Abstract
Objective
Our aim intended to determine the relationship between hematological parameters (neutrophil‐to‐lymphocyte ratio [NLR], platelet‐to‐lymphocyte ratio [PLR], and eosinophil‐to‐lymphocyte ratio [ELR]) and ancylostomiasis.
Methods
There were 140 patients with ancylostomiasis and 159 healthy controls enrolled in this study. All data were collected from electronic medical records of the First Affiliated Hospital of Guangxi Medical University.
Results
The levels of NLR, PLR, and ELR in ancylostomiasis patients were significantly higher than those in the healthy controls (all P = 0.000). A receiver operating characteristic curve was generated to assess the diagnostic efficacy of these three hematological parameters. ELR (AUC = 0.850; sensitivity = 75.00%; specificity = 86.80%) showed the superior AUC than those of NLR (AUC = 0.718; sensitivity = 53.57%; specificity = 88.68%) and PLR (AUC = 0.806; sensitivity = 68.57%; specificity = 86.79%), respectively. A multivariate regression model using the two selected indices (RBC and ELR) was established with the model's sensitivity and specificity reached 82.86% and 96.23%, respectively. In the ancylostomiasis patient group, NLR (r = −0.452, P = 0.000) and PLR (r = −0.357, P = 0.000) were reversely associated with eosinophils.
Conclusion
The pretreatment levels of the three hematological parameters (NLR, PLR, and ELR) may serve as valuable indicators for distinguishing patients with ancylostomiasis from healthy controls. NLR and PLR are negatively associated with the previous indicator, eosinophils.
Keywords: ancylostomiasis, eosinophil‐to‐lymphocyte ratio, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio
1. INTRODUCTION
Ancylostome is one of the most prevalent soil‐borne nematodes in rural areas in China, especially in tropical and subtropical areas with scarce resources and poor sanitary conditions.1, 2, 3 Among the parasitic nematodes in the digestive tract of the human body, ancylostomes are the most harmful, causing severe chronic blood loss and multisystem diseases.4, 5 Ancylostomiasis is one of the five major parasitic diseases in China, where it is more common in environments with a warm climate, abundant rainfall, and fertile soil.6, 7, 8, 9
Inflammatory response plays an indispensable role in the pathogenesis and severity of ancylostomiasis.10 The correlation between a systemic inflammation marker such as C‐reactive protein and ancylostomiasis has been reported.11 More recently, some hematological parameters related to inflammation have received extensive attention, which include the neutrophil‐to‐lymphocyte ratio (NLR), the platelet‐to‐lymphocyte ratio (PLR), and the eosinophil‐to‐lymphocyte ratio (ELR). The predictions and diagnostic significance of these three parameters have been evaluated in a variety of diseases, such as metastatic melanoma, systemic lupus erythematosus (SLE), and Clonorchis sinensis infection.12, 13, 14 Nevertheless, the clinical value of these three hematological parameters (NLR, PLR, and ELR) in ancylostomiasis is still unclear.
Hence, our study intended to investigate the association between these three hematological parameters (NLR, PLR, and ELR) and ancylostomiasis. We established a model with superior AUC, sensitivity, and specificity for differentiating ancylostomiasis patients from healthy controls.
2. MATERIALS AND METHODS
2.1. Patients
There were 140 patients with ancylostomiasis and 159 healthy controls included in this study. All the data were collected from the electronic medical records of the First Affiliated Hospital of Guangxi Medical University from 2013 to 2017. The patient group was diagnosed by microscopic examination of ancylostome ova. Patients were excluded if they met any of the following criteria: (a) with other parasitic infection or co‐infection; (b) HBV, HIV, syphilis, or other viral infections; (c) hypertension or diabetes; (d) neoplastic diseases; (e) potential immune‐related diseases; (f) autoimmune diseases; or (g) blood system diseases such as leukemia; or (h) kidney diseases. The control group was matched with the patient group in gender and age. Additionally, all healthy controls were selected from the Physical Examination Center of our hospital. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, and the informed consent of all participants was obtained.
2.2. Data collection
The following data from healthy controls and patients before treatment were collected: gender, age, white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), neutrophils (NEU), lymphocytes (LYM), and eosinophils (EOS).
2.3. Laboratory measurements
Routine blood parameters were measured using the Beckman Coulter LH 780 blood analyzer (Beckman Coulter, Brea, CA, USA). NLR (neutrophils/lymphocytes), PLR (platelets/lymphocytes), and ELR (eosinophils/lymphocytes) were calculated based on the blood cell counts accordingly. The colloidal gold method was used to detect fecal occult blood.
2.4. Statistical analysis
Kolmogorov‐Smirnov test was used to evaluate the distribution status of the collected data. The normal distribution data were expressed by mean and standard deviation (mean ± SD), and the non‐normal distribution data were expressed by the median and interquartile range (IQR). The clinical characteristics of the ancylostomiasis patients were described by quantity and proportion. To compare the statistical differences between the two groups, a Student t test and Mann‐Whitney U test were used to compare the quantitative data as appropriate, while a chi‐square test was used to compare the categorical variables. Univariate and multivariate analysis served to determine the independent risk factors for ancylostomiasis. Spearman's correlation coefficient was performed for inspecting the relativity between two variables. A model derived from multivariate logic regression. A receiver operating characteristic (ROC) curve and the area under the ROC curve (AUROC) were generated to assess the diagnostic efficiency of NLR, PLR, ELR, and the multivariate regression model. The statistical analysis and graphical processing of all data were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA), GraphPad Prism 5, and MedCalc statistical software (version 11.3.8.0). The value of P < 0.05 (two‐tailed) was considered to be statistically significant.
3. RESULTS
3.1. Clinical characteristics of ancylostomiasis patients
The clinical characteristics of the ancylostomiasis patients were summarized in Table 1. There were 140 patients included in this study with average age of 59 years. Male and female accounted for 36.43% and 63.57%, respectively. Of those, 90 patients (64.28%) had anemia, while only 5.71% of the ancylostomiasis patients showed severe anemia. The positive rate of the fecal occult blood test was only 14.28%. Hypoproteinemia was seen in 74.28% of the ancylostomiasis patients. Ninety‐four (67.14%) patients had clinical manifestations of abdominal discomfort, 81 (57.86%) had weight loss, 61 (43.57%) had hypodynamia, 20 (14.28%) had cough, and 14 (10.00%) had dermatitis.
Table 1.
Characteristics of patients with ancylostomiasis
| Characteristics | Median range or number (%) (n: 140) |
|---|---|
| Gender | |
| Male | 51 (36.43%) |
| Female | 89 (63.57%) |
| Age (y) | 59.00 (1.00‐85.00) |
| Clinical symptoms | |
| Anemia | 90 (64.28%) |
| Mild anemia | 47 (33.57%) |
| Moderate anemia | 35 (25.00%) |
| Severe anemia | 8 (5.71%) |
| Fecal occult blood test | |
| Positive | 20 (14.28%) |
| Negative | 120 (85.71%) |
| Hypoproteinemia | 104 (74.28%) |
| Abdominal discomfort | 94 (67.14%) |
| Weight loss | 81 (57.86%) |
| Hypodynamia | 61 (43.57%) |
| Cough | 20 (14.28%) |
| Dermatitis | 14 (10.00%) |
3.2. Comparison of hematological parameters between ancylostomiasis patients and healthy controls
As shown in Table 2, WBC (P = 0.937) and neutrophils (P = 0.327) were not statistically different between ancylostomiasis patients and the healthy controls. However, for the patient group, the median (25th‐75th IQR) MCV, MCH, and MCHC were 86.88 (73.42, 91.78) fL, 28.36 (22.96, 30.40) pg, and 325.05 (312.50, 332.15) g/L, respectively. For the control group, the median (25th‐75th IQR) MCV, MCH, and MCHC were 90.04 (87.51, 92.22) fL, 29.91 (29.04, 30.82) pg, and 332.80 (329.00, 335.80) g/L, respectively. Therefore, the ancylostomiasis patients showed lower MCV, MCH, and MCHC levels than those observed in healthy controls (P = 0.000). The RBC and HGB values of ancylostomiasis patients were also significantly lower than those of the controls (RBC 3.87 ± 0.80 × 1012/L vs 4.53 ± 0.29 × 1012/L, P = 0.000; HGB 103.44 ± 24.96 g/L vs 135.48 ± 8.31 g/L, P = 0.000, respectively). The PLT, NEU, and EOS counts of ancylostomiasis patients were also considerably higher than those for the healthy controls (PLT 255.30 [209.62, 320.10] × 109/L vs 232.30 [206.70, 260.90] × 109/L, P = 0.002; NEU 3.64 [2.59, 5.33] × 109/L vs 3.58 [2.93, 4.13] × 109/L, P = 0.000; EOS 0.35 [0.15, 0.72] × 109/L vs 0.11 [0.07, 0.20] × 109/L, P = 0.000, respectively), while the LYM counts in the patient group showed a low level compared to the control group (LYM 1.36 [1.06, 1.87] × 109/L vs 2.15 [1.86, 2.40] × 109/L, P = 0.000).
Table 2.
Comparison of laboratory parameters in ancylostomiasis patients with those of healthy controls
| Patients (n = 140) | Controls (n = 159) | P‐value | |
|---|---|---|---|
| Gender(male/female) | 51/89 | 61/98 | 0.730 |
| Age (y) | 59.00 (50.00, 65.00) | 62.00 (42.00, 67.00) | 0.139 |
| WBC (×109/L) | 6.39 (4.88, 8.96) | 6.29 (5.61, 7.28) | 0.937 |
| RBC (×1012/L) | 3.87 ± 0.80 | 4.53 ± 0.29 | 0.000 |
| HGB (g/L) | 103.44 ± 24.96 | 135.48 ± 8.31 | 0.000 |
| MCV (fL) | 86.88 (73.42, 91.78) | 90.04 (87.51, 92.22) | 0.000 |
| MCH (pg) | 28.36 (22.96, 30.40) | 29.91 (29.04, 30.82) | 0.000 |
| MCHC (g/L) | 325.05 (312.50, 332.15) | 332.80 (329.00, 335.80) | 0.000 |
| PLT (×109/L) | 255.30 (209.62, 320.10) | 232.30 (206.70, 260.90) | 0.002 |
| NEU (×109/L) | 3.64 (2.59, 5.33) | 3.58 (2.93, 4.13) | 0.327 |
| LYM (×109/L) | 1.36 (1.06, 1.87) | 2.15 (1.86, 2.40) | 0.000 |
| EOS (×109/L) | 0.35 (0.15, 0.72) | 0.11 (0.07, 0.20) | 0.000 |
| NLR | 2.60 (1.63, 4.47) | 1.61 (1.36, 2.06) | 0.000 |
| PLR | 171.52 (129.74, 269.48) | 106.83 (90.00, 131.76) | 0.000 |
| ELR | 0.26 (0.13, 0.48) | 0.06 (0.03, 0.09) | 0.000 |
ELR, eosinophil‐to‐lymphocyte ratio; EOS, eosinophils; HGB, hemoglobin; LYM, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NEU, neutrophils; NLR, neutrophil‐to‐lymphocyte ratio; Patients, ancylostomiasis patients; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet count; RBC, red blood cells; WBC, white blood cells.
3.3. Comparison of candidate markers (NLR, PLR, and ELR) between ancylostomiasis patients and healthy controls
In the present research, we evaluated the differences in three candidate markers (NLR, PLR, and ELR) between patients and controls. As shown in Figure 1, the levels of the three candidate markers in the disease group increased significantly compared with those of the control group (P = 0.000); in particular, the ancylostomiasis patients had substantially increased ELR levels in comparison with the healthy controls (P = 0.000). The above results indicate that NLR, PLR, and ELR have important significance for the identification of ancylostomiasis.
Figure 1.
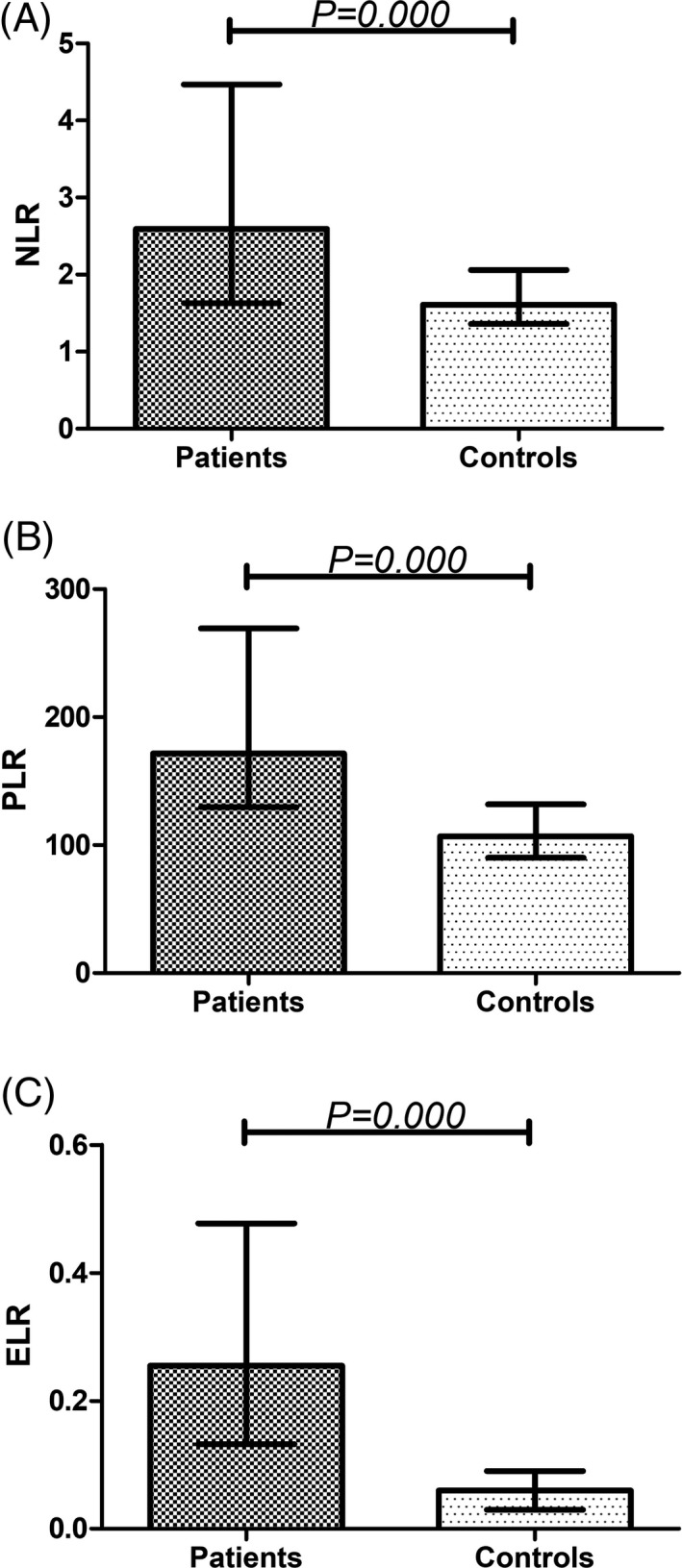
Comparison of NLR (A), PLR (B), ELR (C) in ancylostomiasis patients and healthy controls. ELR, eosinophil‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio
3.4. Establishment of a potential multivariate logistic regression model for distinguishing ancylostomiasis patients from healthy controls
The relationship between ancylostomiasis and hematological parameters was determined by univariate analysis, and then, statistically significant variables were selected for multivariate analysis. The results of the univariate analysis are presented in Table 3, which reveals that RBC (OR = 0.108, 95% CI = 0.059‐0.197, P = 0.000), MCV (OR = 0.899, 95% CI = 0.867‐0.933, P = 0.000), MCH (OR = 0.719, 95% CI = 0.647‐0.800, P = 0.000), MCHC (OR = 0.898, 95% CI = 0.869‐0.928, P = 0.000), NLR (OR = 2.372, 95% CI = 1.769‐3.180, P = 0.000), PLR (OR = 1.025, 95% CI = 1.018‐1.031, P = 0.000), and ELR (OR = 1.134 × 107, 95% CI = 0.015 × 107‐8.510 × 108, P = 0.000) can be used as potential independent predictors of ancylostomiasis epidemics. Then, the seven significant variables were analyzed by multivariate analysis. The results showed that RBC (β = −3.094, P = 0.000) and ELR (β = 20.722, P = 0.000) were significantly correlated with the occurrence of ancylostomiasis; thus, we obtained the optimal model (logit P = 20.722 × ELR−3.094 × RBC + 10.431) for differentiating ancylostomiasis patients from healthy controls.
Table 3.
Univariate and multivariate analyses used for differentiating significant predictors to distinguish ancylostomiasis patients from healthy controls
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Gender | 1.086 | 0.679‐1.738 | 0.730 | |||
| Age (y) | 1.009 | 0.992‐1.025 | 0.139 | |||
| RBC (×1012/L) | 0.108 | 0.059‐0.197 | 0.000 | 0.047 | 0.013‐0.175 | 0.000 |
| MCV (fL) | 0.899 | 0.867‐0.933 | 0.000 | 0.016 | 0.000‐6.471 | 0.177 |
| MCH (pg) | 0.719 | 0.647‐0.800 | 0.000 | 1.790 × 105 | 0.003‐1.261 × 1013 | 0.190 |
| MCHC (g/L) | 0.898 | 0.869‐0.928 | 0.000 | 0.304 | 0.059‐1.564 | 0.154 |
| NLR | 2.372 | 1.769‐3.180 | 0.000 | 2.071 | 0.989‐4.333 | 0.053 |
| PLR | 1.025 | 1.018‐1.031 | 0.000 | 1.015 | 1.000‐1.030 | 0.053 |
| ELR | 1.134 × 107 | 0.015 × 107‐8.510 × 108 | 0.000 | 4.746 × 109 | 1.584 × 106‐1.422 × 1013 | 0.000 |
ELR, eosinophil‐to‐lymphocyte ratio; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; RBC, red blood cells.
3.5. Establishment of the AUC, sensitivity, and specificity of EOS, NLR, PLR, ELR, and logit P for the identification of ancylostomiasis
The ROC curve was created by comparing the hematological parameters of patients with ancylostomiasis with those of healthy controls (Figure 2). The maximum Youden index obtained by the ROC curves determined the optimal cutoff values of five candidate markers. Our results demonstrated that the cutoff values of EOS, NLR, PLR, ELR, and the multivariate regression model were 0.29, 2.38, 145.99, 0.13, and 0.12, respectively. For EOS, the area under the ROC curve (AUC) value was 0.761 (95% CI 0.708‐0.808; sensitivity = 57.14%; specificity = 89.31%). For NLR, the AUC value was 0.718 (95% CI 0.663‐0.768; sensitivity = 53.57%; specificity = 88.68%). The AUC value of PLR was 0.806 (95% CI 0.756‐0.849; sensitivity = 68.57%; specificity = 86.79%). ELR produced an AUC value of 0.850 (95% CI 0.804‐0.888; sensitivity = 75.00%; specificity = 86.80%). In particular, the multivariate logistic regression model (logit P) indicated the best distinction between ancylostomiasis patients and healthy controls, and its AUC value was 0.939 (95% CI 0.906‐0.963; sensitivity = 82.86%; specificity = 96.23%).
Figure 2.
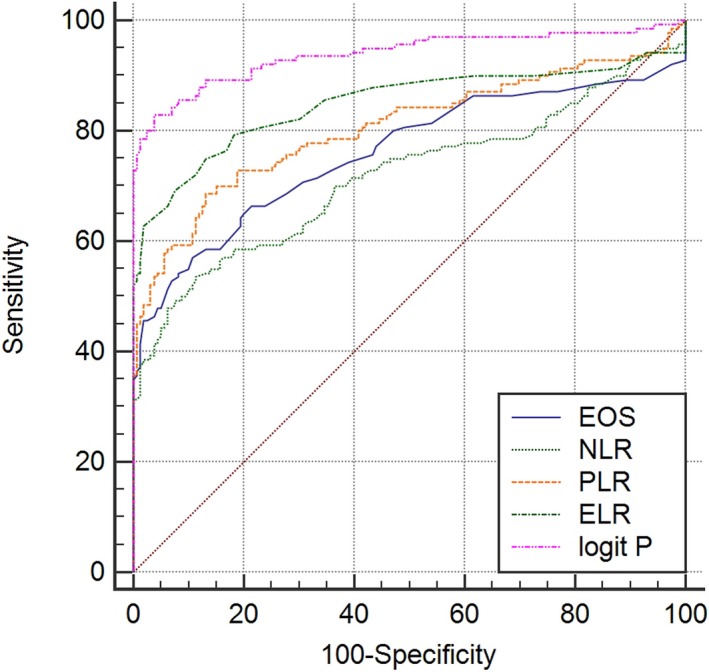
Receiver operating characteristic curves of EOS, NLR, PLR, ELR, and the logit P for distinguishing ancylostomiasis patients from healthy controls. ELR, eosinophil‐to‐lymphocyte ratio; EOS, eosinophils; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio
3.6. Correlations of NLR and PLR with EOS in patients with ancylostomiasis
The association of EOS with NLR and PLR in the patient group was presented in Figure 3. There was a significant negative correlation between NLR and EOS (r = −0.452, P = 0.000, Figure 3A). A significant negative association was also observed between PLR and EOS in ancylostomiasis patients (r = −0.357, P = 0.000, Figure 3B).
Figure 3.
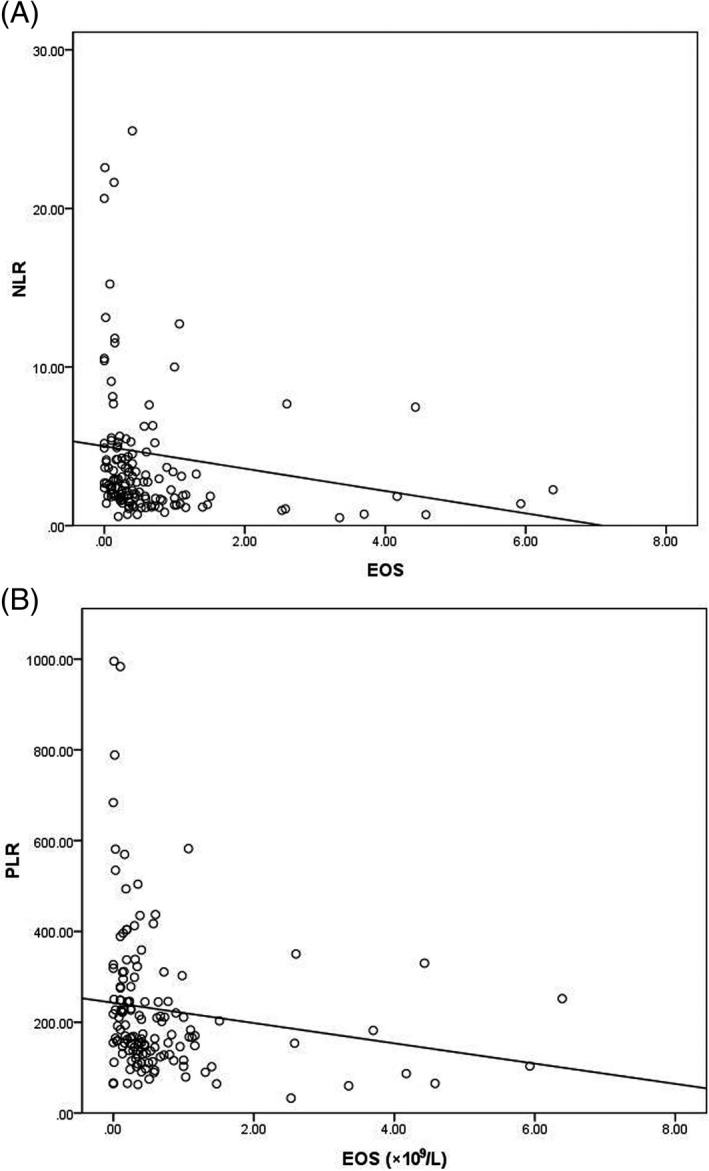
Correlation analysis between NLR and EOS, PLR and EOS in patients with ancylostomiasis. A, NLR and EOS in patients with ancylostomiasis; B, PLR and EOS in patients with ancylostomiasis. ELR, eosinophil‐to‐lymphocyte ratio; EOS, eosinophils; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio
4. DISCUSSION
Ancylostomiasis is characterized by long‐term chronic blood loss and the initiation of multiple systemic diseases (such as diseases of the respiratory, digestive, and cardiovascular systems).4, 5, 15 A definitive diagnosis of ancylostomiasis and early treatment are critical in control the development of the disease and avoiding complications affecting multiple systems, thus further reducing the financial burden of patients.
Inflammation plays a major role in the pathogenesis of ancylostome.10 For our research, the diagnostic efficacy of NLR, PLR, and ELR in ancylostomiasis was investigated. Our findings showed that the NLR, PLR, and ELR values of patients were significantly higher than those of controls. This suggests that these three inflammatory markers (NLR, PLR, and ELR) are statistically significant in differentiating patients with ancylostomiasis from healthy controls.
The mechanisms of the hematological change of these parameters and ancylostomiasis were unclear. Studies by Veraldi et al showed that neutrophils and lymphocytes were involved in the development of the ancylostome infection and that these cells were found in some tissue fluids.16, 17, 18 Wiwanitkit et al19 revealed that platelets were associated with ancylostomiasis. We observed elevated neutrophils and platelets and decreased lymphocytes in the patient group in comparison with the healthy controls. Our findings are consistent with previous studies discussed above. After the organism was infected with the ancylostome, the chemotactic neutrophils concentrated heavily on the local lesion and carried out active phagocytosis and secretion. Platelets were concentrated in hemostasis, and reduced lymphocytes caused a decrease in body immune function. This may explain the clinical value of elevated NLR and PLR for ancylostomiasis.
Eosinophils were closely related to ancylostome infection.20, 21 And from the standard interpretation of the diagnosis of ancylostomiasis (WS 439‐2013), we found that eosinophils were included in the diagnostic criteria for ancylostomiasis. In our research results, NLR, PLR, and EOS were significantly correlated in patients group; therefore, the clinical values of the two inflammatory markers (NLR and PLR) were investigated. More importantly, PLR performed with good identification of ancylostomiasis, which was comparable to or even beyond that of EOS.
Microscopic examination of parasite eggs has been the gold standard for the diagnosis of most parasitic diseases, and ancylostomiasis has been no exception.22, 23 However, a study by Walana et al24 showed that the positive rate for parasite eggs was only 0.3%; this positive rate is hyperbolically low compared to 47 147 patients with intestinal parasite infection. Thus, the miss rate for microscopic examination of parasitic eggs is extremely high. There are also some inadequacies in the microscopic examination of parasitic eggs; this examination method requires some dangerous chemicals, such as concentrated hydrochloric acid and ether, which will cause harm to the human body. Also, a large amount of time is required to complete the examination, which affects the progress of inspectors and doctors in diagnosing the disease. In contrast, blood routine parameters such as ELR and PLR are relatively easy to obtain and fast to detect, and they have a wide range of clinical applications. Hence, ELR and PLR may be alternative or complementary choices.
The diagnostic value of ELR has been rarely studied.14 Our study demonstrates similar findings as shown by others the diagnostic and recurrence prediction value of ELR in nasal diseases, such as nasal polyposis and sinonasal polyps.25, 26 Moreover, ELR (AUC = 0.850; sensitivity = 75.00%; specificity = 86.80%) performed with the most superior AUC compared with NLR (AUC = 0.718; sensitivity = 53.57%; specificity = 88.68%) and PLR (AUC = 0.806; sensitivity = 68.57%; specificity = 86.79%). The clinical significance and application of ELR in different diseases will be an interesting topic to study in the future. After univariate and multivariate analysis, we also found that increased RBC and ELR are risk factors for ancylostomiasis. Based on these two indicators, we established a multivariate regression model: logit P = 20.722 × ELR − 3.094 × RBC + 10.431. The value of this model (AUC = 0.939; sensitivity = 82.86%; specificity = 96.23%) in differentiating ancylostomiasis patients from healthy controls was superior to that of NLR, PLR, and ELR. Our findings demonstrate a number of advantages: Firstly, we first proposed the use of routine blood parameters (NLR, PLR, and ELR) to identify ancylostomiasis. Secondly, for the first time, our research has established a model with excellent clinical performance for distinguishing ancylostomiasis patients from healthy controls. Finally, we are the first to study the relationship between NLR, PLR, and EOS.
Nevertheless, there are also limitations to our study. First of all, our research is a retrospective analysis, so it has some unavoidable inherent defects, such as recall bias and selection bias. Second, the sample size is relatively small with only 140 patients, due to the low prevalence of ancylostomiasis in our region. Thirdly, our research is a single‐center study, which does not reflect the overall situation of ancylostomiasis. Consequently, our findings need to be validated further by multicenter and large population in prospective studies.
In conclusion, our results reveal that the pretreatment values of NLR, PLR, and ELR may be helpful in the identification of ancylostomiasis. NLR and PLR have a negative correlation with the EOS. ELR may be an independent risk factor for ancylostomiasis and serve as the most effective indicator to distinguish patients with ancylostomiasis from healthy controls.
AUTHORS’ CONTRIBUTIONS
XQ and SL drafted the overall design of this paper as the co‐corresponding authors. ZH and HC wrote the article. LH, SC, and ZH collected the laboratory data. SQ and JZ analyzed the data.
Hu Z, Chen H, Huang L, et al. Correlation between hematological parameters and ancylostomiasis: A retrospective study. J Clin Lab Anal. 2019;33:e22705 10.1002/jcla.22705
Funding information
This work is supported by National Natural Science Foundation of China (NO. 81560447) and the Science Study of Guangxi Health and Family Planning Commission (Z2016296).
Contributor Information
Xue Qin, Email: qinxue919@126.com.
Shan Li, Email: lis8858@126.com.
REFERENCES
- 1. Adriko M, Tinkitina B, Arinaitwe M, Kabatereine NB, Nanyunja M, Tukahebwa EM. Impact of a national deworming campaign on the prevalence of soil‐transmitted helminthiasis in Uganda (2004–2016): Implications for national control programs. PLoS Negl Trop Dis. 2018;12(7):e0006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riess H, Clowes P, Kroidl I, et al. Hookworm infection and environmental factors in mbeya region, Tanzania: a cross‐sectional, population‐based study. PLoS Negl Trop Dis. 2013;7(9):e2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarkar R, Rose A, Mohan VR, et al. Study design and baseline results of an open‐label cluster randomized community‐intervention trial to assess the effectiveness of a modified mass deworming program in reducing hookworm infection in a tribal population in southern India. Contemp Clin Trials Commun. 2017;5:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Traub RJ. Ancylostoma ceylanicum, a re‐emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43(12–13):1009‐1015. [DOI] [PubMed] [Google Scholar]
- 5. Mukaya JE, Ddungu H, Ssali F, O'Shea T, Crowther MA. Prevalence and morphological types of anaemia and hookworm infestation in the medical emergency ward, Mulago Hospital, Uganda. S Afr Med J. 2009;99(12):881‐886. [PubMed] [Google Scholar]
- 6. Xiao‐Xiang P, Jin‐Sheng A, Li Z, Hong‐Sheng W. [Surveillance of soil‐borne nematodiasis in population in Hefei City, 2012–2015]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(2):208‐210. [DOI] [PubMed] [Google Scholar]
- 7. Shan‐Shan L, Fei L, Jun X, Yi Y. [Survey and analysis of major human parasitic diseases in Chongqing City]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(2):194‐199. [DOI] [PubMed] [Google Scholar]
- 8. Hu B, Chen GZ, Huang P. [Epidemiology related to soil‐borne nematode disease in Danzhou city, Hainan province]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(4):474‐477. [DOI] [PubMed] [Google Scholar]
- 9. Krause RJ, Koski KG, Pons E, Sandoval N, Sinisterra O, Scott ME. Ascaris and hookworm transmission in preschool children from rural Panama: role of yard environment, soil eggs/larvae and hygiene and play behaviours. Parasitology. 2015;142(12):1543‐1554. [DOI] [PubMed] [Google Scholar]
- 10. Navarro S, Ferreira I, Loukas A. The hookworm pharmacopoeia for inflammatory diseases. Int J Parasitol. 2013;43(3–4):225‐231. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez‐Fernandez D, Pons E, Rueda D, et al. C‐reactive protein is differentially modulated by co‐existing infections, vitamin deficiencies and maternal factors in pregnant and lactating indigenous Panamanian women. Infect Dis Poverty. 2017;6(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khoja L, Atenafu EG, Templeton A, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab‐treated cutaneous metastatic melanoma. Cancer Med. 2016;5(10):2792‐2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Chen S, Huang Z, et al. Relationship between blood parameters and Clonorchis sinensis infection: a retrospective single center study. Int Immunopharmacol. 2018;59:120‐126. [DOI] [PubMed] [Google Scholar]
- 14. Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372‐376. [DOI] [PubMed] [Google Scholar]
- 15. Jonker FA, Calis JC, Phiri K, et al. Real‐time PCR demonstrates Ancylostoma duodenale is a key factor in the etiology of severe anemia and iron deficiency in Malawian pre‐school children. PLoS Negl Trop Dis. 2012;6(3):e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shuhua X, Hotez PJ, Binggui S, et al. Electron and light microscopy of neutrophil responses in mice vaccinated and challenged with third‐stage infective hookworm (Ancylostoma caninum) larvae. Parasitol Int. 2001;50(4):241‐248. [DOI] [PubMed] [Google Scholar]
- 17. Veraldi S, Cuka E, Pontini P, Vaira F. Bullous cutaneous larva migrans: case series and review of atypical clinical presentations. G Ital Dermatol Venereol. 2017;152(5):516‐519. [DOI] [PubMed] [Google Scholar]
- 18. Bower MA, Constant SL, Mendez S. Necator Americanus: the Na‐ASP‐2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Exp Parasitol. 2008;118(4):569‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiwanitkit V, Soogarun S, Saksirisampant W, Suwansaksri J. Platelet parameters in subjects infected with hookworm. Platelets. 2003;14(6):391‐393. [DOI] [PubMed] [Google Scholar]
- 20. Alkazmi LM, Behnke JM. The mucosal response of hamsters exposed to weekly repeated infections with the hookworm Ancylostoma ceylanicum . J Helminthol. 2013;87(3):309‐317. [DOI] [PubMed] [Google Scholar]
- 21. Marcus AD, Higgins DP, Gray R. Ivermectin treatment of free‐ranging endangered Australian sea lion (Neophoca cinerea) pups: effect on hookworm and lice infection status, haematological parameters, growth, and survival. Parasitol Res. 2015;114(7):2743‐2755. [DOI] [PubMed] [Google Scholar]
- 22. Bharti B, Bharti S, Khurana S. Worm infestation: diagnosis, treatment and prevention. Indian J Pediatr. 2017. 10.1007/s12098-017-2505-z [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Holtfreter MC, Stachs O, Reichard M, Loebermann M, Guthoff RF, Reisinger EC. Confocal laser scanning microscopy for detection of Schistosoma mansoni eggs in the gut of mice. PloS One. 2011;6(4):e18799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walana W, Aidoo EN, Tay SC. Prevalence of hookworm infection: a retrospective study in Kumasi. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S158‐S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brescia G, Barion U, Zanotti C, Parrino D, Marioni G. Pre‐ and postoperative blood neutrophil‐to‐lymphocyte and eosinophil‐to‐lymphocyte ratios in patients with sinonasal polyps: a preliminary investigation. Allergy Asthma Proc. 2017;38(5):64‐69. [DOI] [PubMed] [Google Scholar]
- 26. Yenigun A. Assessment of patients with nasal polyposis by the neutrophil‐to‐lymphocyte ratio and eosinophil‐to‐lymphocyte ratio. Kulak Burun Bogaz Ihtis Derg. 2015;25(4):193‐199. [DOI] [PubMed] [Google Scholar]


