The incidence and prognostic significance of intraoperative capsule rupture in apparent stage IA-B ovarian cancer varies across histologies, and postoperative chemotherapy was not associated with improved survival.
OBJECTIVE:
To examine the incidence and prognostic effects of intraoperative capsule rupture and to assess the effectiveness of postoperative chemotherapy for intraoperative tumor rupture in apparent stage I epithelial ovarian cancer.
METHODS:
This is a society-based retrospective observational study in Japan that examined 15,163 women with stage IA-IC1 epithelial ovarian cancer who underwent primary surgical treatment between 2002 and 2015. Associations between intraoperative capsule rupture and cause-specific survival, and between postoperative chemotherapy and cause-specific survival among intraoperatively ruptured cases were examined by histology type (clear cell n=6,107, endometrioid n=3,910, mucinous n=3,382, and serous n=1,764).
RESULTS:
Clear cell histology had the highest risk of intraoperative capsule rupture (57.3%), followed by endometrioid (48.8%), serous (41.8%), and mucinous (32.0%) histologies (P<.001). On multivariable analysis, clear cell type exhibited the largest effect of intraoperative capsule rupture on cause-specific survival (adjusted hazard ratio [HR] 1.99, 95% CI 1.45–2.75), followed by serous (adjusted HR, 1.61, 95% CI 0.84–3.11), mucinous (adjusted HR 1.28, 95% CI 0.79–2.09), and endometrioid (adjusted HR, 1.14, 95% CI 0.64–2.01) tumors. Postoperative chemotherapy for intraoperatively ruptured cases did not improve cause-specific survival in any histologic types in multivariable analysis: clear cell, adjusted HR 0.86, 95% CI 0.56–1.31; serous, adjusted HR 1.08, 95% CI 0.42–2.74; mucinous, adjusted HR 1.11, 95% CI 0.55–2.27; and endometrioid, adjusted HR 2.81, 95% CI 0.85–9.30 (all, P>.05). In the cohort-level analysis of ruptured cases (n=7,227), postoperative chemotherapy use has significantly decreased in mucinous (16.3% relative decrease), endometrioid (13.1% relative decrease), and clear cell (9.3% relative decrease) (all, P<.05); but, the cohort-level 5-year cause-specific survival rate did not change over time (all, P>.05).
CONCLUSION:
Among apparent stage I epithelial ovarian cancer, the clear cell type possesses a disproportionally high risk of capsule rupture during adnexectomy and is associated with the most adverse effect on survival. A decrease in the use of postoperative chemotherapy for intraoperatively ruptured cases in Japan is likely the result of increasing awareness of the absence of survival benefits.
Worldwide, ovarian cancer is the most deadly gynecologic malignancy in developed countries, with 65,900 deaths estimated in 2012.1 Women with a suspicious ovarian mass typically undergo surgery to confirm the histologic diagnosis and allow for comprehensive staging when a malignancy is identified.2 When adnexectomy is performed for a mass that is apparently confined to the ovary, disruption of the capsule during the process of surgical removal occurs in 20%–48% of cases.3–15 Intraoperative capsule rupture has been recently recognized as an independent category in the International Federation of Gynecology and Obstetrics (FIGO) 2014 ovarian cancer staging system.16
Although intraoperative capsule rupture upstages a woman from stage IA or IB to IC1, the prognostic significance remains controversial. Some studies have shown a survival disadvantage owing to intraoperative capsule rupture, whereas others have not.3–15 Moreover, the role of postoperative chemotherapy for intraoperatively ruptured cases has not been well-studied owing to the relatively infrequent occurrence.3–15 Multiple guidelines and working groups recommend postoperative chemotherapy for high-risk early-stage ovarian cancer with stage IC disease, but their supporting evidence for this recommendation is not solely for intraoperative capsule rupture, and, to date, there is no IC1-specific recommendation for postoperative management.17–19 Because stage IC ovarian cancer also encompasses other substages (IC2 and IC3), each with their own distinct relapse and survival patterns, treatment recommendations specific to stage IC1 disease are needed.4,9,11,15,17,20
The primary objective of this study was to examine the incidences and predictors of intraoperative capsule rupture for apparent stage I epithelial ovarian cancer, stratified by histology subtypes. The secondary objective was to examine the association of intraoperative capsule rupture and survival, and to examine the effectiveness of postoperative chemotherapy for ruptured cases.
METHODS
This is a society-based retrospective observational study that used the Gynecologic Tumor Registry database of the Japan Society of Obstetrics and Gynecology. This benchmark nationwide project was conducted within the scope of the Japan Society of Gynecologic Oncology, and the dataset was provided by the Gynecologic Tumor Committee of Japan Society of Obstetrics and Gynecology, functioned as a Japan Society of Gynecologic Oncology–Japan Society of Obstetrics and Gynecology joint study.
The Japan Society of Obstetrics and Gynecology’s database is an organ-based cancer registry for gynecologic malignancy that records comprehensive information for cancer types, tumor characteristics, treatment types, and survival outcomes.21,22 The registry is maintained by the Gynecologic Tumor Committee of the Japan Society of Obstetrics and Gynecology and comprises 388 local and leading regional hospitals, which cover approximately 50% of all new patients with gynecologic malignancy in Japan. Institutional review board approval was obtained at the Japan Society of Obstetrics and Gynecology’s Clinical Research Committee (2018-36-67) and the hosting institution, the Tokai University School of Medicine (17R-100). Each participating institution reviewed the protocol and obtained their own approval, as appropriate. Data entry, accuracy and consistency checks were performed and maintained by clinicians in each participating site.
Women eligible for the analysis were those with stage IA-IC1 epithelial ovarian cancer who underwent adnexectomy-based primary surgical treatment from 2002 to 2015 with the four major histology types (serous, mucinous, endometrioid, and clear cell). Specifically, cases that met criteria for the 2014 FIGO staging with T1a-1c1, N0-x, M0-x were examined. Exclusion criteria included other subgroups of stage I disease (IC2 and IC3), stage II–IV, unknown stage, or ovarian tumors other than the four histology types (serous, mucinous, endometrioid, and clear cell), nonsurgical management, and neoadjuvant therapy before the surgical treatment.
Patient demographics, tumor characteristics, and treatment type were abstracted from the database. Patient demographics included age (younger than 40, 40–49, 50–59, 60–69, and 70 or older), year (2002–2005, 2006–2010, and 2011–2015), and registry area (East, Central, West, and North). Tumor characteristics included histology type and cancer stage, as above. Treatment types included hysterectomy use (yes vs no), lymphadenectomy use (yes vs no), and postoperative chemotherapy use (yes vs no). Survival outcomes included follow-up time, vital status, and cause of death.
Clinical demographics were classified and grouped per our prior study.22 Cause-specific survival was defined as the time interval between diagnosis and death from ovarian cancer. Cases without a survival event or cases that were lost to follow-up were censored at the last visit with known vital status.
Per the 2014 FIGO staging system, stage IC1 disease is defined as ovarian tumor confined in the ovary with the intraoperative capsule rupture due to surgical spill; stage IC2 disease is defined as spontaneous capsule rupture before surgery or ovarian surface tumor involvement; and stage IC3 disease is defined as presence of malignant cells in ascites or peritoneal washings.16
The intraoperative capsule rupture rate was determined as the fractional proportion of stage IC1 cases among stage IA-IC1 cases. The Japan Society of Gynecologic Oncology staging system had been used for the cases before implementation of the 2014 FIGO staging system, and stage 1C(b) in the Japan Society of Gynecologic Oncology staging system corresponds to intraoperative capsule rupture for stage IC1 in the 2014 FIGO staging system. In brief, stage IC(b) from 2001 to 2013 and stage IC1 from 2014 to 2015 represented the intraoperative capsule rupture cases in our study. Because this study examined the effects of upstaging due to intraoperative capsule rupture in ovarian cancer otherwise confined to the ovarian tissue, cases with stage IC2-3 diseases were excluded, as described above.
Normality of continuous variables was assessed with the Kolmogorov-Smirnov test, expressed with mean and standard deviation or with median and interquartile range, as appropriate. For univariable analysis, statistical differences were assessed by one-way analysis of variance test or Kruskal-Wallis H test as appropriate. For categorical or ordinal variables, statistical differences were assessed with the χ2 test.
A binary logistic regression model was fitted to identify the independent clinical–pathologic factors associated with intraoperative capsule rupture for multivariable analysis. Age, year, registry area, hysterectomy, lymphadenectomy, and histology type were entered in the final model, and the magnitude of statistical significance was expressed with odds ratio (OR) and 95% CI. The Hosmer-Lemeshow test was used to assess the goodness-of-fit, and a P>.05 was interpreted as a good model.
A recursive partitioning analysis was fitted to construct a regression-tree model for clinical–pathologic demographic patterns of intraoperative capsule rupture.23 All preoperative (age, year, and area), intraoperative (hysterectomy and lymphadenectomy), and tumor (histology type) factors were entered in the final model, and the χ2 automatic interaction detector method was used to determine the nodes with stopping rule at the levels of three. Among the determined nodes in this analysis, the incidence of intraoperative capsule rupture was calculated. For trend analyses, linear segmented regression with log-transformation was fitted to assess temporal trends of postoperative chemotherapy use and the 5-year cause-specific survival rates over time using the Joinpoint Regression Program.24
The Kaplan-Meier method was used to construct the survival curves, and differences between the curves were assessed with the log-rank test. The association of intraoperative capsule rupture and cause-specific survival was assessed for each histology type, with adjustments for age, year, registry area, hysterectomy, lymphadenectomy, and postoperative chemotherapy on multivariable models. The Cox proportional hazard regression model was used for the analysis, and the magnitude of statistical significance was expressed with hazard ratio (HR) and 95% CI. At the time of analysis, survival data were available for cases between 2002 and 2011.
Finally, propensity score matching was used to adjust for background differences between the two groups.25 The propensity score for ruptured status (yes vs no) was computed by fitting a binary logistic regression model. All preoperative and intraoperative factors were entered in the propensity score model. An automated algorithm was used for 1-to-1 propensity score matching, and the optimal caliper width for estimating differences was equal to 0.2 of the standard deviation of the logit of the propensity score.26 In the matched model, the standardized difference was assessed to evaluate effect size between the two groups. A standardized difference value of 0.10 or less was considered to indicate good balance between the two groups (ruptured vs nonruptured).
For a sensitivity analysis, the efficacy of postoperative chemotherapy on survival was assessed in intraoperative capsule ruptured cases. This is based on the rationale that women with stage IC disease are recommended to receive postoperative chemotherapy per current guidelines,17–19 whereas the actual benefit of this strategy remains understudied among cases with intraoperative capsule rupture (stage IC1-specific). Similarly, outcomes were evaluated for those who had lymphadenectomy, as microscopic metastasis can be seen in 5%–20% of apparent stage I epithelial ovarian cancer cases, and the omission of a staging procedure is associated with decreased survival.27,28 Finally, a doubly robust estimator calculation was used in the matched model to account for unspecified unbalanced variables for outcomes.
All statistical analyses were based on two-sided hypothesis, and a P<.05 was considered statistically significant. Statistical Package for Social Sciences (version 24.0, Armonk, NY) was used for all the analyses. The STROBE guidelines were consulted to display the results of observational cohort study.29
RESULTS
Among 74,291 cases of ovarian malignancy in the database, there were 48,640 cases of epithelial ovarian cancer of the four major histology subtypes. Of those, there were 15,163 cases with stage IA-IC1 disease that had primary surgical treatment and met inclusion criteria for this study. The most common histology type was clear cell (n=6,107, 40.3%), followed by endometrioid (n=3,910, 25.8%), mucinous (n=3,382, 22.3%), and serous (n=1,764, 11.6%).
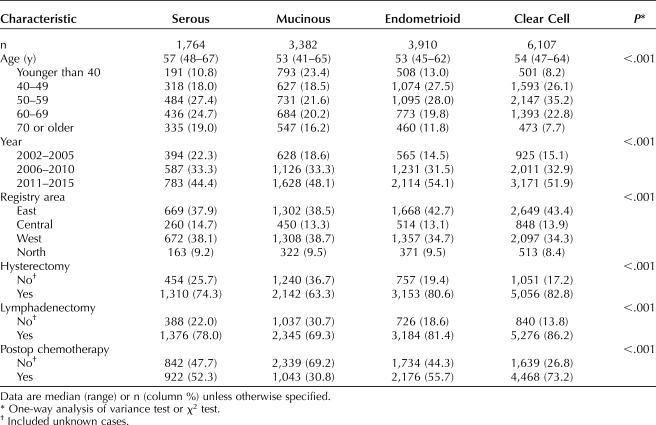
Patient demographics per the histology types are shown in Table 1. Women with serous histology were more likely to be older, compared with the other histologies (median 57 vs 53–54, P<.001). Women with endometrioid and clear cell histologies were more likely to have a recent year of diagnosis, have been diagnosed in the East and Central regions of Japan, and have undergone both a hysterectomy and lymphadenectomy at the time of surgery, compared with other histologies (all, P<.001). Women with clear cell histology had the highest rate of having received postoperative chemotherapy, compared with the other histologies (73.2% vs 30.8–55.7%, P<.001).
Table 1.
Patient Demographics by Histology Type
In the entire cohort, there were 7,227 (47.7%, 95% CI 46.9–48.5) cases of intraoperative capsule rupture, with clear cell histology (57.3%) exhibiting the highest rate, followed by endometrioid (48.8%), serous (41.8%), and mucinous (32.0%) histologies (absolute difference, 25.3%; P<.001; Fig. 1). There was no change in the number of intraoperative capsule rupture cases during the study period in any of the histology types (all, P>.05).
Fig. 1. Incidence of intraoperative capsule rupture (A) and trend of postoperative chemotherapy use for ruptured cases (B) are shown. Postoperative chemotherapy use was assessed in 6-month increments: serous annual percentage change: −0.7, 95% CI –1.6 to 0.2, P=.131; mucinous annual percentage change: −1.3, 95% CI –2.4 to –0.2, P=.024; endometrioid annual percentage change: –1.0, 95% CI –1.7 to –0.4, P=.005; and clear cell annual percentage change: –0.7, 95% CI –1.2 to –0.3, P=.003. Bars represent 95% CI. Dots represent observed value. Bold lines represented model values.
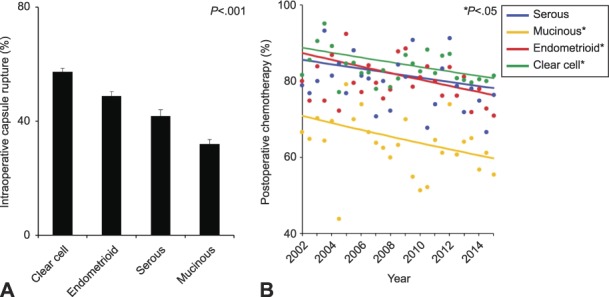
Matsuo. Intraoperative Capsule Rupture in Ovarian Cancer. Obstet Gynecol 2019.
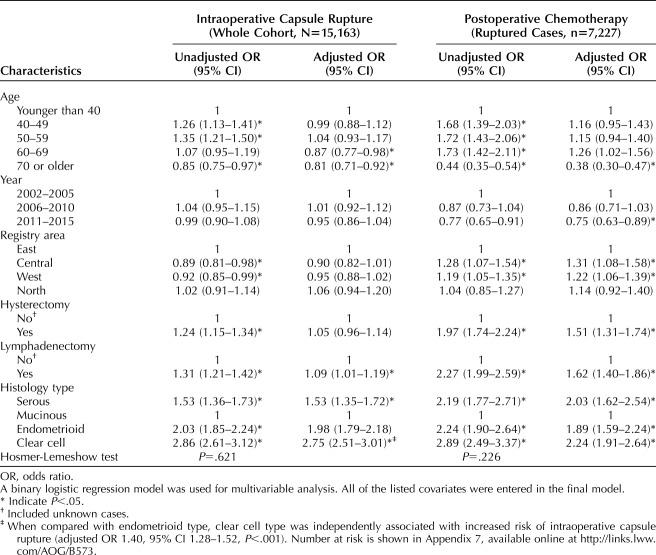
On multivariable analysis (Table 2), a younger age at diagnosis, lymphadenectomy use, and histology type remained independent predictors for intraoperative capsule rupture (both, P<.001). Specifically, clear cell histology was associated with nearly three-fold increased odds of intraoperative capsule rupture compared with mucinous histology (adjusted OR 2.75, 95% CI 2.51–3.01, P<.001). Even compared with endometrioid histology, the histology with the second highest rate of intraoperative capsule rupture, clear cell histology was associated with approximately 40% increased odds of intraoperative capsule rupture (adjusted OR 1.40, 95% CI 1.28–1.52, P<.001).
Table 2.
Independent Factors for Intraoperative Capsule Rupture and Postoperative Chemotherapy Use
When demographic patterns associated with intraoperative capsule rupture were examined (Appendices 1 and 2, available online at http://links.lww.com/AOG/B573), women aged 57 years or younger who had adnexectomy and hysterectomy for ovarian clear cell carcinoma accounted for 20.9% of the study population and had the highest rate of intraoperative capsule rupture (60.9%); women aged older than 47 years with mucinous tumors who had adnexectomy in Central Japan made up 1.8% of the study population and had the lowest rate of intraoperative capsule rupture (24.5%; absolute difference 36.4%, P<.05).
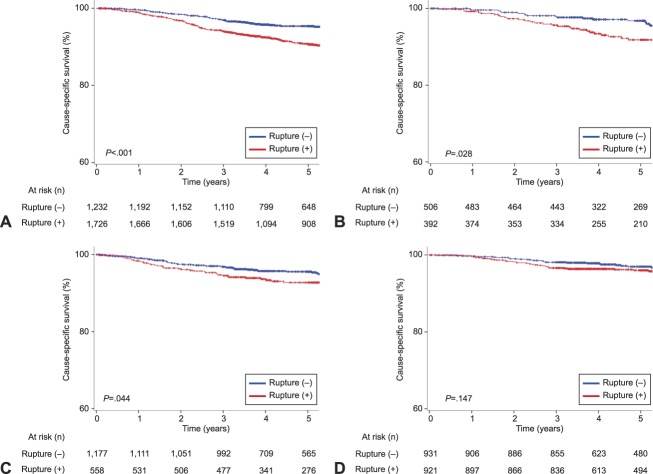
Survival analyses were assessed in 7,484 women diagnosed between 2002 and 2011. The median follow-up time was 5.1 (interquartile range 3.7–5.6) years, and there were 403 deaths from ovarian cancer during follow-up. On univariable analysis without adjustment for other characteristics, intraoperative capsule rupture was significantly associated with decreased cause-specific survival in clear cell (5-year rates, 90.7% vs 95.3%, P<.001; Fig. 2A), serous (91.8% vs 96.8%, P=.028; Fig. 2B), and mucinous (92.7% vs 95.6%, P=.044; Fig. 2C) types, but not for endometrioid type (96.0% vs 97.0%, P=.147; Fig. 2D).
Fig. 2. Cause-specific survival related to intraoperative capsule rupture based on histology types. Cause-specific survival is shown based on intraoperative rupture status for clear cell (A), serous (B), mucinous (C), and endometrioid (D) histology. Log-rank test for P-value. Y-axis is truncated to 60–100%.
Matsuo. Intraoperative Capsule Rupture in Ovarian Cancer. Obstet Gynecol 2019.
After controlling for age, year of diagnosis, registry area, performance of lymphadenectomy and hysterectomy, and postoperative chemotherapy use (Fig. 3A), clear cell type exhibited the largest effect of intraoperative capsule rupture on cause-specific survival (adjusted HR 1.99, 95% CI 1.45–2.75), followed by serous (adjusted HR, 1.61, 95% CI 0.84–3.11), mucinous (adjusted HR 1.28, 95% CI 0.79–2.09), and endometrioid (adjusted HR, 1.14, 95% CI 0.64–2.01) tumors. Similar results were observed in the propensity score matched model (Fig. 3B and Appendix 3, available online at http://links.lww.com/AOG/B573).
Fig. 3. Effect of intraoperative capsule rupture on cause-specific survival per histology type. Nonmatched model (A) and propensity score matched model (B) are shown. Clear cell type demonstrated the largest effect of intraoperative capsule rupture on cause-specific survival followed by serous, mucinous, and endometrioid type. Circles represent adjusted HR, and bars represent 95% CI. *Adjusted for age and year of diagnosis, area, hysterectomy use, performance of lymphadenectomy, and postoperative chemotherapy use.
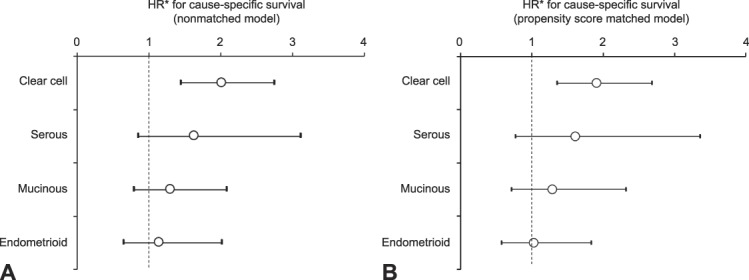
Matsuo. Intraoperative Capsule Rupture in Ovarian Cancer. Obstet Gynecol 2019.
Among those with intraoperative capsule rupture (n=7,227), there were 5,646 (78.1%, 95% CI 77.2–79.1) women who received postoperative chemotherapy. Clear cell (82.7%) exhibited the highest rate of postoperative chemotherapy use followed by endometrioid (78.7%), serous (78.3%) and mucinous (62.3%) histology on multivariable analysis (P<.001; Table 2). In addition, older women were associated with infrequent use of postoperative chemotherapy, whereas management in the Central and West regions as well as hysterectomy and lymphadenectomy use were associated with increased use of postoperative chemotherapy (all, P<.05).
The effectiveness of postoperative chemotherapy on cause-specific survival among intraoperatively ruptured cases was examined by histology type. On multivariable analysis (Fig. 4), none of the four histology types benefited from postoperative chemotherapy (all, P>.05), and women who received postoperative chemotherapy had cause-specific survival similar to those who did not for clear cell (adjusted HR 0.86, 95% CI 0.56–1.31), serous (adjusted HR 1.08, 95% CI 0.42–2.74), mucinous (adjusted HR 1.11, 95% CI 0.55–2.27), and endometrioid (adjusted HR 2.81, 95% CI 0.85–9.30). Similarly, when cases were limited to those who had intraoperative capsule rupture and lymphadenectomy, postoperative chemotherapy did not improve cause-specific survival in any of the histology types (all, P>.05; Appendix 4, available online at http://links.lww.com/AOG/B573). Similar results were observed for unstaged cases (all, P>.05).
Fig. 4. Effectiveness of postoperative chemotherapy for intraoperative capsule rupture cases. Among cases with intraoperative capsule rupture, effect of postoperative chemotherapy on cause-specific survival is shown per histology type. *Adjusted for age and year of diagnosis, area, hysterectomy use, and performance of lymphadenectomy. Circles represent adjusted HR, and bars represent 95% CI.
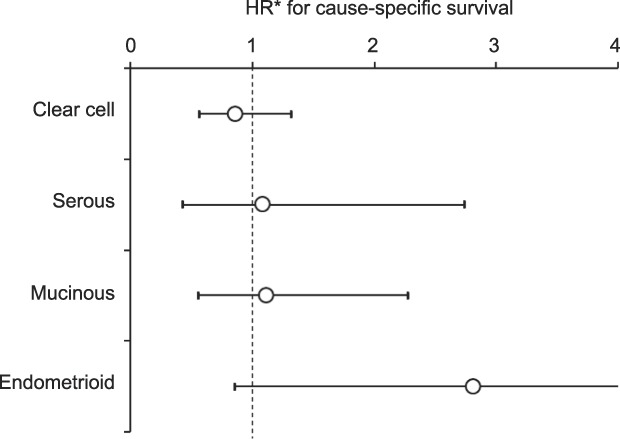
Matsuo. Intraoperative Capsule Rupture in Ovarian Cancer. Obstet Gynecol 2019.
Finally, trends of postoperative chemotherapy use were examined among ruptured cases. During the study period, the cohort-level use of postoperative chemotherapy significantly decreased for mucinous (16.3% relative decrease), endometrioid (13.1% relative decrease), and clear cell (9.3% relative decrease) types between 2002 and 2015 (all, P<.05; Fig. 1B). Postoperative chemotherapy use also decreased in serous type, but this did not reach statistical significance (9.0% relative decrease, P=.131). The cohort-level 5-year cause-specific survival rates were unchanged in the four histology types (all, P>.05; Appendix 5, available online at http://links.lww.com/AOG/B573).
DISCUSSION
Key findings of the study are that both patient and tumor factors contribute to the risk of intraoperative capsule rupture for ovarian cancer. Among those, tumors with clear cell histology had the largest risk for intraoperative capsule rupture. Moreover, our study found that the prognostic effect of intraoperative capsule rupture varies across the histology types, and again, clear cell histology was most associated with ovarian cancer death. Importantly, none of the histologic subtypes benefited from postoperative chemotherapy after intraoperative tumor rupture.
Age and histology type were independently associated with intraoperative capsule rupture. This implies that intraoperative capsule rupture is not a single entity reflecting one factor. A remarkable finding in our study is that young women with clear cell ovarian cancer had the highest risk of intraoperative capsule rupture, exceeding 60%. This may be a result of the association of this histology with endometriosis. Clear cell ovarian cancer has a strong epidemiologic and biological link to endometriosis, which is a disease of young, reproductive-aged women.30 As endometriomas form a pseudo-capsule and surrounding adhesions, there is a high risk of intraoperative capsule rupture during surgical removal.
Our study also showed an association between registry area and intraoperative capsule rupture. This is most likely the result of the difference in histology type across the areas (post hoc analysis, absolute difference 3.3%, P<.001) (Appendix 6, available online at http://links.lww.com/AOG/B573). This association may possibly propose a hypothetical link between ovarian cancer etiology and geographic disparity, and merits further investigations.
Our study showed that the prognostic significance of intraoperative capsule rupture varies by histology type. More specifically, clear cell type displayed the largest effect of intraoperative capsule rupture on decreased ovarian cancer survival followed by serous, mucinous, and endometrioid. This variable survival effect is likely the reason why prior studies have had heterogeneous results reflecting the unique histology characteristics present in different populations.3–15 By examining histology types separately, our study provides more meaningful information regarding the significance of intraoperative capsule rupture. In addition, the majority of prior studies have had limited sample sizes, which risk type II errors. By analyzing more than 15,000 cases, our study likely minimizes this problem.
This study showed that survival of women whose ovarian tumors had ruptured during surgery was similar between those who received postoperative chemotherapy and those who did not. It is possible that the residual in situ tumor burden in such spilled cases is likely smaller compared with higher stage disease, and therefore, the benefit of chemotherapy is also smaller compared with such high tumor burden disease. In fact, even in the ruptured cases, the prognosis is already favorable (5-year cause-specific survival rates: 90.7–96.0%), so it is unlikely that chemotherapy is going to add further survival advantage.
We speculate that growing awareness of this absence of a survival benefit is the most likely reason for the decrease in the use of postoperative chemotherapy for stage IC1 epithelial ovarian cancer in Japan. This nationwide cohort-level analysis showed that the 5-year cause-specific survival rates did not change over time despite the decrease in postoperative chemotherapy use. Although one may suggest that observation can be an alternative option for women with stage IC1 epithelial ovarian cancer, this clinical question needs to be addressed in a prospective study.
Currently, the Japanese Gynecologic Oncology Group is conducting a phase III randomized controlled trial with noninferiority design, examining the effectiveness of postoperative chemotherapy for stage IA-IC1 epithelial ovarian cancer (JGOG-3020).31 The analysis for stage IC1 disease will ultimately address the necessity of postoperative chemotherapy in this disease.
Strengths of the study include the large sample size. Histology-specific analysis provided useful information to clinicians as even the common tumor types display variable differences in characteristics and outcomes. Multiple sensitivity analyses and propensity score matching enriched the robustness of study. There are also several limitations in our study. First, as is inherent to retrospective studies, there will be unmeasured bias which possibly confounded the analysis. For instance, information regarding the mode of surgery (minimally invasive vs laparotomy), surgeon type and experience (gynecologic oncologist vs gynecologist), institution's surgical volume, patient body habitus, tumor size, the presence of peri-adnexal adhesions, and endometriosis, were not available in the database but likely influenced surgical performance.32,33
Similarly, the decision-making process for postoperative chemotherapy was not available. Thus, it is unknown whether a lack of postoperative chemotherapy was due to patient or surgeon's choice. Among those who received postoperative chemotherapy, chemotherapy regimens and the number of administered cycles were not known. As clear cell and mucinous ovarian cancer are known to have a lack of response to taxane and platinum doublet, the standard chemotherapy choice in epithelial ovarian cancer, lack of this information prevents us from analyzing the chemotherapy type-specific benefit in this study.34,35 Information regarding disease recurrence was not available in this study, but this endpoint is an alternative key outcome in any oncology study. As this study was conducted in Japan, generalizability of the study results in other populations is unknown.
Tumor differentiation is not available in this database, and it is not feasible to distinguish high-grade from low-grade serous tumors. Low rates of postoperative chemotherapy use in the serous group may thus be partly due to this misclassification, because women with low-grade serous tumor typically do not receive postoperative chemotherapy.17 Although this database represents the largest tumor registry for ovarian cancer in Japan, it captures only 50% of new cases and it is unknown whether uncaptured cases are similar to the cases in the tumor registry. The database does not have specific information regarding the use of peritoneal cytology testing; however, it is the standard practice in Japan to perform a peritoneal cytology evaluation at the beginning of surgical exploration. Lastly, adverse effects from chemotherapy were not available in this study, and composite endpoint analysis together with survival endpoint was not assessable.
There are multiple clinical utilities of the study. Intraoperatively, if surgeons face impending capsule rupture, care and appropriate arrangement should be made. For example, it would be important to acknowledge that in any clinically suspicious ovarian mass, peritoneal washings should first be obtained for staging purposes. Then, all measures should be used to prevent rupture because, if it is upstaged to IC1, it does have prognostic implications of lower survival. Such consideration may include early decision-making for conversion to a laparotomy if surgeons encounter technical difficulty during the laparoscopic approach when the suspected ovarian tumor is otherwise confined to the ovary.32
Specific to the clear cell type, albeit statistically nonsignificant, the laparoscopic approach had clinically higher incidences of intraoperative capsule rupture compared with laparotomy (42.3% vs 34.7%).33 Further study is warranted to better clarify the association of the laparoscopic approach with intraoperative capsule rupture in ovarian cancer and whether early conversion to achieve intact ovarian removal is indeed beneficial for survival.
Postoperatively, balanced counseling for the pros and cons of systemic chemotherapy is necessary as chemotherapy for stage IC1 epithelial ovarian cancer may not improve survival, and long-term toxicity rates can be high as 60%.36
Footnotes
Supported by the Ensign Endowment for Gynecologic Cancer Research (K.M.), and in part by a Grant-in-aid for scientific research from Ministry of Education, Culture, Sports, Science and Technology (No. 17H04340) to Mikio Mikami.
Financial Disclosure Takayuki Enomoto received honoraria from Chugai and Astra Zeneca. Koji Matsuo received honoraria from Chugai, editorial contribution expense for a textbook from Springer, and investigator meeting attendance expense from VBL Therapeutics. The other authors did not report any potential conflicts of interest.
The authors thank the member institutions of the JSOG for their cooperation in providing data on patients with gynecological tumors. The authors also thank all members of the Committee on Gynecological Oncology of JSOG and Dr. Brendan H. Grubbs, MD for his scientific input.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/B574.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet 2019;393:1240–53. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed FY, Wiltshaw E, A'Hern RP, Nicol B, Shepherd J, Blake P, et al. Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol 1996;14:2968–75. [DOI] [PubMed] [Google Scholar]
- 4.Bakkum-Gamez JN, Richardson DL, Seamon LG, Aletti GD, Powless CA, Keeney GL, et al. Influence of intraoperative capsule rupture on outcomes in stage I epithelial ovarian cancer. Obstet Gynecol 2009;113:11–17. [DOI] [PubMed] [Google Scholar]
- 5.Sainz de la Cuesta R, Goff BA, Fuller AF, Jr, Nikrui N, Eichhorn JH, Rice LW. Prognostic importance of intraoperative rupture of malignant ovarian epithelial neoplasms. Obstet Gynecol 1994;84:1–7. [PubMed] [Google Scholar]
- 6.Sjövall K, Nilsson B, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer 1994;4:333–6. [DOI] [PubMed] [Google Scholar]
- 7.Higashi M, Kajiyama H, Shibata K, Mizuno M, Mizuno K, Hosono S, et al. Survival impact of capsule rupture in stage I clear cell carcinoma of the ovary in comparison with other histological types. Gynecol Oncol 2011;123:474–8. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Ahn JH, Chung HH, Kim JW, Park NH, Song YS, et al. Impact of intraoperative rupture of the ovarian capsule on prognosis in patients with early-stage epithelial ovarian cancer: a meta-analysis. Eur J Surg Oncol 2013;39:279–89. [DOI] [PubMed] [Google Scholar]
- 9.Suh DH, Kim TH, Kim JW, Kim SY, Kim HS, Lee TS, et al. Improvements to the FIGO staging for ovarian cancer: reconsideration of lymphatic spread and intraoperative tumor rupture. J Gynecol Oncol 2013;24:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu D, Sato N, Sato T, Makino K, Kito M, Shirasawa H, et al. Impact of adjuvant chemotherapy for stage I ovarian carcinoma with intraoperative tumor capsule rupture. J Obstet Gynaecol Res 2015;41:432–9. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Yoshino K, Hasegawa K, Murakami R, Ikeda Y, Adachi S, et al. Survival outcome of stage I ovarian clear cell carcinoma with lympho-vascular space invasion. Gynecol Oncol 2015;136:198–204. [DOI] [PubMed] [Google Scholar]
- 12.Tang H, Liu Y, Wang X, Guan L, Chen W, Jiang H, et al. Clear cell carcinoma of the ovary: clinicopathologic features and outcomes in a Chinese cohort. Medicine (Baltimore) 2018;97:e10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiyama H, Suzuki S, Yoshikawa N, Kawai M, Nagasaka T, Kikkawa F. Survival impact of capsule status in stage I ovarian mucinous carcinoma-A multicentric retrospective study. Eur J Obstet Gynecol Reprod Biol 2019;234:131–6. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo K, Huang Y, Zivanovic O, Shimada M, Machida H, Grubbs BH, et al. Effectiveness of postoperative chemotherapy for stage IC mucinous ovarian cancer. Gynecol Oncol 2019 July 4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno M, Kajiyama H, Shibata K, Mizuno K, Yamamuro O, Kawai M, et al. Adjuvant chemotherapy for stage i ovarian clear cell carcinoma: is it necessary for stage IA? Int J Gynecol Cancer 2012;22:1143–9. [DOI] [PubMed] [Google Scholar]
- 16.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1–5. [DOI] [PubMed] [Google Scholar]
- 17.Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN Clinical Practice Guidelines in oncology (NCCN Guidelines). Available at: https://www.nccn.org/. Retrieved May 16, 2019.
- 18.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 2019;30:672–705. [DOI] [PubMed] [Google Scholar]
- 19.Komiyama S, Katabuchi H, Mikami M, Nagase S, Okamoto A, Ito K, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol 2016;21:435–46. [DOI] [PubMed] [Google Scholar]
- 20.Paik ES, Lee YY, Lee EJ, Choi CH, Kim TJ, Lee JW, et al. Survival analysis of revised 2013 FIGO staging classification of epithelial ovarian cancer and comparison with previous FIGO staging classification. Obstet Gynecol Sci 2015;58:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol 2017;28:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO-JSOG joint study. Gynecol Oncol 2019;153:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745–51. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Joinpoint trend analysis software. Available at: https://surveillance.cancer.gov/joinpoint/. Retrieved May 13, 2019. [Google Scholar]
- 25.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol 2012;30:4215–22. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2010;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuo K, Sood AK, Gershenson DM. Management of early-stage ovarian cancer. In: Bristow RE, Karlan BY, Chi DS, editors. Surgery for ovarian cancer. 3rd ed Boca Raton, FL: CRC Press; 2015:67–104. [Google Scholar]
- 28.Matsuo K, Machida H, Mariani A, Mandelbaum RS, Glaser GE, Gostout BS, et al. Adequate pelvic lymphadenectomy and survival of women with early-stage epithelial ovarian cancer. J Gynecol Oncol 2018;29:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol 2019;151:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical trial. Japanese Gynecologic Oncology Group. Available at: https://www.jgog.gr.jp/en/clinical_trial.html. Retrieved May 16, 2019. [Google Scholar]
- 32.Smorgick N, Barel O, Halperin R, Schneider D, Pansky M. Laparoscopic removal of adnexal cysts: is it possible to decrease inadvertent intraoperative rupture rate? Am J Obstet Gynecol 2009;200:237.e1–3. [DOI] [PubMed] [Google Scholar]
- 33.Suh DH, Park JY, Lee JY, Kim BG, Lim MC, Kim JW, et al. The clinical value of surgeons' efforts of preventing intraoperative tumor rupture in stage I clear cell carcinoma of the ovary: a Korean multicenter study. Gynecol Oncol 2015;137:412–17. [DOI] [PubMed] [Google Scholar]
- 34.Gore M, Hackshaw A, Brady WE, Penson RT, Zaino R, McCluggage WG, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol 2019;153:541–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol 2016;27:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelen MJA, Snel BJ, Schaapveld M, Pras E, de Vries EGE, Gietema JA, et al. Long-term morbidity of adjuvant whole abdominal radiotherapy (WART) or chemotherapy for early stage ovarian cancer. Eur J Cancer 2009;45:1193–200. [DOI] [PubMed] [Google Scholar]