Abstract
Background
We compared daily pain, home analgesic use, and utilization, among ambulatory adults in the randomized Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH). We related the fetal hemoglobin (HbF) hydroxyurea response to these response variables.
Methods
Patients rated daily their sickle cell pain intensity (0–9), use of analgesics, and visits for pain. Diaries were collected biweekly, and intensity was collapsed into single interval ratings. The interval proportions of days of analgesic use and medical visits for pain were also calculated. Group comparisons were made by intention to treat as well as by HbF change levels from baseline to 2 years of treatment (placebo, and low, medium, high, or very high response).
Results
A total of 134 (44.8%) enrollees completed two years of follow-up. Pain intensity correlated with analgesic use (r=.83, p.>0001) and utilization (r=.50, p<.0001). Pain intensity was lower for patients on hydroxyurea (2.51 ± 0.062 vs. 2.82 ± 0.063 placebo, F(1,270)=11.65, p=.0007). The difference, though small, appeared early and was sustained. Analgesic use and utilization were also slightly lower (analgesic use: F(1,270)=11.97, p=.0006; utilization: F(1,270)=32.0, p<.0001). Each were each statistically significantly lower among hydroxyurea patients with higher HbF treatment responses to hydroxyurea.
Conclusions
Hydroxyurea use led to a small, statistically significant reduction in daily pain, analgesic use, and utilization in adults in MSH, corroborating previously shown larger reductions in crises and mortality. The degree of daily symptomatic reduction was related to the size of the HbF treatment response, further confirming HbF response as a useful laboratory correlate.
Introduction
Pain is the most prominent feature of sickle cell disease (SCD). Pain is responsible for the majority of SCD medical visits (utilization).1 The often episodic nature of sickle cell pain and the sometimes infrequent utilization have led caregivers to use the term “crisis” to describe utilization provoked by pain. Higher-utilizing adults are at higher risk of death, so measuring crises has clinical meaning.2
However, most SCD pain, even “crisis” pain, is managed at home, without utilization, based on results of longitudinal epidemiologic studies.3,4,5,6,7
Hydroxyurea is the only Food and Drug Administration-approved remittive agent for homozygous SCD (HbSS, sickle cell anemia). Its benefit was tested in the Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH).8 This randomized, double-blind placebo controlled study clearly showed that hydroxyurea reduced the rate of painful crises in adult sickle cell anemia patients with at least three painful crises per year.9
In practice, differences in crises, which may occur infrequently for many patients, may be subtle and hard to gauge. When clinicians prescribe hydroxyurea to individual patients, and when patients are deciding whether to continue to take this lifelong treatment, they may be more interested in the relationship of hydroxyurea therapy to pain managed at home and to home analgesic use. It is unclear whether the reduction in crises found in the MSH was paralleled by a similar reduction in daily pain or analgesic use. It is also unclear whether any possible daily pain or analgesic use reduction predated the reduction in crises.
Although earlier results questioned its value,10,11 later publications confirmed an increase in fetal hemoglobin (HbF) level as one common and likely important laboratory measure of responsiveness to hydroxyurea in SCD.12,13,14,15,16 Independent of other predictors, low levels of fetal hemoglobin were associated with higher mortality in the Cooperative Study of Sickle Cell Disease.17 In the MSH trial, the proportion of red cells containing HbF (F cells) rose between 8 and 24 weeks among patients on hydroxyurea vs. placebo.9 However, subtle and early markers of response to HU included changes in most of the RBC and reticulocyte indices, first detected 10 weeks after starting HU and before the increase in Hb F levels.18
In the MSH nonrandomized follow-up phase.. survival at 9 years was improved among patients showing increasing HbF response to HU,19 Further, at 17.5 years, the mortality rate was increasingly lower among groups of patients who reported taking HU for increasingly larger proportions of the folllowup period, up to ≥ 15 years,, although stroke, organ dysfunction, and infection were similar in all groups. Survival curves demonstrated a significant reduction in deaths with long-term HU exposure.20 Responders to HU used analgesics on fewer days, and their average length of hospital stay was about two days less.21 MSH Patients on HU demonstrated a trend for more consistent employment, even though all MSH patients had moderate to severe disease.22
Still, the relationship between the HbF response to hydroxyurea and the daily pain and analgesic use response is unknown.
The MSH dataset afforded us the ability to examine the association between hydroxyurea treatment and daily pain of SCD, specifically measured as daily pain intensity ratings, daily analgesic use, and daily pain-related utilization. We also assessed how the overall and temporal effects of hydroxyurea on each of these daily symptomatic response variables related to the HbF response.
Methods
The MSH sample
The MSH procedures and study inclusion and exclusion criteria have been previously published.8 Briefly, to be eligible for the MSH study, patients had to be at least 18 years old and have sickle cell anemia. They also had to have at least 3 reported painful crises in the year prior to entry into the study. A total of 299 patients were enrolled from 21 sites (20 in the U.S. and 1 in Canada). Patients were overwhelmingly (95%) African-American. There were no significant differences between the hydroxyurea and placebo groups in terms of sex, race, age, or blood counts.9 Analysis of detailed demographic characteristics of this sample are beyond the bounds of this manuscript, but briefly, the sample was equally male and female, with an average age of 30 years at study entry baseline. While nearly half had completed at least some college, most were unemployed (61%). Table 1 summarizes demographic characteristics of the MSH participants.
Table 1.
Demographics of the MSH sample (N=299)
| Group | Hydroxyurea (n=152) |
Placebo (n=147) |
|---|---|---|
| % | % | |
| Sex: | ||
| Male | 49.3 | 48.3 |
| Female | 50.7 | 51.7 |
| Χ2(1)=0.033, p=.86 | ||
| Education: | ||
| Less than high school | 17.8 | 18.4 |
| High school/GED | 33.6 | 38.1 |
| Some college | 44.1 | 38.8 |
| B.A./postgraduate degree | 4.6 | 4.8 |
| Χ2(3)=0.96, p=.81 | ||
| Personal income: | ||
| Less than 10,000 | 65.3 | 73.3 |
| 10,000–19,999 | 20.4 | 18.5 |
| 20,000–29,999 | 7.5 | 3.4 |
| 30,000–39,999 | 4.8 | 3.4 |
| 40,000–49,999 | 2.0 | 1.4 |
| Χ2(4)=3.53, p=.47 | ||
| Employment | ||
| Full-time | 19.5 | 17.4 |
| Part-time | 13.4 | 18.1 |
| Unemployed/disabled | 62.4 | 58.3 |
| Student (full or part-time) | 4.7 | 6.3 |
| Χ2(3)=1.70, p=.64 | ||
| Age at qualifying visit | ||
| Mean (standard deviation) | 30.6 (7.54) | 29.8 (7.47) |
| Range | 18–59 | 18–54 |
| t(297)=0.93, p=.35 | ||
Study procedures, measures
Once enrolled, patients had biweekly study-related visits throughout the duration of the study. As noted above, frequency of painful crises (defined as medical facility visits with no apparent cause other than an exacerbation of SCD pain, lasting ≥ 4 hours, for which the patient was treated with opioid or nonsteroidal antiinflammatory drugs) was the primary endpoint of the study. But aside from the frequency of painful crises, several other pain-related measures were collected and served as the outcomes of interest in this study.
While out of the hospital, at each biweekly clinic visit, patients returned a pain diary in which they rated their daily pain intensity on a 0–9 scale; indicated whether or not analgesics were taken that day; and indicated whether any medical facility was visited for pain-related reasons (this included both unscheduled acute-care contacts such as emergency department use or clinic visits, and in-patient hospitalization). Daily pain intensity ratings from each diary were averaged to provide a single average pain intensity rating for each 2-week period (pain intensity). Almost always, ratings represented 14 days of diaries, because diary recordings of the severity of pain, use of analgesics, and visits to medical facilities were reviewed every 2 weeks at a clinic visit by members of the clinic staff. When (rarely) data were missing from one or more days, pain intensity was calculated from the reduced number of days. The measure of analgesic usage (analgesic use) was the proportion of days with reported analgesic use in each diary, calculated by dividing the number of days with analgesic use by the total number of days. As with pain intensity, when data were missing from one or more days analgesic use was calculated from the available data. The measure of pain-related medical visits (utilization) was calculated in the same way as analgesic use; the number of days with visits was divided by the total number of days in each diary. Thus, values for pain intensity ranged from 0 to 9, and values for analgesic use and utilization ranged from 0 to 1.
In addition to demographic characteristics of patients, a measure of health-related limitations on physical activity, obtained once at the beginning of the study, was also included as an adjustment variable. This score was based on a multi-item questionnaire on which participants indicated whether or not their health limited their participation in ten different activities ‘a lot,’ ‘a little,’ or ‘not at all.’ Because some of these items were very similar except for how strenuous they were (e.g., climbing several flights of stairs versus one flight of stairs), the final score was based on six different items that included only the most strenuous of closely related activities, with higher scores indicating more health limitations on physical activities.
Drug assignment and drug HbF response
Drug assignment at study entry (hydroxyurea versus placebo) was a key predictor of interest for pain outcomes. But in prior analyses, hydroxyurea recipients were also coded into four response groups based on quartiles of HbF response to treatment: the change in HbF levels from baseline to the 2-year measurement.12 HbF response levels were defined as ‘low’ (a decrease of 0.3 or more in %HbF; n=34), ‘medium’ (a decrease of less than 0.3 to an increase of 1.7 in %HbF; n=38), ‘high’ (an increase of 1.7 to 6.5 in %HbF; n=36), and ‘very high’ (an increase greater than 6.5 in %HbF; n=35). For additional exploration of outcomes, a second HbF response variable was coded that collapsed the low and medium groups into a single ‘lower’ response group (n=69), and the high and very high groups into a single ‘higher’ response group (n=68). Both HbF response measures were also coded ordinally (1–4 for the four-group categorization and 1–2 for the two-group categorization, with the placebo group coded as 0), to allow testing of linear associations between daily symptomatic response measures and HbF response.
Statistical methods
To examine the magnitude of association between pain intensity, analgesic use, and utilization, overall average values were calculated across all pain diaries for each subject, and then used to obtain Pearson correlations between each variable.
Mixed models regression analyses (PROC MIXED in SAS 9.1.3) were used to model pain intensity, analgesic use, and utilization. Mixed modeling allowed for inclusion of both between- and within-subject measures, and both fixed and random factors, in a single model. The models of each of the three daily symptomatic response measures were run three times, substituting treatment assignment, the five-group HbF response classification, and the three-group HbF response classification as the predictors of interest.
Patient age and sex were included in the models as adjustment variables. Variables for drug assignment (hydroxyurea versus placebo), age and sex were appropriately centered; study site was dummy-coded and centered also, to control for possible differences between sites. Personal income was also included (preliminary analyses showed a stronger correlation of pain measures with personal income than with household income). Finally, the health limitations variable was also included. A random statement allowed for individual subject variation in intercepts and slopes, and a repeated statement controlled for repeated measures on the same subject. The matrix structure for correlations between visits was defined as 1st-order autoregressive. Preliminary analyses indicated that other matrix structures (such as compound symmetry) resulted in an inferior fit for the model.
Results
Although originally intended to last 24 months for all participants, the trial was stopped early because of the beneficial effects observed. Only 134 of the 299 patients completed the full two years of follow-up, and the mean follow-up was 21 months.
Pain intensity and analgesic use were highly correlated, r=.83, p.<.0001. Correlations of both of these measures with utilization were lower, but still highly significant (r=.50, p<.0001, for pain intensity and r=.48, p<.0001 for analgesic use).
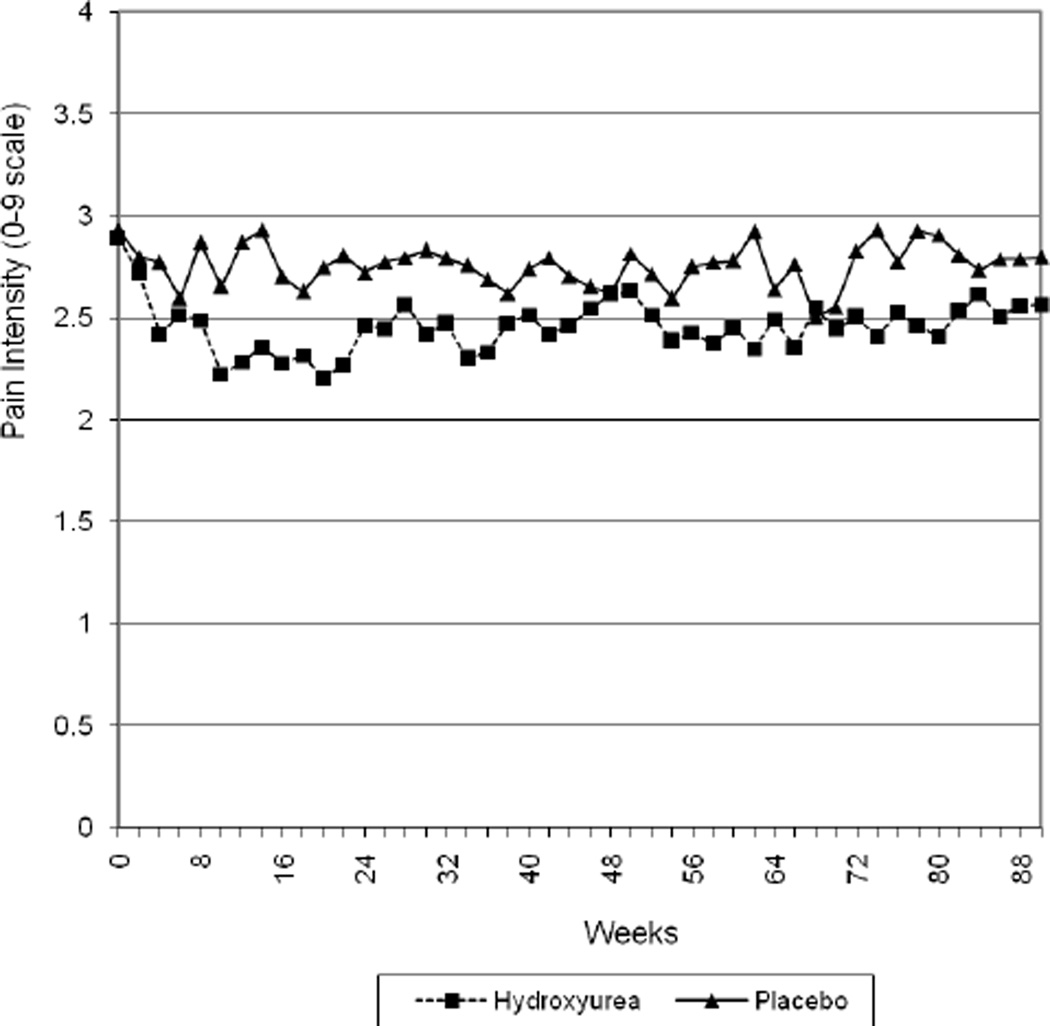
Table 2 shows least squares means estimates and standard errors for each of the three daily symptomatic response variables from each of three models, and the statistical significance of all pairwise group comparisons. The first set of models tested treatment assignment (hydroxyurea and placebo) as a predictor of pain intensity, analgesic use, and utilization. We found significant differences for all three variables. Hydroxyurea patients had lower pain intensity over time, F(1,270)=11.65, p=.0007; lower analgesic use, F(1,270)=11.97, p=.0006; and lower utilization, F(1,270)=32.0, p<.0001. Figure 1 shows pain intensity through 21 months (the average follow-up period) for the hydroxyurea and placebo groups.
Table 2.
Least squares means estimates and standard errors for hydroxyurea daily symptomatic response variables, by model.
| Model | Na | Least Squares Means Estimate (Standard Error), by Daily symptomatic Response Variable |
||
|---|---|---|---|---|
| Hydroxyurea and placebo |
Average pain ratingsb |
Proportion of days with analgesic useb |
Proportion of days with utilizationb |
|
| Placebo | 146 | 2.82 (0.063) | 0.44 (0.008) | 0.11 (0.003) |
| Hydroxyurea | 147 | 2.51 (0.062) | 0.40 (0.008) | 0.08 (0.003) |
| Overall p-value | p=.0007 | p=.0006 | p<.0001 | |
|
HbF response (4 levels) and placebo |
||||
| Placebo | 146 | 2.82 (0.062)A | 0.44 (0.008)A | 0.11 (0.003)A |
| Low response | 33 | 2.71 (0.134)AB | 0.41 (0.018)A | 0.10 (0.006)A |
| Moderate response | 38 | 3.01 (0.122)A | 0.45 (0.016)A | 0.11 (0.006)A |
| High response | 36 | 2.47 (0..126)B | 0.42 (0.017)A | 0.07 (0.006)B |
| Very high | 34 | 1.85 (0..125)C | 0.32 (0.017)B | 0.05 (0.006)B |
| Overall p-value | p<.0001 | p<.0001 | p<.0001 | |
|
Model p Values by Hb F response |
Groups | p-value | ||
| Average pain intensity: | ||||
| ‘Low’ vs. ‘Very High’ responders | p<.0001 | |||
| ‘Moderate’ vs. ‘High’ responders | p=.003 | |||
| ‘Moderate’ vs. ‘Very High’ responders | p<.0001 | |||
| ‘High’ vs. ‘Very High’ responders | p=.001 | |||
| Proportion of days with analgesic use: |
||||
| Placebo vs. ‘Very High’ responders | p<.0001 | |||
| ‘Low’ vs. ‘Very High’ responders | p<.0001 | |||
| ‘Moderate’ vs. ‘Very High’ responders | p<.0001 | |||
| ‘High’ vs. ‘Very High’ responders | p<.0001 | |||
| Proportion of days with utilization: | ||||
| Placebo vs. ‘High’ responders | p<.0001 | |||
| Placebo vs. ‘Very High’ responders | p<.0001 | |||
| ‘Low’ vs. ‘High’ responders | p=.003 | |||
| ‘Low’ vs. ‘Very High’ responders | p<.0001 | |||
| ‘Moderate’ vs. ‘High’ responders | p<.0001 | |||
| ‘Moderate’ vs. ‘Very High’ responders | p<.0001 | |||
Abbreviations: HbF= fetal hemoglobin.
Within each model, n’s for subgroups are consistent across the three daily symptomatic response variables. 146 of 147 placebo patients and 147 of 152 hydroxyurea patients had data for the daily symptomatic response variables; 141 of 152 hydroxyurea patients had data on HbF response to treatment.
In the model incorporating HbF response, different uppercase superscripts within a column (A,B,C) indicate that least squares means differ at p<.05 (e.g., in the model for proportion of days with utilization, the placebo, low response, and moderate response groups do not significantly differ from each other, but have a significantly higher proportion of days with utilization than either the high or very high groups, which in turn do not differ from each other).
Figure 1.
Treatment group and average pain scores by visit, through week 90 (21 months)
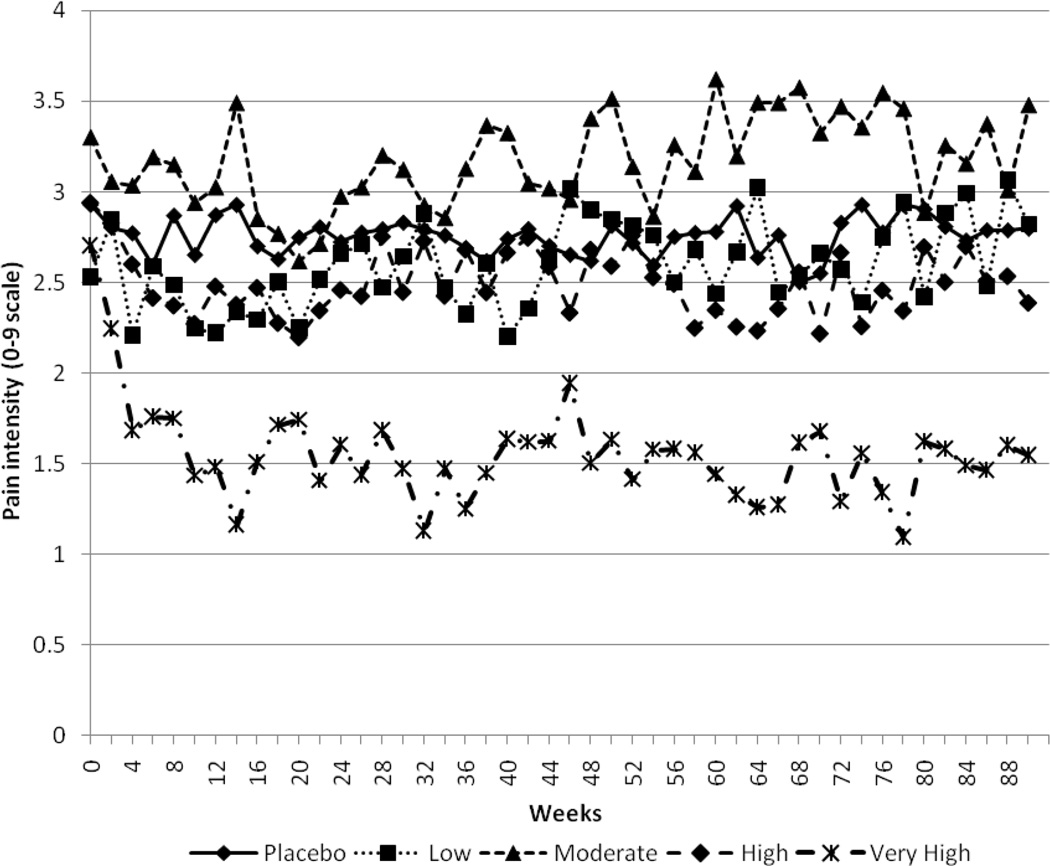
The second set of models tested the five-group hydroxyurea HbF response classification variable (four levels of HbF response plus the placebo group). HbF response was modeled both as an ordinal variable (using the 0 to 5 scoring described above) to test linear trends, and as a categorical variable to obtain least squares means estimates. As a predictor of pain intensity, the overall HbF response to hydroxyurea was significant, F(1,264)=33.64, p<.0001. Pairwise comparisons of least squares means between all groups indicated that the ‘very high’ responders had lower pain intensity than all other groups, and that ‘high’ responders had lower pain intensity than either the placebo patients or the ‘moderate’ responders. Figure 2 displays, by response group, pain intensity through the average follow-up period of 21 months.
Figure 2.
Treatment group and average pain scores by visit, through week 90 (21 months)
HbF response was also a significant predictor of analgesic use, F(1,264)=27.52, p<.0001. The ‘very high’ responders had lower analgesic use than all other groups; other pairwise differences were nonsignificant.
Similarly, HbF response significantly predicted utilization, F(1,264)=66.34, p<.0001. The ‘very high’ and ‘high’ responders, who did not differ from each other, had significantly lower utilization than the placebo patients or the other two response groups, which did not significantly differ from each other.
Finally, the third set of models tested the three-group rather than the five-group hydroxyurea HbF response classification variable. This HbF response variable again significantly predicted pain intensity, F(1,264)=29.32, p<.0001. Pairwise comparisons showed that the ‘higher’ response group had lower pain intensity than either the ‘lower’ response or placebo groups, which did not differ from each other. Results for analgesic use were similar, F(1,264)=22.68, p<.0001, with the ‘higher’ response group using analgesics less often than the other two. Finally, the three-group HbF response significantly predicted utilization, F(1,264)=66.29, p<.0001, with the ‘higher’ response group reporting significantly lower utilization than either the placebo or ‘lower’ response groups, which did not differ.
Discussion
Our results used mixed models to show that in a randomized controlled trial of hydroxyurea in sickle cell anemia, all three hydroxyurea daily symptomatic pain response variables, constructed using data from a biweekly diary, differed statistically significantly between the hydroxyurea and placebo groups, and correlated with one another. Our results also subdivided patients by treatment response based on quartiles of change in HbF in response to hydroxyurea, to show that the hydroxyurea-related HbF response appears to drive the hydroxyurea-related pain response.
These findings are an extension of our previous MSH study results, and contrast with these results. Our first results showed that hydroxyurea treatment was associated with a dramatic, statistically significant reduction in painful crises.9 Later results showed a similarly dramatic reduction in mortality, in the non-randomized MSH extension study.16 However, the reduction in the rate of crises among hydroxyurea users was based on adjudication of crises by a board of blinded investigators. A crisis was adjudicated if patients spent four or more hours in a medical facility due to sickle cell pain.
Both the aims and results herein are different from those of prior analyses of the MSH. For this analysis, we instead focused on whether hydroxyurea caused a reduction in daily pain intensity, in the proportion of days with analgesic use, and in the proportion of days with utilization due to pain. Our observed reductions were small compared to the minimal important difference (MID)23, or clinically significant difference on a Visual Analog Scale (VAS) in studies of acute sickle cell pain and of post-operative pain(13 mm, or 13% change on a 100 mm scale).24,25 But we did not measure acute pain, nor did we use a VAS in this study. Without studies that determine the MID for chronic pain in SCD using our 0–9 scale, we cannot say conclusively whether our observed differences in pain intensity were clinically significant. However, for comparison, the differences we found between groups were approximately 3–5% of the range from 0–9. Similarly, we found 3–4% differences in analgesic use and utilization.
The meaning of the strong correlation between pain intensity and analgesic use is of interest. We note that on most days, at-home analgesic use was not reported. Elsewhere, we report that analgesic use was dependent on age, and temporally on whether or not patients were in crisis.26 We suspect that the correlation indicates that analgesics were not used on the days with no or minimal pain, and the frequency of use simply increased as at-home pain levels increased. The correlations between analgesic use and utilization and between pain scores and utilization raise hope that pain and analgesic use measures, with desirable psychometric properties such as scalability, high frequency, and high resolution, can be used as a measure of remittive drug effect in clinical trials of SCD. Using these measures would obviate the need to conduct large, long, expensive studies required to measure the rarer, correlated event, utilization, which is in turn correlated with mortality. However, on most days the pain score was low, though pain days were frequent, and opioid use frequent. Similarly, on most days utilization did not occur, though there was pain. The correlations we observed may thus be driven by no-utilization, no-pain days, or similar “no-no” days for the other measures. Further, we believe that before these measures ultimately are deemed useful in clinical trials of other chronic remittive SCD drugs or analgesics, other testing must be done, including determining the MID of these measures (see above).
The differences in our three daily symptomatic pain response variables between hydroxyurea and placebo patients occurred early, by six weeks of therapy, paralleling the time course found previously in improvements in F cells in MSH. Further, the daily symptomatic differences were each sustained during the followup period, in a pattern that persisted through almost every month of followup. In addition, it is possible that the differences observed may be conservative due to our methodology. As noted in the primary MSH paper, there were periods off of hydroxyurea for the treatment group. This was not controlled in our analyses, and it is possible that pain frequency and intensity increased for the hydroxyurea group during these periods. This would have the effect of reducing differences in these outcomes between the treatment and placebo groups.
When we examined the full four quartiles of hydroxyurea response previously reported in MSH,12 results showed that the “very high” responders, in particular, were driving the significant differences found. Not only did this group have significantly lower scores on all pain response measures than the placebo group, but it also had significantly lower pain intensity than both the “low” and “moderate” response groups, and lower analgesic use and utilization than the “moderate” response group. The differences in averages between the “very high” response group and the placebo group were larger: approximately 10–12% on the 0–9 pain scale, 12% in daily probability of analgesic use, and 6% in daily probability of a medical contact. For some, daily symptomatic response to HU was no doubt dramatic. There were also inconsistent, smaller differences in the three daily symptomatic response variables between “very high” response group and the “high” HbF responders, or between either of these groups and the “moderate” and “low” HbF responders or patients on placebo.
One explanation for our finding of small differences in analgesic use between hydroxyurea vs. placebo groups is a possible “analgesia displacement effect” resulting from avoidance of hospitalizations by use of hydroxyurea. MSH showed that SCD patients using hydroxyurea are admitted less often to the hospital. Hence, these patients stayed at home for a higher proportion of MSH study time. During these days, they may have taken analgesics for mild to moderate pain. Consequently their utilization of home analgesics may have increased, and may not have been significantly different from patients not taking hydroxyurea. Placebo patients may have taken large amounts of analgesics for more severe pain, but over a shorter period of time.
Another explanation for some of our findings is that ambulatory pain intensity ratings may exhibit a "ceiling effect" – days with higher pain ratings may have been excluded because they met a threshold associated with a medical visit. The effective range of pain ratings for ambulatory pain may have been 0–6 or 0–7, rather than 0–9. If this were true, our patients’ average ambulatory pain response difference may well have been >5%.
We believe these results are vitally important, and in support of of a recent NIH consensus statement on hydroxyurea in SCD.27,28 This statement recommended wider, more consistent use of hydroxyurea in adults with SCD, and possible use in children, but noted a problem with achieving effectiveness of hydroxyurea--consistent adherence is required to demonstrate effectiveness. The statement mentioned that 3 to 6 months of consistent treatment are required for a clinical response to hydroxyurea, and that this delay decreases adherence.
Our results imply that decreased use of or adherence with hydroxyurea by SCD patients and clinicians may be mainly due to lack of perceived effect on daily symptomatic response: daily pain intensity, percent of days with analgesic use, or percent of days with utilization. It is understandable that early, significant improvement in pain is one benefit of hydroxyurea treatment that patents and clinicians desire, and that they might begin or continue hydroxyurea therapy based on this benefit. Our results do suggest that any perceived daily symptomatic benefits persist over time as therapy is continued.
However, our results, combined with earlier research, suggest that clinicians and patients should make a final judgment about hydroxyurea benefit based on whether there has been a positive HbF hydroxyurea response after several months of therapy.9 Even when patients report no perceptible daily symptomatic benefit of hydroxyurea, it would be unwise to discontinue hydroxyurea without checking for a HbF response. SCD mortality has been related to the level of HbF in a dose-response fashion, as well as to frequency of utilization.2,29 Even small increases in HbF are beneficial to SCD mortality.2 Further, there is a statistically significantly higher HbF response to hydroxyurea among patients who are ≥80% adherent overall, based on capsule counts at study visits.12 All of these findings suggest discontinuation of hydroxyurea should only be based on demonstration of no HbF response to hydroxyurea after a several-month trial of therapy during which the patient is adherent.
On the other hand, our results suggest that clinicians may appropriately accept early positive daily symptomatic responses to hydroxyurea as evidence of a positive HbF hydroxyurea response. The results show that fetal hemoglobin responsiveness to hydroxyurea, particularly very high responsiveness, may be heralded by or correlated with a daily symptomatic response of reduced pain, reduced analgesic use, and reduced utilization. For the fortunate circumstance when patients show an early daily symptomatic response, clinicians may reasonably conclude HbF responsiveness, or may choose to continue hydroxyurea with less followup of HbF response (although regular measurements of neutrophil, reticulocyte, and platelet counts are recommended to monitor for bone marrow suppression). Future studies that identify patient characteristics associated with HbF responsiveness may be of benefit in allowing physicians to better predict the outcomes of hydroxyurea treatment.
Pain reduction is not the only indication for hydroxyurea treatment in sickle cell anemia. Recent pediatric hydroxyurea studies have shown promise that hydroxyurea can prevent organ damage in children,30,31,32,33,34,35,36,37,38,39.
Important limitations of the meaning of our findings are worth noting. First, putting aside reductions in hospitalization for pain and in mortality among users of hydroxyurea previously reported in MSH, the improvements in pain, analgesic use, and utilization we report herein leave great room for improvement in treatment of SCD pain. Users of hydroxyurea still often require opioids for palliation of acute and chronic SCD pain. Second, we studied a very select population. Only patients with more than three hospitalizations per year were included in MSH. Our results may not extrapolate to less severely ill SCD patients. Also, MSH study participants were enrolled nearly 20 years ago, and followed strenuous study procedures, including adherence with hydroxyurea, for a mean follow-up of 21 months. Differences in the availability of alternative treatments and in patients’ motivation and social support may yield different adherence and different outcomes in patients beginning hydroxyurea today.
Acknowledgments
The authors wish to acknowledge the assistance of the staff of Maryland Medical Research Institutes for their invaluable contributions to this study and manuscript.
This study was supported by National Heart, Lung, and Blood Institute contract NO1-HB-67129.
*Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia (RCT)
Clinical Centers:
University of North Carolina, Chapel Hill, NC: E Orringer, S Jones, D Strayhorn
Duke University, Durham, NC: W Rosse, G. Phillips, D Peace, A Johnson-Telfair
Medical College of Georgia, Augusta, GA: P Milner, A Kutlar, A Tracy
Thomas Jefferson University, Philadelphia, PA: SK Ballas, GE Allen, J Moshang, B Scott
University of Mississippi, Jackson, MS: M Steinberg, A Anderson, V Sabahi
University of Miami, Miami, FL: C Pegelow, D Temple, E Case, R Harrell, S Childerie
San Francisco General Hospital, San Francisco, CA: S Embury, B Schmidt, D Davies
University of Illinois, Chicago, IL: M Koshy, N Talischy-Zahed, L Dorn, G Pendarvis, M McGee
Michael Reese Hospital, Chicago, IL: M Telfer, A Davis
Howard University, Washington, DC: O Castro, H Finke, E Perlin, J Siteman
University of Medicine and Dentistry of New Jersey, Newark, NJ: P Gascon, P di Paolo, S Gargiulo
Emory University, Atlanta, GA: J Eckman, JH Bailey, A Platt, L Waller
St. Luke’s – Roosevelt Medical Center, New York, NY: G Ramirez, V Knors, S Hernandez, EM Rodriguez, E Wilkes
Children’s Hospital of Oakland, Oakland, CA: E Vichinsky, S Claster, A Earles, K Kleman, K McLaughlin
Medical College of Virginia, Richmond, VA: P Swerdlow, W Smith, B Maddox, L Usry, A Brenner, K Williams, R O’Brien, K Genther
Case Western Reserve University, Cleveland, OH: S Shurin, B Berman, K Chiarucci, L Keverline
Hospital for Sick Children, Toronto, Ontario: N Olivieri, D Shaw, N Lewis
Brigham and Women’s Hospital, Boston, MA: K Bridges, B Tynan, C Winograd
Interfaith Medical Center, Brooklyn, NY: R Bellevue, H Dosik, M Sheikhai, P Ryans, H Souffrant
University of Alabama, Birmingham, AL: J Prchal, J Braddock, T McArdle
University of Pittsburgh, Pittsburgh, PA: T Carlos, A Schmotzer, D Gardner
Central Office Staff (Johns Hopkins University, Baltimore, MD): S Charache, R Moore, G Dover, M Bergner, C Ewart, S Eckert, C Lent, J Ullrich, L Fishpaw, G Tirado, J Gibson, T Moeller, T Nagel
Data Coordinating Center: (Maryland Medical Research Institute, Baltimore, MD): M Terrin, FB Barton, RP McMahon, C Handy, D Harris, M Canner, J Depkin, N Meinert, M Carroll, R Giro, S Karabelas, C Kelly
Crisis Review Committee: M Heyman, P Beilinson, M Druskin, P Ellis, WA Flood, S Kravitz, S Lanzkron, V Lorica, A Moliterno, A Nahum, JA Nesbitt III, L Rosenthal, W Sharfman, M Streiff, M Wachsman, P Bray, C Van Dang, J Casella, M McGuire, L Patrick, H Schaad, C Steiner
Data and Safety Monitoring Board: C Johnson, A Bank, G Cutter, CE Davis, O Huntley, L Lessin, O Platt, M Gray-Secundy
Project Office (National Heart, Lung, and Blood Institute, Bethesda, MD): D Bonds, C Reid, N Geller, M Waclawiw
Footnotes
None of the authors of this manuscript have reported conflicts of interest with regard to the conduct or findings of this study
References
- 1.Charache Samuel, Terrin Michael L, Moore Richard D, Dover George J, Barton Franca B, Eckert Susan V, McMahon Robert P, Bonds Duane R the Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 3.Dampier C, Setty BN, Eggleston B, Brodecki D, O'neal P, Stuart M. Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates. J Pediatr Hematol Oncol. 2004 Dec;26(12):785–790. [PubMed] [Google Scholar]
- 4.Dampier C, Ely E, Eggleston B, Brodecki D, O'Neal P. Physical and cognitive-behavioral activities used in the home management of sickle pain: a daily diary study in children and adolescents. Pediatr Blood Cancer. 2004 Nov;43(6):674–678. doi: 10.1002/pbc.20162. [DOI] [PubMed] [Google Scholar]
- 5.Dampier C, Ely B, Brodecki D, O'Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002 Dec;3(6):461–470. doi: 10.1054/jpai.2002.128064. [DOI] [PubMed] [Google Scholar]
- 6.Dampier C, Ely E, Brodecki D, O'Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002 Nov;24(8):643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Charache Samuel, Terrin Michael L, Moore Richard D, Dover George J, McMahon Robert P, Barton Franca B, Waclawiw Myron, Eckert Susan V the Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Design of the Multicenter Study of Hydroxyurea in sickle cell anemia. Controlled Clinical Trials. 1995;16:432–446. doi: 10.1016/s0197-2456(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 9.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 10.Powars DR, Schroeder WA, Weiss JN, Chan LS, Azen SP. Lack of influence of fetal hemoglobin levels or erythrocyte indices on the severity of sickle cell anemia. J Clin Invest. 1980 Mar;65(3):732–740. doi: 10.1172/JCI109720. PubMed PMID: 6153392; PubMed Central PMCID: PMC371416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984 Apr;63(4):921–926. PubMed PMID: 6200161. [PubMed] [Google Scholar]
- 12.Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: Effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- 13.Charache S, Dover GJ, Moyer MA, Moore JW. Hydroxyurea-induced augmentation of fetal hemoglobin production in patients with sickle cell anemia. Blood. 1987;69:109–116. [PubMed] [Google Scholar]
- 14.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: Determinants of response to hydroxyurea. Blood. 1997;89:1078–1088. [PubMed] [Google Scholar]
- 15.Goldberg MA, Brugnara C, Dover GJ, Schapira L, Charache S, Bunn HF. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N Engl J Med. 1990;323:366–372. doi: 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- 16.Dover GJ, Charache S. Hydroxyurea induction of fetal hemoglobin synthesis in sickle-cell disease. Semin Oncol. 1992;19:61–66. [PubMed] [Google Scholar]
- 17.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 18.Ballas SK, McCarthy WF, Guo N, Brugnara C, Kling G, Bauserman RL, Waclawiw MA. Early detection of response to hydroxyurea therapy in patients with sickle cell anemia. Hemoglobin. 2010;34(5):424–429. doi: 10.3109/03630269.2010.513638. PubMed PMID: 20854115. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, Ataga K, Swerdlow P, Kutlar A, DeCastro L, Waclawiw MA. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia and MSH Patients' Follow-Up. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010 Jun;85(6):403–408. doi: 10.1002/ajh.21699. PubMed PMID: 20513116; PubMed Central PMCID: PMC2879711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Hydroxyurea and acute painful crises in sickle cell anemia: effects on hospital length of stay and opioid utilization during hospitalization, outpatient acute care contacts, and at home. J Pain Symptom Manage. 2010 Dec;40(6):870–882. doi: 10.1016/j.jpainsymman.2010.03.020. PubMed PMID: 20864308 PubMed Central PMCID: PMC3005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballas SK, Bauserman RL, McCarthy WF, Waclawiw MA. Multicenter Study of Hydroxyurea in Sickle Cell Anemia. The impact of hydroxyurea on career and employment of patients with sickle cell anemia. J Natl Med Assoc. 2010 Nov;102(11):993–999. PubMed PMID: 21141286. [PubMed] [Google Scholar]
- 23.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008 Feb;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. Epub 2007 Aug 3. Review. PubMed PMID:18177782. [DOI] [PubMed] [Google Scholar]
- 24.Lopes BL, Flenders P, Davis-Moon L, Corbin T, Ballas SK. Clinically significant differences in the visual analog pain scale in acute vasoocclusive sickle cell crisis. Hemoglobin. 2007;31(4):427–432. doi: 10.1080/03630260701587810. [DOI] [PubMed] [Google Scholar]
- 25.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;4(4):485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 26.Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Utilization of analgesics in the multicenter study of hydroxyurea in sickle cell anemia: effect of sex, age, and geographical location. Am J Hematol. 2010 Aug;85(8):613–616. doi: 10.1002/ajh.21750. PubMed PMID: 20568300. [DOI] [PubMed] [Google Scholar]
- 27.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi CE, James AH, Laraque D, Mendez MH, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. NIH consensus development statement on hydroxyurea treatment for sickle cell disease. NIH Consens State Sci Statements. 2008 Feb 27–29;25(1):1–30. [PubMed] [Google Scholar]
- 28.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008 Jun 17;148(12):932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 29.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 30.Thornburg CD, Rogers ZR, Jeng MR, Rana SR, Iyer RV, Faughnan L, Hassen L, Marshall J, McDonald RP, Wang WC, Huang X, Rees RC. BABY HUG Investigators.--Adherence to study medication and visits: data from the BABY HUG trial. Pediatr--Blood Cancer. 2010 Feb;54(2):260–264. doi: 10.1002/pbc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien SH, Hankins JS. Decision analysis of treatment strategies in children with severe sickle cell disease. J Pediatr Hematol Oncol. 2009 Nov;31(11):873–878. doi: 10.1097/MPH.0b013e3181b83cab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson BW, Miller ST, Rogers ZR, Rees RC, Ware RE, Waclawiw MA, Iyer RV, Casella JF, Luchtman-Jones L, Rana S, Thornburg CD, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik S, Howard TH, Luck L, Wang WC. The pediatric hydroxyurea phase III clinical trial (BABY HUG): challenges of study design. Pediatr Blood Cancer. 2010 Feb;54(2):250–255. doi: 10.1002/pbc.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller ST, Wang WC, Iyer R, Rana S, Lane P, Ware RE, Li D, Rees RC BABY-HUG Investigators. Urine concentrating ability in infants with sickle cell disease: baseline data from the phase III trial of hydroxyurea (BABY HUG) Pediatr Blood--Cancer. 2010 Feb;54(2):265–268. doi: 10.1002/pbc.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukusa AK, Vermylen C, Vanabelle B, Curaba M, Brichard B, Chantrain C, Dupont S, Ferrant A, Wyns C. Bone marrow transplantation or hydroxyurea for sickle cell anemia: long-term effects on semen variables and hormone profiles. Pediatr Hematol Oncol. 2009 Jun;26(4):186–194. doi: 10.1080/07357900902892780. [DOI] [PubMed] [Google Scholar]
- 35.Thornburg CD, Dixon N, Burgett S, Mortier NA, Schultz WH, Zimmerman SA, Bonner M, Hardy KK, Calatroni A, Ware RE. A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer. 2009 May;52(5):609–615. doi: 10.1002/pbc.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strouse JJ, Lanzkron S, Beach MC, Haywood C, Park H, Witkop C, Wilson RF, Bass EB, Segal JB. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008 Dec;122(6):1332–1342. doi: 10.1542/peds.2008-0441. [DOI] [PubMed] [Google Scholar]
- 37.Singh SA, Koumbourlis AC, Aygun B. Resolution of chronic hypoxemia in pediatric sickle cell patients after treatment with hydroxyurea. Pediatr Blood Cancer. 2008 Jun;50(6):1258–1260. doi: 10.1002/pbc.21480. [DOI] [PubMed] [Google Scholar]
- 38.Hankins JS, Helton KJ, McCarville MB, Li CS, Wang WC, Ware RE. Preservation of spleen and brain function in children with sickle cell anemia treated with hydroxyurea. Pediatr Blood Cancer. 2008 Feb;50(2):293–297. doi: 10.1002/pbc.21271. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007 Aug 1;110(3):1043–1047. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]