Abstract
Objective.
To compare the perioperative morbidity and survival between abdominal radical hysterectomy (ARH) and robotic radical hysterectomy (RRH).
Methods.
A retrospective cohort of patients undergoing radical hysterectomy for cervical cancer from 2010 to 2016 was identified. Patients with stage IB1 cervical cancer were included and were grouped by ARH vs. RRH. Tumor characteristics, perioperative complications, recurrence rate, progression-free survival (PFS), and overall survival (OS) were compared between groups.
Results.
105 patients were identified; 56 underwent ARH and 49 underwent RRH. Those who had ARH were more likely to have lesions that were ≥2 cm (62% vs. 39%, p = 0.02) and that were higher grade (p = 0.048). Other tumor characteristics were similar between groups. There was no difference in perioperative complication rates between groups. Additionally, there were no differences in recurrence risk (RR) (14% vs. 24%, p = 0.22), progression-free survival (PFS) (p = 0.28), or overall survival (OS) (p = 0.16). However, in those with tumors ≥2 cm there was a higher risk of recurrence in the overall cohort (30% vs. 8%, p = 0.006), and a shorter PFS in the RRH group (HR 0.31, p = 0.04). On multivariate analysis patients that underwent ARH or had tumors < 2 cm had a lower likelihood of recurrence (HR 0.38, p = 0.04; HR 0.175, p = 0.002) and death (HR 0.21, p = 0.029; HR 0.15, p = 0.02).
Conclusion.
Perioperative morbidity was similar between those undergoing ARH vs. RRH for IB1 cervical cancer. Patients with tumors ≥ 2 cm undergoing RRH had a shorter PFS compared to ARH. On multivariate analysis, RRH and tumor size ≥ 2 cm were independently associated with recurrence and death in this population.
Keywords: Radical hysterectomy, surgical techniques, cervical cancer, patient outcomes
1. Introduction
Despite improvements in cervical cancer screening and prevention, an estimated 13,240 new cases of invasive cervical cancer will be diagnosed in the US in 2018, and 4170 women will die from this disease [1]. The standard of care for early stage cervical cancer remains surgical management with radical hysterectomy [2]. Originally performed exclusively via an abdominal approach, the advent of laparoscopic and robotic surgery has resulted in a shift toward minimally invasive techniques to accomplish this task [3]. Robotic surgery has been shown to have several advantages over laparotomy in the treatment of gynecologic malignancies, including shorter hospitalizations, fewer major complications, decreased blood loss, and higher lymph node counts [4–9]. Recently, however, results from the Laparoscopic Approach to Cervical Cancer (LACC) trial were published indicating that patients undergoing minimally invasive robotic hysterectomy have a lower rate of disease-free survival and overall survival than those who undergo a laparotomy [10]. This has the potential to cause a paradigm shift in how cervical cancer is managed and therefore led us to further evaluate the data from our institution. Our objective was to compare tumor characteristics, perioperative complications, risks of recurrence, and survival in a cohort of women undergoing abdominal radical hysterectomy (ARH) versus robotic radical hysterectomy (RRH) for early stage cervical cancer at a single institution that cares for a high volume of cervical cancer patients.
2. Materials and methods
After obtaining approval from the University of Alabama at Birmingham (UAB) Institutional Review Board, a retrospective cohort of patients who underwent radical hysterectomy for cervical cancer from 2010 to 2016 at UAB was identified. Using the International Federation of Gynecology and Obstetrics (FIGO) staging system (prior to the revision in 2018), all patients with a preoperative diagnosis of IB1 cervical cancer of squamous cell, adenocarcinoma, or adenosquamous histologies were included [11]. Patients with microscopic lesions < 5 mm deep or < 7 mm wide, or macroscopic lesions >4 cm in greatest dimension as determined by preoperative examination were excluded from the study. Preoperative imaging was at the discretion of the surgeon. All patients in this cohort underwent type III radical hysterectomy (removal of the uterus, cervix, parametria, proximal uterosacral ligaments, cardinal ligaments, and upper 1/3 of the vagina), as defined by the Gynecologic Cancer Group of the European Organization of Research and Treatment of Cancer [12]. Patients also underwent a systematic pelvic lymphadenectomy, which included removal of the internal iliac nodes, external iliac nodes, obturator nodes, and common iliac nodes. Some patients with larger tumors (≥2 cm) also underwent para-aortic lymphad-enectomy to remove lymphatic tissue surrounding the aorta and inferior vena cava to the level of the inferior mesenteric artery. The VCare® uterine manipulator was used routinely for uterine manipulation in robotic cases. The vaginal cuff was typically closed with two running 0 polydioxanone (PDS) sutures or a single running absorbable barbed suture per surgeon preference in robotic cases. The vaginal cuff was closed with either 1 chromic gut suture or 0 polyglactin (Vicryl) suture per surgeon preference in open cases. All surgeries were performed by board certified gynecologic oncologists at a single tertiary referral center. The majority of surgeries were assisted by gynecologic oncology fellows.
Patients were stratified by surgical approach depending on whether they underwent abdominal or robotic radical hysterectomy. Surgical approach was determined on an individual basis with each patient after discussion of the risks and benefits of both options. Provider recommendations were based on multiple factors, including BMI, medical comorbidities, surgical history, tumor characteristics, and provider preference. A post-hoc subgroup survival analyses of patients with tumors ≥ 2 cm or <2 cm was also performed based on recently presented data suggesting that those with tumors ≥ 2 cm may have shorter OS with robotic surgery [13]. Those who did not have definitive surgical treatment, whose data was incomplete, or who had histologies other than squamous cell, adenosquamous, or adenocarcinoma were excluded from the study. Clinical data including patient demographics, perioperative complications, tumor characteristics, and clinical outcomes were abstracted from the electronic medical record.
Differences in demographic, behavioral, and clinical characteristics were compared between the surgical approach groups using the t-test, and chi-square or Fisher’s exact test where appropriate. Progression free survival (PFS) was defined as the duration of time between surgery and the initial disease recurrence. Overall survival (OS) was defined as the amount of time between surgery and patient death. Patients were censored at the time of their last follow up as documented in the medical record. Survival analysis was performed using the Kaplan Meier method, and curves were compared with the Mantel-Cox log-rank test. Cox proportional hazards regression was used to estimate hazard ratios (HRs), 95% confidence intervals (CIs) and associated p-values. P-values of ≤0.05 (two-sided) were considered statistically significant. Multivariate logistic regression analysis examining recurrence and death was used to adjust for statistically significant clinical and pathologic differences between groups. Statistical analyses were performed using SAS version 9.4 (Cary, NC).
3. Results
A total 105 patients were identified who met the above criteria; 56 underwent abdominal radical hysterectomy and 49 underwent robotic radical hysterectomy. There were no significant differences between groups in age, BMI, race, or smoking status (Table 1). However, those who underwent ARH were more likely to have tumors that were ≥ 2 cm (62% vs 39%, p = 0.02), and had more grade 3 and unknown-grade tumors (p = 0.048). The rest of the tumor characteristics were similar between groups, with no differences in depth of invasion (p = 0.43), histology (p = 0.95), the presence of lymphovascular space invasion (LVSI) (35% vs. 26%, p = 0.38), or the presence of positive lymph nodes (15% vs. 6%, p = 0.21). In addition, there were no differences in parametrial involvement (5% vs. 6%, p = 1.0), vaginal margin involvement (4% vs. 8%, p = 0.41), or adjuvant therapy received (p = 0.17).
Table 1.
Patient information and tumor characteristics of patients undergoing radical hysterectomy from 2010 to 2016.
| Abdominal radical hysterectomy |
Robotic radical hysterectomy |
p-Value | |
|---|---|---|---|
| Number of cases | 56 | 49 | |
| Mean age (years) | 40.6 (±10.7) | 44.1 (±10.7) | 0.09 |
| Mean BMI | 27.6 (±7.1) | 28.7 (±6.7) | 0.41 |
| Race | 0.09 | ||
| African American | 7 (13%) | 14 (29%) | |
| Caucasian | 46 (82%) | 34 (69%) | |
| Other | 3 (5%) | 1 (2%) | |
| Smoker | 0.40 | ||
| No | 28 (50%) | 20 (41%) | |
| Yes | 19 (34%) | 23 (47%) | |
| Former | 9 (16%) | 6 (12%) | |
| LVSI | 0.38 | ||
| No | 34 (65%) | 35 (74%) | |
| Yes | 18 (35%) | 12 (26%) | |
| Histology | 0.95 | ||
| Squamous | 32 (57%) | 30 (61%) | |
| Adenocarcinoma | 20 (36%) | 16 (33%) | |
| Adenosquamous | 4(7%) | 3 (6%) | |
| Grade | 0.048 | ||
| FIGO Grade 1 | 2 (4%) | 8 (16%) | |
| FIGO Grade 2 | 37 (66%) | 34 (69%) | |
| FIGO Grade 3 | 9 (16%) | 5 (10%) | |
| Unknown | 8 (14%) | 2 (4%) | |
| Size of primary lesion (cm) | 0.02 | ||
| < 2 cm | 21 (38%) | 30 (61%) | |
| ≥ 2 cm | 35 (62%) | 19 (39%) | |
| Depth of invasion (cm) | 0.43 | ||
| Superficial (<0.5cm) | 15 (27%) | 19 (39%) | |
| Middle (0.5–1 .0cm) | 25 (45%) | 19 (39%) | |
| Deep (>1.0cm) | 16 (29%) | 11 (22%) | |
| Positive Lymph Nodes | 0.21 | ||
| No | 47 (85%) | 46 (94%) | |
| Yes | 8 (15%) | 3 (6%) | |
| Parametrial involvement | 1.0 | ||
| No | 53 (95%) | 46 (94%) | |
| Yes | 3 (5%) | 3 (6%) | |
| Vaginal margin involvement | 0.41 | ||
| No | 54 (96%) | 45 (93%) | |
| Yes | 2 (4%) | 4 (8%) | |
Bold indicates p-Value of < 0.05
Perioperatively, there were no differences between the groups in the total complication risk (23% vs. 29%, p = 0.66) (Table 2). Additionally, there were no differences in individual complications. This included intraoperative complications (2% vs. 0%,p = 1.0), GI complications (7% vs. 4%, p = 0.68), urinary complications, (5% vs. 4%, p = 1.0), UTI/pyelonephritis (4% vs. 4%, p = 1.0), pelvic abscess (2% vs. 6%, p = 0.34), wound infection (2% vs. 2%, p = 1.0), wound dehiscence (0% vs. 0%, p = 1.0), pneumonia (5% vs 0%, p = 0.25), vaginal cuff dehiscence (0% vs 6%, p = 0.10), fistula formation (5% vs. 6%, p = 1.0), venous thromboembolism (VTE) (2% vs. 4%, p = 0.60), lymphedema (2% vs 2%, p = 1.0), and hernias (2% vs. 6%, p = 0.34).
Table 2.
Perioperative complications.
| Abdominal radical hysterectomy |
Robotic radical hysterectomy |
p-Value | |
|---|---|---|---|
| Perioperative complications | |||
| Any complication | 0.66 | ||
| No | 43 (77%) | 35 (71%) | |
| Yes | 13(23%) | 14 (29%) | |
| Intraoperative complication | 1.0 | ||
| No | 55 (98%) | 49 (100%) | |
| Yes | 1 (2%) | 0 (0%) | |
| GI complication | 0.68 | ||
| No | 52 (93%) | 47 (96%) | |
| Yes | 4(7%) | 2 (4%) | |
| Urinary complication | 1.0 | ||
| No | 53 (95%) | 47 (96%) | |
| Yes | 3 (5%) | 2 (4%) | |
| UTI/Pyelonephritis | 1.0 | ||
| No | 54 (96%) | 47 (96%) | |
| Yes | 2 (4%) | 2 (4%) | |
| Pelvic abscess | 0.34 | ||
| No | 55 (98%) | 46 (94%) | |
| Yes | 1 (2%) | 3 (6%) | |
| Wound infection | 1.0 | ||
| No | 55 (98%) | 48 (98%) | |
| Yes | 1 (2%) | 1 (2%) | |
| Wound dehiscence | 1.0 | ||
| No | 56 (100%) | 49 (100%) | |
| Yes | 0 (0%) | 0 (0%) | |
| Pneumonia | 0.25 | ||
| No | 53 (95%) | 49 (100%) | |
| Yes | 3 (5%) | 0 (0%) | |
| Cuff dehiscence | 0.10 | ||
| No | 56 (100%) | 46 (94%) | |
| Yes | 0 (0%) | 3 (6%) | |
| Fistula | 1.0 | ||
| No | 53 (95%) | 46 (94%) | |
| Yes | 3 (5%) | 3 (6%) | |
| VTE | 0.60 | ||
| No | 55 (98%) | 47 (96%) | |
| Yes | 1 (2%) | 2 (4%) | |
| Lymphedema | 1.0 | ||
| No | 55 (98%) | 48 (98%) | |
| Yes | 1 (2%) | 1 (2%) | |
| Hernia | 0.34 | ||
| No | 55 (98%) | 46 (94%) | |
| Yes | 1 (2%) | 3 (6%) | |
When evaluating recurrence and survival outcomes, we found no statistical difference in recurrence risk (14% vs. 24%, p = 0.22) or recurrence patterns (p = 0.32) between ARH and RRH groups, with a mean follow-up time of 25.4 months (range, 0.2–95.1) (Table 3). Most recurrences were either vaginal or multifocal, with multifocal recurrences most frequently comprising a combination of pelvic and extrapelvic disease. There was also no difference in risk of death between surgical groups (5% vs. 14%, p = 0.18). When stratifying risk of recurrence and death by tumor size, those with <2 cm tumors had a lower risk of recurrence than those with ≥2 cm tumors (8% vs. 30%, p = 0.006), but there was no statistical difference in risk of death (4% vs. 15%, p = 0.09).
Table 3.
Adjuvant therapy, recurrence, and survival.
| Abdominal radical hysterectomy | Robotic radical hysterectomy | p-Value | |
|---|---|---|---|
| Adjuvant therapy | 0.17 | ||
| None | 37(66%) | 40 (82%) | |
| Radiation | 7 (13%) | 2 (4%) | |
| Chemoradiation | 12 (21%) | 7 (14%) | |
| Recurrence | 0.22 | ||
| No | 48(86%) | 37 (76%) | |
| Yes | 8 (14%) | 12 (24%) | |
| Recurrence site | 0.32 | ||
| Vault | 3 (5%) | 3 (6%) | |
| Pelvis | 1 (2%) | 0 (0%) | |
| Abdomen | 1 (2%) | 1 (2%) | |
| Distant | 0 (0%) | 0 (0%) | |
| Multiple | 3 (5%) | 8 (16%) | |
| Death | 0.18 | ||
| No | 53(95%) | 42 (86%) | |
| yes | 3 (5%) | 7 (14%) | |
| Tumor size < 2 cm | Tumor size ≥ 2 cm | p-Value | |
| Recurrence | 0.006 | ||
| No | 47 (92%) | 38 (70%) | |
| yes | 4 (8%) | 16 (30%) | |
| Death | 0.09 | ||
| No | 49 (96%) | 46 (85%) | |
| Yes | 2 (4%) | 8 (15%) | |
Bold indicates p-Value of < 0.05
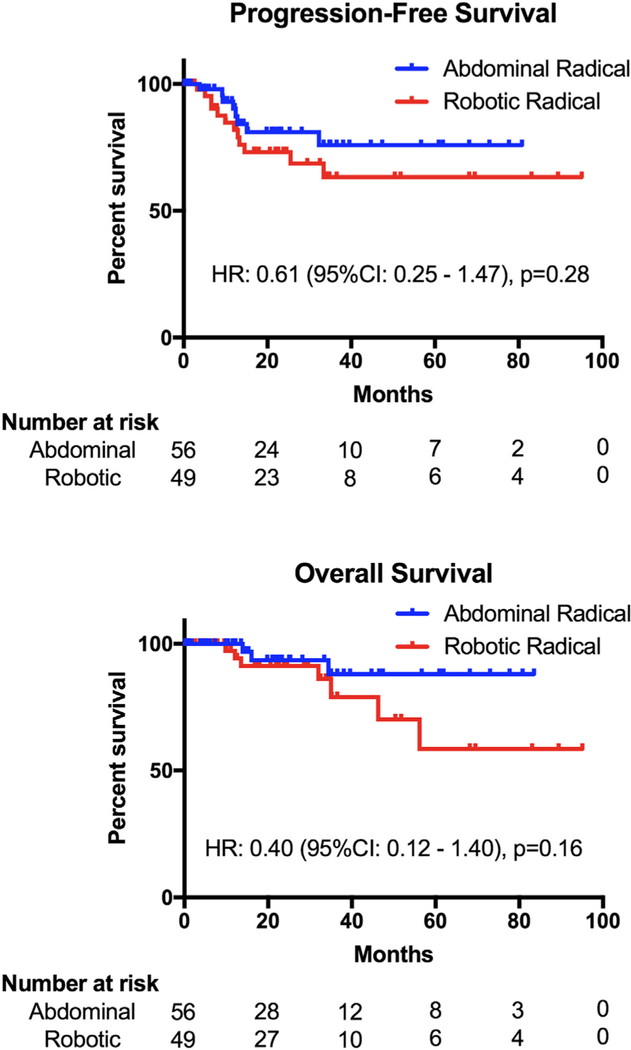
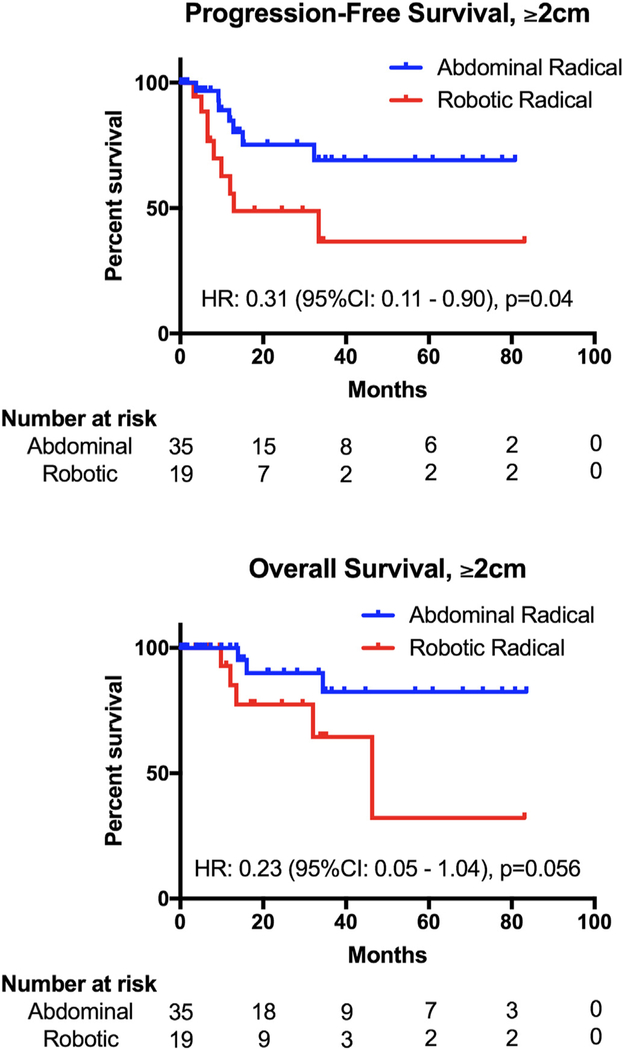
In terms of survival, PFS (HR 0.61, 95% CI: 0.25–1.47, p = 0.28) and OS (HR 0.40,95% CI: 0.12–1.40, p = 0.16) (Fig. 1) were statistically similar between the two surgical groups. When stratifying by tumor size, patients with <2 cm tumors had no difference in PFS (HR 0.72, 95% CI 0.09–5.87, p = 0.76) or OS (HR 0.26, 95% CI 0.01–7.07, p = 0.45) (Supplemental Fig. S1); however, those with ≥2 cm tumors who underwent ARH had a PFS advantage over RRH (HR 0.31, 95% CI 0.11–0.90, p = 0.04) (Fig. 2). OS in this population also favored of ARH (HR 0.23, 95% CI 0.05–1.04, p = 0.056). Using multivariate analysis to control for statistically significant clinical and pathologic differences between the groups, patients who had ARH had a lower risk of recurrence (HR 0.38, 95% CI 0.15–0.95, p = 0.039) and a lower risk of death (HR 0.21, 95% CI 0.05–0.85, p = 0.029) than those who had RRH (Table 4). Similarly, patients with tumors < 2 cm had a lower risk of recurrence (HR 0.175, 95% CI 0.06–0.54, p = 0.002) and death (HR 0.15, 95% CI 0.03–0.74, p = 0.02) than those with larger tumors.
Fig. 1.
Progression-free and overall survival of the entire cohort.
Fig. 2.
Progression-free and overall survival of patients with tumors ≥ 2 cm.
Table 4.
Multivariate analysis for recurrence and death.
| HR | 95% Cl | p-Value | |
|---|---|---|---|
| Recurrence | |||
| Abdominal RH | 0.38 | 0.15–0.95 | 0.04 |
| Tumor size < 2 cm | 0.175 | 0.06–0.54 | 0.002 |
| Death | |||
| Abdominal RH | 0.21 | 0.05–0.85 | 0.03 |
| Tumor size < 2 cm | 0.15 | 0.03–0.74 | 0.02 |
4. Discussion
When comparing robotic surgery to laparotomy for radical hysterectomy, our data demonstrates similar overall safety and complication rates between the two surgical approaches. This is consistent with previous retrospective reports comparing operative outcomes between these two groups [14–16]. Regarding disease recurrence and survival in our cohort, we did not observe a difference in recurrence risk, PFS, or OS in this patient population based on mode of hysterectomy. When stratifying by tumor size, however, patients with tumors ≥2 cm who underwent RRH had a shorter PFS than those who underwent ARH. Additionally, the difference in OS for larger tumors favored ARH (p = 0.056). In order to control for differences between surgical groups, a multivariate analysis was performed it determine risk factors for recurrence or death. We found that RRH and tumor size ≥ 2 cm were independently associated with recurrence and death in our overall study population.
Previous retrospective studies have not shown a survival difference between these two approaches [17–20]. Similarly, studies that have examined oncologic outcomes of laparoscopic radical hysterectomy have noted no difference between laparoscopic and abdominal approaches, even when stratified by tumor size [21,22]. Recently, however, results from the LACC trial, an international randomized phase III trial examining long-term survival for patients undergoing minimally invasive radical hysterectomy vs. abdominal radical hysterectomy, were published [10]. This study included patients with stages IA1 with LVSI, IA2, and IB1 disease and randomized 631 patients to radical hysterectomy using minimally invasive surgery (MIS) or laparotomy. The LACC trial was inconclusive with respect to its primary objective of disease-free survival as the confidence interval (CI) crossed the predetermined non-inferiority margin of 7.2 percentage points for minimally invasive surgery (95% CI −16.4 to −4.7, p = 0.87 for noninferiority). However, secondary endpoints of disease-free survival and overall survival favored the open surgery group. These results were unexpected and have already led to a change in practice patterns at many institutions that have now eliminated or significantly reduced their use of minimally invasive radical hysterectomy. Nonetheless, there are a few points of caution as to how this data is interpreted. Despite including a mix of both traditional laparoscopy and robotic surgery in the minimally invasive arm, it was heavily skewed toward laparoscopy with only 15.6% of patients receiving robotic surgery (n = 45). Moreover, a large proportion of patients had unknown grade (29%), or unknown depth of invasion (33%). The authors also concede that their results may not be generalizable to patients with <2 cm tumors. Despite these limitations, these results suggest that the two surgical approaches may be fundamentally different in regards to the risk of recurrence after radical hysterectomy.
In addition to the LACC trial, a large database study was also recently published addressing the question of survival based on surgical technique using the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database. In this evaluation, minimally invasive surgery was associated with an increased probability of death within 4 years compared to open radical hysterectomy (9.1 vs. 5.3%) [23]. However, in subgroup analysis, the hazard ratio for death was statistically similar between the two surgical approaches for patients that had tumors < 2 cm. Another study that was presented using data from the NCDB and the Premier Healthcare Database concluded that MIS was associated with decreased survival in women who had tumors ≥ 2 cm [13]. Our data is in agreement with these results, which suggest worse outcomes for patients who undergo RRH for cervical cancer, particularly those with ≥2 cm tumors. Robotic radical surgery for smaller tumors appears to be safer, but the data does not definitively favor a particular approach. It is important to note, however, that the size cutoff of 2 cm is somewhat arbitrary, and the true criteria for minimally invasive surgery may need to include a different size altogether, and probably should also include other tumor characteristics. These considerations should be taken into account in further investigations of this question in the future.
It remains unclear why a minimally invasive approach may potentially confer worse survival in cervical cancer patients undergoing radical hysterectomy. Possible explanations for this discrepancy may involve the use of insufflation, which may cause dispersion of tumor cells, Trendelenburg positioning, or the use of a uterine manipulator, which may disrupt larger tumors and cause them to fracture and spread during surgery. Another theory could be that two sets of clamps placed across the vagina below the cervix in an open radical hysterectomy may be sealing the cancer cells within the specimen, limiting the chance of intra-abdominal seeding during removal. There is no similar way to seal the vagina during uterine removal using the uterine manipulator. If this indeed were the culprit, it would be consistent with the finding that larger tumors have a shorter PFS in minimally invasive surgery, as the chance for cancer seeding in this situation is likely higher with a bulkier exposed tumor. Possible solutions would include novel ways to quarantine the tumor from exposure to the abdomen at the time of amputation from the vagina, such as suturing closed the vagina distal to the cervix prior to amputation or performing the colpotomy vaginally followed by immediate removal of the uterus.
Strengths of this study were that it only included robotic surgeries, was performed at an institution that conducts a high volume of robotic surgery for cervical cancer, and that it found similar results to the LACC trial in this patient population. For this reason it may clear up concerns regarding the applicability of the LACC trial to robotic radical hysterectomy since the LACC trial included relatively few RRHs. Limitations to this study include those inherent to any retrospective study, including selection bias and unmeasured confounders. The shorter follow-up time for patients that underwent surgery toward the end of the sample period also limits the survival analysis for those patients who have not yet had an opportunity for recurrence. Additionally, the two groups were not equal in terms of tumor characteristics, as the abdominal radical hysterectomy group had larger tumors and skewed slightly toward higher-grade pathology. As a result, these patients had a higher rate of adjuvant radiation, which may have resulted in a lower recurrence rate in this group. Although there is not a formal policy in our practice to triage those with larger tumors to open surgery, some surgeons do routinely choose ARH for these patients. This is due to several concerns – that disruption of a larger tumor may occur when dilating the cervix prior to manipulator placement, that the manipulator may not fit properly around larger lesions, and that tactile feedback is lost in robotic surgery, which some feel is necessary for large tumors. Additionally, providers tended to be more comfortable with an abdominal approach for larger tumors, particularly earlier in this series when robotic surgery was a relatively new modality. When controlling for this discrepancy in tumor size we saw that robotic surgery was an independent predictor of recurrence and death.
Another concern about our data is that we had a higher recurrence rate than what has been reported in the LACC trial and other case series. Upon further examination of the patients who recurred, 3/20 patients met intermediate risk criteria as defined by GOG 92 [24], 1/20 met high risk criteria as defined by GOG 109 [25], and 10/20 met both intermediate and high risk criteria. Of these, 6 did not receive adjuvant treatment, 4 of which had initially undergone RRH. Reasons for not receiving or completing adjuvant treatment were primarily due to patient factors (lost to follow up, refused treatment, missed appointments). Had all 6 of these patients been treated with adjuvant radiation it would have potentially decreased the recurrence rate in this study and may have reduced the survival advantage that is seen in the ARH group.
Other considerations for the higher recurrence rate in this study, particularly in the ≥2 cm tumor group, may be due to the fact that several recurrent patients wound up having tumors that were larger than 4 cm on final pathology or had occult vaginal involvement or positive lymph nodes. While it is our current practice to obtain pre-operative imaging on all cervical cancer patients, during this study period that was not the case and several recurrent patients may have been triaged to primary chemoradiation had they had a PET/CT at diagnosis. Other potential reasons for our higher recurrence rate could be related to demographics: African American ethnicity and Southern region are both associated with higher cervical cancer incidence and mortality [26]. In addition, as a public hospital and tertiary referral center we tend to see a high proportion of uninsured and indigent patients, who are at increased risk of poor outcome. Our study also only included patients with a preoperative diagnosis of IB1 disease, while previous studies included IA1 (with LVSI) and IA2 patients, effectively increasing our recurrence risk in comparison.
It is apparent from these results that the optimal surgical management algorithm for patients with early stage cervical cancer is still being determined. Based on our data, as well as published data from the LACC trial and other database analyses, we believe that robotic radical hysterectomy should be avoided in cervical cancer patients that have ≥2 cm lesions in greatest diameter. The data is not as clear when addressing smaller tumors, for which a minimally invasive approach still is a reasonable option when taken in consideration with the other advantages of minimally invasive surgery. These concerns and a review of the data should be included when discussing surgical treatment options for patients with cervical cancer. Hopefully the cause of the higher recurrence risk after RRH will be discovered and eliminated in future studies so that minimally invasive surgery can once again be considered an option for all cervical cancer patients who are otherwise appropriate surgical candidates.
Supplementary Material
HIGHLIGHTS.
Robotic and abdominal radical hysterectomies have similar complication rates.
Robotic radical hysterectomy has shorter PFS for tumors ≥ 2 cm.
Robotic radical hysterectomy is independently associated with recurrence and death.
Acknowledgments
Grant support
CAL was supported in part by the 3P30CA013148-43S3, U10CA18055 and P50 CA098252. WKH was supported in part by the 5P50 CA098252-13.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.03.001.
Conflict of interest statement
WKH reports serving as an expert witness for Intuitive Surgical and consultant to Medtronic. The remaining authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin. 68 (1) (2018) 7–30 January. [DOI] [PubMed] [Google Scholar]
- [2].Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD, Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation, Am. J. Obstet. Gynecol. 201 (5) (2009. November) 485 e1–9. [DOI] [PubMed] [Google Scholar]
- [3].Advincula AP, Song A, The role of robotic surgery in gynecology, Curr. Opin. Obstet. Gynecol. 19 (4) (2007. August) 331–336. [DOI] [PubMed] [Google Scholar]
- [4].Holloway RW, Ahmad S, Robotic-assisted surgery in the management of endometrial cancer, J. Obstet. Gynaecol. Res. 38 (1) (2012) 1–8 January. [DOI] [PubMed] [Google Scholar]
- [5].Paley PJ, Veljovich DS, Shah CA, Everett EN, Bondurant AE, Drescher CW, et al. Surgical outcomes in gynecologic oncology in the era of robotics: analysis of first 1000 cases. Am. J. Obstet. Gynecol. 2011. June;204(6): 551 e1–9. [DOI] [PubMed] [Google Scholar]
- [6].Shafer A, Boggess JF, Robotic-assisted endometrial cancer staging and radical hysterectomy with the da Vinci surgical system, Gynecol. Oncol. 111 (2 Suppl) (2008. November) S18–S23. [DOI] [PubMed] [Google Scholar]
- [7].Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA 3rd, Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging, Am. J. Obstet. Gynecol. 198 (6) (2008. June) 679 e1–9 (discussion e9–10). [DOI] [PubMed] [Google Scholar]
- [8].Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009. November 10;27 (32): 5331–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brudie LA, Backes FJ, Ahmad S, Zhu X, Finkler NJ, Bigsby GEt, et al. Analysis of disease recurrence and survival for women with uterine malignancies undergoing robotic surgery. Gynecol. Oncol. 2013. February;128(2):309–315. [DOI] [PubMed] [Google Scholar]
- [10].Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 2018. November 15; 379(20): 1895–1904. [DOI] [PubMed] [Google Scholar]
- [11].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet. 105 (2) (2009) 103–104 May. [DOI] [PubMed] [Google Scholar]
- [12].Verleye L, Vergote I, Reed N, Ottevanger PB, Quality assurance for radical hysterectomy for cervical cancer: the view of the European Organization for Research and Treatment of Cancer—Gynecological Cancer Group (EORTC-GCG), Ann. Oncol. 20 (2009) 1631–1638 October. [DOI] [PubMed] [Google Scholar]
- [13].Margul DJ, Yang J, Seagle BL, Kocherginsky MSS, Outcomes and costs of open, robotic, and laparoscopic radical hystrectomy for stage IB1 cervical cancer, 2018 American Society of Clinical Oncology Annual Meeting; 2018; Chicago, IL, 2018. [Google Scholar]
- [14].Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am. J. Obstet. Gynecol. 2008. October;199(4):357 e1–7. [DOI] [PubMed] [Google Scholar]
- [15].Estape R, Lambrou N, Diaz R, Estape E, Dunkin N, Rivera A, A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy, Gynecol. Oncol. 113 (3) (2009) 357–361 June. [DOI] [PubMed] [Google Scholar]
- [16].Ko EM, Muto MG, Berkowitz RS, Feltmate CM, Robotic versus open radical hysterectomy: a comparative study at a single institution, Gynecol. Oncol. 111 (3) (2008) 425–430 December. [DOI] [PubMed] [Google Scholar]
- [17].Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV 3rd, Micha JP, Lopez KL, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg. Oncol. 2016. March; 25(1): 66–71. [DOI] [PubMed] [Google Scholar]
- [18].CA Shah T Beck JB. Liao NV Giannakopoulos D Veljovich P Paley, Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer, J. Gynecol. Oncol. 28 (6) (2017. November) e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Eur.J. Surg. Oncol. 2016. April; 42(4): 513–522. [DOI] [PubMed] [Google Scholar]
- [20].Cantrell LA, Mendivil A, Gehrig PA, Boggess JF, Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience, Gynecol. Oncol.117 (2) (2010) 260–265 May. [DOI] [PubMed] [Google Scholar]
- [21].Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study, Ann.Oncol.23 (4) (2012) 903–911 April. [DOI] [PubMed] [Google Scholar]
- [22].Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ, Laparoscopy versus laparotomy for the management of early stage cervical cancer, BMC Cancer 15 (2015. November 24) 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N. Engl. J. Med. 2018. November 15; 379(20): 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ, A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymph-adenectomy: a gynecologic oncology group study, Gynecol. Oncol. 73 (2) (1999) 177–183 May. [DOI] [PubMed] [Google Scholar]
- [25].Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000. April; 18(8): 1606–1613. [DOI] [PubMed] [Google Scholar]
- [26].Yoo W, Kim S, Huh WK, Dilley S, Coughlin SS, Partridge EE, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS One 2017; 12(2): e0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.