Abstract
Background:
Prior studies have suggested that patients with cognitive impairment are at increased risk for adverse post-hospitalization outcomes. We aimed to determine if cognitive status assessed by the Mini-Cog, a quick bedside screening test, is associated with long-term outcomes.
Methods:
In this secondary analysis of data from a prospective cohort study, 668 patients >65 years of age admitted to a tertiary care academic hospital over a two-year period were screened for cognitive impairment with the Mini-Cog within 24 hours of admission. We performed multivariable regression adjusting for demographics, comorbidities, principal diagnoses and functional status to determine association between cognitive impairment and discharge to post-acute care, 90-day readmission and one-year mortality.
Results:
Overall 35% screened positive for cognitive impairment. Those with impairment were older (median age 83 versus 78), less likely to be admitted from home and had lower functional independence and self-reported performance scores (p<0.001 for all). Patients with cognitive impairment were more likely to be discharged to post-acute care facilities (54% versus 39%, p<0.001). 90-day readmission rate of patients with and without cognitive impairment was 35% versus 27%; one-year survival 77% versus 84% and median length-of-stay was 4 days for both groups. Differences in readmission and mortality were not statistically significant after adjusting for covariates.
Conclusion:
Cognitive impairment as screened for by the Mini-Cog was not associated with readmission, length-of-stay, or 1-year mortality but was associated with discharge to post-acute care. Other tools such as frailty assessment may be more useful in predicting these outcomes in hospitalized older adults.
Keywords: cognition, readmission, mortality, dementia
INTRODUCTION
Cognitive impairment is common in elderly patients 1. In the US population in 2010, the estimated rate of dementia was 14.7% for those over 70 years of age with an attributable total cost of 157 to 215 billion USD 2. Although several screening tools for cognitive impairment exist, studies have shown that clinicians often fail to identify cognitive impairment 3,4. These tools include the Montreal Cognitive Assessment test (MOCA), the Saint Louis University Mental Status exam (SLUMS) and the Folstein Mini Mental Status Examination (MMSE) among others. The simplest assessment tool for cognitive impairment is the Mini-Cog screening test which consists of a three-item recall test and clock draw; it is a validated tool which is readily accessible to clinicians and can be performed quickly at bedside 3,5,6. The maximum score is five, with a score of two or less suggesting cognitive impairment. It screens accurately for mild to severe dementia and may also predict functional abilities in terms of self-management tasks at home 7. Given its simplicity some studies have assessed its value as a predictor for hospital readmission or mortality. In a recent study of 720 patients hospitalized with congestive heart failure, patients with poor Mini Cog performance had almost twice the risk for readmission 8. In surgical elderly patients, cognitive impairment was associated with longer length of stay, greater rates of post-surgical complications and increased hospital mortality 9-11. However, limited data exist on the relationship between cognitive impairment and long-term outcomes post hospitalization in a broad range of diagnoses of medical or surgical patients. In the present study, we aimed to determine whether cognitive impairment as identified using the Mini-Cog test is associated with readmission, length of stay or one-year mortality for older adults. Based on the prior literature we hypothesized that patients with cognitive impairment will have worse outcomes. If this assumption was true, then including the Mini-Cog in the electronic medical record as a recommended assessment could help discussing risks and planning appropriately for this group of patients.
METHODS
Setting and subjects:
This was a secondary analysis of the data from a two-year prospective study examining frailty in elderly adults (≥ 65 years of age) who were admitted for a non-elective medical or surgical diagnosis from June 2014 to August 2016 at a 716 bed academic tertiary medical center in Western Massachusetts. Frailty was assessed using the Reported Edmonton Frailty scale, a validated tool to assess frailty which examines nine geriatric domains including functionality 12,13.
Patients were excluded if they had existing diagnosis of advanced dementia, if they did not speak English and if they were admitted to the intensive care unit. Observations with missing cognitive assessment or outcomes data were excluded. Patients who expired during the index admission (as we report on post-discharge outcomes) or were discharged to hospice were also excluded. We extracted patient characteristics including age, sex, comorbidities, principal admission diagnosis (ICD-9 diagnosis code), length of stay, discharge disposition, and readmission dates from the hospital’s billing database (McKesson Performance Analytics version 18.0 [2014]). To determine the comorbidity burden we calculated the Elixhauser comorbidity score 14. Of note, the Elixhauser score does not include dementia as one of the comorbidities. Principal diagnoses were grouped in categories as best described by their active condition in categories such as cardiac, gastrointestinal, infectious, pulmonary, renal, hematological, and primary surgical admissions. We also included patient characteristics collected as part of the Reported Edmonton Frail Scale assessment 12,13, including: 1) functional independence which is grouped in 3 categories based on number of tasks requiring assistance out of eight daily tasks (excellent = 0-1, fair = 2-4, poor = 5-8); and 2) self-reported performance score (score 0-3, lower is better). We recorded discharge status as post-acute care when patient was discharged to any of the following: skilled nursing facility, intermediate care facility, long term care hospital, rehab facility, or short term hospital. Mortality data was obtained from the National Death Index (NDI) maintained by the Center for Disease Control 15.
Mini-Cog testing:
Trained research assistants (RAs) interviewed patients and/or caregivers within 24 hours of admission and administered Mini-Cog together with frailty assessment, collected information on the patient's living situation 16. The RAs were trained by a geriatrician and one of the authors (MS) of the study using the hospital elderly life program delirium training manual and coding guide for short confusion assessment method 17. For each of the instruments, as specified in our protocol, after the training the PI supervised the RAs for the first 10 patients, and randomly thereafter for at least 4 times a month.
We used the Mini-Cog test as described by Borson, et al. to screen for cognitive impairment (three-item recall test and clock drawing) with a score ≤2 indicating cognitive impairment 6. The recall task contributes 1 point for each of the three items accurately remembered and the clock-draw contributes a total of 2 possible points to the overall score (depending on accuracy) for a total of 5 possible points on the overall test. Although data for both the recall and clock draw sections were collected for all patients in the study, as per the defined scoring system by Borson only those patients correctly recalling 1-2 words have the clock draw scored. The stricter cutoff of ≤ 2 is often used to screen for dementia although scores of ≤ 3 have previously been used in other studies to target patients with mild cognitive impairment (MCI) as well 6.
Outcomes:
Our primary outcomes were 30-, 60-, 90-day readmission. Secondary outcomes included length of stay, discharge disposition, time to first readmission and 1-year post-discharge mortality.
Statistical analysis:
Characteristics of the study cohort were reported as means, medians and inter-quartile range for continuous variables and as counts and percentages for categorical variables. We compared the characteristics and outcomes of patients with and without cognitive impairment, as determined using the Mini-Cog assessment, using Chi-square, Wilcoxon and Kruskal–Wallis tests. A p-value < 0.05 was considered statistically significant and all tests were two-sided. To calculate readmission at 30, 60 and 90 days we excluded admissions where the patient expired. We performed multivariable logistic regression to assess the association between cognitive impairment and readmission rates, discharge disposition, time to readmission, and 1-year survival. An adjusted Cox proportional hazards model was used to assess the relationship between cognitive impairment and time to first readmission. Due to the right-skew of length-of-stay data, its relationship with cognitive impairment was modeled using Poisson regression. We adjusted for age, sex, race, admission source (home or not), and comorbidities (Elixhauser score). We also adjusted for the functional independent score and the self-reported Edmonton performance score as noted above. Because prior studies have shown an association between cognitive impairment and outcomes in patients with heart failure and some surgical specialties, we also investigated the potential for interaction between cognitive impairment and patient type (medical versus surgery), and between those with and without a principal diagnosis of heart failure. Of note, this exploratory analysis of potential heterogeneity was not accounted for in the original sample size calculations.
Results are reported as point estimates and 95% confidence intervals (CI). All data management and statistical analyses were performed using R version 3.5 18.
The study was approved by the Baystate Medical Center Institutional Review Board.
RESULTS
Patient characteristics:
Among the 668 patients enrolled in the study, median age was 80 (IQR 74-86), 88% were of white race and 58% were female. The baseline characteristics are summarized in table 1. There were 482 (72%) patients admitted for medical diagnosis and 186 (28%) were admitted for urgent surgical diagnoses.
Table 1.
Baseline Characteristics of subjects in the study, divided based on screening positive or negative for cognitive impairment by use of the Mini-Cog test.
| Cognitive Impairment | |||||
|---|---|---|---|---|---|
| Overall | Negative | Positive | P-value | ||
| Number | 668 | 436 | 232 | ||
| Age (median [IQR]) | 80.00 [74.00, 86.00] | 78.00 [72.00, 84.00] | 83.00 [77.00, 88.00] | <0.001 | |
| Age Category (%) | 65-74 | 189 (28.3) | 149 (34.2) | 40 (17.2) | <0.001 |
| 75-84 | 275 (41.2) | 186 (42.7) | 89 (38.4) | ||
| 85+ | 204 (30.5) | 101 (23.2) | 103 (44.4) | ||
| Sex (%) | Female | 385 (57.6) | 258 (59.2) | 127 (54.7) | 0.30 |
| Race (%) | White | 586 (87.7) | 387 (88.8) | 199 (85.8) | 0.08 |
| Black | 39 (5.8) | 19 (4.4) | 20 (8.6) | ||
| Hispanic | 35 (5.2) | 23 (5.3) | 12 (5.2) | ||
| Other | 8 (1.2) | 7 (1.6) | 1 (0.4) | ||
| Hospitalization Prior Year (%) | None | 228 (34.2) | 169 (38.8) | 59 (25.5) | <0.001 |
| 1 or 2 | 293 (43.9) | 189 (43.3) | 104 (45.0) | ||
| 3+ | 146 (21.9) | 78 (17.9) | 68 (29.4) | ||
| Encounter Type (%) | Surgery | 186 (27.8) | 140 (32.1) | 46 (19.8) | 0.001 |
| Medical | 482 (72.2) | 296 (67.9) | 186 (80.2) | ||
| Payor Category (%) | Medicare | 597 (89.4) | 387 (88.8) | 210 (90.5) | 0.57 |
| History of Dementia (%) | 67 (10.0) | 19 (4.4) | 48 (20.7) | <0.001 | |
| Elixhauser Score (median [IQR]) | 4.50 [3.00, 6.00] | 4.00 [3.00, 6.00] | 5.00 [3.00, 7.00] | 0.12 | |
| Falls Prior Year (%) | None | 450 (68.0) | 300 (69.3) | 150 (65.5) | 0.61 |
| 1 or 2 | 155 (23.4) | 97 (22.4) | 58 (25.3) | ||
| 3+ | 57 (8.6) | 36 (8.3) | 21 (9.2) | ||
| Live at Home (%) | 579 (86.7) | 392 ( 89.9) | 187 (80.6) | 0.001 | |
| Functional Independence (%) | Excellent | 308 (46.1) | 236 (54.1) | 72 (31.0) | <0.001 |
| Fair | 176 (26.3) | 118 (27.1) | 58 (25.0) | ||
| Poor | 184 (27.5) | 82 ( 18.8) | 102 (44.0) | ||
| Performance Score (mean and standard difference) | 1.46 (1.23) | 1.32 (1.22) | 1.72 (1.20) | <0.001 | |
Overall, 232 (34.7%) patients screened positive for cognitive impairment based on the Mini-Cog test. The cognitive screen results are summarized in table 2. Out of the 668 patients tested, 165 patients (25%) were able to recall all 3 words in the item recall task. Performance on clock draw showed large differences between those who eventually tested positive for cognitive impairment compared with cognitively intact patients, with 82% making errors on the clock draw versus 21% respectively (p<0.001). Those screening positive were significantly older with median age of 83 (IQR 77-88) compared with 78 (IQR 72–84) in those without. Medical patients were more likely than surgical patients to be cognitively impaired (39% vs. 25%; p<0.001). Two hundred thirty-two patients (34.7%) had a Mini-Cog score suggesting cognitive impairment but only 20.7% of those identified with cognitive impairment had a diagnosis of dementia. The median Elixhauser score was 5 (IQR 3-7) for those screening positive, and 4 (IQR 3-6) for those screening negative for cognitive impairment. In addition, patients with cognitive impairment were more likely to have at least one hospitalization in the prior year (74.8% vs 61.2%), to have poor functional independence with only 31% rating their independence as "excellent" versus 54% in those without cognitive impairment (p<0.001). Similarly, mean performance score was significantly lower (1.7 versus 1.3, p<0.001). Those screening in for cognitive impairment were significantly less likely to be admitted from home rather than other facility (81% versus 90%, p<0.001).
Table 2.
Results of Mini-Cog testing in all subjects.
| Cognitive Impairment | |||||
|---|---|---|---|---|---|
| Overall | Negative | Positive | P-value | ||
| N=668 | N=436 | N=232 | |||
| Mini-Cog Words Recalled (%) | All 3 | 165 (24.7) | 165 (37.8) | 0 (0.0) | <0.001 |
| 1 or 2 | 398 (59.6) | 271 (62.2) | 127 (54.7) | ||
| None | 105 (15.7) | 0 (0.0) | 105 (45.3) | ||
| Mini-Cog Clock Draw (%) | No Errors | 386 (57.8) | 343 (78.7) | 43 (18.5) | <0.001 |
| Any Error | 282 (42.2) | 93 (21.3) | 189 (81.5) | ||
Outcomes:
Unadjusted analysis:
When we compared patients with and without cognitive impairment we did not find any significant statistical differences in the proportion with a readmission at 30- or 60-day.. Readmission rate at 90 days was 35% for those with cognitive impairment and 27% for those without (p=0.03), while one year survival rate was 77% for those with cognitive impairment and 84% for those without (p=0.02). Time to readmission was 6 days shorter (95% CI: −1.9 days, 8.0 days) for those with cognitive impairment compared to those without. The readmission-free survival curve is shown in figure 1. Notably, patients with cognitive impairment were more likely than those without to be discharged to a post-acute care facility (54% versus 39%, p<0.001). Median length of stay was 4 days in both groups; though due to difference in IQR (3-7 in those with CI, verses, 3-6 in those without) a statistically significant difference was obtained (p=0.007). These results are summarized in table 3.
Figure 1.
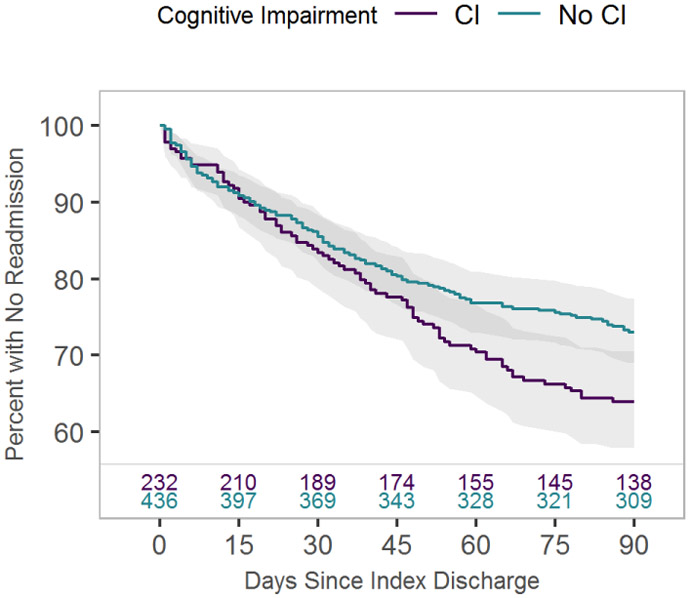
Kaplan-Meier survival curve of time to all cause readmission for subjects screening positive for cognitive impairment (CI) versus negative (no CI).
Table 3.
Unadjusted outcomes of readmission, length of stay, 1 -year survival, and discharge location based on cognitive screening result.
| Cognitive Screening | |||||
|---|---|---|---|---|---|
| Total | Negative | Positive | P-value | ||
| N | 668 | 436 | 232 | ||
| 30 Day Readmission (%) | 101 (15.1) | 63 (14.4) | 38 (16.4) | 0.58 | |
| 60 Day Readmission (%) | 167 (25.0) | 100 (22.9) | 67 ( 28.9) | 0.11 | |
| 90 Day Readmission (%) | 197 (29.5) | 116 (26.6) | 81 (34.9) | 0.03 | |
| Length of Stay (median [IQR]) | 4.00 [3.00, 6.00] | 4.00 [3.00, 6.00] | 4.00 [3.00, 7.00] | 0.007 | |
| 1 Year Survival (%) | 545 (81.6) | 367 ( 84.2) | 178 (76.7) | 0.02 | |
| Discharge Location (% discharged to home) | 375 (56.1) | 268 (61.5) | 107 (46.1) | <0.001 | |
| Discharge location (% discharged to post-acute care) | 293 (43.9) | 168 ( 38.5) | 125 ( 53.9) | ||
Adjusted analyses:
In the multivariable regression model which adjusted for demographics, principal diagnosis, admission from home, functional capacity, self-reported general health and comorbidities, only discharge disposition was significantly different between the two groups. There was no evidence of statistical interaction between cognitive impairment and patient type (medical versus surgery patients), and between cognitive impairment and those with and without a principal diagnosis of heart failure. These results showed lack of heterogeneity of effect of cognitive impairment on the outcomes among those groups. Our adjusted primary outcome results are summarized in table 4.
Table 4.
Adjusted and Unadjusted Odds ratios for included Outcome Measures.
| Unadjusted | Adjusted* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio |
95% CI | ||||||
| 30-Day Readmission | 1.16 | 0.75 | 1.80 | 1.02 | 0.64 | 1.64 | |||
| 60-Day Readmission | 1.33 | 0.92 | 1.92 | 1.18 | 0.79 | 1.75 | |||
| 90-Day Readmission | 1.46 | 1.02 | 2.08 | 1.39 | 0.94 | 2.06 | |||
| 1-year survival | 0.62 | 0.42 | 0.92 | 0.82 | 0.53 | 1.27 | |||
Adjusted for age, sex, race, admission source, Elixhauser score, Edmonton functional independence score, Edmonton self-reported performance score
DISCUSSION
In this study of almost 700 elderly patients admitted with a medical diagnosis or for urgent surgery at our tertiary center we found that cognitive impairment as screened for by the Mini-Cog test was not a predictor of post-hospitalization outcomes including one-year survival, readmission or hospital length-of-stay, but was associated with discharge to post-acute care facilities.
The Mini-Cog test offers a quick, validated tool to screen for the presence of cognitive impairment. The benefit of the Mini-Cog is the ease of administration, requiring less than 5 minutes to administer, yet maintaining a high specificity (76-99%) and sensitivity (89-93%) for the detection of dementia 3,5,6. If this test was predictive for our outcomes, then performing a quick Mini-Cog assessment at admission could help providers in discussion of risks with patient’s families and allow planning appropriately for this group of patients.
To our knowledge, our study is one of the few that has examined the association of the Mini-Cog as a screening tool for cognitive impairment with post-hospitalization outcomes in a broad range of medical and surgical patients admitted to a large tertiary-care hospital. Although this is a largely negative study it provides valuable information to inform future work; negative results also advance the field. We now need studies testing other screening tools and additional factors such as social support at home, clinician awareness of cognitive impairment and subsequent development of delirium and other hazards of hospitalization. Prior to this, a few studies have used the Mini-Cog to identify patients at increased risk for hospital adverse events but focused on specific diagnoses such as heart failure or certain surgical procedures 8,9,19. For example in a study of 720 patients hospitalized with heart failure, the 6-month readmission rate was 46% in those with cognitive impairment versus 22% in those without. In this group of patients, cognitive impairment (as defined by Mini-Cog screening) was the most important predictor for readmission 8. Likewise, studies focused on surgical patients undergoing procedures in cardiothoracic and orthopedic settings have also found strong association between early post-operative readmission rates in patients with cognitive impairment 10,11,19.
Intuitively, patients with dementia will be more likely to have worse outcomes including readmissions to the hospital and post-discharge mortality. In our study, there was a trend for worse outcomes in patients with cognitive impairment (90-days readmission was 8.1 percentage points higher and one-year survival was 7.2 percentage points lower) but the results were not statistically significant after adjusting for other confounders. The differences in findings in our study could be due to several factors: first, only heart failure patients were included in the Patel study with much higher readmission rates and nonspecific principal diagnoses in our study 8; second, exclusion of patients with advanced dementia in our study could have resulted in a less sick population; third, the hospital-culture and processes seeking to optimize care of hospitalized older adults may well have differed among the institutions. For example, some of the patients in this medical cohort were patients in its Acute Care for Elders program (ACE). This may well have affected the outcomes by mitigating excess risk. The lack of interaction between heart failure diagnosis and cognitive impairment could also be due to our limited sample size. Large retrospective studies in Medicare populations have also reported rather conflicting results. A retrospective study of more than 3,000 Medicare beneficiaries with dementia in Rhode Island by Daiello et al found that patients carrying an ICD-9 dementia diagnosis had higher 30-day readmission rates (17.8% vs. 14.5%; p < 0.001)1. However, in another study of nearly 1 million Medicare patients, those with Alzheimer dementia diagnosis were less likely to be admitted to hospital for a diagnosis of heart failure (OR = 0.80; 95% CI 0.50-1.26) but more likely to be admitted for diabetes (OR = 3.61; OR = 2.60) 20. A recently-large retrospective cohort study of elders in Japan (N = 1,834,378) found that risk of hospital readmission in dementia patients showed substantial variation according to the primary admitting diagnoses 21. For instance, the authors highlight that a diagnosis of hip fracture was associated with an increased risk for 30-day hospital readmission (relative risk 1.46; 95% CI: 1.28-1.68), compared to a negligible adjusted relative risk for a diagnosis of cholecystitis (1.03; 95% CI:0.90–1.18) 21. The readmission rates for cognitive impairment patients in our study are comparable with those reported in these studies (16.4% vs 14.4%) and the lack of significance could be due to the smaller sample. Lastly in a recent systematic review, the impact of diagnosed dementia on readmission was only found to be modest as four of five cohort studies in this review showed only small increase in readmission rates 22.
Although we did not find an association between a positive Mini-Cog test and our primary outcomes, we believe that establishing the prevalence of cognitive impairment at admission remains valuable in and of itself. It may be that different screening tools should be employed or that cutoffs need to be adjusted to provide better discrimination of patients at highest risk for poor outcomes. Further studies are needed to assess the best ways to assess for a potential role of cognitive screening in hospitalization outcomes and optimize communication about newly detected cognitive impairment across settings of care.
Our data confirm the high prevalence of cognitive impairment in hospitalized patients with one in three hospitalized elders screening positive. This, in fact, is an underestimate of the extent of more subtle impairment. It is worth noting that only 1 in 4 patients was able to recall all 3 words and for that minority with perfect recall an additional 21% had some errors on the clock-drawing task. Depending on the method of case identification, the prevalence of cognitive impairment among hospitalized elders has been reported as high 30–45% and importantly, at least half of those with significant cognitive deficits may go unrecognized without systematic screening 23. In our study, 79% of the patients with cognitive impairment based on Mini-Cog screening did not have a prior diagnosis of dementia or cognitive impairment, supporting the idea that without structured screening cognitive impairment may not be noticed by the patient's healthcare providers. Notably, we found that patients on the surgical service were less likely to test positive with cognitive screening (25% vs 39%; p<0.001). This may be attributed to decreased likelihood of planning urgent surgical intervention in those with significant cognitive impairment or altered mentation, opting instead for medical management or admission to a medical service with surgery consultation when possible in these cases.
Our study has several limitations. First, the result of the Mini-Cog can be confounded by the occurrence of delirium in hospitalized elders. Delirium is associated with in-hospital and post-discharge negative outcomes and can contribute to outcomes independently and in presence of existing dementia. A large cohort study of over 10,000 patients in the United Kingdom used a structured geriatric assessment within 24 hours to assess the association of a spectrum of cognitive disorders including dementia, delirium and delirium superimposed on dementia with outcomes of readmission, length of stay, and 1 year mortality. Significantly increased risk for adverse outcomes including length of stay, 1 year mortality and one year death or readmission were seen in those with both delirium as well as other forms of cognitive impairment 24. In our study, research assistants performed the confusion assessment and enroll only patients without overt delirium at the time of initial assessment. Some of the prior studies did not specify how they controlled for this situation. However, we should note our purpose in this study was to use the Mini-Cog to assess for cognitive impairment upon admission hypothesizing that impairment predisposes patients to adverse outcomes post discharge. Although we excluded patients with delirium based on the admission CAM, some patients who were not delirious at presentation could have developed delirium subsequently. Since we are unable to identify and control for subsequent delirium this may have contributed to our negative results.
Second, this was a single-center study and hospital wide-specific interventions for elderly adults may differ from other hospitals and could have influence the outcomes. Third, we did not have information on the level of home support, anesthesia or surgical type, the standards of nursing care for ambulation, all of which could impact the outcomes. Fourth, due to limitations in practicality with applying the test in patients speaking other languages, we only included English-speaking patients. Fifth, the population was heterogeneous and although the statistical testing for heterogeneity was negative, our study was not powered to detect this association so this relationship could still exist. Lastly, the results of the Mini-Cog screening were not known to the supervising physician, and therefore we are unable to assess what impact the knowledge of cognitive impairment would have had on their care or management once applied to general hospital care.
CONCLUSION
We found that Mini-Cog test as a screening tool for cognitive impairment was not associated with readmission, length of stay, or 1-year mortality rates but was associated with discharge to post-acute care facilities. The addition of other tools such as frailty assessment may be more useful in predicting these outcomes.
Highlights:
The Mini-Cog test offers a quick and easy bedside screen for cognitive impairment
Testing was done on medical and surgical patients within 24 hours of hospitalization
Mini-Cog performance was not associated with mortality or readmission outcomes
Mini-Cog performance was associated with discharge to post-acute care
Acknowledgments
FUNDING
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST STATEMENT:
All authors declare no conflict of interest in the current work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Daiello LA, Gardner R, Epstein-Lubow G, Butterfield K, Gravenstein S. Association of dementia with early rehospitalization among Medicare beneficiaries. Arch Gerontol Geriatr. 2014;59(1):162–168. [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;369(5):489–490. doi: 10.1056/NEJMc1305541 [DOI] [PubMed] [Google Scholar]
- 3.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21(4):349–355. [DOI] [PubMed] [Google Scholar]
- 4.Raymont V, Bingley W, Buchanan A, et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: cross-sectional study. Lancet. 2004;364(9443):1421–1427. [DOI] [PubMed] [Google Scholar]
- 5.Scanlan J, Borson S. The Mini-Cog: receiver operating characteristics with expert and naïve raters. Int J Geriatr Psychiatry. 2001;16(2):216–222. [DOI] [PubMed] [Google Scholar]
- 6.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10): 1451–1454. [DOI] [PubMed] [Google Scholar]
- 7.Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23(6):459–472. [DOI] [PubMed] [Google Scholar]
- 8.Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8–16. [DOI] [PubMed] [Google Scholar]
- 9.Culley DJ, Flaherty D, Fahey MC, et al. Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology. 2017;127(5):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heng M, Eagen CE, Javedan H, Kodela J, Weaver MJ, Harris MB. Abnormal Mini-Cog Is Associated with Higher Risk of Complications and Delirium in Geriatric Patients with Fracture. J Bone Joint Surg Am. 2016;98(9):742–750. [DOI] [PubMed] [Google Scholar]
- 11.Ketterer MW, Chawa M, Paone G. Prospective correlates of early (30 day) readmissions on a Cardiothoracic Surgery Service. Psychol Health Med. 2017;22(8):947–954. [DOI] [PubMed] [Google Scholar]
- 12.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilmer SN, Perera V, Mitchell S, et al. The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28(4):182–188. doi: 10.1111/j.1741-6612.2009.00367.x [DOI] [PubMed] [Google Scholar]
- 14.Healthcare Cost and Utilization Project. HCUP Elixhauser Comorbidity Software. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.hcupus.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed August 7, 2018. [Google Scholar]
- 15.CDC - National Center for Health Statistics. National Death Index. National Death Index. https://www.cdc.gov/nchs/ndi/index.htm. Published 2017. Accessed July 2, 2019.
- 16.Ramdass SK, Brennan MJ, Starr R, et al. The Association of Frailty with Discharge Disposition for Hospitalized Community Dwelling Elderly Patients. J Hosp Med. 2018;13(3):182–184. doi: 10.12788/jhm.2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye S. The Short Confusion Assessment Method (Short CAM): Training Manual and Coding Guide. 2014. http://www.hospitalelderlifeprogram.org/uploads/disclaimers/Short_CAM_Training_Manual_8-29-14.pdf.
- 18.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 19.Benedetti MG, Ginex V, Mariani E, et al. Cognitive impairment is a negative short-term and long-term prognostic factor in elderly patients with hip fracture. Eur J Phys Rehabil Med. 2015;51(6):815–823. [PubMed] [Google Scholar]
- 20.Lin P-J, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimers Dement. 2013;9(1):30–38. doi: 10.1016/j.jalz.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Sakata N, Okumura Y, Fushimi K, Nakanishi M, Ogawa A. Dementia and Risk of 30-Day Readmission in Older Adults After Discharge from Acute Care Hospitals. J Am Geriatr Soc. 2018;66(5):871–878. [DOI] [PubMed] [Google Scholar]
- 22.Pickens S, Naik AD, Catic A, Kunik ME. Dementia and Hospital Readmission Rates: A Systematic Review. Dement Geriatr Cogn Dis Extra. 2017;7(3):346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor MD, Stephens C, Bowles KH, Bixby MB. Cognitively impaired older adults: from hospital to home. Am J Nurs 2005;105(2):52–61; quiz 61-62. [DOI] [PubMed] [Google Scholar]
- 24.Reynish EL, Hapca SM, De Souza N, Cvoro V, Donnan PT, Guthrie B. Epidemiology and outcomes of people with dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 10,014 admissions. BMC Med. 2017;15(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]


