Abstract
Background
Little is known about the involvement of herpes simplex virus (HSV) or Mycobacterium tuberculosis (MTB) as potentially curable causes of central nervous system (CNS) infections in sub‐Saharan Africa.
Objective
In this study, we developed a PCR assay dedicated to simultaneous testing of HSV1/HSV2 and MTB in Burkina Faso, a country where HSV is neglected as a cause of CNS infection and where TB prevalence is high.
Methods
A consensus HSV1/HSV2 set of primers and probe were designed and combined to primers and probe targeting the IS6110 repetitive insertion sequence of MTB. Analytical performances of the assay were evaluated on reference materials. Cerebrospinal fluid (CSF) collected from subjects with aseptic meningitis was tested for HSV1/HSV2 and MTB DNA.
Results
The UL29 gene was chosen as a highly conserved region targeted by the HSV1/HSV2 nucleic acid test. The lower limits of detection were estimated to be 2.45 copies/µL for HSV1, 1.72 copies/µL for HSV2, and 2.54 IS6110 copies per µL for MTB. The PCR was used in 202 CSF collected from subjects suspected of aseptic meningitis. Five samples (2.46%) tested positive, including two children positive for HSV1 (0.99%) and three adults tested positive for MTB (1.47%).
Conclusion
Using an in‐house real‐time PCR assay, we showed that both HSV and MTB are etiologic pathogens contributing to aseptic meningitis in Burkina Faso. This molecular test may have clinical utility for early diagnosis for those treatable CNS infections.
Keywords: cerebrospinal fluid, herpes simplex virus, meningitis, Mycobacterium tuberculosis, PCR
1. INTRODUCTION
Infections of the central nervous system (CNS) are responsible for approximately 530 000 deaths throughout the world each year.1 Patients with clinical and laboratory evidences for CNS infections but in whom common bacterial agents cannot be identified by conventional bacterial culture in the cerebrospinal fluid (CSF) can be considered as having aseptic meningitis and/or encephalitis. Clinical presentation of aseptic meningitis and of bacterial meningitis is similar, whereas encephalitis manifests with altered cognition, seizures, and focal neurological dysfunction. The distinction between aseptic meningitis and encephalitis may be difficult since patients may have both signs of meningitis and altered mental status with sensory deficit. The clinical presentation of the different types of CNS infections may be nonspecific and can overlap, but viral meningitis is usually less severe, whereas viral encephalitis and bacterial meningitis are associated with a significant mortality. Viruses including those from the Herpesviridae family are the most common cause of acute aseptic inflammation of meninges and brain.2, 3, 4, 5, 6 Among them, herpes simplex virus (HSV) is regularly reported as a current and severe cause of encephalitis in high‐income countries. Other pathogens associated with meningitis and meningoencephalitis include Mycobacterium tuberculosis (MTB)7 which is responsible for a particularly deadly and disabling form of meningitis.8
In African settings, aseptic meningitis is thought to be common, but the relative contribution of potential etiologic pathogens remains largely unexplored.9 In countries located in the “meningitis belt” such as Burkina Faso, CSF samples are generally tested for the three main causative bacteria Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae by culture, antigen detection, and PCR.10, 11 However, only about a quarter of suspected cases are laboratory‐confirmed with these tests.12 Little is known about the prevalence of HSV and MTB as agents responsible for severe but potentially curable aseptic meningoencephalitis in sub‐Saharan Africa.13, 14 Identification of MTB and HSV in CSF is important since a specific chemotherapy is required for these pathogens.
The purpose of the present study was to improve the laboratory diagnosis of HSV1/HSV2 and MTB using an in‐house duplex nucleic acid assay and to screen CSF samples collected from febrile patients clinically suspected of CNS infections in Burkina Faso.
2. MATERIAL AND METHODS
2.1. Standards, controls, and clinical specimens
World Health Organization (WHO) international control for human herpes simplex virus type 1 and 2 was obtained from the National Institute for Biological Standards and Control (NIBSC; Hertfordshire, UK, product number 08/224‐005 and 08/226‐005, respectively). Mycobacterium bovis bacillus Calmette‐Guerin (BCG) strain was obtained from the Montpellier Infectiology Research Institute (IRIM, Montpellier, France). The target sequence of the MTB PCR assay is the IS6110, present in a single copy in BCG but in an average of 10 reiterations in MTB strains.15
A total of 202 archived clinical CSF samples collected between 2014 and 2015 in the context of the nationwide meningitis surveillance in Burkina Faso (Centre Muraz, Bobo‐Dioulasso) from patients with meningitis symptoms were tested for HSV and MTB DNA. Criteria for inclusion were a CSF white cell count >5 cells/mm3, negative CSF Gram stain, negative routine culture using, negative meningococcal PCR, negative latex agglutination assays for S. pneumoniae; H. influenzae type b; N. meningitidis, group A; group B/Escherichia coli K1; group C; group Y/W135; and Streptococcus group B (kit Pastorex Meningitis; Bio‐Rad, Marnes‐la‐Coquette, France).16 All samples were anonymized and stored at −20°C.
2.2. DNA extraction
DNA extraction was performed using Chelex‐100® (Bio‐Rad; 100 Resin #1432832), as previously reported.17, 18 This method chelates cations including Mg2+, which is an essential cofactor for DNases, preserving the DNA released after lysis of bacterial wall. This method was previously used for mycobacterial and HSV DNA extraction from CSF samples.18, 19 In this procedure, 200 µL of CSF samples was added to 100 μL of 20% of a Chelex‐100 suspension prepared in molecular water and mix vigorously. The suspension was heated for 20 minutes at 95°C. The boiled mixture was centrifuged to pellet out the Chelex‐100 resin, and 50 µL of supernatant was retained for PCR. Extracted DNA was kept at −20°C until PCR testing.
2.3. Real‐time PCR duplex
Primers and a probe were designed using the PRIMER 3 PLUS software. Published HSV‐1 and 2 sequences were analyzed using BLAST software sequence alignment tool to identify a single set of primers and a probe for both HSV type 1 and type2. Primers and probe for MTB nucleic acid test were designed within the IS6110 insertion sequence. The IS6110 gene is a multiple repeated sequence frequently used for in‐house and commercial MTB PCR methods.20, 21, 22, 23 The amplified DNA sequences were a 66‐bp region of the IS6110 insertion elements which occur most of the time in multiple copies in the MTB genome.24 For each PCR, 5 µL of DNA extract was added to 15 µL of master mixture incorporating 1.6 µL of HSV forward and reverse primers at 10 µmol/L, 0.4 µL of probe (FAM—TAMRA) at 10 µmol/L, 1.2 µL of MTB IS6110 forward and reverse primers at 10 µmol/L, 0.6 µL of ISP probe (Cy5—BHQ2) at 10 µmol/L, 4 µL of 5× TaqMan Master Mix (Omunis, Clapiers, France), 0.8 µL of DMSO, and 6.4 µL of RNase/DNase‐free water.
Reaction consisted of 2 minutes at 50°C, 10 minutes at 95°C followed by 45 cycles of 15 seconds at 95°C, and 1 minute at 60°C each. The duplex PCR assays were performed using a LightCycler 480 II Real‐Time PCR Instrument (Roche Diagnostics GmbH, Mannheim, Germany), and amplification data were analyzed using the LightCycler 480 Software (Roche Diagnostics). All samples tested positive for HSV using the duplex PCR were tested by the RealStar® HSV PCR Kit 1.0 (Altona Diagnostics, Hamburg, Germany) to discriminate HSV type 1 vs type 2 on the same apparatus. All results are expressed as copies/µL of eluate.
2.4. Determination of the analytical performance of duplex PCR
The limits of detection (LOD) for HSV1/HSV2 and MTB were determined using serial concentrations of HSV1/HSV2 and BCG in at least 10 separate runs. The linearity of duplex quantitative polymerase chain reaction (PCR) technique was established by testing in 24 separate PCR runs performed on serial dilution of the DNA samples ranging from 6.05 × 105 to 6.05 × 101 copies/µL for HSV1, 9.27 × 103 to 9.27 copies/µL for HSV2, and 3.42 × 105 to 3.42 × 101 genomic copies for MTB. Intra‐assay variability of duplex PCR assay was assessed by analyzing a single PCR run with nine replicates of the same serial concentrations of HSV1/HSV2, and BCG DNA. The inter‐assay variability of duplex PCR technique was evaluated with two different operators measured five times on separate PCR runs. Duplex PCR assay specificity was assessed by testing 24 negative CSF samples including two hepatitis B virus (HBV) DNA‐positive samples, two human immunodeficiency virus (HIV) RNA‐positive samples, two enterovirus‐positive RNA samples, two leptospires DNA‐positive samples, two N. meningitidis DNA samples, two S. pneumoniae DNA samples, and two H. influenzae DNA samples.
2.5. Statistical analysis data
HSV1/HSV2 and MTB DNA concentrations were converted to Log10 values before performing statistical analysis. The LOD was defined as the lowest DNA load detected in 95% of the times. The probit regression analysis performed on SPSS was used to determine the LOD. Coefficient of variation ([(SD/Means) × 100]; SD, the standard deviation) was calculated to estimate intra‐assay and inter‐assay variabilities. Continuous variables were described as median and interquartile range (IQR).
3. RESULTS
3.1. Design of HSV1/HSV2 set of primers and probe
Herpes simplex virus UL47, UL40, UL35, UL29, and UL15 were identified as highly conserved HSV genes, and among them, a target sequence nested in UL29 was selected. UL29 HSV1/HSV2 percentage of homology is 52% and 100% in the PCR target sequence. Primers allowed amplification of a 121 nucleic acid sequence (Table 1).
Table 1.
Description of primers and probes
| Microorganisms | Primers’ sequence (5′ 3′) | Genome region amplified | Amplicon, bp |
|---|---|---|---|
| HSV1/HSV2 | |||
| Forward | AAGAGCCGCGTGTTGTTC | UL29 | 121 |
| Reverse | GTCCGAGGAGGATGTCCA | ||
| Probe | CCTACCAGAAGCCCGACAAG | ||
| Mycobacterium tuberculosis | |||
| Forward | CATGTCCGGAGACTCCAGTT | IS6110 | 66 |
| Reverse | GGTACCTCCTCGATGAACCA | ||
| Probe | AAAGGATGGGGTCATGTCAG | ||
HSV, herpes simplex virus.
3.2. Analytical performance of the duplex PCR assays
Using serial concentrations on repeated runs, the lowest DNA concentration giving 95% detection signal was 2.45 HSV1 DNA copies/µL (95% confidence interval [CI]: 2.22‐2.84) (Figure 1A) and 1.72 HSV2 DNA copies/µL (95% CI: 1.55‐1.93) for HSV2 (Figure 1B). The LOD of MTB PCR was 2.54 IS6110 copies/µL (95% CI: 2.27‐2.99; Figure 1C). Good linearity of the measures was observed with coefficients of determination R 2 = 0.9999 (60.5‐650 000 copies/µL) for HSV1 (Figure 2A), R 2 = 0.9989 (9.27‐9270 copies/µL) for HSV2 (Figure 2B), and R 2 = 1 (34.2‐342 000 copies/µL) for MTB (Figure 2C). The coefficients of intra‐run and inter‐run variation for each target with serial concentration are represented in Table 2. All 24 negative samples tested were found negative for HSV1/HSV2 and MTB, conferring an analytical specificity of 100% for the duplex PCR.
Figure 1.
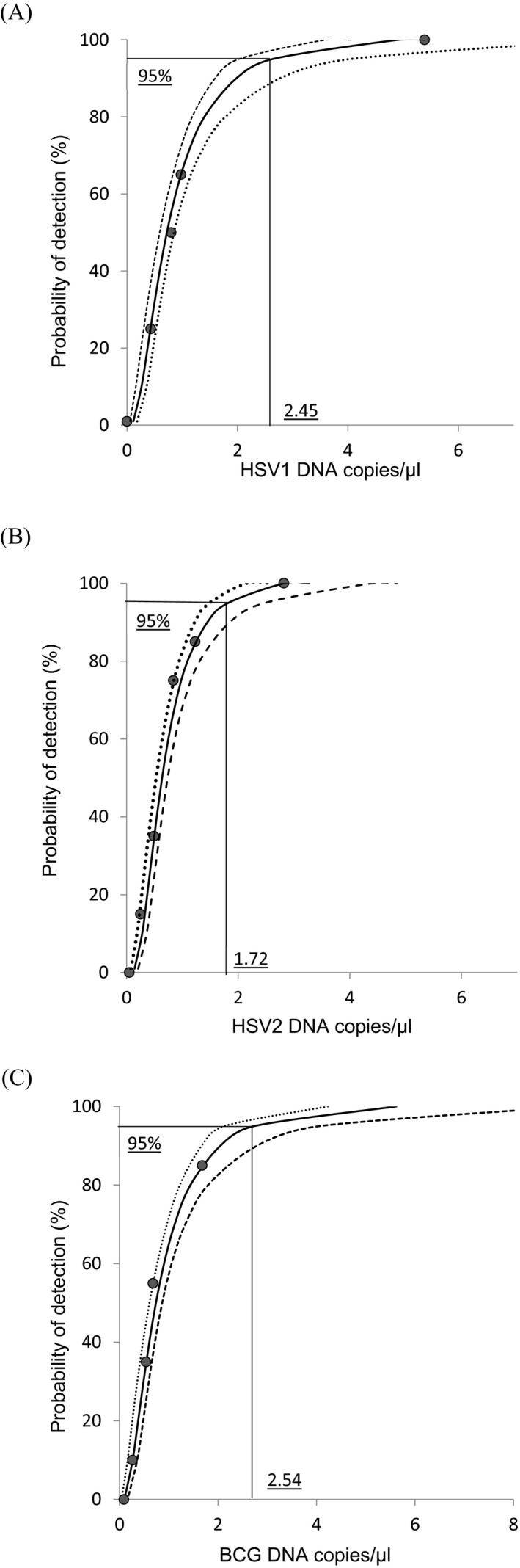
Detection limits of the HSV1/HSV2, and IS61160 PCR assays. Curves were determined by probit analysis (95% probability detection). A, The LOD was estimated at 2.45 copies/µL for HSV1; B, 1.72 copies/µL for HSV2; and C, 2.54 copies/µL for IS6110 using the BCG strain containing a single target copy of IS6110. Dashed lines show 95% confidence interval for the analysis. BCG, bacillus Calmette‐Guerin; HSV, herpes simplex virus; LOD, limits of detection
Figure 2.
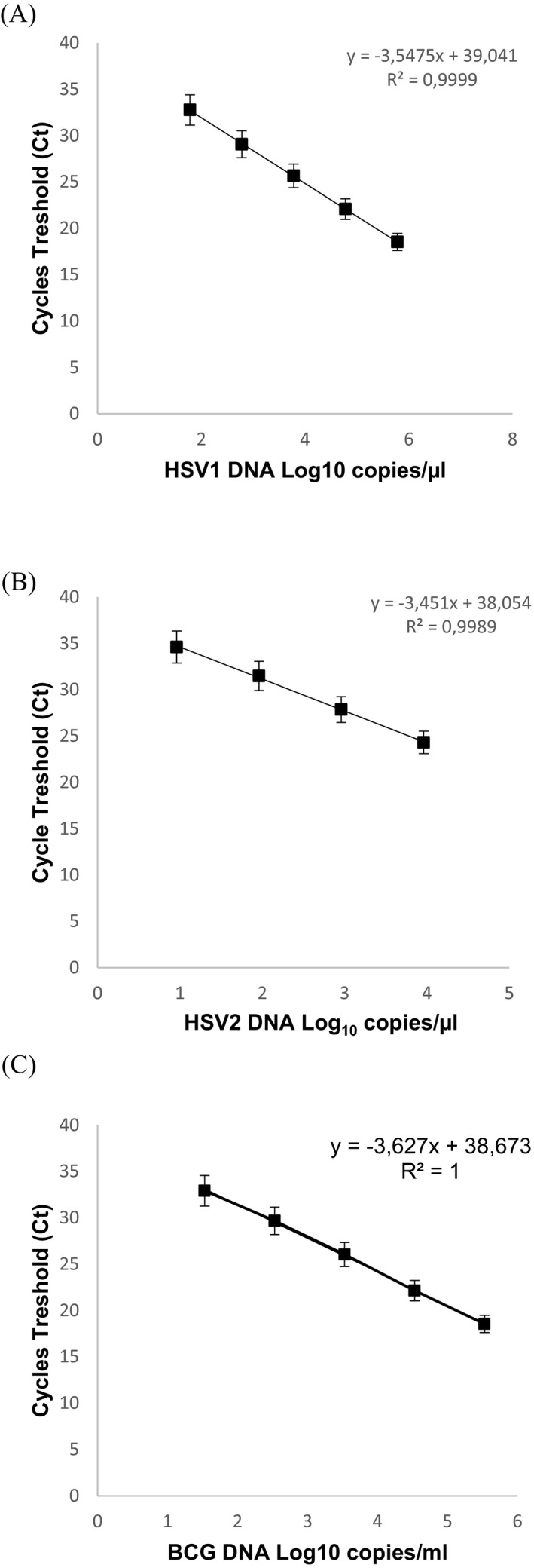
Duplex HSV1/HSV2 and Mycobacterium tuberculosis PCR standard curves established by means of serial dilutions of DNA. The HSV1 standard curve is represented by (A), the HSV2 standard curve by (B), and the BCG DNA standard curve by (C). BCG, bacillus Calmette‐Guerin; HSV, herpes simplex virus
Table 2.
Intra‐ and inter‐assay results of PCR
| DNA log10 copies/µL | %CV | ||
|---|---|---|---|
| Intra‐run | Inter‐run | ||
| HSV1 | |||
| Sample 1 | 4.78 | 0.42 | 2.03 |
| Sample 3 | 3.78 | 0.24 | 1.66 |
| Sample 3 | 2.78 | 0.29 | 1.40 |
| Sample 4 | 1.78 | 0.79 | 0.76 |
| HSV2 | |||
| Sample 1 | 2.96 | 0.38 | 1.08 |
| Sample 2 | 1.96 | 0.86 | 0.5 |
| Sample 3 | 0.96 | 0.77 | 1.41 |
| Mycobacterium tuberculosis | |||
| Sample 1 | 4.53 | 1.49 | 2.01 |
| Sample 2 | 3.53 | 1.86 | 1.52 |
| Sample 3 | 2.53 | 0.59 | 2.29 |
| Sample 4 | 1.53 | 0.86 | 1.14 |
HSV, herpes simplex virus.
3.3. HSV1/HSV2 and MTB testing in CSF from patients with suspected meningitis
A total of 202 CSF samples collected from patients with aseptic meningitis were tested for HSV and MTB DNA (Table 3). Sixty‐five per cent (65.8%) of the subjects were below 16 years of age. CSF cellularity was most of the time moderately elevated with a median (IQR) of 17 (8‐67.75) cells/mm3.
Table 3.
Demographics, CSF characteristics, and detection of herpesviruses and MTB in 202 patients with suspected aseptic meningitis
| Aetiology |
Patient with diagnosis N0 (%) N = 202 |
CSF WCC cell/mm3 (IQR) | Age median (range) | Sex ratio H/F |
|---|---|---|---|---|
| HSV1 | 2 | 17 and 22 | 2 and 3 | 0/2 |
| HSV2 | 0 | – | – | – |
| Mycobacterium tuberculosis | 3 | 103 (54, 551) | 26 (5‐28) | 3/0 |
| Non‐reactive | 197 | 17 (8, 64) | 7 (1‐85) | 1.13 |
CSF, cerebrospinal fluid; HSV, herpes simplex virus; IQR, interquartile range; WCC, white cell count.
Five out of 202 CSF samples (2.46%) were tested positive using the HSV/MTB PCR. Two CSF (0.99%) were tested positive for HSV DNA, and three (1.47%) were tested positive for MTB DNA. The two HSV‐positive CSF were confirmed positive for HSV type 1. These samples were collected from two children aged 2 years and presenting paucicellular CSF. The three samples positive for MTB had been collected from young adults and exhibited higher cellularity ranging from 54 to 551 cells/mm3 (Table 3).
4. DISCUSSION
Meningitis is an important health problem in sub‐Saharan countries. In Burkina Faso, meningitis accounts for 4% of all death representing the 7th most frequent cause of death.25 Only a quarter of suspected meningitis cases with CSF were laboratory‐confirmed as either S. pneumoniae, N. meningitidis, or H. influenza.12 Rapid and accurate diagnosis is mandatory for allowing prompt and appropriate care improving the clinical prognosis and minimizing debilitating sequelae. Furthermore, identification of the causative infectious agents yields valuable epidemiological information. Due to its speed, sensitivity, and specificity, the PCR method is now being established as a diagnostic tool of growing potential for the diagnosis of aseptic meningitis and encephalitis.26, 27
In the first phase of this study, the analytical performance of the PCR was determined using HSV standards and BCG strain. UL29 (ICP8) chosen as a highly conserved HSV gene is a single‐stranded linear DNA binding protein involved in the initiation of HSV replication.28, 29 The assay demonstrated a limit of detection around 2 copies/µL for HSV1/HSV2 and 2.5 IS6110 copies/µL for MTB. Taking into account a median number of ten IS6110 copies per bacteria, the analytical sensitivity of the PCR can be estimated around 250 copies of MTB/mL. The sensitivity of the assay for HSV DNA was comparable to others HSV nucleic acid tests30, 31 and probably sufficient to detect most of the HSV CNS infections regarding viral load generally observed in CSF.32 However, a better sensitivity for HSV detection, able to detect <500‐200 HSV DNA copies/mL, would be preferable to detect some of the specimens with low HSV DNA concentration.33 The cation exchanger Chelex 100 requires less than an hour for DNA extraction and is especially inexpensive.
In the second phase of the evaluation, we explored HSV and MTB as pathogenic agents possibly involved in severe aseptic meningitis in a setting where HSV is neglected as a cause of CNS infection and TB prevalence is high.
Two children aged 2 years were tested positive for HSV in the CSF and confirmed HSV1. HSV1/HSV2 are important causes of CNS infections particularly among children and immunocompromised patients. HSV induces a large spectrum of presentation: meningitis, myelitis, radiculitis, and encephalitis that are associated with a mortality estimated to 70% in absence of acyclovir/valacyclovir treatment.34 HSV1 was identified in CSF collected from of 2% to 4% of adults and children with aseptic meningitis or encephalitis from Malawi35 and Sudan.36 In a prospective study conducted in Malawian children with suspected viral CNS infection, HSV1 was detected in eight out of 513 cases (1.5%).37 A similar study conducted in Bonn University Hospital, Bonn, Germany, reported a prevalence of 0.3% for HSV.38 Other studies reported a prevalence of 1.5% for HSV1 in Korea39 and 1.4% in Georgia.40 HSV1 was also reported in 3.4% and 6.5% in two studies conducted in adults living in Vietnam.41, 42 Children are probably affected by HSV CNS infection during primary infection, whereas HSV reactivation is involved in adults. HSV2 was not detected in our study, but HSV2 has been reported as a cause of recurrent aseptic meningitis in immunosuppressed individuals.43, 44, 45
Regarding MTB, the proportion of aseptic meningitis attributable to this pathogen is thought to vary by location according to the overall frequencies of tuberculosis and HIV.8 Tuberculous meningitis remains an important cause of death in young children and immunocompromised adults living in endemic areas. The highest rates were observed in South Africa with up to 30% of all adult meningitis.46, 47, 48
In a recent study in Uganda, a diagnosis of tuberculous meningitis was confirmed in 4% of children suspected of meningitis.49 Data are missing in West African countries where HIV prevalence is lower. The prevalence of MTB observed in our study (1.5%) is close to a report from Niger where 1.9% of confirmed cases of bacterial meningitis were caused by MTB.50
Multiplex assays for diagnosis of meningitis are commercially available, for example, BioFire,51, 52 SeeGene53 and Fast Track diagnostics,54 and Clart Entherpex Genomica.30 Panels used in these assays include HSV1/HSV2 but not MTB. BioFire and SeeGene panels include S. pneumoniae, H. influenzae, N. meningitidis testing that are requested in Africa, whereas for Fast Track kits, a separate bacterial panel is needed to test these pathogens. Furthermore, some assays cannot be performed on open and polyvalent PCR platform; hence, a dedicated automated apparatus is needed for the BioFire assay while SeeGene use a specific microchip electrophoresis system. The implementation of these syndromic diagnostics tests has just started, but cost of the tests remains one of the limitations for a large scale‐up in routine.
The results presented here show a highly specific, sensitive, and reproducible duplex PCR assay using a fast, simple, and reliable DNA extraction method for the detection of HSV1/HSV2 and MTB. This one‐step assay can be easily implemented in the clinical laboratory equipped with open and polyvalent real‐time PCR apparatus. The assay can also be used as a screening tool when large numbers of samples need to be tested, such as for epidemiological studies. Implementation of the assay in routine practice would request the use of external quality controls. Aseptic meningitis caused by HSV or MTB diagnosed by a molecular test constituted only a small proportion of the CSF samples in our setting. The high proportion of cases meeting the criteria for aseptic meningitis and without etiologies may be due to enteroviruses which represent the most common etiological agents of aseptic meningitis in high‐income countries55, 56, 57 and arbovirus. Consensus enterovirus primers may be included in the stepwise improved format of our multiplex assay. In addition, cases of TB infections have been possibly missed since the performances of MTB PCR assays are not optimal for the diagnosis of tuberculous meningitis.58
Access to nucleic acid tests has slowly increased in low‐income countries, but remains largely insufficient. Molecular diagnosis remains limited to a restricted number of infections such as HIV and tuberculosis. Development of polyvalent PCR platforms may contribute to enlarge the range of pathogens tested by molecular assays, including herpesviruses. These latter tests may also enable reduction of reagent costs. The cost of acquiring an open polyvalent PCR platform with automated extraction and real‐time thermocycler is generally over 30 000$, but these apparatuses are part of the existing equipment in central laboratories. Furthermore, in this study a Chelex®‐based method was used for extraction of HSV and MTB DNA. This method is rapid, efficient with a low risk for laboratory‐induced contamination since all the procedure can be carried out in a single tube as recently reported.59 The expense for Chelex® method is very low as compared to the commercial methods based on silica columns or magnetic beads costing from 2 to 6$ per test, which makes it particularly advantageous in low‐income countries. Future developments may include used of lyophilized reagent for the PCR.
5. CONCLUSION
In this study, we developed a PCR test dedicated to HSV1/2 and MTB detection. Our results show that HSV and MTB are etiologic agents of aseptic meningitis in Burkina Faso, and this molecular tool used under routine conditions has the potential to facilitate early detection and treatment.
ACKNOWLEDGMENTS
S Z was recipient of a PhD fellowship funding from the Mediterranean Infection Foundation (IHU Méditerranée Infection). The authors acknowledge Yalgado Ouédraogo University Hospital Center, Charles de Gaulle University Hospital Center, Sourou Sanou University Hospital Center, and National Public Health Laboratory for their contribution to the collection of cerebrospinal fluid samples.
Zida S, Kolia‐Diafouka P, Kania D, et al. Combined testing for herpes simplex virus and Mycobacterium tuberculosis DNA in cerebrospinal fluid of patients with aseptic meningitis in Burkina Faso, West Africa. J Clin Lab Anal. 2019;33:e22719 10.1002/jcla.22719
REFERENCES
- 1. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, Farrar J. Tuberculous meningitis. J Neurol Neurosurg Psychiatry. 2000;68:289‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Almeida SM, Nogueira MB, Raboni SM, Vidal LR. Laboratorial diagnosis of lymphocytic meningitis. Braz J Infect Dis. 2007;11:489‐495. [DOI] [PubMed] [Google Scholar]
- 4. Mourvillier B. Wolff M. Rev Prat. 2006;56:1927‐1932. [PubMed] [Google Scholar]
- 5. Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: a review. J Infect. 2009;59(3):156‐166. [DOI] [PubMed] [Google Scholar]
- 6. Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285‐298. [DOI] [PubMed] [Google Scholar]
- 7. Irani DN. Aseptic meningitis and viral myelitis. Neurol Clin. 2008;26(3):635‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilkinson RJ, Rohlwink U, Misra UK, et al. Tuberculous meningitis. Nat Rev Neurol. 2017;13:581‐598. [DOI] [PubMed] [Google Scholar]
- 9. Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis. 1995;20:971‐981. [DOI] [PubMed] [Google Scholar]
- 10. Parent du Châtelet I, Traore Y, Gessner BD, et al. Bacterial meningitis in Burkina Faso: surveillance using field‐based polymerase chain reaction testing. Clin Infect Dis. 2005;40:17‐25. [DOI] [PubMed] [Google Scholar]
- 11. Yaro S, Lourd M, Traoré Y, et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clin Infect Dis. 2006;43:693‐700. [DOI] [PubMed] [Google Scholar]
- 12. Diallo AO, Soeters HM, Yameogo I, et al. Bacterial meningitis epidemiology and return of Neisseria meningitidis serogroup A cases in Burkina Faso in the five years following MenAfriVac mass vaccination campaign. PLoS One. 2017;12:e0187466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S‐H, Cho O‐H, Park S‐J, et al. Rapid diagnosis of tuberculous meningitis by T cell‐based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Infect Dis. 2010;50:1349‐1358. [DOI] [PubMed] [Google Scholar]
- 14. James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka MM, Rosenberg NA, Small PM. The control of copy number of IS6110 in Mycobacterium tuberculosis . Mol Biol Evol. 2004;21:2195‐2201. [DOI] [PubMed] [Google Scholar]
- 16. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12:999‐1010. [DOI] [PubMed] [Google Scholar]
- 17. Tapiainen T, Prevots R, Izurieta HS, et al. Aseptic meningitis: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5793‐5802. [DOI] [PubMed] [Google Scholar]
- 18. Nagdev KJ, Kashyap RS, Deshpande PS, Purohit HJ, Taori GM, Daginawala HF. Determination of polymerase chain reaction efficiency for diagnosis of tuberculous meningitis in Chelex‐100 extracted DNA samples. Int J Tuberc Lung Dis. 2010;14:1032‐1038. [PubMed] [Google Scholar]
- 19. Kessler HH, Mühlbauer G, Rinner B, et al. Detection of herpes simplex virus DNA by real‐time PCR. J Clin Microbiol. 2000;38:2638‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thierry D, Brisson‐Noël A, Vincent‐Lévy‐Frébault V, Nguyen S, Guesdon JL, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990;28:2668‐2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deshpande PS, Kashyap RS, Ramteke SS, et al. Evaluation of the IS6110 PCR assay for the rapid diagnosis of tuberculous meningitis. Cerebrospinal Fluid Res. 2007;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazurek GH, Cave MD, Eisenach KD, Wallace RJ, Bates JH, Crawford JT. Chromosomal DNA fingerprint patterns produced with IS6110 as strain‐specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991;29:2030‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1‐196. [DOI] [PubMed] [Google Scholar]
- 24. McEvoy C, Falmer AA, Gey van Pittius NC, Victor TC, van Helden PD, Warren RM. The role of IS6110 in the evolution of Mycobacterium tuberculosis . Tuberculosis (Edinb). 2007;87:393‐404. [DOI] [PubMed] [Google Scholar]
- 25. Country statistics and global health estimates by WHO and UN Partner: Burkina Faso WHO statistical profile. 2015.
- 26. Lee BW, Tan JA, Wong SC, et al. DNA amplification by the polymerase chain reaction for the rapid diagnosis of tuberculous meningitis. Comparison of protocols involving three mycobacterial DNA sequences, IS6110, 65 kDa antigen, and MPB64. J Neurol Sci. 1994;123:173‐179. [DOI] [PubMed] [Google Scholar]
- 27. Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain‐biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857‐863. [DOI] [PubMed] [Google Scholar]
- 28. Szpara ML, Gatherer D, Ochoa A, et al. Evolution and diversity in human herpes simplex virus genomes. J Virol. 2014;88:1209‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aguilar JS, Devi‐Rao GV, Rice MK, Sunabe J, Ghazal P, Wagner EK. Quantitative comparison of the HSV‐1 and HSV‐2 transcriptomes using DNA microarray analysis. Virology. 2006;348:233‐241. [DOI] [PubMed] [Google Scholar]
- 30. Leveque N, Van Haecke A, Renois F, Boutolleau D, Talmud D, Andreoletti L. Rapid virological diagnosis of central nervous system infections by use of a multiplex reverse transcription‐PCR DNA microarray. J Clin Microbiol. 2011;49:3874‐3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodrigues D, de‐Paris F, Paiva RM. Minimum detection limit of an in‐house nested‐PCR assay for herpes simplex virus and varicella zoster virus. Rev Soc Bras Med Trop. 2013;46:625‐628. [DOI] [PubMed] [Google Scholar]
- 32. Schloss L, Falk KI, Skoog E, Brytting M, Linde A, Aurelius E. Monitoring of herpes simplex virus DNA types 1 and 2 viral load in cerebrospinal fluid by real‐time PCR in patients with herpes simplex encephalitis. J Med Virol. 2009;81:1432‐1437. [DOI] [PubMed] [Google Scholar]
- 33. Pillet S, Verhoeven PO, Epercieux A, Bourlet T, Pozzetto B. Development and validation of a laboratory‐developed multiplex real‐time PCR assay on the BD max system for detection of herpes simplex virus and varicella‐zoster virus DNA in various clinical specimens. J Clin Microbiol. 2015;53:1921‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alford CA, Dolin R, Hirsch MS, Karchmer AW, Whitley RJ. Herpes simplex encephalitis and clinical trial design. Lancet. 1982;1:1013. [DOI] [PubMed] [Google Scholar]
- 35. Benjamin LA, Kelly M, Cohen D, et al. Detection of herpes viruses in the cerebrospinal fluid of adults with suspected viral meningitis in Malawi. Infection. 2013;41:27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El‐Amin EO, Elbashir M, Elamin OE, et al. The underlying aetiologies of coma in febrile Sudanese children. Trans R Soc Trop Med Hyg. 2013;107:307‐312. [DOI] [PubMed] [Google Scholar]
- 37. Mallewa M, Vallely P, Faragher B, et al. Viral CNS infections in children from a malaria‐endemic area of Malawi: a prospective cohort study. Lancet Glob Health. 2013;1:e153‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Archimbaud C, Chambon M, Bailly JL, et al. Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis. J Med Virol. 2009;81:42‐48. [DOI] [PubMed] [Google Scholar]
- 39. Vollbach S, Müller A, Drexler JF, et al. Prevalence, type and concentration of human enterovirus and parechovirus in cerebrospinal fluid samples of pediatric patients over a 10‐year period: a retrospective study. Virol J. 2015;12(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akhvlediani T, Bautista CT, Shakarishvili R, et al. Etiologic Agents of central nervous system infections among febrile hospitalized patients in the country of Georgia. PLoS One. 2014;9:e111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor WR, Nguyen K, Nguyen D, et al. The spectrum of central nervous system infections in an adult referral hospital in Hanoi, Vietnam. PLoS One. 2012;7(8):e42099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan LV, Thai LH, Phu NH, et al. Viral Aetiology of central nervous system infections in adults admitted to a tertiary referral hospital in Southern Vietnam over 12 years. PLoS Negl Trop Dis. 2014;8:e3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi R, Kim G‐M, Jo IJ, et al. Incidence and clinical features of herpes simplex viruses (1 and 2) and varicella‐zoster virus infections in an adult Korean population with aseptic meningitis or encephalitis. J Med Virol. 2014;86:957‐962. [DOI] [PubMed] [Google Scholar]
- 44. Franzen‐Röhl E, Tiveljung‐Lindell A, Grillner L, Aurelius E. Increased detection rate in diagnosis of herpes simplex virus type 2 meningitis by real‐time PCR using cerebrospinal fluid samples. J Clin Microbiol. 2007;45:2516‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forsgren M, Sköldenberg B, Aurelius E, Gille E. Neurologic morbidity after herpes simplex virus type 2 meningitis: a retrospective study of 40 patients. Scand J Infect Dis. 2002;34:278‐283. [DOI] [PubMed] [Google Scholar]
- 46. Schutte CM, Van der Meyden CH, Magazi DS. The impact of HIV on meningitis as seen at a South African Academic Hospital (1994 to 1998). Infection. 2000;28:3‐7. [DOI] [PubMed] [Google Scholar]
- 47. Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nanoo A, Izu A, Ismail NA, et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004–12: a time series analysis. Lancet Infect Dis. 2015;15:1066‐1076. [DOI] [PubMed] [Google Scholar]
- 49. Page A‐L, Boum Ii Y, Kemigisha E, et al. Aetiology and outcomes of suspected infections of the central nervous system in children in Mbarara, Uganda. Sci Rep. 2017;7:2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mindadou H, Sidikou F, Boisier P, Chanteau S. Prevalence of tuberculous meningitis in Niamey’s Hospital, Niger. Bull Soc Pathol Exot. 1990;2006(99):39‐40. [DOI] [PubMed] [Google Scholar]
- 51. Leber AL, Everhart K, Balada‐Llasat J‐M, et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanson KE, Slechta ES, Killpack JA, et al. Preclinical assessment of a fully automated multiplex PCR panel for detection of central nervous system pathogens. J Clin Microbiol. 2016;54:785‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin SY, Kwon KC, Park JW, Kim JM, Shin SY, Koo SH. Evaluation of the Seeplex® meningitis ACE detection kit for the detection of 12 common bacterial and viral pathogens of acute meningitis. Ann Lab Med. 2012;32:44‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walls T, McSweeney A, Anderson T, Jennings LC. Multiplex‐PCR for the detection of viruses in the CSF of infants and young children. J Med Virol. 2017;89:559‐561. [DOI] [PubMed] [Google Scholar]
- 55. Read SJ, Jeffery KJ, Bangham CR. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95:e2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dewan M, Zorc JJ, Hodinka RL, Shah SS. Cerebrospinal fluid enterovirus testing in infants 56 days or younger. Arch Pediatr Adolesc Med. 2010;164:824‐830. [DOI] [PubMed] [Google Scholar]
- 58. Solomons RS, van Elsland SL, Visser DH, et al. Commercial nucleic acid amplification tests in tuberculous meningitis–a meta‐analysis. Diagn Microbiol Infect Dis. 2014;78:398‐403. [DOI] [PubMed] [Google Scholar]
- 59. Kolia‐Diafouka P, Godreuil S, Bourdin A, et al. Lysis‐extraction method combined with IS6110‐amplification for detection of Mycobacterium tuberculosis in paucibacillary sputum specimens. Front Microbiol. 2018;9:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]


