Objective:
The main objectives of this article were to assess the effect of preoperative transdermal fentanyl patch (TFP) on interleukin (IL)-6 and IL-8 levels and pain after laparoscopic cholecystectomy.
Materials and Methods:
Patients received a TFP (25 μg/h) (patch group, n=30) or a placebo patch (control group, n=30) applied 14 hours before operation. After surgery, control group received intravenous continuous fentanyl (25 μg/h) with loading dose (25 μg). IL-6 and IL-8 levels were measured at admission and 1, 6, 12, 24, and 48 hours postoperatively. Pain score and consumption of rescue analgesic were evaluated too.
Results:
At 24 hours postoperatively, IL-6 and IL-8 reached a peak and then decreased. The peak IL-6 levels were 21.92(±6.22) and 24.91(±6.81) pg/mL in the patch and control group. The significant differences of IL-6 between groups were shown at 6 and 12 hours postoperatively (P=0.032, 0.0001). There were no significant differences in IL-8 levels and pain score.
Conclusions:
Preoperative TFP attenuated the increase in IL-6 levels after surgery and provided similar analgesia to continuous fentanyl infusion. Preemptive TFP may have influence on proinflammatory reactions and pain control after surgery.
Key Words: interleukin-6, interleukin-8, preemptive, surgery, transdermal fentanyl patch
Anesthesiologists and surgeons are responsible for providing adequate perioperative pain management. Although multimodal analgesia and preventive analgesia have recently been emphasized,1 opioids are still the primary pharmacotherapy for intraoperative and postoperative analgesia, despite their significant adverse effects.2,3 Multimodal analgesia and ideal regimens are undefined and there is a need for the reevaluation of guidelines for perioperative pain management.2,4
There is still controversy on the value and efficacy of preemptive analgesia, despite many clinical studies on this topic; however, it is essential to consider inflammatory reactions.5,6 It has also been reported that there is a relationship between proinflammatory cytokine production and postoperative pain. Preoperative preventable pain management can provide adequate pain control and can have a positive effect on inflammatory reactions after operations. The transdermal fentanyl patch (TFP) has been used for chronic pain in cancer patients.7 Recently, the efficacy of TFP for acute postoperative pain management has been reported.8–11
In this study, we evaluated the effects of preoperative TFP on interleukin (IL)-6 and IL-8 production after operation. The secondary aim was to assess the postoperative pain score and consumption of rescue analgesic.
MATERIALS AND METHODS
Subjects
The criteria for inclusion in this study were patients between 20 and 60 years of age, who were scheduled for elective single-port laparoscopic cholecystectomy under general anesthesia by one surgeon, with an American Society of Anesthesiologists physical status of 1 to 2. Patients who had a preoperative pain and a history of allergy to opioids, history of drug abuse, were on immune-suppressive medications, or had a body mass index <20 or >30 were excluded. This prospective study was reviewed and approved by the Institutional Review Board of the Chuncheon Sacred Heart Hospital, School of Medicine, Hallym University (Gangwon, Korea), and written informed consent was obtained from all participants.
Study Design
Upon admission, patient randomization was performed using online randomization software. Patients in the patch group received a single TFP (Duragesic, 25 μg/h matrix fentanyl patch; Janssen Pharmaceutica, Beerse, Belgium) affixed to the anterior chest wall 14 hours before surgery. The control group received a placebo patch affixed in the same manner. General anesthesia was induced with propofol of 2 mg/kg and rocuronium of 0.8 mg/kg. After tracheal intubation, anesthesia was maintained with sevoflurane-O2 (2 L/min) and N2O (2 L/min). After the operation, patients were transferred to the postanesthetic care unit. Then intravenous fentanyl infusion (25 μg/h) was started with a loading dose of 25 μg in the control group and only normal saline in the patch group, administered via a disposable balloon pump for 48 hours. Drug preparation was performed by a nurse who was blinded to this study. The TFP and disposable balloon pump were removed 48 hours after the operation.
IL-6 and IL-8 Analyses
Blood samples for IL-6 and IL-8 levels were taken from the peripheral vein at admission (base) and 1, 6, 12, 24, and 48 hours postoperatively. Samples were centrifuged at 3000 rpm for 10 minutes and plasma was frozen within 30 minutes at −70°C before use in the assays. Plasma IL-6 and IL-8 levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Human IL-6 Quantikine ELISA Kit and Human CXCL8/IL-8 Quantikine ELISA Kit; R&D Systems, Minneapolis, MN).
Postoperative Pain and Adverse Effects Analyses
Pain severity was assessed according to an 11-point Numeric Rating Scale (NRS), which ranged from 0 (no pain) to 10 (most severe pain imaginable). Patients were educated on the pain scores before surgery. When a patient complained of pain at any time or when the NRS score was checked at a specified time (1, 6, 24, and 48 h postoperatively), the patient was given 25 mg pethidine. Incidences of nausea, vomiting, dizziness, itching, and respiratory depression (oxygen saturation <90%) were also evaluated.
Statistical Analyses
The sample size was calculated using a power analyses (α=0.05; power=0.8) based on our preliminary study results of IL-6 levels measured 12 hours postoperatively (control group mean=22.144, patch group=17.654, SD=5.75, effect size=0.7808696). Twenty-seven patients were required in each group; 60 patients were recruited to take into account an estimated dropout rate of 10%. SPSS 24.0 (SPSS Inc., Chicago, IL) was used in the statistical analyses of the collected data. Student’s t test was performed for continuous variables with a nearly normal distribution. IL-6 and IL-8 concentrations at each time point were compared between the 2 groups using the t test or Mann-Whitney U test. Categorical variables, including the incidence of adverse effects and use of rescue analgesics, were analyzed using the χ2 test and the Fisher exact test, as appropriate. P-values <0.05 were considered statistically significant.
RESULTS
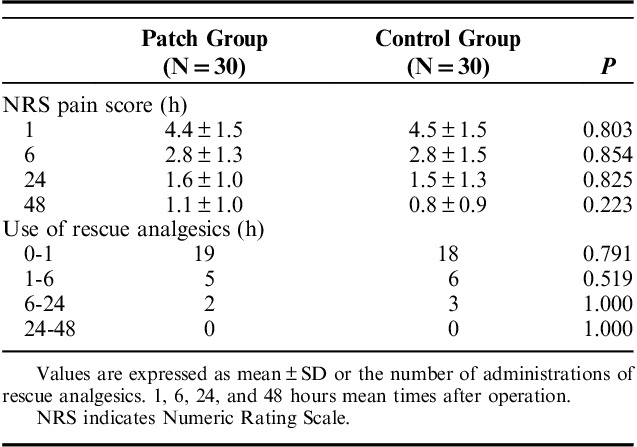
A total of 68 patients undergoing one-port laparoscopic cholecystectomy under general anesthesia were assessed for eligibility. Thereafter, 65 patients were allocated to the patch and control groups. One patient in the patch group was excluded before surgery; the patient suffered from nausea and declined further participation. One patient in each group was excluded after surgery for the same reason. One patient in the patch group was excluded due to change to open cholecystectomy. One patient in the control group was excluded due to a broken blood sample. Therefore, 60 patients were included in the final analyses (Fig. 1). Table 1 shows the demographic data of the patients. IL-6 levels progressively increased after surgery. At 24 hours postoperatively, IL-6 levels reached a peak of 21.92 (±6.22) and 24.91 (±6.81) pg/mL in the patch group and control group, respectively, and declined thereafter. The overall levels were lower for the patch group. Significant differences between the 2 groups were shown at 6 and 12 hours postoperatively (P=0.032, 0.0001; Fig. 2). IL-8 levels also increased after surgery and peaked at 24 hours postoperatively. The levels were 210.86 (±2.34) and 11.97 (±4.50) pg/mL in the patch group and control group, respectively. The overall levels were also low in the patch group, but did not reach statistical significance at any time point (Fig. 3). The postoperative pain score and use of rescue analgesics were similar between the 2 groups (Table 2). Respiratory depression, itching, vomiting, and dizziness did not occur during the whole study period. Nausea occurred in one patient in the patch group before surgery and one patient in each group after surgery.
FIGURE 1.
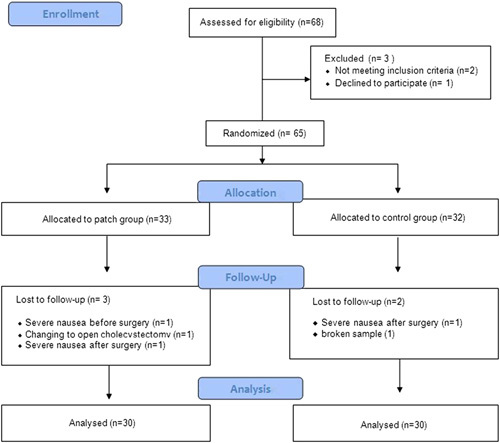
Flow diagram of study.
TABLE 1.
Patient Demographics and Clinical Characteristics
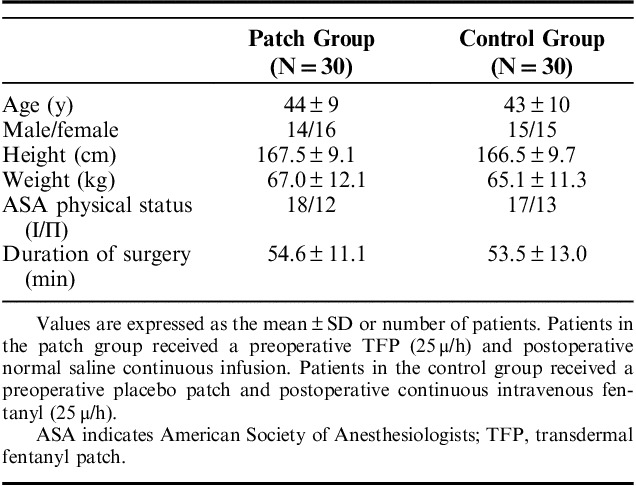
FIGURE 2.
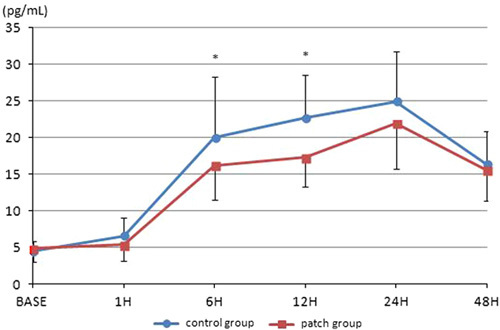
The levels of IL-6. IL-6 increased after surgery and peaked at 24 hours postoperatively. IL indicates interleukin. *P=0.0001.
FIGURE 3.
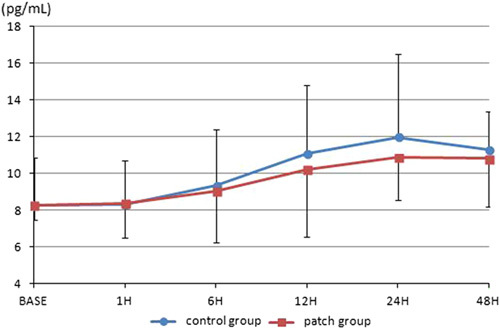
The levels of interleukin-8. There is no significant difference between 2 groups at each time point.
TABLE 2.
Postoperative Pain Score and the Use of Rescue Analgesics
DISCUSSION
Optimal perioperative pain management is an important factor for a good outcome after surgery. Despite their significant adverse effects, opioids are typically the primary pharmacotherapy for postoperative analgesia, particularly for moderate to severe pain.3
TFP is considered inappropriate for acute pain management due to its slow onset time. To achieve a timely effect, TFP should be applied 15 hours before the end of surgery.12 The efficacy of TFP on postoperative pain control has been reported. TFP can attenuate the intensity of postoperative pain and reduce the use of rescue analgesics safely by affixing the TFP at the optimal time before surgery, by considering onset time and optimizing the dose.8–11 In this study, we applied the TFP 14 hours before surgery, considering the duration of the surgery, and used the same dose regardless of patient body weight. The dose of intravenous continuous fentanyl in control group was also fixed regardless of body weight. The analgesia dose may be considered inadequate in some patients. There were no significant differences between groups with regard to the consumption of rescue analgesic and pain score. There was no clinical inflammation in both groups. This can explain the small difference of IL-6 levels between the 2 groups.
Many studies have shown the relationship between pain and proinflammatory cytokine levels. IL-6 and IL-8 have proinflammatory actions in various conditions. Plasma IL-6 is an important mediator in host defense following trauma and is an early marker of tissue damage after surgery. Therefore, IL-6 may serve as a useful indicator of surgical stress during the perioperative period.13–17 IL-6 levels vary according to the surgical procedure performed16 and appear to be proportional to the extent of tissue injury during surgery, more than to the duration of the surgery itself.14 Sakamoto et al proposed that the factors influencing the level of IL-6 after surgery were operation time, volume of blood loss, and thoracotomy.16 In this study, IL-6 and IL-8 peaked at 24 hours after laparoscopic cholecystectomy. This result was similar to the result found after laparoscopic hysterectomy.18 A low postoperative IL-6 level is a reliable indicator of an abating inflammatory process.19 IL-8 may be a humoral link between tissue injury of various origins and the sensitization of pain receptors in the development of sympathetic hyperalgesia, and has been proposed as an additional biomarker for the risk of multiple organ failure.20,21
There have been reports that preemptive analgesia reduces postoperative pain and attenuates production of IL-6.6,18,22,23 Preoperative management for reducing the production of proinflammatory cytokines, which augment pain sensitivity, can improve the quality of postoperative pain relief, and preemptive analgesic management is also associated with reduced postoperative pain and attenuates the production of proinflammatory cytokines.6,13 The preemptive analgesic effects of TFP are worthy of attention. In this study, preemptive TFP reduced the IL-6 levels throughout the postoperative period, and IL-6 levels significantly differed compared with the control group, at 6 and 12 hours postoperatively. IL-8 levels in the TFP group also showed low levels throughout the postoperative period, although there were no statistically significant differences between the 2 groups. Both IL-6 and IL-8 have also been implicated in pain levels, and patients with high pain scores exhibited significantly increased levels of IL-6 and IL-8.24–27 However, in this study, which compared TFP with constant intravenous fentanyl infusion rather than placebo normal saline, the pain score was similar between the 2 groups.
Although inflammatory cytokines are indispensable for wound healing and the restoration of homeostasis, exaggerated production of proinflammatory cytokines at injury sites can induce systemically hemodynamic instability or metabolic derangements.13–15 Therefore, the effects of preemptive TFP on proinflammatory cytokines are worth evaluating in a variety of ways.
TFP has several advantages compared with patient-controlled analgesia. TFP costs less than patient-controlled analgesia, and there is no need for intravenous access, so the risk of infection is decreased, and the patient’s comfort is improved. TFP can also prevent programming errors.11 However, the disadvantages of TFP are delayed onset, large interindividual variability in pharmacokinetics, and the inability to adjust the dose during the period of application.28 Respiratory depression is the most serious adverse effect of TFP that must be observed and controlled.
If the appropriate dose of TFP can be used according to individual patient needs, and attention is given to the management of side effects, TFP is a potentially good option among multimodal analgesics, and is considered efficient as a background analgesia, although it may not be adequate alone.9,29
In conclusion, preoperative TFP attenuated the increase in IL-6 levels after surgery and provided similar analgesic effects to continuous intravenous fentanyl infusion. Therefore, preemptive TFP may have influence on proinflammatory reactions during the perioperative period, and may be effective background analgesia.
Footnotes
S.M.H. and J.J.L. contributed equally.
There is no funding to report. The authors declare no conflicts of interest.
REFERENCES
- 1.Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol. 2014;28:191–201. [DOI] [PubMed] [Google Scholar]
- 2.Joshi GP, Kehlet H. Guidelines for perioperative pain management: need for reevaluation. Br J Anaesth. 2017;119:720–722. [DOI] [PubMed] [Google Scholar]
- 3.Kharasch ED, Brunt LM. Perioperative opioids and public health. Anesthesiology. 2017;124:960–965. [DOI] [PubMed] [Google Scholar]
- 4.Kharasch ED, Eisenach JC. Wherefore gabapentinoids? Was there rush too soon to judgment? Anesthesiology. 2016;124:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissin I. Preemptive analgesia. Anesthesiology. 2000;93:1138–1143. [DOI] [PubMed] [Google Scholar]
- 6.Beilin B, Bessler H, Mayburd E, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003;98:151–155. [DOI] [PubMed] [Google Scholar]
- 7.Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathitkarnmanee T, Tribuddharat S, Noiphitak K, et al. Transdermal fentanyl patch for postoperative analgesia in total knee arthroplasty: a randomized double-blind controlled trial. J Pain Res. 2014;7:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merivirta R, Pitkänen M, Alanen J, et al. Postoperative pain management with transdermal fentanyl after forefoot surgery: a randomized, placebo-controlled study. J Pain Res. 2015;8:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merivirta R, Äärimaa V, Aantaa R, et al. Postoperative fentanyl patch versus subacromial bupivacaine infusion in arthroscopic shoulder surgery. Arthroscopy. 2013;29:1129–1134. [DOI] [PubMed] [Google Scholar]
- 11.Minville V, Lubrano V, Bounes V, et al. Postoperative analgesia after total hip arthroplasty: patient-controlled analgesia versus transdermal fentanyl patch. J Clin Anesth. 2008;20:280–283. [DOI] [PubMed] [Google Scholar]
- 12.Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000;38:59–89. [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Chao PC, Borel CO, et al. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth Analg. 2004;99:1465–1471. [DOI] [PubMed] [Google Scholar]
- 14.Lin E, Calvano SE, Lowry SF. Inflammaatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Suzuki H, Murakami M, et al. Elevated plasma levels of interleukin-6, interleukin-8, and granuocyte colony-stimulating factor during and after major abdominal surgery. J Clin Anesth. 1997;9:293–298. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6:181–186. [DOI] [PubMed] [Google Scholar]
- 17.Cruickshank AM, Fraser WD, Burns HJG, et al. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci. 1990;79:161–165. [DOI] [PubMed] [Google Scholar]
- 18.Hong JY, Lim KT. Effect of preemptive epidural analgesia on cytokine response and postoperative pain in laparoscopic radical hysterectomy for cervical cancer. Reg Anesth Pain Med. 2008;33:44–51. [DOI] [PubMed] [Google Scholar]
- 19.Ni Choileain N, Redmond HP. The immunological consequences of injury. Surgeon. 2006;4:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Cunha FQ, Lorenzetti BB, Poole S, et al. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991;104:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partrick DA, Moore FA, Moore EE, et al. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg. 1996;172:425–429. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Ju H, Yang B, et al. Effects of a selective cyclooxygenase-2 inhibitor on postoperative inflammatory reaction and pain after total knee replacement. J Pain. 2008;9:45–52. [DOI] [PubMed] [Google Scholar]
- 23.Buvanendram A, Kroin JS, Berger RA, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mazidi S, Farhat K, Nedjadi T, et al. Association of interleukin-6 and other cytokines with self-reported pain in prostate cancer patients receiving chemotherapy. Pain Med. 2018;19:1058–1066. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira CMB, Sakata RK, Issy AM, et al. Cytokines and pain. Rev Bras Anestesiol. 2011;61:255–265. [DOI] [PubMed] [Google Scholar]
- 26.Edwards RR, Kronfli T, Haythornthwaite JA, et al. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada A, Sekido N, Akahoshi T, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 28.Gourlay GK, Kowalski SR, Plummer JL, et al. The efficacy of transdermal fentanyl in the treatment of postoperative pain: a double-blind comparison of fentanyl and placebo systems. Pain. 1990;40:21–28. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann LJ, DeSio JM, Radvany T, et al. Transdermal fentanyl in postoperative pain. Reg Anesth. 1997;22:24–28. [DOI] [PubMed] [Google Scholar]


