Abstract
Posttraumatic epilepsy (PTE) is a major neurodegenerative disease accounting for 20% of symptomatic epilepsy cases. A long latent phase offers a potential window for prophylactic treatment strategies to prevent epilepsy onset, provided that the patients at risk can be identified. Some promising imaging biomarker candidates for posttraumatic epileptogenesis have been identified, but more are required to provide the specificity and sensitivity for accurate prediction. Experimental models and preclinical longitudinal, multimodal imaging studies allow follow-up of complex cascade of events initiated by traumatic brain injury, as well as monitoring of treatment effects. Preclinical imaging data from the posttraumatic brain are rich in information, yet examination of their specific relevance to epilepsy is lacking. Accumulating evidence from ongoing preclinical studies in TBI support insight into processes involved in epileptogenesis, e.g. inflammation and changes in functional and structural brain-wide connectivity. These efforts are likely to produce both new biomarkers and treatment targets for PTE.
Keywords: Atrophy, Axonal injury, Connectivity, Epileptogenesis, Inflammation, Magnetic resonance imaging (MRI), Plasticity, Positron emission tomography (PET), Vascular injury
1. Initial injury to the brain, final outcome epilepsy and the chaos in between -neuroimaging unveiling patterns and order from the chaos
Traumatic brain injury is caused by external mechanical forces to the brain. The initial impact causes both focal injury (contusion) and diffuse injuries (axonal injury, vascular injury), and launches secondary injury cascades that may progress for months and years (neurodegeneration, axonal plasticity, hemodynamic deficits, inflammation). The chronic outcome can be different types of cognitive disorders, or epilepsy. The exact mechanisms of how traumatic brain injury (TBI) leads to the development of epilepsy are still unknown, although several pathways and pathologies have been identified to play a role. Different animal models of TBI can recapitulate at least to some degree both pathophysiological mechanisms and outcomes (Pitkänen et al., 2017). Investigation of these pathways using multimodal imaging allows interrogation of the brain network as an entity and its evolution in preclinical models with translational capability. The challenge for imaging science is to use the variety of available non-invasive methods to reveal the temporal order of events and topographical distribution of pathologies that comprise the evolving determinants of epileptogenicity. Investigation of the interactions, mismatches and correlations of these imaging findings with the electrophysiological determinants may reveal the basis for PTE. In the text herein, we address point-by-point the preclinical radiological presentation of TBI, and describe how imaging studies have shown correlation between various pathologies to either the concurrent or downstream development of hyperexcitability or seizure activity.
Direct evidence from the specificity of an imaging biomarker to the posttraumatic epileptogenecity is still lacking regarding many of the modalities listed in this review; however there are (i) demonstrated correlations between TBI pathology and epileptic outcome and ii) evidence in models of acquired temporal lobe epilepsy (TLE) that imaging techniques do probe the relevant brain pathology. Here we review this evidence as well as identify the next questions to be asked. We also expand the discussion to the potential of newer cutting-edge techniques that can further the future efforts.
While this review focuses on preclinical imaging, we also briefly discus the utility of these biomarkers in the clinical setting.
2. Atrophy and structural diaschisis after TBI
A hallmark of moderate to severe traumatic brain injury is a progressive cortical lesion at the primary contusion site after experimental TBI. The location of the atrophic lesion is dictated by location of the primary impact site and the direction of the impact forces, and thus varies across different animal models of PTE. The primary contusion and subsequent atrophic lesion are robustly detectable by structural MRI acutely after impact. In particular, the T2-weighted (T2-wt) images highlight the acute contusional complex composed of edema and intracerebral hemorrhage. The later developing atrophied lesion cavity filled with cerebrospinal fluid (CSF) can be accurately delineated using structural MRI with several different MRI tissue contrasts (Fig. 1 A–D).
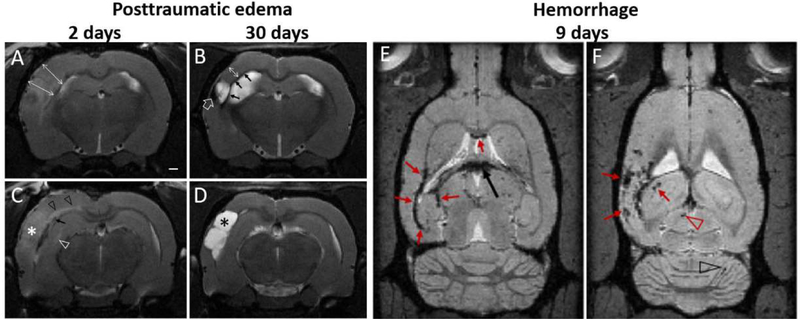
Fig. 1.
Progression of posttraumatic edema, atrophy and microbleeds. (A-D). Acute edema 2 days after LFPI (A,C) is evident in T2-wt images as hyperintensity of the contusional complex (white asterisk) and cortical swelling (double headed arrows in A). Structural MRI 2 days post-injury also shows diffuse axonal injury-related microbleeds (black arrow in C), axonal edema-associated hyperintensity of the external capsule and corpus callosum (black arrow heads), hyperintense midline of hippocampus (white arrow head) and hippocampal swelling. (B-D) In the same animal 28 days later, there is atrophy in the contusional area forming a CSF-filled cavity within the cortex (black asterisk D), cortical thickness is reduced (double arrow in B), and there is iron residues along the white matter tracts (arrows in B) as well as within the lesion (open arrow in B). Subacute hemorrhages (E-F) in T2* weighted images are shown at two horizontal levels along the white matter (red arrows in E), in choroid plexus (black arrow), as well as within the contused cortex, and in hippocampus (arrows in F), superior colliculus (red arrowhead) and cerebellum (black arrowhead). Data from EpiBioS4Rx project, courtesy of R. Immonen, University of Eastern Finland.
2.1. Cortical injury
2.1.1. Acute posttraumatic edema determined by MRI is associated with injury severity and with later seizure susceptibility
While acute and subacute edema are associated with the subsequent development of atrophy, attempts to use edema as a biomarker for later epileptogenecity have provided contradictory results. These differences may arise from the variation in models used, methods of quantification, and post-injury time used to define the edema, but the differences in the underlying causes of evolving post TBI tissue edema certainly play a role. The mechanical forces of head injury evoke edema from numerous origins and through various mechanism: vasogenic, cytotoxic, intracranial pressure, disruption of blood brain barrier, failed interstitial fluid clearance, thrombin, heme oxygenase, microglial activation and leukocyte infiltration etc.
Imaging metrics of post-traumatic edema are increased T2 signal, first decreased (cytotoxic) and then increased (vasogenic) apparent diffusion coefficient (ADC), midline shift, swollen cortex and swollen hippocampus, and these peak in 2–3 days after experimental TBI (Fig. 1 A,C). Acute diffusivity increases at 72h post-injury and subacute post-injury edema at 1 week have been negatively correlated with mean seizure duration when initiated with pentylenetetrazol (PTZ) at 3 months (Frey et al., 2014). Decreases in hippocampal ADC indicative of cytotoxic edema, at 3 hours post LFPI, correlated with seizure susceptibility and mossy fiber sprouting 1 year later (Kharatishvili et al., 2007). Some of the therapies alleviating acute edema (hypothermia, energy substrates) have been found to alleviate the later hyperexcitability in animal models (Pitkanen & Immonen, 2014), and clinically the midline shift due to brain swelling is considered as a strong risk factor for developing PTE (Xu et al., 2017).
2.1.2. Cortical lesion location and size and correlations with epileptogenecity
In patients with PTE the presence of cortical or subcortical lesions visible on T2-weighted scans 1 year after injury has been associated with an increased risk for development of epilepsy with an odds ratio of 3.3, n = 104 (Angeleri et al., 1999). Although the severity and type of injury (penetrating, hemorrhagic) correlates with the incidence of PTE (Diaz-Arrastia et al., 2009; Eftekhar et al., 2009; Pitkänen et al., n.d.), the association with lesion size or location is not fully understood.
In the rat model of PTE, the lesion size and caudal extent has been shown to be correlated with epileptogenecity: the greater the damage extent caudally, the higher the number of spikes and number of epileptiform discharges in seizure threshold test by PTZ 12months post LFPI (Kharatishvili & Pitkanen, 2010). Damage to the parahippocampal cortical areas in particular (entorhinal, perirhinal and postrhinal cortices) was linked with lower seizure threshold.
2.1.3. Persistent lesion growth, glia scar formation and progressive modulation of the perilesional cortex
After LFPI or controlled cortical impact (CCI) injury, there is continual expansion of the cortical lesion for up to 6–12 months post-injury (Immonen et al., 2009; Pischiutta et al., 2018). The glia scar that surrounds the lesion matures slowly and the perilesional cortex displays a gradient of imaging abnormalities with variety of underlying causes. [We refer to the pericontusional cortex as perilesional cortex, a term better applicable to the chronic stages as well.] The rate of lesion expansion can be used to classify injured animals into two categories: those with slowly expanding versus those with rapidly expanding lesions, and this rate if expansion may provide an indicator of underlying CNS responsiveness and putative vulnerability to epileptogenesis (Yasmin, Immonen, unpublished). Perilesional cortex appears intact in conventional imaging, but has been shown to undergo progressive structural modulation that is detectable by quantitative relaxation and diffusion mapping (Immonen et al., 2009; Li et al., 2016). These pericontusional/perilesional findings have been shown to serve as MRI biomarkers for epileptogenecity both in humans and in experimental models (Gupta et al., 2005; Immonen et al., 2013a).
In chronic posttraumatic patients, abnormalities in diffusion metrics within perilesional regions-of-interest, normal appearing tissue adjacent to the T2 or FLAIR visible lesion, were found to have decreased FA and a trend toward increased MD. Furthermore, affected areas was wider and more significantly changed in patients with epilepsy compared to those without (Gupta et al., 2005).
In animal models, subacute tissue water relaxivity (T2, T1ρ), and diffusivity in perilesional cortex have demonstrated power as indicators of seizure susceptibility 12 months after LFPI with PTZ treatment (Immonen et al., 2013b). At 9 and 23 days post-TBI, a change in T1ρ of the perilesional cortex showed the greatest predictive value for epileptogenecity in ROC analysis [area under the curve (AUC), 0.929 and 0.952, respectively; p < 0.01].
However, once the injury had progressed to the thalamus via axonal die-back at 2 months post-TBI, thalamic ADC was shown to be the best predictive biomarkers (AUC, 0.988; p < 0.05) [see also Chapter 1.2 for thalamic injury]. The specificity and sensitivity for predicting the development of seizures was further improved by combining the measurement of elevated ADC in the perilesional cortex and the thalamus at 2 months post-TBI (AUC, 1.000; p < 0.01). The perilesional abnormalities presented as a gradient that attenuated with distance from the core, so that there are no clear lesion boarders. As a result of this, there is likely to be a large variation in the definition of perilesional cortex among reports in the field.
In addition to brain atrophy due to the cell death, the perilesional cortex suffers from significant pathophysiological changes in extracellular matrix, perfusion deficits, metabolic deficits, inflammation, edema, as well as functional deficits. These are discussed extensively in upcoming sections. It is notable that progressive volumetric atrophy can be detected far beyond the perilesional cortex: Wright et al. demonstrated decreased volume of motor cortices bilaterally 12 weeks after CCI (Wright et al., 2017a).
2.2. Thalamic injury
The force of impact injury penetrates the cortex and reaches deep into the underlying thalamus (Pena 2005) and the resulting primary damage can often be observed as edema acutely post-injury. In experimental TBI models, cortico-thalamic deafferentation also causes secondary thalamic damage. Microgliosis, aggregated calcifications and iron are observed to accumulate into the ipsilateral thalamus during the following weeks and months. The location of the evolving thalamic injury depends on the location of the cortical lesion. In lateral fluid percussion injury (LFPI), the primary lesion covers the auditory cortex, while the secondary thalamic injury covers ventral posteromedial (VPM), ventral posterolateral (VPL), and Reticular (Rt) thalamic nuclei (Fig. 2B). The exact location of the thalamic injury is more dorsal (centered within the Po) in controlled cortical impact injury (CCI) model where the cortical lesion encompasses sensorimotor cortex (S1) (Onyszchuk et al., 2009). Since multiple cortical regions receive afferents from a single, compromised thalamic nucleus (Herrero et al., 2002), a third step of the cascade is likely to be the loss of input from damaged thalamic neurons that gradually leads to diaschisis in sensorimotor cortex distant from the lesion ipsilaterally, and contralaterally via cortico-cortical circuitry. Some of these areas of functional diaschisis have been suggested to act as a seizure foci in posttraumatic models (Reid et al., 2016), and they may well have a wider impact in modulating the structural and functional connectivity within the associated circuitry as well as the global network obtained by resting state fMRI (Harris et al., 2016a). MRI-mapping of thalamic injury may serve as surrogate marker to indicate MRI-negative, subtle pathology in the cortical area to which the nuclei project (ie. diaschisis).
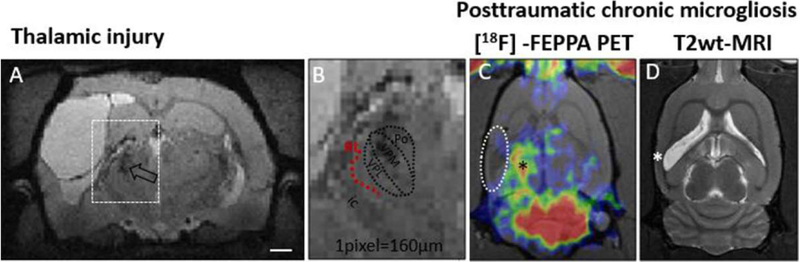
Fig. 2.
Thalamic injury identified by structural MR1 and thalamic chronic inflammation by [18F]-FEPPA-PET in chronic post-trauma rats. (A-B) Structural MRI (multi-echo gradient echo, isotropic 160μm3) 5 months post-LFPI and typical location of the thalamic calcifications and iron (both hypointense here, open arrow in A; B shows magnified view). In the LFP1 model, the calcifications are known to form within VPM and VPL nuclei (approximately outlined in B) that project to the S1 barrel field. Thalamic injury leads to deafferentation of projection neurons. Despite the high spatial resolution of the MRI, the shape deformation of ipsilateral thalamus hinders the analysis of any specific nuclei. The damage in reticular nuclei (Rt, red dotted arc) along the lateral border of the thalami facing the capsula interna (ic) is thus difficult to assess. (C-D) A PET marker of reactive microglia (18kDa Translocator protein (TSPO) radiotracer [18F]-FEPPA) shows prominent, sustained inflammation in ipsilateral thalamus (black asterisk in C). This representative animal was scanned 1 month post-LFPI and shows only negligible TSPO expression in injured cortex (dashed oval in PET image; white asterisk depicts the primary contusion location in corresponding T2-wt image). Courtesy of R.Immonen unpublished, University of Eastern Finland.
Accumulation of iron and calcium makes the thalamic damage highly visible using several MRI techniques. Differentiating between calcifications and iron can be accomplished with quantitative susceptibility mapping (QSM) and other phase contrast MRI approaches (Aggarwal et al., 2018). Calcifications and their delayed accumulation pattern has been well characterized in LFPI model (Lehto et al., 2012; Lehto et al., 2017). Calcifications are presumed to be functionally inert per se, but iron is also present in the same thalamic nuclei during the ‘maturation’ of calcifications and is associated with on-going microgliosis (Lehto et al., 2012). The source of thalamic iron in chronic stages after TBI is suggested to be the neuronal and oligodendrocyte cell death that release tightly bound iron from sites where it is normally utilized, mainly mitochondria (Benkovic & Connor, 1993; Onyszchuk et al., 2009). In addition, increased thalamic vascular density and branching, that have been quantified at 8 months after LFPI (Hayward et al., 2010), may also lead to increased uptake of iron from the blood. It should be noted here, that thalamus suffers from aggressive, sustained inflammation (reactive microglia / microgliosis) that can be seen months (in animals) and years or even decades (in humans) after TBI by dedicated PET tracers. This is discussed in Section 4 (Chronic inflammation) and is shown in Fig. 2C.
2.2.1. Structural MRI of the thalamic damage and correlations with epileptogenecity
Thalamic injury is a common finding in several brain insults leading to epilepsy (e.g. stroke). The suppression of thalamic activity can suppress the on-going seizures, and functional and structural analysis of network changes in epilepsy indicate disruption and modulation of thalamic connections. Recent studies using optogenetics have demonstrated seizure suppression and circuitry modification by thalamic (or corticothalamic) stimulus (Mantoan Ritter et al., 2014; Paz et al., 2013; Tonnesen & Kokaia, 2017; Tung et al., 2018). EEG evidence of the involvement of the thalamic reticular nuclei in seizure generation in a LFPI model has been recently provided by Andrade et al., (Andrade et al., 2017) who observed seizures in chronic posttraumatic rats to occur systematically at the transition into Rapid-Eye-Movement sleep. Thalamic reticular nuclei are well known to control that transition, and is severely atrophied ipsilaterally in posttraumatic rats.
The utility of thalamic injury as an imaging biomarker of posttraumatic epileptogenecity is yet to be established. Observations in several LFPI cohorts have shown that thalamic injury is both evident in non-epileptic animals, as well as absent in some animals that display lowered seizure threshold to the PTZ test (Immonen, Yasmin unpublished data). Thus, it is unlikely that the presence of thalamic iron and calcifications chronically after traumatic brain injury, per se, will have high specificity as an imaging biomarker for epileptogenesis. However, the potential characteristics of thalamic injury in combination with abnormalities in other nodes of the epileptic network may well show prognostic power.
2.3. Hippocampal injury
Hippocampal sclerosis in chronic PTE patients is associated with the underlying seizure pattern and is a crucial factor in the clinical diagnosis of temporal lobe epilepsy (Diaz-Arrastia et al., 2009). Posttraumatic hippocampal injury is a characteristic of the LFPI model (D’Ambrosio et al., 2005; Kharatishvili et al., 2006) and the weight drop model (Golarai et al., 2001) and has been linked with epileptogenecity. Imaging findings of hippocampal sclerosis/atrophy serve as biomarkers, and have been directly correlated with posttraumatic epileptogenecity as follows:
Hippocampal surface shape analysis utilizing large-deformation, high-dimensional mapping 1 week after LFPI in rats was able to prognostically differentiate those that would develop epilepsy (spontaneous seizures or epileptiform activity 7months post injury) from those that did not. Simple volume analysis failed to differentiate these animals (Shultz et al., 2013).
Different time points in the time-course of MR diffusional changes after LFPI have been correlated with seizure susceptibility assessed 1 year after LFPI (Kharatishvili et al., 2007). These changes include: acute decrease in diffusion (ADC) within hippocampus at 3 hours after injury that is associated with cytotoxic edema, and elevated diffusion thereafter at 23 days, and more chronically from 2 months onwards up to 11 months post-injury.
Quantitative mapping of T2, T1ρ and ADC in ipsilateral hippocampus in LFPI model has revealed that subacute T1ρ at 9 days, and ADC at 23 days to 2 months post-TBI, were predictive for increased seizure susceptibility in ROC analysis (AUC > 0.857; p < 0.05), when epileptiform activity was assessed 12 months post LFPI by PTZ challenge. Notably, all the hippocampal MRI biomarkers alone or a combination of them were lower in sensitivity and specificity compared to the best cortical and thalamic MRI biomarkers at similar post-injury time points (Immonen et al., 2013b). On the other hand, another study using the LFPI model reported that the relative signal intensity changes in T2-wt images in these same regions and at several time points after LFPI failed to identify differences in non-epileptic and epileptic groups (Shultz et al., 2013).
3. Diffuse axonal injury
Shear stress of the impact force results in diffuse axonal injury (DAI) across the brain - predominantly along the thickest white matter bundles such as corpus callosum, external capsule, internal capsule, fimbria and fornix. Information extracted from diffusion-weighted imaging (DWI) data using a range of methodologies can report on axon and myelin integrity as well as general tissue microstructure including cell size and packing densities. Accumulating evidence has shown that axonal pathology in human patients persists for years after traumatic brain injury (Johnson et al., 2013), and is detectable chronically even after mild TBI (mTBI) (Lipton et al., 2008). Multifocal axonal injury with low fractional anisotrophy (FA) and associated high mean diffusivity (MD) have been found in chronic mTBI subjects with cognitive disability, both at group level (Lipton et al., 2009), and systematically at the subject level (Lipton et al., 2008). DAI imaging findings of altered diffusion tensor imaging (DTI) metrics of white matter (WM) tracts are serving as biomarker for post-traumatic cognitive deficits (Irimia et al., 2012), and are currently being investigated for their potential as biomarkers of post-traumatic epilepsy (EpiBioS4Rx). The molecular and cellular features of epileptogenesis may differ between focal and diffuse injury mechanisms. In DAI the circuitry is modified by the Wallerian degeneration of some of the original connections and the sprouting of aberrant, new connections.
3.1. Abnormalities in DTI and tractography metrics
DTI tractography reveals changes in structural connections, and in epilepsy patients tractography was found to be useful in surgical management of temporal lobe epilepsy (Sivakanthan et al., 2016), detection of disrupted structural connectivity in childhood absence epilepsy (Xue et al., 2014), as well as in focal epilepsies (Deleo et al., 2018; Jeong et al., 2015). In adolescents with absence seizures, the genu of the corpus callosum was marked by decreased FA and increased radial diffusivity (RD) and MD values, while tractography revealed significantly elevated tract asymmetry within fiber tracts passing through the genu of the corpus callosum in patients with epilepsy (Liang et al., 2016). In TBI patients, tractography metrics have been shown to serve as a biomarker for cognitive impairment (Marquez de la Plata et al., 2011), while there are only a few studies associating tractography with PTE.
In a recent case study of an active duty male soldier with sleep-related hypermotor epilepsy and a history of repetitive mTBI, multi-shell tractography revealed changes in the right superior longitudinal fasciculus including reduced tract density and lower FA values compared to the left side (Tchopev et al., 2018). These changes were hypothesized to be related to hyperkinetic behavior. EEG also revealed pathological changes to the right frontal lobe suggesting this location as a potential nidus for the patient’s seizures. Tractography-based metrics are sensitive enough to indicate changes along the entire affected pathway, e.g. reduced path length in the genu of corpus callosum and its projections into the frontal lobe were shown in clinical TBI study (Pannek et al., 2011).
DTI tractography measures have found to be sensitive in detecting the persistent changes in tissue microstructure related to diffuse axonal injury in TBI animal models (Fig. 3). Three months post-LFPI, the tract-based metrics (tract density, path length and curvature) revealed wider injury than traditional DTI metrics (Wright et al., 2017b). Even in the absence of any observable abnormalities in T2* or DTI metrics, the reduced mean curvature in the ipsi- and contralateral corpus callosum could be detected 30 days after mild FPI (Wright et al., 2016). Bilateral changes in diffusion metrics have been reported after rodent CCI injury (Harris et al., 2016b) with increased tract number in contralateral circuits possibly reflecting compensatory effect. Decreased FA and increased RD are associated with reduced myelin or axon fiber density and has been observed in the anterior corpus callosum of rats with spike-wave epilepsy (genetic absence epilepsy rats of Strasbourg, GAERS), with more severely affected animals exhibiting more pronounced changes. Streamlines originating in these areas connected somatosensory cortical regions shown to be involved in seizure discharges (Chahboune et al., 2009). While tractography-metrics derived from the properties of the streamlines have not yet been associated with post-traumatic epileptogenecity, they are incorporated in currently on-going trials (EpiBioS4Rx).
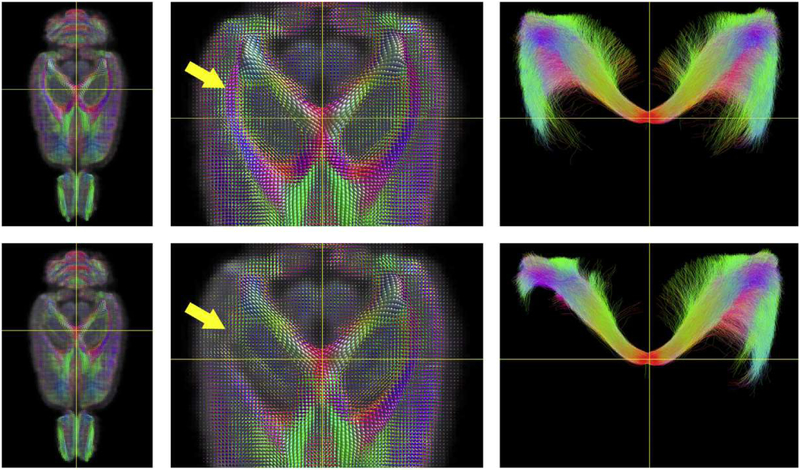
Fig. 3.
Fiber orientated distribution (FOD) and tractography template images generated for rats after sham operation (top row) and lateral fluid percussion injury (LFPI, bottom row). Damage to the white matter is reflected by reduced FOD amplitudes (yellow arrow, middle column). The FOD images were used to guide streamlines which were seeded from a spherical ROI positioned at the intersection of the two yellow lines. The resulting tractography images are shown at right and include all of the streamlines contained within the full superior/inferior slab. Following moderate LFPI, rats exhibit truncating of streamlines ipsilateral to the injury site. FODs and tractography streamlines are color-encoded according to orientation: red, medial/lateral; blue, superior/inferior; and green, anterior/posterior. Courtesy of D. Wright, Monash University.
Microbleeds are often used as marker for diffuse axonal injury, even though in reality they are markers of vascular injury that are often, but not always co-localized with axonal injury. Imaging of microbleeds is addressed below.
3.2. Microbleeds and other iron residues are easy to detect by susceptibility weighted imaging (SWI) but can they aid in epilepsy prognosis?
Microbleeds of diffuse vascular injury and resulting iron residues distributed along the injured WM/along the corticomedullary junction (i.e. at the interface of GM and WM bundles) are robustly detected by susceptibility weighted imaging and other gradient echo methods. Microbleeds are common clinical radiological finding in DAI, where the biomarker potential of both DW lesions and SW lesions have been widely studied (Abu Hamdeh et al., 2017; Benson et al., 2012; Liu et al., 2014). Microbleeds occur only among subset of injured tracts, and they are often but not always associated with inflammatory cells (see paragraph 2.3). Hemosiderin contained within microbleeds may evoke local tissue hyperexcitability (von Essen et al., 1996; Williamson et al., 2003; Willmore & Rubin, 1981), and intracranial hemorrhages are a risk factor for post-traumatic epilepsy. Messori et al. showed that hemorrhagic contusions surrounded by an incomplete glial scar are a PTE risk factor, while those with complete glia scar are not (Messori et al., 2005). Surgical excision of the hemosiderin fringe surrounding the cerebral cavernous malformations (in addition to the vascular malformation itself) has been shown to reduce the occurrence of post-surgical seizures (Baumann et al., 2006; Ruan et al., 2015). Although microbleeds have been shown to be present in posttraumatic epileptic and non-epileptic animals, as yet they have not been explicitly linked to seizure susceptibility. Importantly, a significant number of susceptibility-weighted imaging (SWI)-observable iron residues along WM tracts may originate from dying oligodendrocytes and not from the microbleeds (Connor & Menzies, 1995; Zhang et al., 2016). Iron containing debris of inflammatory cells and degenerating neurons and oligodendrocytes is scavenged by macrophages and microglia, thereby preventing the detrimental increase in neuronal iron (Connor & Menzies, 1995). In PTE animal models the role of residual iron pattern in epileptogenecity is still unknown, and while SWI cannot yet serve as biomarker of PTE, it is a useful tool in mapping the sporadic iron accumulation across the brain and monitoring treatments that reduce the iron load such as iron chelators.
4. Imaging of BBB disruption and findings in TBI or epilepsy models other than posttraumatic epilepsy
The significance of blood-brain barrier (BBB) permeability in PTE is discussed and reviewed in greater detail in another contribution of this special issue (‘Breakdown of blood brain barrier as a mechanism of posttraumatic epilepsy’ by Dadas and Janigro (Dadas & Janigro, 2018). Here we briefly summarize the related imaging findings in animal models:
Van Vliet utilized a slow infusion paradigm of gadolinium to image the sporadic BBB during epileptogenesis in status model of TLE (van Vliet et al., 2014) in which increased BBB permeability in latent and chronic phase had been correlated with later seizure frequency (van Vliet et al., 2014). BBB leakage has been assessed in experimental TBI at different time points by dynamic contrast enhanced MRI utilizing gadolinium (Frey et al., 2014; Li et al., 2016). The biomarker potential of gadolinium based BBB imaging for experimental post-traumatic epilepsy has been suggested by Van Vliet (van Vliet et al., 2017) and also by Frey, who showed that the relative signal enhancement of injured cortex after Gd administration 72 hours post LFPI correlated positively with total number of seizures, and negatively with latency to the first seizure after PTZ administration 3 months later (Frey et al., 2014) (Marchi et al., 2016).
5. Imaging of chronic inflammation by PET tracers
Inflammatory signaling cascades in epileptogenesis after TBI were recently reviewed by Webster et al. (Webster et al., 2017) and role of glia in epilepsy more broadly by Devinsky et al. (Devinsky et al., 2013). MR imaging biomarkers of neuroinflammation relating to TBI were covered by Koepp et al. highlighting several techniques that either directly or indirectly serve as a probe for inflammatory components (Koepp et al., 2017). Animal studies have provided a priori information about the topography of chronic posttraumatic inflammation, which is widespread across cortical regions, far beyond the actual glia scar. In chronic stage, months after the injury, the cortical inflammation becomes more subtle, and thus challenging to detect in vivo, but it has been demonstrated to persist through the lifespan, worsen upon an additional stressor or injury, and manifest even bilaterally (Chen et al., 2003a; Kyyriainen et al., 2017; Loane et al., 2014; Ndode-Ekane et al., 2018; Susarla et al., 2014; Webster et al., 2017). In vivo imaging of inflammatory cells and mediators that are known to be present in posttraumatic brain is a challenge that needs to be overcome, in order to more efficiently identify the optimal window for intervention. If immunosuppressive treatment are applied too early after TBI, they may suppress endogenous regenerative processes. Delaying anti-inflammatory intervention to a later stage could potentially modify or prevent the epileptogenesis.
Strategies to image inflammatory processes include indirect MRI approaches (e.g. dynamic contrast enhanced imaging of BBB leaks), MR spectroscopy of myoInositol or glutathione (covered in chapter 8), MR molecular imaging probes (beyond the scope of this review), and PET approaches. Several PET radiotracers have been developed to target 18-kDa translocator protein (TSPO) - also known as peripheral benzodiazepine receptor (PBR) - expressed on the mitochondrial membrane of reactive astro- and microglia. The use of TSPO-PET in epilepsy studies both clinically and pre-clinically have been recently reviewed (Amhaoul et al., 2014; Scott et al., 2017), but their utility as a biomarker of PTE is unknown. In temporal lobe epilepsy patients the binding of TSPO has been shown to increase both ipsilateral and contralateral to seizure origin (Gershen et al., 2015). Wang and colleagues followed post-injury cortical inflammation after CCI in rat during the acute stage and along the first month post injury with in vivo microPET utilizing [18F]-DPA-714 tracer. The TSPO expression at the cortical lesion site was shown to peak at day 6 post-injury and decay to control level by 1 month. Importantly, the cellular source of TSPO expression was activated (amoeba shaped) microglia (CD68) at day 6 post injury, while 10days post injury the main contributor to the TSPO signal was reactive astroglia (GFAP) (Wang et al., 2014). Similarly, the cortical expression was shown to peak at 1week after LFPI, and to decay by 9 weeks when monitored by in vivo PET (Yu et al., 2010). However, an autoradiography study showed details of [18F]-FE-DAA1106 binding from 3days to 12 weeks after LFPI demonstrating TSPO expression along the lesion border, along the subcortical white matter tracts (external capsule), and within the thalamus ipsilaterally, and showed notable expression even at 12 weeks post-injury (Yu et al., 2010). Furthermore, a weight-drop, closed head injury study in mice demonstrated TSPO tracer [18F]-DPA-714 binding to be evident after moderate/severe TBI 7–16d post-injury, but not after mild TBI (Israel et al., 2016). However, other studies using histology have shown inflammation to persist for several months after TBI in rodent models (Chen et al., 2003b; Kyyriainen et al., 2017; Loane et al., 2014; Ndode-Ekane et al., 2018; Susarla et al., 2014; Webster et al., 2017) indicating that current in vivo techniques might not possess the required sensitivity and/or spatial resolution for a full longitudinal analysis to map persistent inflammation. Thus, while current in vivo microPET techniques may provide useful information on the acute inflammatory response, readouts on the persistent but more subtle inflammation that is present chronically after moderate or mild TBI at all times, may, as yet, be unavailable.
On the other hand, TSPO-PET studies robustly highlight how inflammation in thalamus is sustained for months in rodents (Vallez Garcia et al., 2016), and years or even decades in humans (Ramlackhansingh et al., 2011) after TBI (Fig. 2C shows [18F]-FEPPA-PET 1mo post LFPI). The far-reaching demyelination-associated inflammation along the white matter tracts is also sustained chronically (1yr after CCI in mice, (Pischiutta et al., 2018)), and also contralaterally, possibly indicating a role modulating circuit changes leading to epileptic activity. PET studies combining in vivo PET and autoradiography with TSPO radiotracers have reported the TSPO expression along the ipsilateral white matter structures and lesion edges in LFPI model, yet none of the studies have correlated the findings with electrophysiology. Data proposing the TSPO-PET (by [18F]-GE-180 radiotracer) of epileptogenic network to be a biomarker of epileptogenecity were reported in amygdala stimulation induced status rat model of TLE. TSPO expression (2 weeks after the status i.e. during the epileptogenesis) of a cluster VOI comprising of parts of hippocampus, parietal cortex, thalamus, somatosensory cortex, amygdala etc. was found to correlate positively with later seizure frequency (Russmann et al., 2017). In TLE epilepsy patients, a recent study utilizing [11C]-PBR28 radioligand showed TSPO overexpression both ipsi- and contralaterally to seizure foci (Gershen et al., 2015). The biomarker-capability of TSPO-PET for posttraumatic epileptogenecity is yet to be explored.
6. Blood flow deficits
Hemodynamic regulation (autoregulation) fails after injury and long-lasting blood flow deficits are found in trauma patients and in animal models of TBI (Hayward et al., 2011; Kenney et al., 2016; Toth et al., 2016). Vascular dilatation and hyper/hypotension present post trauma are also contributing factors to cascades leading to chronic hemodynamic imbalance. Hypertension leads to compromised integrity of the BBB, and the resulting hypoperfusion in turn compromises the blood supply and can lead to energy crisis.
6.1. Cerebral blood flow -MRI as biomarker for posttraumatic epileptogenecity in animals
There are several imaging methods to probe the flow and vasculature by MRI and CT (MRI-BOLD, arterial spin labeling, dynamic contrast enhanced MRI, etc. (Griffith & Jain, 2016; Lavina, 2016)). Posttraumatic vascular imaging findings have been linked with seizure outcome. In chronic LFPI rats the cerebral blood flow (CBF) measured by arterial spin labeling MRI 8 months after injury correlated with seizure susceptibility induced by PTZ one month later: the lower the ipsilateral hippocampal CBF (ipsi) the shorter the latency to the first spike after PTZ administration, and further, the higher the thalamic CBF the shorter the latency to the first spike. High thalamic CBF was associated with higher vessel density, and the high vessel density was also correlated with increased seizure susceptibility. However, no such correlations were found in cortex. (Hayward et al., 2010) Thus, the chronic posttraumatic CBF abnormalities have been linked with epileptogenesis. Cerebrovascular reactivity (CVR) is probed by adding a challenge (e.g. CO2 inhalation) to the MRI paradigm, and CVR has been shown to correlate with chronic neurobehavioral symptoms better than the CBF per se in TBI patients (Amyot et al., 2018). Association between CVR and tissue hyperexcitability is not yet understood, and studies on PTE are called for.
7. Plasticity, axonal sprouting and astrocyte mediated circuit shaping
Beneficial plasticity and epileptogenic plasticity are the ‘ying and yang’ of brain in the post-injury period. Spontaneous plasticity recovers a portion of brain function after TBI. Plasticity can also be aberrant and detrimental to function since it results in adverse outcomes, for example, the occurrence of spontaneous seizures. Local structural network alterations due to axonal sprouting in hippocampus and in perilesional cortex are putative causes for seizure activity. Some forms of plasticity can be imaged in vivo.
7.1. MRI (MEMR1 and DTI) detects axonal sprouting both after TBI and other epileptogenic insults, and sprouting in turn correlates with seizure activity
Mossy fiber sprouting in the dentate gyrus of the hippocampus correlates with seizure susceptibility in LFPI (Kharatishvili et al., 2007), and in SE animal models (Lowenstein et al., 1993; Mathem et al., 1993). It is one of the most consistent findings in surgical specimens from patients with mesial temporal lobe epilepsy, while the exact role in epileptogenesis is still under debate (Godale & Danzer, 2018). Axonal sprouting has been imaged in pre-clinical models by manganese enhanced MRI (MEMRI), in LFPI (Bouilleret et al., 2009) and other post insult epilepsy models such as kainic acid induced status epilepticus (Dedeurwaerdere et al., 2013; Immonen et al., 2008). Hippocampal manganese accumulation has been found to be associated with seizure activity and have value as a biomarker for epileptogenesis (Dedeurwaerdere et al., 2013). Mossy fiber sprouting after TBI is far less pronounced than after kainic acid induced status. After TBI the area of MEMRI enhancement within the CA3 region of the hippocampus has been correlated with corresponding infra-pyramidal sprouting (LFPI rat, 6 months post injury (Bouilleret et al., 2009)), but the study could not examine the correlation with epileptic outcome, due to low number of animals with epileptiform activity. Hence, currently there is a lack of evidence to support the potency of MEMRI as a biomarker for PTE. It should be understood however, that the technique is confounded by the fact that manganese also accumulates into glia and endothelial cells as demonstrated in both the FPI (Immonen et al., 2008) and the CCI model (Talley Watts et al., 2015). MEMRI has actually been utilized to visualize reactive gliosis surrounding the ischemic core in stroke model (Kawai et al., 2010), and moreover, the inflammation has been suggested to stimulate the neuronal manganese uptake (Bade et al., 2013).
Diffusion imaging (DTI, HARDI): Very high-resolution DTI can be used to visualize layer-specific changes in the hippocampus after various epileptogenic brain insults (Sierra et al., 2015). Increased FA in the rat dentate gyrus several months after status epilepticus is associated with mossy fiber sprouting and reorganization of axons in the outer molecular layer, indicating that DTI has the potential to visualize structural plasticity at the axonal level during epileptogenesis in vivo (Grohn et al., 2011; Salo et al., 2017). Layer specific changes were also detected after TBI altogether indicating that hippocampal network plasticity can be an important target for PTE biomarker studies (Laitinen et al., 2015). Application of more advanced HARDI type diffusion imaging that go beyond simple tensor model will provide opportunities to address microstructural changes associated with plasticity in more detail in future.
7.2. Diffusion tractography maps structural circuitry modifications across the brain networks
As was discussed in chapter 2.1, diffusion tractography is highly sensitive to post-injury white matter pathology both in TBI animals and in patients. Often this pathology occurs as a loss of connectivity due to axonal injury. However, tractography can also detect other forms of circuit changes and can be utilized for whole brain connectomic analysis of structural connectivity. Structural network data from diffusion imaging can then be incorporated with functional connectivity assessed by resting state fMRI. Such combined approach enables characterization of brain-wide epileptic network. Initial studies have already demonstrated the differences in the large-scale structural connectivity between epileptic and non-epileptic TBI subjects.
Dennis et al found network differences in pediatric patients who had suffered a moderate to severe TBI and who experienced early post-traumatic seizures (EPTS), compared to those who did not (Dennis et al., 2017). The authors performed tractography on fiber orientation distributions and created a 68 × 68 connectivity matrix describing the number of fibers connecting each label. TBI patients who suffered EPTS had significantly greater clustering and significantly lower modularity (degree to which the network can be divided into modules) in their network compared to those who did not. Furthermore, these patients were also found to have significantly shorter normalized pathlengths. Connectivity deficits mapped by tractography have already been demonstrated to provide robust biomarkers of cognitive deficits after TBI (Irimia et al., 2012). The challenge now is to identify the epileptogenic changes.
8. Functional MRI of whole brain: functional connectivity and seizure onset and spread
Correlations of fluctuations in resting state fMRI (rs-fMRI) signal can be used to indicate functional connectivity between brain regions. The major benefit of the approach is that it allows large-scale network level assessment of post-traumatic alterations, in whole brain (Fig. 4). As epilepsy is increasingly recognized to be a network-level disorder, it is highly intriguing concept to try to evaluate how local lesion and modification of white matter pathology modulates the connectivity patterns, and how that is related to epileptogenesis, or to other functional outcomes after TBI. In a recent study, fluid percussion injured rats with increased seizure susceptibility under PTZ, were studied using rs-fMRI (Mishra et al., 2014). Group level decreases in connectivity were found between the ipsilateral and contralateral parietal cortex, and between the ipsilateral parietal cortex and hippocampus when compared to sham-operated animals. In addition, there were injury-related decreases in connectivity between the ipsilateral and contralateral parietal cortex to other regions. However, connectivity at the subject level did not correlate with hyperexcitability. Topological features of functional networks, were recently estimated by graph theory analysis in an adult rat CCI model of TBI at days 7–28 post injury. Global network analysis revealed alterations in network parameters indicative of possible acutely increased random connectivity and temporary reductions in modularity that were matched by local increases in connectedness and increased efficiency among more weakly connected regions. The global network parameters: shortest path-length, clustering coefficient and modularity, were most affected by trauma, and also scaled with the severity of injury (Harris et al., 2016c).
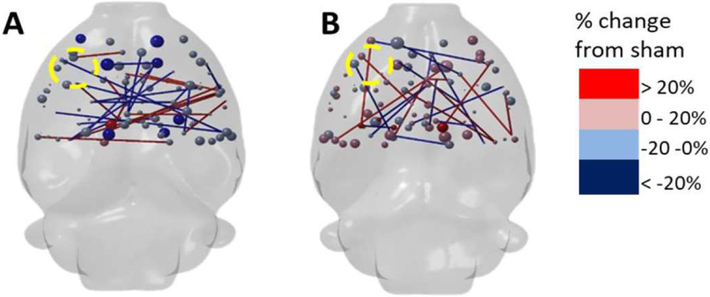
Fig. 4.
Controlled cortical impact injury (CCI) results in both decreases (blue) and increases (red) in resting state fMRI (rs-fMRI) functional connectivity. Data are at (A) 1 week and (B) 4 weeks post-injury, calculated at 35% network density. Data show that nodal and edge strength (spheres and sticks, respectively) are decreased (blue) in most regions at 1 week after injury by greater than 20% change from sham. There were some increases in connectivity above sham levels at 4 weeks post-injury (red), but not in the primary injury site (left side) which remained decreased. Courtesy of N.G. Harris and A. Paydar, David Geffen School of Medicine at University of California at Los Angeles.
As rs-fMRI usually relies on group level analysis, a major challenge for future rs-fMRI studies is to be able to reliably and reproducibly detect alterations in functional connectivity at the subject level. This is prerequisite to be able to utilize functional connectivity as biomarker for post-traumatic epilepsy, in the future.
9. Inhibitory / excitatory imbalance by proton spectroscopy
Magnetic resonance spectroscopic imaging (MRSI) and magnetic resonance spectroscopy (MRS) measurements of GABA, glutamate or glutamate + glutamine concentrations or their ratios as indicators of brain excitatory-inhibitory balance have not succeeded in providing a link to epileptogenecity in experimental PTE models. Although decreases in GABA have been found in ipsilateral hippocampus 5 months after LFPI (Immonen et al., 2009), this drop did not correlate with seizure susceptibility 6 months later (unpublished). Hippocampal glutamate was found to be decreased along with decreased neuronal marker NAA in the same study, while glia marker myo-inositol was elevated. The meaning of these results are not yet clear and only underscore the complexity of the disease in both the spatial as well as temporal extent.
Numerous metabolites within the hippocampal neurochemical profile measured by 1H-MRS in models of TLE have been examined in association with seizure activity: antioxidant glutathione (GSH/Cr) concentrations during epileptogenesis at 7 days post pilocarpine induced status correlated negatively with the frequency of spontaneous seizures, while myo-inositol (mIns/Cr), lactate (Lac/Cr) or NAA (NAA/Cr) did not (Filibian et al., 2012). In a more chronic follow-up it was shown that the elevated level of hippocampal myo-inositol persisted up to 65 days post SE, that is, until the occurrence of spontaneous seizures in those animals that developed epilepsy (62–70%), while showing only transient increase at 15 days post-injury in those not developing epilepsy (Pascente et al., 2016). No correlations were reported between neurochemical concentrations and electrophysiology. Elevated myo-inositol was associated with elevated number of S100β-immunopositive astrocytes, while Ox-42 staining showed microglia to be in a resting state.
10. Hypometabolism associated with seizure focus lateralization
[18F]-Fluorodeoxyglucose PET ([18F]-FDG PET) is used to lateralize the seizure foci in temporal lobe epilepsy patients and metabolic dysregulation is one major factor in epileptogenesis (Bazzigaluppi et al., 2017). Seizure focus shows inter-ictal hypometabolism and ictal hypermetabolism (Sarikaya, 2015). Also, chronic inhibition of brain glycolysis by daily injections of non-metabolizable glucose analog 2-deoxy-D-glucose initiates epileptogenesis (Samokhina et al., 2017). In TBI models the ipsilateral hemisphere displays widespread hypometablism (Lee et al., 1999; Moore et al., 2000).
10.1. FDG-PET and glucose uptake as biomarker for posttraumatic epilepsy
Shultz and colleagues (Shultz et al., 2013) found that a multivariate regression model that incorporated the PET findings 1week, 1month and 3 months post LFPI in ipsilateral hippocampus was able to predict the epileptogenecity, although no differences between epileptic and non-epileptic group were found in the univariate analysis. All TBIs showed trend of cortical and hippocampal hypometabolism, but the epileptic group showed steadily low FDG uptake as compared to the partial recovery of FDG uptake at 3 months of non-epileptic TBIs. Widespread ipsilateral hypometabolism is detectable several months after severe LFPI in majority of injured animals, regardless of whether they show signs of lowered seizure threshold in PTZ-EEG 6 months after injury (Yasmin, Immonen, unpublished). Reduced glucose uptake is both cause and consequence of progressive pathology, and likely contributing to the epileptogenesis in chronic trauma brain. Network analysis is required to determine the specific regions that are causally involved in epileptogenesis more precisely, and could serve as biomarkers for epileptogenecity.
10.2. GlucoCEST/GlcCEST, GlycoCEST, GluCEST - emerging tools to complete the picture
In order to improve the spatial readout of regional deficits in glucose uptake, a novel chemical exchange saturation transfer method called glucoCEST (Chan et al., 2012; Nasrallah et al., 2013) can be utilized. Alternative approach is a spin-lock version, called glucoCESL (Jin et al., 2014). GlucoCEST can map the glucose uptake in brain with high resolution upon oral administration of regular D-glucose, i.e. sugar. By tuning the saturation frequency, the method can be sensitized to other compounds: e.g. glycogen storages of astrocytes could be probed by GlycoCEST (van Zijl et al., 2007), while excitatory neurotransmitter glutamate concentrations could be mapped by GluCEST (Cai et al., 2012). GluCEST can lateralize the epileptic foci in non-lesional TLE (Davis et al., 2015). It should be noted though, that these methods have inherently low signal and specificity can be easily hampered by methodological imperfections. Applications on PTE are pending.
11. Conclusions
The numerous pathophysiological features described in this review (local or diffuse inflammation, vascular injury, diffuse axonal injury and network plasticity) are among the consequences of the head injury and considered as mechanisms contributing to the development of posttraumatic epilepsy. The lesion at the contusion site per se is not the direct cause for tissue hyperexcitability but instead the secondary cascades and the interplay of the many brain alterations and extra stressors are. That means that there are wide variety of promising imaging biomarkers candidates for posttraumatic epilepsy. MRI is one of the few tools that can assess the entire brain network and connectivity, discriminate focal and diffuse injury mechanisms, probe micro-structure, metabolism and relate these to physiological function within the same subject. However, publications using imaging methods to investigate PTE to date are proof-of-concept studies, and further investigations are required to establish robust biomarkers for posttraumatic epileptogenecity. In a complex disease such as PTE, combinatory biomarkers using imaging, electrophysiology and metabolomics/proteomics will likely be necessary, and current major trials (e.g. EpiBioS4Rx) are incorporating this view.
Acknowledgements
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) Center without Walls of the National Institutes of Health (NIH) under Award Number U54NS100064 (EpiBioS4Rx).
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- Abu Hamdeh S, Marklund N, Lannsjo M, Howells T, Raininko R, Wikstrom J, Enblad P, 2017. Extended anatomical grading in diffuse axonal injury using MRI: hemorrhagic lesions in the substantia nigra and mesencephalic tegmentum indicate poor long-term outcome. J. Neurotrauma 34, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal M, Li X, Grohn O, Sierra A, 2018. Nuclei-specific deposits of iron and calcium in the rat thalamus after status epilepticus revealed with quantitative susceptibility mapping (QSM). J. Magn. Reson. Imaging 47, 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amhaoul H, Staelens S, Dedeurwaerdere S, 2014. Imaging brain inflammation in epilepsy. Neuroscience 279, 238–252. [DOI] [PubMed] [Google Scholar]
- Amyot F, Kenney K, Moore C, Haber M, Turtzo LC, Shenouda C, Silverman E, Gong Y, Qu BX, Harburg L, Lu HY, Wassermann EM, Diaz-Arrastia R, 2018. Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 35, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade P, Nissinen J, Pitkanen A, 2017. Generalized seizures after experimental traumatic brain injury occur at the transition from slow-wave to rapid-eye-movement sleep. J. Neurotrauma 34 (7), 1482–1487. 10.1089/neu.2016.4675. Apr 1. [DOI] [PubMed] [Google Scholar]
- Angeleri F, Majkowski J, Cacchio G, Sobieszek A, D’Acunto S, Gesuita R, Bachleda A, Polonara G, Krolicki L, Signorino M, Salvolini U, 1999. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia 40, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Bade AN, Zhou B, Epstein AA, Gorantla S, Poluektova LY, Luo J, Gendelman HE, Boska MD, Liu Y, 2013. Improved visualization of neuronal injury following glial activation by manganese enhanced MRI. J. Neuroimmune Pharmacol. 8, 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, Siegel AM, 2006. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia 47, 563–566. [DOI] [PubMed] [Google Scholar]
- Bazzigaluppi P, Ebrahim Amini A, Weisspapir I, Stefanovic B, Carlen PL, 2017. Hungry neurons: metabolic insights on seizure dynamics. Int. J. Mol. Sci 18 10.3390/ijms18112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic SA, Connor JR, 1993. Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J. Comp. Neurol 338, 97–113. [DOI] [PubMed] [Google Scholar]
- Benson RR, Gattu R, Sewick B, Kou Z, Zakariah N, Cavanaugh JM, Haacke EM, 2012. Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: implications for neurorehabilitation. NeuroRehabilitation 31, 261–279. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Cardamone L, Liu YR, Fang K, Myers DE, O’Brien TJ, 2009. Progressive brain changes on serial manganese-enhanced MRI following traumatic brain injury in the rat. J. Neurotrauma 26, 1999–2013. [DOI] [PubMed] [Google Scholar]
- Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R, 2012. Magnetic resonance imaging of glutamate. Nat. Med 18, 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML., Crunelli V, Hyder F, Blumenfeld H, 2009. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage 47, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, Artemov D, van Zijl PC, 2012. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med 68, 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG, 2003a. Time course of cellular pathology after controlled cortical impact injury. Exp. Neurol 182, 87–102. [DOI] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG, 2003b. Time course of cellular pathology after controlled cortical impact injury. Exp. Neurol 182, 87–102. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, 1995. Cellular management of iron in the brain. J Neurol. Sci 134 (Suppl), 33–44. [DOI] [PubMed] [Google Scholar]
- Dadas A, Janigro D, 2018. Breakdown of blood brain barrier as a mechanism of post-traumatic epilepsy. Neurobiol. Dis 10.1016/j.nbd.2018.06.022. (Jul 18. pii: S0969–9961(18)30201–8. [Epub ahead of print]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW, 2005. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain 128, 174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Nanga RP, Das S, Chen SH, Hadar PN, Pollard JR, Lucas TH, Shinohara RT, Litt B, Hariharan H, Elliott MA, Detre JA, Reddy R, 2015. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci. Transl. Med 7, 309ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerdere S, Fang K, Chow M, Shen YT, Noordman I, van Raay L, Faggian N, Porritt M, Egan GF, O’Brien TJ, 2013. Manganese-enhanced MRI reflects seizure outcome in a model for mesial temporal lobe epilepsy. Neuroimage 68, 30–38. [DOI] [PubMed] [Google Scholar]
- Deleo F, Thom M, Concha L, Bemasconi A, Bernhardt BC, Bemasconi N, 2018. Histological and MRI markers of white matter damage in focal epilepsy. Epilepsy Res. 140, 29–38. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Rashid F, Jahanshad N, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Asamow RF, Thompson PM, 2017. A network approach to examining injury severity in pediatric Tbi. Proc. IEEE Int. Symp. Biomed. Imaging 2017, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA, 2013. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Madden CJ, Van Ness PC, 2009. Posttraumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia 50 (Suppl. 2), 14–20. [DOI] [PubMed] [Google Scholar]
- Eftekhar B, Sahraian MA, Nouralishahi B, Khaji A, Vahabi Z, Ghodsi M, Araghizadeh H, Soroush MR, Esmaeili SK, Masoumi M, 2009. Prognostic factors in the persistence of posttraumatic epilepsy after penetrating head injuries sustained in war. J. Neurosurg 110, 319–326. [DOI] [PubMed] [Google Scholar]
- Filibian M, Frasca A, Maggioni D, Micotti E, Vezzani A, Ravizza T, 2012. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 53, 1907–1916. [DOI] [PubMed] [Google Scholar]
- Frey L, Lepkin A, Schickedanz A, Huber K, Brown MS, Serkova N, 2014. ADC mapping and T1-weighted signal changes on post-injury MRI predict seizure susceptibility after experimental traumatic brain injury. Neurol. Res 36, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow JS, Hirvonen J, Kreisl WC, Jenko KJ, Inati SK, Fujita M, Morse CL, Brouwer C, Hong JS, Pike VW, Zoghbi SS, Innis RB, Theodore WH, 2015. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol. 72, 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godale CM, Danzer SC, 2018. Signaling pathways and cellular mechanisms regulating mossy fiber sprouting in the development of epilepsy. Front. Neurol 9, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA, 2001. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci 21, 8523–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith B, Jain R, 2016. Perfusion imaging in neuro-oncology. Basic Tech. Clin. Appl 24, 765–779. [DOI] [PubMed] [Google Scholar]
- Grohn O, Sierra A, Immonen R, Laitinen T, Lehtimaki K, Airaksinen A, Hayward N, Nairismagi J, Lehto L, Pitkanen A, 2011. Multimodal MRI assessment of damage and plasticity caused by status epilepticus in the rat brain. Epilepsia 52 (Suppl. 8), 57–60. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Saksena S, Agarwal A, Hasan KM, Husain M, Gupta V, Narayana PA, 2005. Diffusion tensor imaging in late posttraumatic epilepsy. Epilepsia 46, 1465–1471. [DOI] [PubMed] [Google Scholar]
- Harris NG, Verley DR, Gutman BA, Thompson PM, Yeh HJ, Brown JA, 2016a. Disconnection and hyper-connectivity underlie reorganization after TBI: a rodent functional connectomic analysis. Exp Neurol 277, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Verley DR, Gutman BA, Sutton RL, 2016b. Bi-directional changes in fractional anisotropy after experiment TBI: disorganization and reorganization? Neuroimage 133, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Verley DR, Gutman BA, Thompson PM, Yeh HJ, Brown JA, 2016c. Disconnection and hyper-connectivity underlie reorganization after TBI: a rodent functional connectomic analysis. Exp. Neurol 277, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward NM, Immonen R, Tuunanen PI, Ndode-Ekane XE, Grohn O, Pitkanen A, 2010. Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J. Neurotrauma 27, 2203–2219. [DOI] [PubMed] [Google Scholar]
- Hayward NM, Tuunanen PI, Immonen R, Ndode-Ekane XE, Pitkanen A, Grohn O, 2011. Magnetic resonance imaging of regional hemodynamic and cerebrovascular recovery after lateral fluid-percussion brain injury in rats. J. Cereb. Blood Flow Metab 31, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM, 2002. Functional anatomy of thalamus and basal ganglia. Childs Nerv. Syst 18, 386–404. [DOI] [PubMed] [Google Scholar]
- Immonen RJ, Kharatishvili I, Sierra A, Einula C, Pitkänen A, Gröhn OHJ, 2008. Manganese enhanced MRI detects mossy fiber sprouting rather than neurodegeneration, gliosis or seizure-activity in the epileptic rat hippocampus. Neuroimage 40, 1718–1730. [DOI] [PubMed] [Google Scholar]
- Immonen RJ, Kharatishvili I, Niskanen JP, Grohn H, Pitkanen A, Grohn OH, 2009. Distinct MRI pattern in lesional and perilesional area after traumatic brain injury in rat-11 months follow-up. Exp. Neurol 215, 29–40. [DOI] [PubMed] [Google Scholar]
- Immonen R, Kharatishvili I, Grohn O, Pitkanen A, 2013a. MRI biomarkers for post-traumatic epileptogenesis. J. Neurotrauma 30, 1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen R, Kharatishvili I, Grohn O, Pitkanen A, 2013b. MRI biomarkers for post-traumatic epileptogenesis. J. Neurotrauma 30, 1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Wang B, Aylward SR, Prastawa MW, Pace DF, Gerig G, Hovda DA, Kikinis R, Vespa PM, Van Horn JD, 2012. Neuroimaging of structural pathology and connectomics in traumatic brain injury: toward personalized outcome prediction. Neuroimage Clin. 1, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel I, Ohsiek A, Al-Momani E, Albert-Weissenberger C, Stetter C, Mend S, Buck AK, Kleinschnitz C, Samnick S, Siren AL, 2016. Combined [(18)F]DPA-714 micro-positron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. J. Neuroinflammation 13 (140–016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Asano E, Juhasz C, Chugani HT, 2015. Localization of specific language pathways using diffusion-weighted imaging tractography for presurgical planning of children with intractable epilepsy. Epilepsia 56, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Mehrens H, Hendrich KS, Kim SG, 2014. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J. Cereb. Blood Flow Metab 34, 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W, 2013. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Aoki I, Umeda M, Higuchi T, Kershaw J, Higuchi M, Silva AC, Tanaka C, 2010. In vivo visualization of reactive gliosis using manganese-enhanced magnetic resonance imaging. Neuroimage 49, 3122–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, Diaz-Arrastia R, 2016. Cerebral Vascular Injury in Traumatic Brain Injury. Exp. Neurol 275 (Pt 3), 353–366 Jan. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A, 2010. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 90, 47–59. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A, 2006. A model of post-traumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Grohn O, Pitkanen A, 2007. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain 130, 3155–3168. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Arstad E, Bankstahl JP, Dedeurwaerdere S, Friedman A, Potschka H, Ravizza T, Theodore WH, Baram TZ, 2017. Neuroinflammation imaging markers for epileptogenesis. Epilepsia 58 (Suppl. 3), 11–19. [DOI] [PubMed] [Google Scholar]
- Kyyriainen J, Ekolle Ndode-Ekane X, Pitkanen A, 2017. Dynamics of PDGFRbeta expression in different cell types after brain injury. Glia 65 (2), 322–341. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Sierra A, Bolkvadze T, Pitkanen A, Grohn O, 2015. Diffusion tensor imaging detects chronic microstructural changes in white and gray matter after traumatic brain injury in rat. Front. Neurosci 9, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavina B, 2016. Brain vascular imaging techniques. Int. J. Mol. Sci 18 10.3390/ijms18010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Wong MD, Samii A, Hovda DA, 1999. Evidence for energy failure following irreversible traumatic brain injury. Ann. N Y Acad. Sci 893, 337–340. [DOI] [PubMed] [Google Scholar]
- Lehto LJ, Sierra A, Corum CA, Zhang J, Idiyatullin D, Pitkanen A, Garwood M, Grohn O, 2012. Detection of calcifications in vivo and ex vivo after brain injury in rat using SWIFT. Neuroimage 61, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto LJ, Sierra A, Grohn O, 2017. Magnetization transfer SWIFT MRI consistently detects histologically verified myelin loss in the thalamocortical pathway after a traumatic brain injury in rat. NMR Biomed. 30 10.1002/nbm.3678. [DOI] [PubMed] [Google Scholar]
- Li W, Long JA, Watts L, Shen Q, Liu Y, Jiang Z, Duong TQ, 2016. Spatiotemporal changes in diffusion, T2 and susceptibility of white matter following mild traumatic brain injury. NMR Biomed. 29, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JS, Lee SP, Pulli B, Chen JW, Kao SC, Tsang YM, Hsieh KL, 2016. Microstructural changes in absence seizure children: a diffusion tensor magnetic resonance imaging study. Pediatr. Neonatol 57, 318–325. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA, 2008. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA, 2009. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 252, 816–824. [DOI] [PubMed] [Google Scholar]
- Liu J, Kou Z, Tian Y, 2014. Diffuse axonal injury after traumatic cerebral microbleeds: an evaluation of imaging techniques. Neural. Regen. Res 9, 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA., Cabatbat R, Faden AI, 2014. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol 73, 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Seren MS, Longo FM, 1993. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience 56, 597–604. [DOI] [PubMed] [Google Scholar]
- Mantoan Ritter L, Golshani P, Takahashi K, Dufour S, Valiante T, Kokaia M, 2014. WONOEP appraisal: optogenetic tools to suppress seizures and explore the mechanisms of epileptogenesis. Epilepsia 55, 1693–1702. [DOI] [PubMed] [Google Scholar]
- Marchi N, Banjara M, Janigro D, 2016. Blood-brain barrier, bulk flow, and interstitial clearance in epilepsy. J. Neurosci. Methods 260, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata CD, Yang FG, Wang JY, Krishnan K, Bakhadirov K, Paliotta C, Aslan S, Devous MD, Moore C, Harper C, McColl R, Munro Cullum C, Diaz-Arrastia R, 2011. Diffusion tensor imaging biomarkers for traumatic axonal injury: analysis of three analytic methods. J. Int. Neuropsychol. Soc 17, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathem GW, Cifuentes F, Leite JP, Pretorius JK, Babb TL, 1993. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr. Clin. Neurophysiol 87, 326–339. [DOI] [PubMed] [Google Scholar]
- Messori A, Polonara G, Carle F, Gesuita R, Salvolini U, 2005. Predicting posttraumatic epilepsy with MRI: prospective longitudinal morphologic study in adults. Epilepsia 46, 1472–1481. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Bai X, Sanganahalli BG, Waxman SG, Shatillo O, Grohn O, Hyder F, Pitkanen A, Blumenfeld H, 2014. Decreased resting functional connectivity after traumatic brain injury in the rat. PLoS One 9, e95280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Osteen CL, Chatziioannou AF, Hovda DA, Cherry SR, 2000. Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J. Cereb. Blood Flow Metab 20, 1492–1501. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Pages G, Kuchel PW, Golay X, Chuang KH, 2013. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J. Cereb. Blood Flow Metab 33, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndode-Ekane XE, Matthiesen L, Banuelos-Cabrera I, Palminha CAP, Pitkanen A, 2018. T-cell infiltration into the perilesional cortex is long-lasting and associates with poor somatomotor recovery after experimental traumatic brain injury. Restor. Neurol. Neurosci 36, 485–501. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, LeVine SM, Brooks WM, Berman NE, 2009. Post-acute pathological changes in the thalamus and internal capsule in aged mice following controlled cortical impact injury: a magnetic resonance imaging, iron histochemical, and glial immunohistochemical study. Neurosci. Lett 452, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannek K, Mathias JL, Bigler ED, Brown G, Taylor JD, Rose SE, 2011. The average pathlength map: a diffusion MRI tractography-derived index for studying brain pathology. Neuroimage 55, 133–141. [DOI] [PubMed] [Google Scholar]
- Pascente R, Frigerio F, Rizzi M, Porcu L, Boido M, Davids J, Zaben M, Tolomeo D, Filibian M, Gray WP, Vezzani A, Ravizza T, 2016. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis 93, 146–155. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR, 2013. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci 16, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischiutta F, Micotti E, Hay JR, Marongiu I, Sammali E, Tolomeo D, Vegliante G, Stocchetti N, Forloni G, De Simoni MG, Stewart W, Zanier ER, 2018. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp. Neurol 300, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Immonen R, 2014. Epilepsy related to traumatic brain injury. Neurotherapeutics 11, 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshé SL, 2017. Models of Seizure and Epilepsy. Academic Press. [Google Scholar]
- Pitkänen A, Bolkvadze T, Immonen R: Anti-epileptogenesis in rodent post-traumatic epilepsy models. Neurosci. Lett DOI: 10.1016/j.neulet.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ, 2011. Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol 70, 374–383. [DOI] [PubMed] [Google Scholar]
- Reid AY, Bragin A, Giza CC, Staba RJ, Engel J, 2016. The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia 57 (10), 1558–1567 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan D, Yu XB, Shrestha S, Wang L, Chen G, 2015. The role of hemosiderin excision in seizure outcome in cerebral cavernous malformation surgery: a systematic review and meta-analysis. PLoS One 10, e0136619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russmann V, Brendel M, Mille E, Helm-Vicidomini A, Beck R, Gunther L, Lindner S, Rominger A, Keck M, Salvamoser JD, Albert NL, Bartenstein P, Potschka H, 2017. Identification of brain regions predicting epileptogenesis by serial [(18)F]GE-180 positron emission tomography imaging of neuroinflammation in a rat model of temporal lobe epilepsy. Neuroimage Clin. 15, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo RA, Miettinen T, Laitinen T, Grohn O, Sierra A, 2017. Diffusion tensor MRI shows progressive changes in the hippocampus and dentate gyrus after status epilepticus in rat - histological validation with Fourier-based analysis. Neuroimage 152, 221–236. [DOI] [PubMed] [Google Scholar]
- Samokhina E, Popova I, Malkov A, Ivanov AI, Papadia D, Osypov A, Molchanov M, Paskevich S, Fisahn A, Zilberter M, Zilberter Y, 2017. Chronic inhibition of brain glycolysis initiates epileptogenesis. J. Neurosci. Res 95, 2195–2206. [DOI] [PubMed] [Google Scholar]
- Sarikaya I, 2015. PET studies in epilepsy. Am. J. Nucl. Med. Mol. Imaging 5, 416–430. [PMC free article] [PubMed] [Google Scholar]
- Scott G, Mahmud M, Owen DR, Johnson MR, 2017. Microglial positron emission tomography (PET) imaging in epilepsy: applications, opportunities and pitfalls. Seizure 44, 42–47. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Cardamone L, Liu YR, Hogan RE, Maccotta L, Wright DK, Zheng P, Koe A, Gregoire MC, Williams JP, Hicks RJ, Jones NC, Myers DE, O’Brien TJ, Bouilleret V, 2013. Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome? Epilepsia 54, 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Laitinen T, Grohn O, Pitkanen A, 2015. Diffusion tensor imaging of hippocampal network plasticity. Brain Struct. Funct 220, 781–801. [DOI] [PubMed] [Google Scholar]
- Sivakanthan S, Neal E, Murtagh R, Vale FL, 2016. The evolving utility of diffusion tensor tractography in the surgical management of temporal lobe epilepsy: a review. Acta Neurochir. (Wien.) 158, 2185–2193. [DOI] [PubMed] [Google Scholar]
- Susarla BT, Villapol S, Yi JH, Geller HM, Symes AJ, 2014. Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro. 6, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley Watts L, Shen Q, Deng S, Chemello J, Duong TQ, 2015. Manganese-enhanced magnetic resonance imaging of traumatic brain injury. J. Neurotrauma 32, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchopev ZN, Yeh PH, Morgan GW, Meyer E, Wolf JM, Ollinger JM, Riedy GP, Young LC, 2018. Acquired sleep-related hypermotor epilepsy with disrupted white matter tracts assessed by multishell diffusion magnetic resonance imaging. Front. Neurol 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen J, Kokaia M, 2017. Epilepsy and optogenetics: can seizures be controlled by light? Clin. Sci. (Lond.) 131, 1605–1616. [DOI] [PubMed] [Google Scholar]
- Toth P, Szarka N, Farkas E, Ezer E, Czeiter E, Amrein K, Ungvari Z, Hartings JA, Buki A, Koller A, 2016. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol 311, H1118–H1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JK, Shiu FH, Ding K, Gross RE, 2018. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol. Dis 109, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallez Garcia D, Otte A, Dierckx RA, Doorduin J, 2016. Three month follow-up of rat mild traumatic brain injury: a combined [18F]FDG and [11C]pk11195 positron emission study. J. Neurotrauma 33, 1855–1865. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Gorter JA, Dijkhuizen RM, Wadman WJ, 2014. Longitudinal assessment of blood-brain barrier leakage during epileptogenesis in rats. A quantitative MRI study. Neurobiol. Dis 63, 74–84. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Dedeurwaerdere S, Cole AJ, Friedman A, Koepp MJ, Potschka H, Immonen R, Pitkanen A, Federico P, 2017. WONOEP appraisal: imaging biomarkers in epilepsy. Epilepsia 58, 315–330. [DOI] [PubMed] [Google Scholar]
- van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD, 2007. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc. Natl. Acad. Sci. USA 104, 4359–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Essen C, Rydenhag B, Nystrom B, Mozzi R, van Gelder N, Hamberger A, 1996. High levels of glycine and serine as a cause of the seizure symptoms of cavernous angiomas? J. Neurochem 67, 260–264. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yue X, Kiesewetter DO, Niu G, Teng G, Chen X, 2014. PET imaging of neuroinflammation in a rat traumatic brain injury model with radiolabeled TSPO ligand DPA-714. Eur. J. Nucl. Med. Mol. Imaging 41, 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KM, Sun M, Crack P, O’Brien TJ, Shultz SR, Semple BD, 2017. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation 14, 10–016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Lee S, Spencer DD, 2003. Physiology of human cortical neurons adjacent to cavernous malformations and tumors. Epilepsia 44, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Rubin JJ, 1981. Antiperoxidant pretreatment and iron-induced epileptiform discharges in the rat: EEG and histopathologic studies. Neurology 31, 63–69. [DOI] [PubMed] [Google Scholar]
- Wright DK, Trezise J, Kamnaksh A, Bekdash R, Johnston LA, Ordidge R, Semple BD, Gardner AJ, Stanwell P, O’Brien TJ, Agoston DV, Shultz SR, 2016. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Sci. Rep 6, 28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, Liu S, van der Poel C, McDonald SJ, Brady RD, Taylor L, Yang L, Gardner AJ, Ordidge R, O’Brien TJ, Johnston LA, Shultz SR, 2017aTraumatic brain injury results in cellular, structural and functional changes resembling motor neuron disease. Cereb. Cortex 27, 4503–4515. [DOI] [PubMed] [Google Scholar]
- Wright DK, Johnston LA, Kershaw J, Ordidge R, O’Brien TJ, Shultz SR, 2017b. Changes in apparent fiber density and track-weighted imaging metrics in white matter following experimental traumatic brain injury. J. Neurotrauma 34, 2109–2118. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Ou S, Liu X, Yuan J, Huang H, Yang J, He L, Chen Y, 2017. Risk factors for posttraumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav. 67, 1–6. [DOI] [PubMed] [Google Scholar]
- Xue K, Luo C, Zhang D, Yang T, Li J, Gong D, Chen L, Medina YI, Gotman J, Zhou D, Yao D, 2014. Diffusion tensor tractography reveals disrupted structural connectivity in childhood absence epilepsy. Epilepsy Res. 108, 125–138. [DOI] [PubMed] [Google Scholar]
- Yu I, Inaji M, Maeda J, Okauchi T, Nariai T, Ohno K, Higuchi M, Suhara T, 2010. Glial cell-mediated deterioration and repair of the nervous system after traumatic brain injury in a rat model as assessed by positron emission tomography. J. Neurotrauma 27, 1463–1475. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gauthier SA, Gupta A, Chen W, Comunale J, Chiang GC, Zhou D, Askin G, Zhu W, Pitt D, Wang Y, 2016. Quantitative susceptibility mapping and R2* measured changes during white matter lesion development in multiple sclerosis: myelin breakdown, myelin debris degradation and removal, and iron accumulation. AJNR Am J. Neuroradiol 37, 1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]