Abstract
Background
The association between adiponectin and nonalcoholic fatty liver disease (NAFLD) has been studied before, but most of the studies are cross‐sectional and cannot prove a causal link.
Objective
To prospectively investigate the relationship between serum adiponectin levels and the incidence of NAFLD in 3 years.
Subjects and methods.
A total of 1325 subjects aged 40 to 70 from the Chongming District of Shanghai, China, were included. All of them did not have fatty liver according to the liver ultrasound examination at entry; alcohol abuse and hepatitis were also excluded. Serum adiponectin levels and other indices were measured at baseline. After 3 years of follow‐up, hepatic ultrasound examination was performed on each participant again to detect fatty liver.
Results
The serum adiponectin levels at entry were significantly lower in subjects who developed NAFLD compared with those who did not develop NAFLD after 3 years (1.75 ± 0.89 ug/mL vs 2.37 ± 1.01 ug/mL, P < 0.001). After multiple adjustments, the highest odds ratios for NAFLD were in the second adiponectin quartile, the adjusted ORs were 1.89 (95% confidence interval [CI] 1.25 to 2.86) compared with those in the highest quartile. Multivariate logistic regression analysis showing variables at entry independently associated with NAFLD after 3 years was adiponectin (P < 0.01), sex (P < 0.01), BMI (P < 0.001), insulin (P < 0.001), HOMA‐IR (P < 0.01), GGT (P = 0.001), TG (P < 0.001), and WBC (P < 0.001).
Conclusions
Lower serum adiponectin level is a predictor of NAFLD among middle‐aged and elderly subjects.
Keywords: Adiponectin, insulin resistance, NAFLD, prospective
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent chronic liver diseases, which is pathologically characterized by more than 5% of triglyceride accumulation in the hepatocytes. Over the past 20 years, the prevalence of NAFLD has dramatically increased, affecting nearly 24% to 42% of the population in Western countries and 5% to 30% in Asian countries.1, 2, 3, 4 The pathogenesis of NAFLD is still elusive; the current assumption of the pathogenesis of NAFLD is the “multiple hit hypothesis.”5, 6 In line with this theory, the first hit is the appearance of fatty liver (steatosis), followed by a second episode leading to the progression of nonalcoholic steatohepatitis (NASH). The potential secondary hits may include fatty acid beta‐oxidation enhancement, oxidative stress, pro‐inflammatory cytokines, and endotoxin exposure.6, 7 Accumulating evidence suggests that one of the white adipose tissue‐derived adipokines, adiponectin seems to play essential roles in the pathogenesis of NASH.8
Adiponectin (also known as ACRP30) is a 30 kDa protein almost exclusively derived from white adipose tissue. It is one of the most important adipocytokines, which is involved in the regulation of insulin sensitivity, glucose metabolism, and lipid metabolism.9 In addition, adiponectin has potent anti‐inflammatory and antiatherogenic characteristics.10 As one of the most abundant adipokines, adiponectin exists in a relatively high concentration in circulation and is negatively related to plasma triglycerides and low‐density lipoprotein and positively related to high‐density lipoprotein. Hypoadiponectinemia has been recognized as a possible risk factor for NAFLD.11 Cross‐sectional clinical studies had demonstrated that serum adiponectin is lower in NAFLD patients than in healthy controls and is inversely associated with hepatic fat content and hepatic insulin resistance.12, 13, 14, 15, 16 However, all these studies could not prove a causal link between adiponectin and NAFLD. In the present study, we conducted a prospective study for 3 years to investigate the relationship between serum adiponectin levels and the incidence of NAFLD.
2. SUBJECTS AND METHODS
2.1. Subjects
The present work was one part of the survey from Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study, which was conducted among 259 657 adults, aged 40 years and older in 25 communities across mainland China, from 2011 to 2014. The studied subjects in this study were from the Chongming District of Shanghai, one of the 25 communities. The detailed research design was as in the previous studies.17, 18 Informed consent was obtained from every participant, and the approval was given by the Institutional Review Board of Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine. At baseline, a total of 9930 subjects aged 40‐70 with full information were included. Of them, 5089 were without fatty liver according to the liver ultrasound examination. A total of 1325 subjects (396 men and 929 women) were randomly selected from the 5089 non‐NAFLD participants. The exclusion criteria also included 1) those with a known history of liver diseases such as hepatitis, cirrhosis, or malignancy; 2) those with a consumption of alcohol greater than 140 g/wk for men and 70 g/wk for women; and 3) those with more than 3 times the normal range of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), or γ‐glutamyltransferase (GGT). Hepatic ultrasound examination was performed on each participant again at the end of the 3 years of follow‐up.
2.2. Data collection
Peripheral venous blood samples were collected after fasting overnight and tested for fasting plasma glucose, fasting serum insulin concentrations, white blood cell (WBC) counts, lipid profile including triglycerides (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL), low‐density lipoprotein cholesterol (LDL), ALT, AST, and GGT. Homeostasis model assessment for insulin resistance (HOMA‐IR) was calculated according to the equation described by Matthews et al19 Body mass index (BMI) was defined as weight in kilograms divided by the square of height in meters. The blood pressure was measured three times from the right arm of the participant in a sitting position after resting for 30 minutes; measurements were taken in 5‐min intervals, and mean values of blood pressure were used.
2.3. Detection of Adiponectin
Serum samples were separated from fasting subjects and preserved at −80℃ prior to analysis. The serum adiponectin was detected in duplicate using Duoset ELISA kit (DY008; R&D Systems) according to the manufacturer. The ELISA system had an interassay coefficient of variation of 4%‐10% and an intra‐assay coefficient of variation of 3%‐9%, respectively.
2.4. Definition of NAFLD
Liver ultrasound examination was carried out on all participants by two experienced ultrasonographists without knowledge of the clinical and laboratory information using a high‐resolution B‐mode tomographic ultrasound system (Esaote Biomedica SpA, Italy) with a 3.5‐MHz probe. Diagnostic criteria of fatty liver by ultrasonography were the existence of at least two of the three abnormal findings: diffusely enhanced echogenicity in the liver compared with the kidney, deep ultrasonic attenuation, and vascular blurring.20 NAFLD was defined by hepatic ultrasound after the exclusion of alcohol abuse and other liver diseases.
2.5. Statistical analysis
Data were presented as means ±SD. The unpaired Student's t test was used to compare the differences between groups. Correlation coefficients between adiponectin and clinical parameters were calculated by Pearson correlation analysis. The association of serum adiponectin and other parameters was tested using multiple stepwise regression analysis. Multivariate logistic regression models were used to calculate the odds ratios (ORs) for NAFLD. Potential confounders including age, sex, blood pressure, lipids, and BMI were adjusted in the regression models. All statistical analysis was conducted using the SPSS Statistical Package (version 18.0; SPSS Inc, Chicago, IL). P values <0.05 were considered statistically significant.
3. RESULTS
3.1. Characteristics at entry according to NAFLD status after three years
Of the studied 1325 participants who were non‐NAFLD initially, 453 (110 men, 343 women) developed NAFLD 3 years later. As shown in Figure 1, the baseline serum adiponectin levels were significantly lower in subjects who developed NAFLD compared with those who did not develop NAFLD (1.75 ± 0.89 ug/mL vs 2.37 ± 1.01 ug/mL, P < 0.001). The clinical characteristics of participating subjects at entry according to NAFLD status 3 years later are shown in Table 1. When compared with those without NAFLD, the subjects with NAFLD showed significant differences in several clinical and laboratory characteristics in this study (Table 1).
Figure 1.
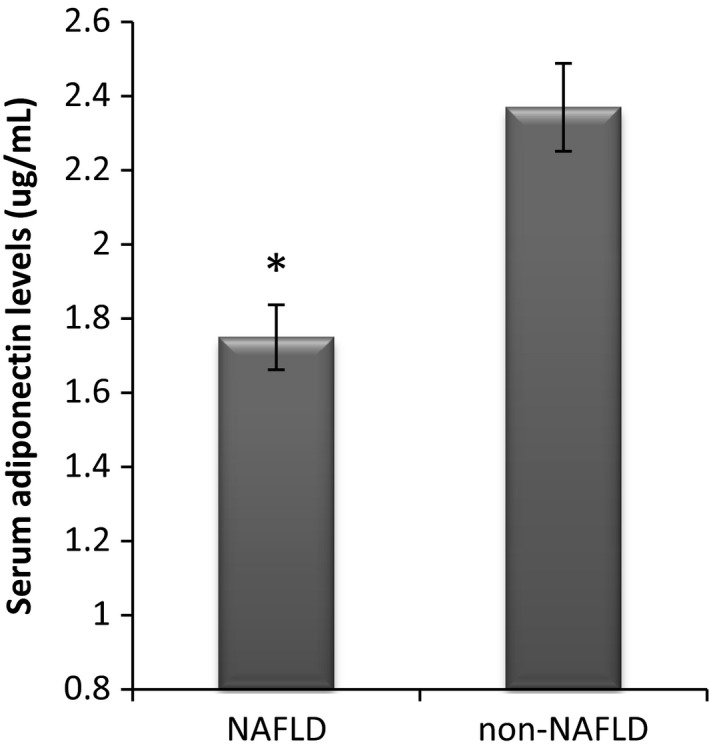
Serum adiponectin levels at entry according to NAFLD status after 3 years in subjects who were initially non‐NAFLD, *P < 0.001
Table 1.
Clinical and laboratory characteristics at entry according to NAFLD status after 3 years in subjects who were initially non‐NAFLD
| Characteristics | NAFLD | Non‐NAFLD | P value |
|---|---|---|---|
| N | 453 | 872 | |
| Age (y) | 55.81 ± 7.38 | 55.89 ± 7.99 | 0.883 |
| Sex (Male/Female) | 110/343 | 286/586 | 0.004 |
| BMI (kg/m2) | 24.05 ± 3.89 | 22.29 ± 4.52 | <0.001 |
| SBP (mm Hg) | 130.05 ± 18.06 | 127.16 ± 18.83 | 0.018 |
| DBP (mm Hg) | 81.12 ± 10.23 | 78.65 ± 10.24 | <0.001 |
| WBC (109/L) | 6.25 ± 1.53 | 5.76 ± 1.35 | <0.001 |
| FBG (mmol/L) | 6.28 ± 1.54 | 6.12 ± 1.70 | 0.134 |
| HbA1C (%) | 5.86 ± 1.03 | 5.76 ± 0.88 | 0.086 |
| Insulin (pmol/L) | 7.50 ± 2.61 | 5.32 ± 2.87 | <0.001 |
| HOMA‐IR | 2.22 ± 0.92 | 1.58 ± 0.73 | <0.001 |
| HDL (mmol/l) | 1.29 ± 0.30 | 1.37 ± 0.32 | <0.001 |
| LDL (mmol/L) | 2.74 ± 0.82 | 2.66 ± 0.76 | 0.102 |
| TC (mmol/L) | 4.88 ± 1.09 | 4.70 ± 0.96 | 0.006 |
| TG (mmol/L) | 1.72 ± 0.91 | 1.24 ± 0.70 | <0.001 |
| ALT (U/L) | 15.87 ± 10.45 | 14.50 ± 10.85 | 0.051 |
| AST (U/L) | 20.45 ± 7.80 | 20.82 ± 10.29 | 0.551 |
| GGT (U/L) | 29.03 ± 14.02 | 20.60 ± 10.47 | <0.001 |
Data are means ±SD or number (percent).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; GGT, γ‐glutamyltransferase; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WBC, white blood cell.
3.2. Association between adiponectin and NAFLD
As seen in Table 2, multivariate logistic regression analysis showing that adiponectin at baseline was independently associated with NAFLD after 3 years of follow‐up (P < 0.01). Other variables associated with NAFLD also included sex (P < 0.01), BMI (P < 0.001), insulin (P < 0.001), HOMA‐IR (P < 0.01), GGT (P = 0.01), TG (P < 0.001), and WBC (P < 0.001). Table 3 displays the ORs for NAFLD by adiponectin quartiles. As expected, increased ORs for NAFLD were observed with the decreased adiponectin levels (P < 0.001 for trend). The highest odds ratios for NAFLD were in the second adiponectin quartile, and the adjusted ORs were 1.89 (95% confidence interval [CI] 1.25 to 2.86) (Table 3).
Table 2.
Logistic regression analysis showing baseline variables independently associated with NAFLD after 3 years
| Independent variables | β | Exp (β) | P value |
|---|---|---|---|
| Adiponectin | −0.072 | 0.931 | <0.01 |
| Gender | 0.605 | 1.831 | <0.01 |
| BMI | 0.153 | 1.166 | <0.001 |
| Insulin | 0.218 | 1.243 | <0.001 |
| HOMA‐IR | 1.191 | 1.211 | <0.01 |
| GGT | 0.012 | 1.012 | 0.001 |
| TG | 0.373 | 1.452 | <0.001 |
| WBC | 0.127 | 1.135 | <0.001 |
The variables entered in the analysis also included age, SBP, DBP, TC, HDL, and LDL which were all excluded from the model.
Table 3.
Adjusted ORs and 95% CIs for NAFLD according to adiponectin quartiles
| ORs (95% CI) | P value for trend | ||||
|---|---|---|---|---|---|
|
Q4 ≥2.741(ug/mL) |
Q3 1.284‐2.740(ug/mL) |
Q2 0.478‐1.283(ug/mL) |
Q1 <0.478(ug/mL) |
||
| NAFLD/non‐NAFLD | 79/252 | 110/221 | 137/195 | 128/203 | |
| Model 1 | 1 | 2.02 (1.38‐2.96) | 2.26 (1.55‐3.29) | 1.58 (1.08‐2.31) | <0.001 |
| Model 2 | 1 | 2.24 (1.52‐3.31) | 2.51 (1.71‐3.69) | 1.64 (1.12‐2.41) | <0.001 |
| Model 3 | 1 | 1.68 (1.10‐2.57) | 1.89 (1.25‐2.86) | 1.523 (1.01‐2.30) | <0.001 |
Q, Quartile
Model 1 not adjusted.
Model 2 adjusted for age and gender.
Model 3 further adjusted for BMI, Insulin, HOMA‐IR, TG, GGT, and WBC.
Correlation analysis demonstrated a remarkable correlation between adiponectin and age (r = 0.139, P < 0.001); adiponectin and BMI (r = 0.056, P < 0.01); adiponectin and WBC (r = −0.098, P < 0.001); adiponectin and insulin (r = −0.070, P < 0.001); adiponectin and HOMA‐IR (r = 0.063, P < 0.01); adiponectin and TG (r = −0.155, P < 0.001); and adiponectin and HDL (r = 0.174, P < 0.001) (see Table 4). A multiple stepwise regression analysis was used to assess the independent variables that may affect serum adiponectin levels. The variables entered in the model were the following: age, BMI, insulin, HOMA‐IR, TG, and HDL. The main determinants of adiponectin are age (β = 0.144, P < 0.001), TG (β = −0.120, P < 0.001), HDL (β = 0.159, P < 0.001), HOMA‐IR (β = −0.059, P < 0.01), and WBC (β = −0.116, P < 0.001) (Table 5).
Table 4.
Crude and partial correlation between plasma adiponectin and clinical parameters in the studied subjects
| Variable | Crude r | Partial r a |
|---|---|---|
| Age (y) | 0.139** | ‐ |
| BMI (kg/m2) | −0.056* | −0.056* |
| SBP (mm Hg) | −0.007 | −0.035 |
| DBP (mm Hg) | −0.032 | −0.026 |
| WBC(109/L) | −0.106** | −0.098** |
| FBS (mmol/L) | −0.036 | −0.050 |
| HbA1c (%) | −0.089 | −0.039 |
| Insulin (pmol/L) | −0.056* | −0.070** |
| HOMA_IR | −0.048 | −0.063* |
| HDL (mmol/L) | 0.190** | 0.174** |
| LDL (mmol/L) | 0.051 | 0.021 |
| TC (mmol/L) | 0.067* | 0.028 |
| TG (mmol/L) | −0.147** | −0.155** |
| ALT (U/L) | −0.055* | −0.042 |
| AST (U/L) | 0.027 | 0.019 |
| GGT (U/L) | −0.004 | 0.027 |
adjusted for age, gender
P < 0.01
P < 0.001
Table 5.
Multiple stepwise regression analysis showing variables independently associated with the serum adiponectin levels
| Independent variables | Standardized β | t | P value |
|---|---|---|---|
| Age | 0.144 | 5.339 | <0.001 |
| TG | −0.120 | −4.281 | <0.001 |
| HDL | 0.159 | 5.664 | <0.001 |
| HOMA_IR | −0.059 | −2.148 | <0.01 |
| WBC | −0.116 | −5.538 | <0.001 |
The analysis also included insulin and BMI which were excluded.
4. DISCUSSION
Although the pathogenesis of NAFLD is still not very clear, insulin resistance, oxidative stress, and inflammation may be involved in the development and progression of NAFLD.21 Adiponectin, one of the adipocytokines, is regarded as a major regulatory link between insulin resistance, disorganized substrate oxidation, and mitochondrial dysfunction in multiple organs.22 The liver is a main target organ of adiponectin, and adiponectin represents the best‐studied adipocytokine in NAFLD. Adiponectin has demonstrable effects on the pathophysiology of NAFLD by regulating hepatic fat deposition, insulin resistance, inflammation, and fibrosis.9, 23 The relationship between NAFLD and plasma adiponectin levels has been investigated in latest years, and hypoadiponectinemia was identified as an independent risk factor for NAFLD.23, 24 Some research have indicated that adiponectin levels are reduced from 20% to 40% during the development of NAFLD.25 The mean adiponectin levels in subjects with NASH were half of the controls.26
The strong connection between insulin resistance and NAFLD has been broadly demonstrated, and several authors have suggested to include NAFLD as one component of the metabolic syndrome.27 Adiponectin can protect liver from injury. In the liver, adiponectin enhances fatty acid β‐oxidation, thus reducing hepatic triacylglycerol content and hepatic insulin resistance.28 Adiponectin was negatively correlated with HOMA‐IR, which indicated its role in the link between insulin resistance and NAFLD. The patients with NAFLD have decreased serum adiponectin, and this decrease is related to insulin resistance.16 Unlike previous studies, the present study is a prospective study; we are able to prove a causal link between adiponectin and NAFLD. We found that hypoadiponectinemia was an independent risk factor for developing NAFLD, and serum adiponectin was inversely related to insulin resistance. Adiponectin is the link of insulin resistance and NAFLD.
In addition to decreasing hepatic and systematic insulin resistance, adiponectin also attenuates liver inflammation and fibrosis. Adiponectin levels are inversely related to inflammatory mediators, such as IL‐6 and C‐reactive protein (CRP), but positively associated with anti‐inflammatory cytokine IL‐10.29, 30 Adiponectin inhibits TNF‐alpha functions by suppressing the expression of TNF‐alpha and antagonizing its activities.31, 32 NAFLD is associated with mitochondrial dysfunction and decreased activity of the respiratory chain complexes which is connected to overproduction of free radicals.33 Adiponectin might also protect the liver via regulating mitochondrial function.9 Low levels of plasma adiponectin may be related to declined hepatic anti‐inflammatory ability, intensified oxidative stress and inflammation, and the progression of NAFLD. In our present study, we have found that serum adiponectin levels are negatively associated with WBC counts and WBC count is an independent risk factor for NAFLD; there existed a connection between adiponectin, inflammation, and NAFLD.
A limitation of the present study may be the lack of cytological and/or histopathological information because we could not carry out liver biopsy; ultrasonic examination was used to detect NAFLD. Another limitation is that other environmental confounders may influence our conclusions and such factors, if discovered, need to be considered in future studies.
5. CONCLUSION
In summary, our study shows low serum adiponectin levels are a predictor of progressing to NAFLD among middle‐aged and elderly subjects. Measurement of serum adiponectin could be an indicator for NAFLD risk stratification. It is interesting and important to confirm that adiponectin plays a causal role during the pathogenesis of NAFLD in humans.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by the grants from National Key R&D Program of China (2016YFC0901200, 2016YFC0901203), the Shanghai Science and Technology Commission (15411953200, 10411956600, 14ZR1427400), National Natural Science Foundation of China (81370935, 81670743), the National Clinical Research Center for Metabolic Diseases (2013BAI09B13), the National Key New Drug Creation, and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006‐001). We are grateful to the REACTION group.
Zhang H, Niu Y, Gu H, et al. Low serum adiponectin is a predictor of progressing to nonalcoholic fatty liver disease. J Clin Lab Anal. 2019;33:e22709 10.1002/jcla.22709
Contributor Information
Li Qin, Email: qinli@xinhuamed.com.cn.
Qing Su, Email: suqing@xinhuamed.com.cn.
REFERENCES
- 1. Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non‐alcoholic fatty liver disease in the Asia‐Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22(6):788‐793. [DOI] [PubMed] [Google Scholar]
- 2. Bedogni G, Nobili V, Tiribelli C. Epidemiology of fatty liver: an update. World J Gastroenterol. 2014;20(27):9050‐9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu X, Huang Y, Bao Z, et al. Prevalence and factors associated with nonalcoholic fatty liver disease in Shanghai work‐units. BMC Gastroenterol. 2012;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaBrecque DR, Abbas Z, Anania F, et al. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48(6):467‐473. [DOI] [PubMed] [Google Scholar]
- 5. te Sligte K, Bourass I, Sels JP, Driessen A, Stockbrugger RW, Koek GH. Non‐alcoholic steatohepatitis: review of a growing medical problem. Eur J Intern Med. 2004;15(1):10‐21. [DOI] [PubMed] [Google Scholar]
- 6. Edmison J, McCullough AJ . Pathogenesis of non‐alcoholic steatohepatitis: human data. Clin Liver Dis. 2007;11(1):75‐104. [DOI] [PubMed] [Google Scholar]
- 7. Mulhall BP, Ong JP, Younossi ZM. Non‐alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 2002;17(11):1136‐1143. [DOI] [PubMed] [Google Scholar]
- 8. Jarrar MH, Baranova A, Collantes R, et al. Adipokines and cytokines in non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27(5):412‐421. [DOI] [PubMed] [Google Scholar]
- 9. Moschen AR, Wieser V, Tilg H. Adiponectin: key player in the adipose tissue‐liver crosstalk. Curr Med Chem. 2012;19(32):5467‐5473. [DOI] [PubMed] [Google Scholar]
- 10. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772‐783. [DOI] [PubMed] [Google Scholar]
- 11. Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends in endocrinology and metabolism: TEM. 2010;21(6):345‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bugianesi E, Pagotto U, Manini R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J clin Endocrinol Metab. 2005;90(6):3498‐3504. [DOI] [PubMed] [Google Scholar]
- 13. Kaser S, Moschen A, Cayon A, et al. Adiponectin and its receptors in non‐alcoholic steatohepatitis. Gut. 2005;54(1):117‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musso G, Gambino R, Biroli G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta‐cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100(11):2438‐2446. [DOI] [PubMed] [Google Scholar]
- 15. Vuppalanchi R, Marri S, Kolwankar D, Considine RV, Chalasani N. Is adiponectin involved in the pathogenesis of nonalcoholic steatohepatitis? A preliminary human study. J Clin Gastroenterol. 2005;39(3):237‐242. [DOI] [PubMed] [Google Scholar]
- 16. Pagano C, Soardo G, Esposito W, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152(1):113‐118. [DOI] [PubMed] [Google Scholar]
- 17. Ning G, Reaction Study G . Risk evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172‐173. [DOI] [PubMed] [Google Scholar]
- 18. Bi Y, Lu J, Wang W, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6(2):147‐157. [DOI] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 20. Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver‐kidney contrast. Tohoku J Exp Med. 1983;139(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 21. Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calton EK, Miller VS, Soares MJ. Factors determining the risk of the metabolic syndrome: is there a central role for adiponectin? Eur J Clin Nutr. 2013;67(5):485‐491. [DOI] [PubMed] [Google Scholar]
- 23. Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non‐alcoholic fatty liver disease: multiple interactions. Int J Mol sci. 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Metabolism. 2011;60(3):313‐326. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity‐related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009;53(2):201‐212. [DOI] [PubMed] [Google Scholar]
- 26. Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF‐alpha or adiponectin? Hepatology. 2004;40(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 27. Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917‐923. [DOI] [PubMed] [Google Scholar]
- 28. Balmer ML, Joneli J, Schoepfer A, Stickel F, Thormann W, Dufour JF. Significance of serum adiponectin levels in patients with chronic liver disease. Clin Sci (Lond). 2010;119(10):431‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi KM, Ryu OH, Lee KW, et al. Serum adiponectin, interleukin‐10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75(2):235‐240. [DOI] [PubMed] [Google Scholar]
- 30. Engeli S, Feldpausch M, Gorzelniak K, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52(4):942‐947. [DOI] [PubMed] [Google Scholar]
- 31. Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS‐stimulated TNF‐alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G998–G1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat‐derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Investig. 2003;112(1):91‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez‐Carreras M, Del Hoyo P, Martin MA, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999‐1007. [DOI] [PubMed] [Google Scholar]


