, then performed a systematic analysis. The following keywords were used: “human anti‐mouse antibody” or “HAMA” or “heterophile” or “heterophilic” in combination with “D‐dimer”. In addition, other relevant literature references were reviewed. All articles identified according to these search criteria were evaluated by two authors (Wu Y and Zhang XY).
3. RESULTS
3.1. Comparative Test
The results measured by another instrument and testing method (HemosIL D‐dimer HS for ACL TOP, Latex‐enhanced immunoturbidimetric immunoassay) were within the normal reference range (Table 1).
Table 1.
D‐dimer comparative test
| Patient | Patient condition | Test | Comparative test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Clinical manifestations | Assay name and methodology | D‐dimer (μg/L FEU) | FDP (μg/mL) | Assay name and methodology | D‐dimer (μg/mL) | FDP (μg/mL) | |||||
| Value | Reference range | Value | Reference range | Value | Reference range | Value | Reference range | ||||||
| A | Female | 86 | Repeated dizziness and limb weakness | INNOVANCE D‐Dimer, latex‐enhanced immunoturbidimetric immunoassay | 336360 | <500 | 3.7 | 0‐5 | HemosIL D‐Dimer, latex‐enhanced immunoturbidimetric immunoassay | 0.49 | 0‐0.5 | 1.1 | 0‐5 |
| B | Female | 70 | Asthma | 4580 | 1.5 | 0.15 | 0.7 | ||||||
FEU, fibrinogen equivalent units; FDP, fibrinogen degradation products.
3.2. Dilution Test
From Figure 3 there was no linear relationship between the D‐dimer value and the dilution factor. The D‐dimer value decreased after dilution and then fluctuated above the reference value.
Figure 3.

Dilution values for patients A and B. After diluting serum samples 2, 4, 8, 16, and 32 times for patient A and 2, 4, 8, and 16 times for patient B, the D‐dimer assay was performed and the results were plotted as shown in the figure
3.3. Heterophilic antibody blocking reagent (HBR)
The changes in D‐dimer values for patients A and B, measured after addition of HBR, and the control groups are shown in Figure 4. The D‐dimer value of patient A was 229470 μg/L FEU, compared with 369490 μg/L FEU before adding HBR (reduced by 38%), and the control group was 353700 μg/L FEU (reduced by 4.3%). Patient B's D‐dimer level was 2920 μg/L FEU compared with 4580 μg/L FEU before adding HBR (reduced by 36%), with the control group value being 5950 μg/L FEU (reduced by −29.9%).
Figure 4.
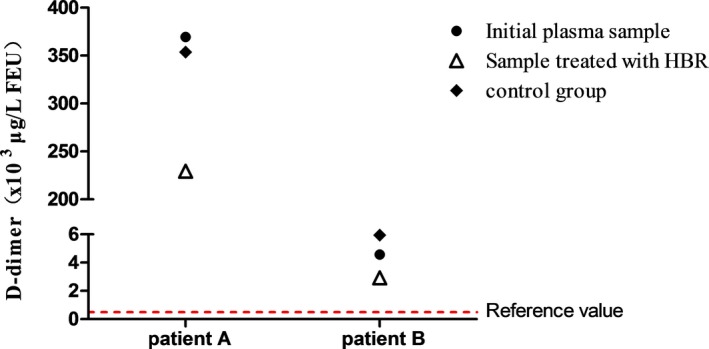
Changes in D‐dimer values of patient A and B. Black circls (●) indicate the results obtained with the initial plasma sample, white triangle (△) represents the results obtained after treating the sample with the heterophilic blocking reagent (HBR), and diamond pattern (◆) refers to the control group (measurement of D‐dimer after incubation under the same conditions as the sample groups except that no HBR was added)
3.4. Systematic review and analysis
Articles that met the conditions were collected and summarized, including six articles, of which four were in English, one in French, and one in Chinese. According to literature data, there were 66.7% (4/6) references showed the interference were heterophilic antibody. (Table 2).
Table 2.
Cases of factors influencing abnormal D‐dimer levels in related articles
| reference | Patient Condition | Test | Comparative Test | Influencing factors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Clinical manifestations | Assay name and methodology | D‐dimer | Reference range | Assay name and methodology | D‐dimer | Reference range | ||
| Lippi G13 | Female | 3 | varicella‐zoster virus infection | HemosIL D‐Dimer HS, Latex‐enhanced immunoturbidimetric | 8998 ng/mL | <243 ng/mL | dealt with heterophilic antibodies blocking reagent (HBT) | 232 ng/mL | <240 ng/mL | heterophilic antibodies |
| Rouviere JA23 | Female | 24 | chest trauma, asthma | STA Liatest D‐Dimer, Quantitative, latex‐enhanced immunoturbidimetric | 2680 ng/mL | <500 ng/mL | VIDAS D‐Dimer, Quantitative, ELISA, sandwich type | 201 ng/mL | <500 ng/mL | heterophilic antibodies |
| Gardiner C19 | Female | 14 | acute pain and swelling in left ankle and calf | HemosIL D‐Dimer, Latex‐enhanced immunoturbidimetric | 2749 ng/mL | <235 ng/mL | MDAW D‐Dimer, Quantitative, latex‐enhanced immunoturbidimetric | 395 ng/mL FEU | <500 ng/mL FEU | heterophilic antibodies |
| Dade Behring D‐dimer Plus, latex agglutination immunoassay | 1022 ng/mL | <112 ng/mL | ||||||||
| AMAX Auto D‐dimer, latex agglutination immunoassay | 195 ng/mL | <75 ng/mL | ||||||||
| Biopool MiniQuant D‐dimer, latex agglutination immunoassay | 1290 ng/mL | <254 ng/mL | ||||||||
| Biopool AutoDimer, latex agglutination immunoassay | 430 ng/mL | <255 ng/mL | ||||||||
| VIDAS D‐dimer, enzyme‐linked fluorometric assay | 94 ng/mL FEU | <500 ng/mL FEU | ||||||||
| NycoCard D‐dimer, immunogold filtration assay | 3100 ng/mL | <300 ng/mL | ||||||||
| Agen dimer test Gold, enzyme‐linked immunosorbent assay | 16 ng/mL | <129 ng/mL | ||||||||
| Robier C24 | Male | 42 | retrosternal pain and vertigo | INNOVANCE D‐Dimer, latex‐enhanced immunoturbidimetric | 34470 ng/mL | <500 ng/mL | NycoCard D‐Dimer; semiquantitative, immunofiltration and DIMERTEST latex, qualitative or semiquantitative latex agglutination | within the normal range | heterophilic antibodies | |
| Roller RE17 | Male | 34 | thoracic pain | Advanced D‐Dimer, Quantitative, latex‐enhanced immunoturbidimetric | 3342 μg/L | <200 μg/L) | NycoCard D‐Dimer; semiquantitative, immunofiltration and Agen dimer test Gold, enzyme‐linked immunosorbent assay | within the normal range | unidentified cause | |
| Zhao QY18 | Female | 64 | Hashimoto; thyroiditis, coronary heart disease; cerebral infarction; vertigo | HemosIL D‐Dimer, Latex‐enhanced immunoturbidimetric | 23640 ng/mL | 37‐429 ng/mL | INNOVANCE D‐Dimer, Quantitative, latex‐enhanced immunoturbidimetric | 0.48 mg/L | <0.5 mg/L | Anti‐rheumatoid factor |
4. DISCUSSION
Heterophilic antibody is a kind of multispecific immunoglobulin produced when a known or unknown antigenic substance stimulates the human body, it has sufficient titer and can bind weakly to immunoglobulins of multiple species. The prevalence of heterophilic antibodies in the general population is 0.17%‐40%.12 Sources are recommended for inducing heterophilic antibodies including exposure to animals and contaminated products, immunization vaccines, blood transfusions, autoimmune diseases, and maternal metastases.13, 14 D‐dimer tests in patient plasma have been widely used in clinical practice for decades.15 A false‐positive D‐dimer results may lead to misdiagnosis, overtreatment or missed treatment timing, impact on treatment evaluation, and psychological stress. From the two examples we provided, it can be seen that the initial D‐dimer results measured by our hospital's equipment and methods were significantly higher than the normal range and even exceeding the FDP value (Table. 1), but mechanisms of interference are not well characterized.16 Based on the experimental results above, the identical serum can be displayed distinctly different D‐dimer measurements by different instruments and methods, and the further comparative dilution experiment showed that there was no linearity and parallelism between the D‐dimer measurements and the dilution factor (Figure 3), which further confirms that the D‐dimer results measured originally are not reliable.6 After the addition of heterophilic antibody blockers, the measured levels of D‐dimer in the sera of the two patients decreased although the results were still higher than normal (Figure 4). This indicates that the heterophilic antibodies do indeed interfere with immunoturbidimetric test results and there may exist other impact mechanisms.
From a review and analysis of prior literature (Table 2), it is clear that apart from the earlier articles mentioning the factors causing the D‐dimer abnormality was unclear17 or the influence of anti‐rheumatoid factor in the sample,18 the more recent four articles (66.7%) refer to interference by heterophilic antibodies. The reported cases span a relatively large age range, with or without underlying disease (including acute injury, hypertension, diabetes, cerebral embolism, and coronary heart disease). Furthermore, the results obtained by different instruments and testing methods are quite different. Most of the comparative tests were distinct from the original methods. In the six articles, an abnormal increase of D‐dimer levels was initially detected by immunoturbidimetric methods. Among them, three articles (50%) showed that this kind of false positive can be eliminated by enzyme‐linked immunosorbent assay, and two articles (34%) were tested by another immunoturbidimetric assay to make a comparative test and acquired results within the normal range, only one (17%) article reported that a heterophilic antibody blocking reagent can be used to eliminate this effect. Moreover, one of the articles compared eight different commercial kits with different methodologies suggesting that D‐dimer was elevated, but obtained variable results using turbidimetry and by immunogold filtration not by enzyme‐linked fluorescent or enzyme‐linked immunosorbent assays.19
In our two cases, we show evidence of interference by heterophilic antibody, for the D‐dimer levels became lower after adding a heterophilic antibody blocker, but did not return to normal levels, as Lippi previously reported.6 It has been reported that D‐dimer commercial kits detect various cross‐linked fibrin degradation products with different molecular weights through different monoclonal antibodies,2, 5 which may be the reason for the large difference between the results of the different instruments and methods. However, as a non‐invasive and rapid diagnostic tool, D‐dimer detection plays an indispensable role in clinical examination for the diagnosis of DVT, PE, or DIC, and may also become an important biomarker of coronary and carotid atherosclerosis and aortic disease.20, 21 Therefore, accurate diagnosis of coagulopathy requires the standardization of D‐dimer reagents, although the development of “universal” calibrators can be very difficult,22 and the “standardization” of the specificity of the assay antibodies may be considered to be a first and most important step.2 Before the D‐dimer assay standardization, when results are suspicious, use a combination of instrumental methods when measure, deal with heterophilic antibody blockers in necessity and connect with the clinical performance and imaging data can rule out most of the interference.
CONFLICT OF INTEREST
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Informed consent: Informed consent was obtained from patient A and patient B.
ETHICAL APPROVAL
This study was approved by the Privacy Office of the First Affiliated Hospital of Shantou University Medical College.
Wu Y, Xiao Y‐X, Huang T‐Y, et al. What makes D‐dimer assays suspicious–heterophilic antibodies?. J Clin Lab Anal. 2019;33:e22687 10.1002/jcla.22687
Ying Wu and Ying‐Xiu Xiao contributed equally to this research and are co‐first authors.
Funding information
This study was funded by the National Natural Science Foundation of China (No. 81302540); by the Supported by Science and Technology Planning Project of Guangdong Province, China (No.2014A020212456).
REFERENCES
- 1. Stefanelli VL, Barker TH. The evolution of fibrin‐specific targeting strategies. J Mater Chem B. 2015;3(7):1177‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kogan AE, Mukharyamova KS, Bereznikova AV, et al. Monoclonal antibodies with equal specificity to D‐dimer and high‐molecular‐weight fibrin degradation products. Blood Coagul Fibrinolysis. 2016;27(5):542‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linkins LA, Takach Lapner S. Review of D‐dimer testing: good, Bad, and Ugly. Int J Lab Hematol. 2017;39:98–103. [DOI] [PubMed] [Google Scholar]
- 4. Longstaff C, Adcock D, Olson JD, et al. Harmonisation of D‐dimer — a call for action. Thromb Res. 2016;137:219–220. [DOI] [PubMed] [Google Scholar]
- 5. Riley RS, Gilbert AR, Dalton JB, Pai S, McPherson RA. Widely used types and clinical applications of D‐dimer assay. Lab Med. 2016;47(2):90–102. [DOI] [PubMed] [Google Scholar]
- 6. Bolstad N, Warren DJ, Nustad K. Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol & Metab. 2013;27(5):647–661. [DOI] [PubMed] [Google Scholar]
- 7. Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false‐positive human chorionic gonadotropin concentrations. Lancet. 2000;355(9205):712–715. [DOI] [PubMed] [Google Scholar]
- 8. Anderson CB, Pyle AL, Woodworth A, Cookson MS, Smith JA, Barocas DA. Spurious elevation of serum PSA after curative treatment for prostate cancer: clinical consequences and the role of heterophilic antibodies. Prostate Cancer and Prostatic Dis. 2011;15(2):182–188. [DOI] [PubMed] [Google Scholar]
- 9. Lippi G, Aloe R, Meschi T, Borghi L. Cervellin G. Interference from heterophilic antibodies in troponin testing. Case report and systematic review of the literature. Clin Chim Acta. 2013;426:79–84. [DOI] [PubMed] [Google Scholar]
- 10. Ismail AA, Barth JH. Wrong biochemistry results. BMJ (Clinical research ed). 2001;323(7315):705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Preissner CM, Dodge LA, O'Kane DJ, Singh RJ, Grebe SK. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin chem. 2005;51(1):208–210. [DOI] [PubMed] [Google Scholar]
- 12. Narasimhan S, Clausen D. Heterophile antibodies and troponin results: implications in rural setting. N Z Med J. 2009;122(1306):130–132. [PubMed] [Google Scholar]
- 13. Lippi G, Ippolito L, Tondelli MT, Favaloro EJ. Interference from heterophilic antibodies in D‐dimer assessment. A case report. Blood Coagul Fibrinolysis. 2014;25(3):277–279. [DOI] [PubMed] [Google Scholar]
- 14. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43:391–393. [PubMed] [Google Scholar]
- 15. Bates S. D‐Dimer Assays in Diagnosis and Management of Thrombotic and Bleeding Disorders. Semin Thromb Hemost. 2012;38(07):673–682. [DOI] [PubMed] [Google Scholar]
- 16. Bjerner J, Bolstad N, Piehler A. Belief is only half the truth ‐ or why screening for heterophilic antibody interference in certain assays makes double sense. Ann Clin Biochem. 2012;49(4):381–386. [DOI] [PubMed] [Google Scholar]
- 17. Roller RE, Lahousen T, Lipp RW, Korninger C, Schnedl WJ. Elevated D‐dimer results in a healthy patient. Blood Coagul Fibrinolysis. 2001;12(6):501–502. [DOI] [PubMed] [Google Scholar]
- 18. Zhao QY, Zhang Y, Yang M. Investigation and analysis of false‐increased D‐dimer results: a case report. Int J Lab Med. 2013;34(24):3439–3440. (Chinese). [Google Scholar]
- 19. Gardiner C, Pennaneac'h C, Mackie IJ, Sheldrake A, Harrison J, Machin SJ. Falsely elevated D‐dimer results in a healthy patient on account of heterophiletul antibodies. Br J Haematol. 2003;122(5):871–873. [DOI] [PubMed] [Google Scholar]
- 20. Soomro AY, Guerchicoff A, Nichols DJ, Suleman J, Dangas GD. The current role and future prospects of D‐dimer biomarker. Eur Heart J ‐ Cardiovasc Pharmacother. 2016;2(3):175–184. [DOI] [PubMed] [Google Scholar]
- 21. Adam SS, Key NS, Greenberg CS. D‐dimer antigen: current concepts and future prospects. Blood. 2008;113(13):2878–2887. [DOI] [PubMed] [Google Scholar]
- 22. Madoiwa S, Kitajima I, Ohmori T, Sakata Y, Mimuro J. Distinct reactivity of the commercially available monoclonal antibodies of D‐dimer and plasma FDP testing to the molecular variants of fibrin degradation products. Thromb Res. 2013;132(4):457–464. [DOI] [PubMed] [Google Scholar]
- 23. Rouviere JA, Devignes J, de Maistre E, Kennel A, Chabot F, Lecompte T. Discrepancy between two methods of D‐dimers measurement: one case of human anti‐mouse antibody interference. Ann De Bio Clin. 2008;66(4):441–446. [DOI] [PubMed] [Google Scholar]
- 24. Robier C, Edler E, Klescher D, Neubauer M. False‐positive D‐dimer result in a latex‐enhanced immunoassay caused by interfering human anti‐mouse antibodies. Clin Chem Lab Med. 2014;52(11):e253–e255. [DOI] [PubMed] [Google Scholar]


