Abstract
Background
Slow gait speed is a powerful predictor of disability in activities of daily living and mortality. Muscle strength and body composition change over time, but their changes differ by sex. How these parameters jointly affect gait speed decline is unknown. Understanding this association could help develop and evaluate the sex‐specific effects of lifestyle interventions to delay gait speed decline in older adults. We assessed whether changes in strength (Δstrength), appendicular lean mass (ΔALM), and fat mass (Δfat) jointly relate to change in gait speed and whether the association differs by sex.
Methods
The analytic sample comprised 575 women and 539 men aged 22–95 years enrolled in the Baltimore Longitudinal Study of Aging. Mean follow‐up was 4.0 years. Measures included isometric knee extension strength, dual‐energy X‐ray absorptiometry‐assessed ALM and fat mass, and gait speed from the 400 m fast pace walk. Sex‐specific linear mixed models were adjusted for follow‐up time and baseline age, race, height, ALM, fat mass, peak torque, and gait speed. We also included second‐order interaction terms of the key predictive variables (e.g. Δstrength × ΔALM). To interpret the interactions, we estimated average gait declines using the 25th or 75th percentile of the two significant predictive variables and then assessed which condition relates to larger decline in gait speed.
Results
In both sexes, independent of ΔALM and Δfat, larger decline in strength significantly related to larger decline in gait speed (P = 0.01 for both sexes). In men, interactions between Δstrength × ΔALM and Δfat by ΔALM were associated with change in gait speed; men with greater declines in both muscle strength and ALM or greater declines in both ALM and fat have steeper gait speed decline. In contrast, in women, the interaction between Δfat and ΔALM was associated with change in gait speed; women with an increase in fat mass combined with less decline in ALM have steeper gait speed decline.
Conclusions
While change in strength affects change in gait speed in both sexes, the effects of body composition change differ by sex. Dual‐energy X‐ray absorptiometry‐based estimates of lean mass may be confounded by intramuscular fat. Future studies should examine sex‐specific combined effects of change in strength and body composition on mobility using multiple techniques to measure body composition. Intervention studies should consider testing sex‐specific interventions on body composition.
Keywords: Ageing, Strength, Walking, Body composition
Introduction
Poor mobility performance, often assessed as slow gait speed, is a powerful predictor of disability in activities of daily living, nursing home admission, and mortality.1, 2, 3, 4 Although a wide variety of demographic, environmental, social, and behavioural risk factors have been related to more rapid decline in gait speed with ageing, there is a robust literature suggesting that poor muscle strength, low muscle mass, and higher adiposity are important predictors of poor mobility performance.5, 6 In comparison with muscle mass, muscle strength is a better predictor of mobility decline and disability, and fat mass is a better predictor of disability and mortality.5, 7, 8 However, the joint association of changes in muscle strength, muscle mass, and fat mass with age‐associated mobility decline remains unclear and has not been fully explored in a longitudinal perspective. Understanding how changes in strength and body composition among older adults jointly relate to changes in mobility is important for developing and tracking the effect of lifestyle‐based interventions aimed at preventing mobility disability in older adults.
Most studies that assessed the associations between muscle strength, muscle mass, fat mass, and mobility performance measured strength and body composition at a single time point to predict the onset of mobility limitation or subsequent rates of mobility decline. In the InCHIANTI study, Hicks et al.6 found that muscle strength predicts future changes in mobility performance and the previous rate of decline in muscle strength adds no significant information. However, subsequent changes in mobility may be strongly conditioned by parallel changes in muscle and fat variables, which were not considered. Auyeung et al.9 described changes of handgrip strength, muscle mass, and gait speed during a 4 year follow‐up in Asian women and men over 65 years of age but did not consider the effect of changes in adiposity. Thus, whether and how parallel changes over time of muscle strength, muscle mass, and adiposity jointly relate to simultaneous changes in mobility has not been fully elucidated, even though muscle strength and body composition are known to change dynamically across the lifespan, especially in later life.5
Throughout adult life, muscle strength and body composition differ by sex, and both muscle strength and muscle mass decline with ageing faster in men than in women.9, 10, 11, 12 While in both sexes, fat mass increases until 70 years and then declines, women tend to have higher per cent fat mass than men, and the pattern of adipose tissue accumulation also differs between sexes.13 Age‐associated loss in muscle mass and increase in fat mass are related to each other. From a biomechanical perspective, a combined decline in muscle mass and increase in adiposity cause a smaller ‘engine’ having to carry a heavier load. Also, several adipokines secreted from adipose tissue affect insulin resistance, energy metabolism, and growth hormone secretion, which may subsequently contribute to a decrease in muscle mass and strength.14
Our aim was to investigate whether changes in muscle strength (Δpeak torque), appendicular lean mass (ΔALM), and whole‐body fat mass (Δfat) independently or jointly relate to simultaneous change in gait speed, after adjusting for cross‐sectional and longitudinal covariates, and how these relationships differ between men and women. Given the substantial differences in muscle strength and body composition between men and women, we hypothesized that changes in muscle strength and body composition influence changes in gait performance differently in men and women.
Methods
Participants
This longitudinal study used data from the Baltimore Longitudinal Study of Aging (BLSA), a prospective observational cohort study aimed at describing the effects of normal ageing on multiple aspects of the human anatomy and physiology.15 BLSA participants undergo 3 day comprehensive examinations, including muscle strength tests, body composition assessments, and mobility performance measures. The interval between follow‐up visits depends on participants' age: 20‐ to 59‐year‐old participants are studied every 4 years, 60–79‐year‐olds every 2 years, and over 80 years every year. In the present analysis, we selected visits when participants had complete data on the isometric knee extension test, measures of body composition, and the 400‐m‐long corridor walk test. Between March 2007 and September 2017, a total of 1114 BLSA participants (22‐ to 95‐year‐olds at first visit; women, n = 575; men, n = 539) were eligible for this study, providing 2440 person‐visits. Mean follow‐up time was 3.99 ± 3.00 years [median, 3.00 years, 1–11 years]. In this analytic samples, 38.8% of the participants were examined only once. Supporting Information, Table S1 shows the number of visits for each participant and the number of participants by length of follow‐up. The BLSA protocol was approved by the Institutional Review Board of record at the time of data collection (National Institute of Environmental Health Sciences, NC), and written informed consent was obtained from all participants.
Knee extension peak torque
Until February 2011, the BLSA measured isometric knee extension with the Kin‐Com isokinetic dynamometer (Kin‐Com model 125E, version 3.2, Chattanooga Group, Chattanooga, TN). From February 2010 to present (data included through l September 2017), BLSA used the Biodex Multi‐Joint System‐Pro dynamometer (Biodex Medical System, Advantage Software V.4X, Inc., Shirley, NY). Between February 2010 and January 2011, a total of 108 participants (women, n = 54; 66.7 ± 12.0 [37–94] years old) performed isometric knee extension strength testing by both the Kin‐Com and the Biodex technology at the same visit, and a conversion equation was estimated that allowed the harmonization of data collected with the two assessment methods (Supporting Information, Figure S1).
For both the Kin‐Com and the Biodex knee extension testing, participants were asked to extend their left knee for 3 s as hard as possible for three trials with an instructor's verbal encouragement.16, 17 The starting knee joint position was in 120° extension for the Kin‐Com and 70° less than the full extension (130° extension) for the Biodex. Participants rested for 15 s between trials. Peak torque was defined as the highest trial value.
Mobility performance
In the 400‐m‐long corridor walk, examiners asked participants to walk as fast as possible without running.18, 19 A 20 m walking course was set with two fluorescent orange traffic cones at each end and tape marking each meter between the cones. Participants completed 10 round trips, for a total of 400 m. Gait speed was calculated as distance divided by total time to complete the 400 m walk (m/s). In our analysis, the distribution of time to complete 400 m was skewed, while that of gait speed was more normally distributed. Thus, we used the calculated gait speed from the 400 m walk for further analyses.
Body composition
Whole‐body fat mass and appendicular lean mass (sum of arm and leg lean mass) (ALM) were measured using whole‐body dual‐energy X‐ray absorptiometry (Prodigy Dual Photon X‐ray Absorptiometry unit, General Electric, Milwaukee, WI) with enCORE 2016 or version 16 SP2.17
Statistical analysis
Descriptive data were reported by the mean ± standard deviation or percentages. Differences in age, race (Black vs. non‐Black) between sexes were assessed with an unpaired t‐test and χ 2 test, respectively. Sex differences in baseline variables and slope of gait speed were tested by generalized linear regression models with adjustment for age to account for ageing effects on anthropometric, morphological, and performance measures.
Because we consistently observed significant sex differences in baseline characteristics, we explored sex‐specific mean trajectory change in gait speed in participants who had data at a minimum of three time points. After estimating each participant's gait speed slope and intercept by generalized linear regression model, we obtained mean gait speed change per year in each sex. Furthermore, to visualize how Δpeak torque, ΔALM, and Δfat jointly relate to Δgait speed, we divided participants into eight groups in each sex by using sex‐specific median values of Δpeak torque, ΔALM, and Δfat.
To account for inhomogeneous follow‐up visit intervals between and within participants, generalized linear mixed‐effects models were used to assess the longitudinal association between absolute changes in peak torque and body composition and absolute change in gait speed adjusted for covariates.20 In exploratory analyses, we also consistently observed a significant interaction between sex and follow‐up time (years) (‘sex × time’), suggesting that the slope of absolute change in gait speed differs by sex; thus, all analyses were sex stratified.
Absolute change in gait speed (Δgait speed) was calculated by the following equation: gait speedx minus gait speed1, where subscript x and 1 represent xth and the first visit, respectively. A larger negative value indicates larger decline between xth and the first visit. We similarly calculated absolute change in peak torque (Δpeak torque), ALM (ΔALM), and fat mass (Δfat).
In Model 1, we regressed Δgait speed on Δpeak torque. The model included covariates; race (Black vs. non‐Black), baseline age, height, gait speed, and baseline peak torque, and follow‐up time (years). In Model 2, we regressed Δgait speed on ΔALM and Δfat by using similar linear mixed model as Model 1. In Model 3, all predictors included in Models 1 and 2 were modelled together to test whether Δpeak torque, ΔALM, and Δfat relate to Δgait speed are independent of each other. We additionally included second‐order interaction terms between these predictors into Models 4–7.
To visualize the relationships of independent variables with change in gait speed, we used the models to compute predicted values of gait speed change at the mean follow‐up time at different values of Δpeak torque, ΔALM, and Δfat. Specifically, we used the 25th and 75th percentile change in one of the independent variables (Δpeak torque or ΔALM or Δfat), after adjusting for sex‐specific averages of the other two change variables and other covariates. We used three scenarios: (i) 25th (greater decline) vs. 75th (less decline) percentile of Δpeak torque, after adjusting for ΔALM and Δfat (sex‐specific averages); (ii) 25th (greater decline) vs. 75th (less decline) percentile of ΔALM, after adjusting for sex‐specific averages of Δpeak torque and Δfat; and (iii) 25th (fat decrease) vs. 75th (fat increase) percentile of Δfat, after adjusting for sex‐specific average of Δpeak torque and ΔALM (see Model 7 in Tables 3A and 3B).
Table 3A.
Sex‐specific longitudinal association of peak torque and body composition with change in gait speed in men
| Men (n = 539) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δgait speed in 400 m (m/s) | ||||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | ||||||||
| P‐value | P‐value | P‐value | P‐value | P‐value | P‐value | P‐value | ||||||||
| Time (years) | ||||||||||||||
| β | −0.031 | <0.0001 | −0.031 | <0.0001 | −0.03 | <0.0001 | −0.03 | <0.0001 | −0.03 | <0.0001 | −0.03 | <0.0001 | −0.03 | <0.0001 |
| SE | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | |||||||
| Δpeak torque (Nm) | ||||||||||||||
| β | 0.0004 | 0.004 | 0.0004 | 0.01 | 0.0003 | 0.02 | 0.0004 | 0.01 | 0.0004 | 0.01 | 0.0004 | 0.01 | ||
| SE | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||||||
| ΔALM (kg) | ||||||||||||||
| β | 0.007 | 0.01 | 0.006 | 0.01 | 0.004 | 0.15 | 0.006 | 0.01 | 0.006 | 0.01 | 0.005 | 0.09 | ||
| SE | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | 0.003 | ||||||||
| Δfat mass (kg) | ||||||||||||||
| β | 0.001 | 0.31 | 0.0007 | 0.52 | 0.0007 | 0.52 | −0.00003 | 0.98 | −0.0001 | 0.95 | 0.0001 | 0.94 | ||
| SE | 0.001 | 0.001 | 0.0011 | 0.0014 | 0.001 | 0.0012 | ||||||||
| Δpeak torque (Nm) × ΔALM (kg) | ||||||||||||||
| β | −0.0002 | 0.04 | −0.0001 | 0.12 | ||||||||||
| SE | 0.0001 | 0.0001 | ||||||||||||
| Δpeak torque (Nm) × Δfat mass (kg) | ||||||||||||||
| β | −0.00004 | 0.31 | ||||||||||||
| SE | 0.00004 | |||||||||||||
| ΔALM (kg) × Δfat mass (kg) | ||||||||||||||
| β | −0.001 | 0.03 | −0.001 | 0.09 | ||||||||||
| SE | 0.001 | 0.001 | ||||||||||||
β = unstandardized beta coefficients; Δgait speed in 400 m walk = gait speed at each visit minus gait speed at first visit; Δpeak torque (Nm) = peak torque at each visit minus peak torque at first visit; ΔALM (kg) = appendicular lean mass at each visit minus ALM at first visit; Δfat mass (kg) = whole‐body fat mass at each visit minus whole‐body fat mass at first visit. SE, standard error.
All models were adjusted for race (Black vs. non‐Black), baseline age, baseline gait speed, baseline height, and baseline gait speed. In addition, Model 1 was further adjusted for baseline peak torque. Model 2 was adjusted for baseline ALM and fat mass. Models 3 to 7 were adjusted for baseline peak torque, ALM, and fat mass. Of note, no significant associations were observed between these covariates and Δgait speed in any models (P < 0.05).
Table 3B.
Sex‐specific longitudinal association of peak torque and body composition with change in gait speed in women
| Women (n = 575) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δgait speed in 400 m (m/s) | ||||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | ||||||||
| P‐value | P‐value | P‐value | P‐value | P‐value | P‐value | P‐value | ||||||||
| Time (years) | ||||||||||||||
| β | −0.023 | <0.0001 | −0.024 | <0.0001 | −0.022 | <0.0001 | −0.022 | <0.0001 | −0.022 | <0.0001 | −0.022 | <0.0001 | −0.022 | <0.0001 |
| SE | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |||||||
| Δpeak torque (Nm) | ||||||||||||||
| β | 0.0004 | 0.02 | 0.0005 | 0.01 | 0.0005 | 0.01 | 0.0005 | 0.01 | 0.0005 | 0.01 | 0.0005 | 0.01 | ||
| SE | 0.0000 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | ||||||||
| ΔALM (kg) | ||||||||||||||
| β | 0.0035 | 0.17 | 0.0028 | 0.27 | 0.0028 | 0.29 | 0.0029 | 0.26 | 0.003 | 0.24 | 0.004 | 0.17 | ||
| SE | 0.003 | 0.0026 | 0.0026 | 0.0026 | 0.003 | 0.003 | ||||||||
| Δfat mass (kg) | ||||||||||||||
| β | −0.0011 | 0.28 | −0.0017 | 0.10 | −0.0017 | 0.11 | −0.0019 | 0.11 | −0.0015 | 0.14 | −0.0016 | 0.13 | ||
| SE | 0.001 | 0.0011 | 0.0011 | 0.0012 | 0.0011 | 0.0011 | ||||||||
| Δpeak torque (Nm) × ΔALM (kg) | ||||||||||||||
| β | −0.000003 | 0.98 | 0.0001 | 0.37 | ||||||||||
| SE | 0.0001 | 0.0001 | ||||||||||||
| Δpeak torque (Nm) × Δfat mass (kg) | ||||||||||||||
| β | −0.00002 | 0.73 | ||||||||||||
| SE | 0.00005 | |||||||||||||
| ΔALM (kg) × Δfat mass (kg) | ||||||||||||||
| β | −0.0013 | 0.050 | −0.0016 | 0.03 | ||||||||||
| SE | 0.0007 | 0.0007 | ||||||||||||
β = unstandardized beta coefficients; Δgait speed in 400 m walk = gait speed at each visit minus gait speed at first visit; Δpeak torque (Nm) = peak torque at each visit minus peak torque at first visit; ΔALM (kg) = appendicular lean mass at each visit minus ALM at first visit; Δfat mass (kg) = whole‐body fat mass at each visit minus whole‐body fat mass at first visit. SE, standard error.
All models were adjusted for race (Black vs. non‐Black), baseline age, baseline gait speed, baseline height, and baseline gait speed. In addition, Model 1 was further adjusted for baseline peak torque. Model 2 was adjusted for baseline ALM and fat mass. Models 3 to 7 were adjusted for baseline peak torque, ALM, and fat mass. Of note, no significant associations were observed between these covariates and Δgait speed in any models (P < 0.05).
SAS software version 9.4 for Windows (SAS Institute, Inc., Cary, NC) was used for all data processing and statistical analyses. Statistical significance was defined as P < 0.05 (two sided).
Results
A total of 1114 participants (women, 51.6%) were included in the analysis. Baseline participant characteristics are presented in Table 1. Significant sex differences were observed in all characteristics.
Table 1.
Participant characteristics
| Men (n = 539) | Women (n = 575) | P‐value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 67.24 ± 14.64 | 65.43 ± 13.31 | 0.031 |
| Race (Black, %) | 21.9 | 32.4 | <0.0001 |
| Height (cm) | 175.52 ± 7.17 | 162.14 ± 6.06 | <0.0001a |
| Weight (kg) | 84.31 ± 14.46 | 69.59 ± 13.94 | 0.010a |
| ALM (kg) | 25.82 ± 3.91 | 17.43 ± 2.73 | <0.0001a |
| Fat mass (kg) | 24.87 ± 9.63 | 27.44 ± 10.26 | <0.0001a |
| Gait speed in 400 m (m/s) | 1.61 ± 0.29 | 1.51 ± 0.25 | <0.0001a |
| Peak torque (Nm)b | 171.44 ± 49.62 | 115.04 ± 32.81 | <0.0001a |
ALM, appendicular lean mass; SD, standard deviation.
Age‐adjusted P‐value.
Measured by isometric knee extension.
Figure 1 shows the sex‐specific mean trajectory of change in gait speed. Compared with women, men had higher baseline walking speed but a steeper rate of decline.
Figure 1.
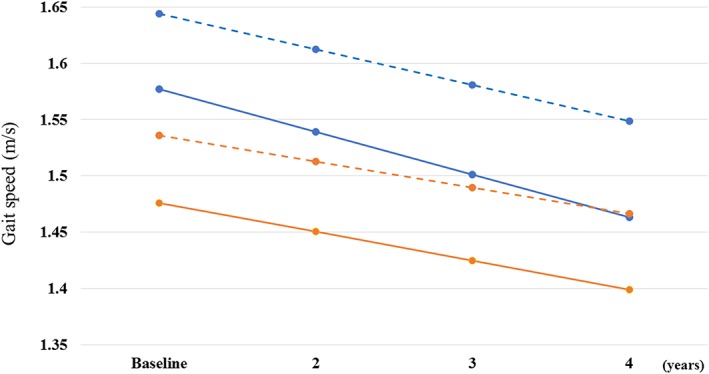
Mean trajectory of change in gait speed in men and women. Solid blue (men) and orange (women) lines were mean baseline and slopes of gait speed, which were obtained in participants who had more than three visits over the follow‐up (182 men, mean age, 71.0 ± 10 years; 183 women, mean age, 69.6 ± 9.8 years). A significant difference was observed in slopes between women and men (P < 0.0001). Dotted blue (men) and orange (women) lines were mean baseline and slopes of gait speed, estimated from a linear mixed model including all participants (539 men, mean age, 67.2 ± 14.6 years; 575 women, mean age, 65.4 ± 13.3 years).
In exploratory, stratified analyses, in both men and women, those with a smaller decline in peak torque had higher baseline gait speed and smaller decline in gait speed over follow‐up, except in the subset of men who simultaneously lost more ALM and gained more fat (Table 2). This subgroup was slower at baseline and experienced accelerated speed decline. In general, men and women with larger decline in peak torque were slower at baseline and experienced larger decline in gait speed over follow‐up, with the group who lost more peak torque and ALM and gained fat mass showing the largest decline in gait speed (Table 2).
Table 2.
Mean trajectory of changes in gait speed in men stratified by median Δpeak torque, ΔALM, and Δfat
| PT | ALM | FAT | Men | Women | ||||
|---|---|---|---|---|---|---|---|---|
| Mean gait speed at baseline (m/s) | Mean slope of gait speed (m/s) | n | Mean gait speed at baseline (m/s) | Mean slope of gait speed (m/s) | n | |||
| Less decline | Less decline | Increase | 1.648 | −0.027 | 27 | 1.581 | −0.018 | 25 |
| Less decline | Greater decline | Increase | 1.607 | −0.028 | 23 | 1.546 | −0.015 | 24 |
| Less decline | Less decline | Decrease | 1.609 | −0.031 | 25 | 1.523 | −0.020 | 20 |
| Greater decline | Greater decline | Decrease | 1.533 | −0.039 | 28 | 1.492 | −0.033 | 21 |
| Greater decline | Less decline | Increase | 1.584 | −0.041 | 17 | 1.466 | −0.036 | 20 |
| Greater decline | Less decline | Decrease | 1.556 | −0.045 | 22 | 1.396 | −0.028 | 27 |
| Less decline | Greater decline | Decrease | 1.541 | −0.045 | 16 | 1.528 | −0.020 | 23 |
| Greater decline | Greater decline | Increase | 1.527 | −0.053 | 24 | 1.488 | −0.037 | 23 |
Median slopes of key variables are as follows: Δpeak torque, −3.31 Nm/year; ΔALM, −0.14 kg/year; Δfat, 0.13 kg/year. Change in gait speed is in m/s/year. In peak torque (PT) and appendicular lean mass (ALM), ‘greater decline’ is defined as larger than median value, while ‘less decline’ means smaller than median value. In fat, ‘increase’ is defined as larger than median value because median value of Δfat is 0.13 kg/year, whereas ‘decrease’ means smaller than median value.
Tables 3A and 3B show the relationships of Δpeak torque, ΔALM, and Δfat with Δgait speed after adjusting for covariates. In all seven models, baseline values of peak torque, ALM, and fat were not significantly associated with Δgait speed. The relationships between Δpeak torque and Δgait speed in men are shown in Model 1 (Table 3A). For Model 1, an additional decline of 10 Nm of peak torque is associated with an additional 0.004 m/s decline in gait speed (P = 0.004). In Model 2, a significant association of ΔALM with Δgait speed is also evident. For Model 2, an additional decline of 1 kg of ALM is associated with an additional 0.007 m/s decline in gait speed (P = 0.01). When all three predictors were included in the same model (Model 3), Δpeak torque and ΔALM were significantly and independently associated with Δgait speed, while Δfat was not. Significant interactions were found for Δpeak torque with ΔALM and for Δfat with ΔALM (Model 4 and Model 6 in Table 3A). Each interaction suggests that both the effect of Δpeak torque on Δgait speed and the effect of Δfat on Δgait speed differ by ΔALM. However, significance was attenuated when all factors were included in the same model (Model 7 in Table 3A).
Although these interaction terms were of marginal significance, they appeared to be physiologically meaningful; therefore, we explored the joint relationships of Δpeak torque, ΔALM, and Δfat with Δgait by estimating the change in gait speed associated with the 25th (greater decline) or 75th (less decline) of Δpeak torque among under the assumption of 25th (greater decline) or 75th (less decline) percentile of ΔALM. Then we repeated the same analysis for Δfat (25th, decrease, or 75th, increase) instead of Δpeak torque.
For the Δpeak torque by ΔALM interaction, the significant interaction term implies that the effect of Δpeak torque on Δgait speed is stronger for larger ALM declines. For example, for participants with ΔALM in the 25th percentile (greater decline), the estimated difference in change in gait speed comparing Δpeak torque in 25th (greater decline) and 75th (less decline) percentile was −0.0072 m/s/year, whereas for participants with less decline in ALM (ΔALM 75th percentile), the relationship of change in Δpeak torque with change in gait speed was −0.0060 m/s/year (Supporting Information, Figure S2A). Thus, the largest gait speed decline occurs when both declines in peak torque and ALM are steeper.
Next, when we plugged 25th or 75th of ΔALM and Δfat, the model showed that the effect of Δfat on Δgait speed was stronger when ALM decline is steeper. For example, for participants with greater decline in ALM (ΔALM 25th percentile), the estimated change in gait speed for the difference between Δfat in 25th (fat decrease) and 75th (fat increase) percentile was −0.0006 m/s/year, whereas for participants with less decline in ALM (ΔALM 75th percentile), the effect of change in Δfat on change in gait speed was close to zero. Of note, the largest gait speed decline was observed under the combination of larger decline of ALM coupled with decrease in fat mass (Supporting Information, Figure S2B).
In women, the associations between Δpeak torque, ΔALM, Δfat, and Δgait speed are shown in Table 3B. As in men, baseline values of peak torque, lean mass, and fat mass were not significant predictors of Δgait speed in any model. The Δpeak torque was significantly associated with Δgait speed (Model 1). In Model 2, neither ΔALM nor Δfat was significantly associated with Δgait speed (P > 0.05). In the presence of all predictors (Model 3), Δpeak torque remained significant, suggesting that a larger decline of peak torque was associated with larger gait speed decline. In Model 7, Δpeak torque remained a significant predictor of change in gait speed,s and there was a significant interaction between ΔALM and Δfat. The sign and size of the interaction term suggest that the relationship of Δfat with Δgait speed depends on how much ΔALM declines. When ALM declines are larger (25th percentile), fat decrease (Δfat 25th percentile) relates to greater gait speed decline compared with fat increase (Δfat 75th percentile). In contrast, when ALM declines are smaller (75th percentile), fat increase (Δfat 75th percentile) is associated with a greater speed decline compared with fat decrease (Δfat 25th percentile). Of note, the largest gait speed decline was observed under the combination of ΔALM 75th (greater decline) and Δfat 75th (fat increase) (Supporting Information, Figure S2B).
Figure 2 visualizes the overall, mutually adjusted effects of Δpeak torque, ΔALM, and Δfat on Δgait speed (in Model 7 in Table 2). Larger differences in change in gait speed between 25th and 75th percentiles in one predictor suggests that the change in that predictor has a larger effect than other two variables. In men, while Δpeak torque and ΔALM have clear influenced on Δgait speed, Δfat rarely influenced Δgait speed. In women, only Δpeak torque affected Δgait speed.
Figure 2.
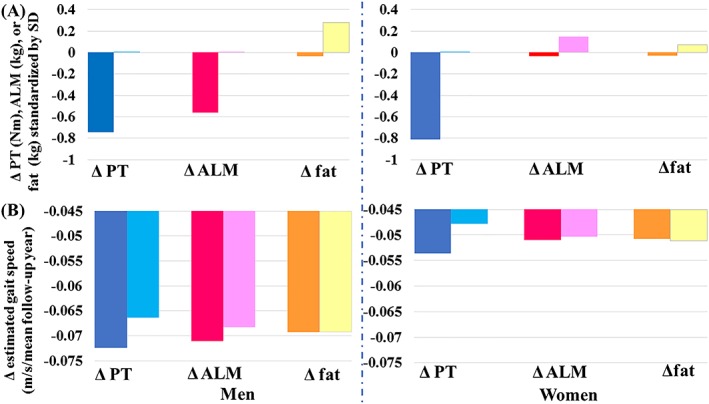
Magnitudes of changes in peak torque, appendicular lean mass (ALM), and fat mass and their effects on change in gait speed in men and women. (A) Twenty‐fifth (dark‐coloured bars) and 75th (light‐coloured bars) percentiles of z‐transformed Δpeak torque, ΔALM, and Δfat in sex‐stratified groups. Note that for visualization, a value of 0.01 was plugged for value equal to zero. In Δpeak torque and ΔALM, 25th percentile represents ‘greater decline’, while 75th percentile means ‘less decline’. In Δfat, 25th percentile represents ‘decrease’ in fat mass, while 75th percentile means ‘increase’ in fat mass. (B) Estimated average gait declines estimated by plugging 25th or 75th percentile of either one of Δpeak torque, ΔALM, and Δfat during mean follow‐up period. Dark‐coloured bars (25th percentile) and light‐coloured bars (75th percentile) are average gait speed changes estimated by plugging into Model 7 (Table 3AA and 3AB), respectively; the 25th and 75th percentile values for the variable are indicated below the bars, while mean values are plugged in all other covariates. Mean follow‐up period was 3.2 years in men and 3.3 years in women. SD, standard deviation.
Of note, all analyses were repeated only among participants with at least two visits, and results did not change substantially (data not shown).
Discussion
Using longitudinal data collected in the BLSA, we studied to what extent changes over time of muscle strength, lean body mass (ALM), and fat mass relate to parallel changes in gait speed in men and women after adjusting for covariates. We found that in both sexes, loss of muscle strength most consistently co‐occurs with gait speed decline, while baseline status of muscle strength and body composition have little predictive value on change in gait speed. Interestingly, change in ALM was related to change in gait speed in men but not in women. In men, decline in peak torque and increase in adiposity most strongly related to changes in gait speed in participants who experienced a steeper decline in ALM and weakly in participants in whom ALM remained stable or declined less. In women, a larger gait speed decline occurred in participants who experienced fat increase with less decline in ALM.
The innovation of this study is that it demonstrated that change in knee extension peak torque relates to simultaneous change in gait speed independent of baseline peak torque, body composition, and changes in body composition. Interestingly, the magnitude of association between change in peak torque and change in gait speed was similar in men and women.
The physiological reasons for the more consistent association of muscle strength with change in gait speed compared with muscle mass and the relatively small difference in predictors in men compared with women are, at this time, only speculative. Age‐related anatomical and physiological changes occur in the supra‐spinal regions of the central nervous system, such as cortical atrophy and decrease in motor cortical excitation.21, 22 In parallel, a number of age‐associated changes occur at the peripheral level, including muscle atrophy, defective excitation–contraction coupling regulation, increase in co‐activation of antagonist muscles to stabilize the involved joint, lower pennation angles in quadriceps muscles, and higher likelihood of knee osteoarthritis.23, 24, 25, 26 Age‐associated changes in these central and peripheral properties would cause decline of muscle strength and mobility dysfunctions but may not substantially affect muscle mass. Our results suggest that muscle strength is essential to predict age‐related decline in gait speed in men and women. Over the past few decades, cross‐sectional studies have shown that lower muscle mass is not associated with poor physical function after adjusting for fat or muscle strength.8, 27 From these studies, a concept has emerged that muscle strength and fat are the main parameters affecting mobility performance. Our results are consistent with the findings that muscle strength is more sensitive to the effect of ageing than muscle mass5 and also consistent with findings that exercise programmes, especially resistance exercise, positively affect muscle strength before any change in muscle mass is detected.28 We confirmed that change in muscle strength significantly relates to simultaneous change in gait speed, independent of cross‐sectional and longitudinal covariates. Consistent with our findings, we propose that interventions on muscle that are aimed to maintain mobility should be focused on improving muscle strength. Further studies are needed to test what type of exercise intervention is effective for preventing mobility dysfunctions and understand whether monitoring muscle mass and fat may also be important.
A somewhat unexpected result of our study was that changes in muscle mass, at least as measured by dual‐energy X‐ray absorptiometry, also related to changes of mobility performance, although only in men and much less than muscle strength. It is difficult to explain these findings based on the available data, although the presence of significant interaction of change in muscle mass with change in muscle strength and change in muscle mass with change in fat mass offers some clue in this regard. It is possible that parallel combination of changes in muscle strength, muscle mass, and fat identifies different pathways to change in gait speed. For example, the fact that the decline in strength particularly more strongly relates to gait speed in men when combined with decline in lean body mass may suggest pure muscle atrophy or cachexia, which may affect mobility through multiple pathways. Accelerated decline in lean body mass and also parallel decline in adiposity in men suggest changes in body composition that occurs as a consequence of severe deterioration of health status, similarly to what happens in cachexia associated with chronic disease or cancer. On the other hand, in women, the interpretation of the interaction of muscle mass with fat mass is that increased fatness with less decline of lean body mass may be associated with sedentary state and sarcopenic obesity. Women have higher adiposity and accumulation of an even greater adiposity over the lifespan than men.13 In the absence of decline or a lesser decline in lean body mass, an increase in adiposity is associated with weight gain, which for biomechanical reasons, may negatively affect mobility. Although previous studies report age‐related losses of muscle strength and muscle mass and their associations with adverse outcomes,5, 7, 29 none of these studies have investigated how the joint longitudinal changes of muscle strength and muscle atrophy relate to changes in gait speed. Thus, it is difficult to compare the results of this study to previous work.
An important strength of this study is the longitudinal design with large sample size in addition to diversity in sex and race. Our study also has limitations. First, the participants included in these analyses were healthy throughout the follow‐up visits and excluded disabled or frail individuals cannot be made. Thus, generalization of our findings to sicker and more disabled individuals is not possible. Second, we changed isokinetic dynamometers during follow‐up, which may affect trajectory changes in peak torque. Although we developed a conversion equation, we cannot be sure that residual differences exist between the two methods, especially for extreme values in the distribution. Last, although our model was adjusted for possible covariates, level of physical activity and vitamin D status were not adjusted for because of the considerable number of missing data.
Conclusions
There are sex‐specific associations of decline in muscle strength and change in body composition with age‐associated decline in gait speed. Muscle strength decline predicts gait speed decline in both sexes, and the association between changes in body composition and gait speed decline differed by sex. Further longitudinal studies including both women and men and a wide variety of physical performance measures are needed to test the hypothesis that changes in muscle strength and body composition predict disability.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Conflict of interest
None declared.
Supporting information
Figure S1. The association of two peak torques measured by Kin‐Com and Biodex isokinetic dynamometers
Figure S2A. Estimated average gait declines estimated by plugging 25th or 75th percentile of two predictive variables that were found significant interaction in men.
Figure S2B. Estimated average gait declines estimated by plugging 25th or 75th percentile of two predictive variables that were found significant interaction in women.
Table S1A. The number of participants by the number of visits
Table S1B. The number of participants by the length of follow‐up years
Acknowledgement
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.30
Osawa Y., Chiles Shaffer N., Shardell M. D., Studenski S. A., and Ferrucci L. (2019) Changes in knee extension peak torque and body composition and their relationship with change in gait speed, Journal of Cachexia, Sarcopenia and Muscle, 10: 1000–1008. 10.1002/jcsm.12458.
Contributor Information
Yusuke Osawa, Email: yusuke.osawa@nih.gov.
Luigi Ferrucci, Email: ferruccilu@grc.nia.nih.gov.
References
- 1. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6‐min walk test: a quick measure of functional status in elderly adults. Chest 2003;123:387–398. [DOI] [PubMed] [Google Scholar]
- 3. Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006;295:2018–2026. [DOI] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 2012;67:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2012;67:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, et al. Reexamining the sarcopenia hypothesis: muscle mass versus muscle strength. Ann N Y Acad Sci 2000;904:456–461. [PubMed] [Google Scholar]
- 8. Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci 1998;53:M214–M221. [DOI] [PubMed] [Google Scholar]
- 9. Auyeung TW, Lee SW, Leung J, Kwok T, Woo J. Age‐associated decline of muscle mass, grip strength and gait speed: a 4‐year longitudinal study of 3018 community‐dwelling older Chinese. Geriatr Gerontol Int 2014;14:76–84. [DOI] [PubMed] [Google Scholar]
- 10. Metter EJ, Conwit R, Tobin J, Fozard JL. Age‐associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 1997;52:B267–B276. [DOI] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 12. Cruz‐Jentoft AJ, Morley JE. Sarcopenia. The Atrium,Southern Gate, Chichester, West Sussex, PO10 8SQ, UK: John Wiley & Sons; 2012. [Google Scholar]
- 13. Henche SA, Torres RR, Pellico LG. An evaluation of patterns of change in total and regional body fat mass in healthy Spanish subjects using dual‐energy X‐ray absorptiometry (DXA). Eur J Clin Nutr 2008;62:1440–1448. [DOI] [PubMed] [Google Scholar]
- 14. Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2007;102:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50‐year‐long journey and plans for the future. J Gerontol A Biol Sci Med Sci 2008;63:1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age‐associated differences between arm and leg muscle groups. J Appl Physiol 1999;86:188–194. [DOI] [PubMed] [Google Scholar]
- 17. Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 1997;83:1581–1587. [DOI] [PubMed] [Google Scholar]
- 18. Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, et al. Measuring higher level physical function in well‐functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 19. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility‐intact older adults. J Am Geriatr Soc 2014;62:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci 2009;64:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilgour AH, Todd OM, Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr 2014;14:85 10.1186/1471-2318-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fathi D, Ueki Y, Mima T, Koganemaru S, Nagamine T, Tawfik A, et al. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol 2010;121:90–93. [DOI] [PubMed] [Google Scholar]
- 23. Delbono O. Excitation–contraction coupling regulation in aging skeletal muscle In Sarcopenia—Age‐related Muscle Wasting and Weakness. Dordrecht: Springer; 2011. p 113–134. [Google Scholar]
- 24. Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 2013;35:2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist–antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 2002;25:858–863. [DOI] [PubMed] [Google Scholar]
- 26. Oiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta‐analysis. Osteoarthr Cartil 2015;23:171–177. [DOI] [PubMed] [Google Scholar]
- 27. Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, et al. High body fatness, but not low fat‐free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr 1998;68:584–590. [DOI] [PubMed] [Google Scholar]
- 28. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–1359. [DOI] [PubMed] [Google Scholar]
- 29. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 30. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The association of two peak torques measured by Kin‐Com and Biodex isokinetic dynamometers
Figure S2A. Estimated average gait declines estimated by plugging 25th or 75th percentile of two predictive variables that were found significant interaction in men.
Figure S2B. Estimated average gait declines estimated by plugging 25th or 75th percentile of two predictive variables that were found significant interaction in women.
Table S1A. The number of participants by the number of visits
Table S1B. The number of participants by the length of follow‐up years


