Supplemental Digital Content is available in the text.
Background:
Recent technology developed by Tulip Medical Products allows clinicians to mechanically disaggregate fat tissue into small fat particles known as nanofat. The present study aimed to evaluate the cell yield obtained from nanofat generation in comparison to traditional methods involving enzymatic dissociation (stromal vascular fraction).
Methods:
Nanofat preparations were characterized by cell content and viability, based on DNA quantification and image cytometry, respectively. DNA analysis was also used to determine the cell content in unprocessed dry lipoaspirate and native adipose tissue (excised adipose tissue). To evaluate cell yield, the authors compared the number of cells recovered from 1 g of lipoaspirate between stromal vascular fraction and nanofat preparations, and subsequently determined the final cell inoculum obtained following their respective protocols.
Results:
The data showed that nanofat samples presented a cell burden of 7.3 million cells/g, close to 80 percent of unprocessed dry lipoaspirate, and 70 percent of native excised adipose tissue. Moreover, cell viability was not altered by mechanical disaggregation in nanofat samples compared to unprocessed dry lipoaspirate. Nanofat samples exhibited a cell yield of 6.63 million cells/g lipoaspirate, whereas stromal vascular fraction preparations resulted in only 0.68 million cells/g lipoaspirate. The final cell inoculum obtained from stromal vascular fraction isolation was 120 million cells and it required 200 to 250 cc of raw lipoaspirate as starting material, whereas nanofat preparation resulted in 125 million cells with only 20 cc of raw lipoaspirate.
Conclusion:
Mechanical disaggregation offers a better cell inoculum than conventional enzymatic dissociation methods by using 10 times less fat tissue as starting material and delivering a higher cell yield.
Any given tissue contains a specific population of resident cells that maintain tissue homeostasis in a self-regulated steady state.1 From a histologic perspective, tissue resident cells can be divided into two main components: parenchyma and stroma.2 The parenchyma consists of a specialized group of cells that complete a specific tissue function (i.e., cardiomyocytes in the heart, hepatocytes in the liver, or adipocytes in fat), whereas the stroma plays a structural role by supporting the parenchyma under physiologic and pathologic conditions.3 The stroma is composed of a heterogeneous population of cells (i.e., stromal cells) that act as trophic mediators for tissue repair and regeneration by secreting cytokines and growth factors into the extracellular milieu.4,5 On injury, stromal cells initiate a site-specific reparative response consisting of (1) extracellular matrix remodeling, (2) enhanced angiogenesis, (3) modulation of the immune system, and (4) cellular turnover.6 These reparative properties make stromal cells a great therapeutic value for regenerative medicine and cell-based therapies.
Adipose tissue has become one of the most suitable sources of stromal cells, given its abundance in the adult organism along with easy and safe acquisition.7–9 The cellular components of adipose tissue consist predominantly of mature adipocytes (parenchyma) sustained by a cohort of stromal cells (stroma), including fibroblasts, endothelial cells, resident mononuclear cells, wandering blood-derived cells, and progenitor cells.3–5 Traditionally, isolation of stromal cells has been conducted by enzymatic dissociation of lipoaspirate followed by centrifugation to separate mature adipocytes from stromal cells.9–11 Enzymatically released stromal cells consist of a heterogeneous isolated cell population known as stromal vascular fraction. However, the use of enzymes is associated with high costs and presents some conflicts with regulatory agencies.12 Consequently, mechanical methods have emerged as an alternative for stromal vascular fraction isolation, where physical forces break down mature adipocytes. These methods involve vibration, high-speed centrifugation, and shaking, but they all produce a lower cell yield of stromal vascular fraction cells compared with enzymatic methods.13
Recently, Tonnard et al. developed a new method for mechanical processing of lipoaspirate to reduce the size of fat particles needed to obtain an injectable product, known as nanofat.14 Mechanically disaggregated nanofat was obtained by sequential passes through different Luer-lock sizes where the lipoaspirate is exposed to shear forces, resulting in mature adipocyte rupture and sizing down of stromal tissue fragments. As opposed to isolated stromal vascular fraction cells, nanofat contains a stromal cell population organized as cell aggregates, which retain their vasculature while remaining attached to the native cellular matrix.15 It has been shown that cell aggregates maintain cell viability and exhibit an enhanced response to proliferation and differentiation compared with isolated cells.16–18 Most importantly, recent studies have reported a successful outcome of nanofat application in surgical procedures for facial rejuvenation and skin graft reconstruction.19–21 In addition, a condensed form of nanofat has been shown to promote wound healing and improve flap survival in a mouse model.22,23 The present study aimed to evaluate the cell content of nanofat preparations and compare the cell yield and performance of lipoaspirate processing between mechanical disaggregation and enzymatic dissociation methods. For clarity purposes in this article, stromal vascular fraction refers to enzymatic dissociation procedures and nanofat relates to mechanical disaggregation by means of the Tulip Medical (Tulip Medical Products, San Diego, Calif.) system.
PATIENTS AND METHODS
The present study was authorized by the Ethics Committee of the Balearic Isles. All liposuctions were performed under general anesthesia, and an informed consent document was signed by all patients.
Adipose Tissue Harvesting and Processing
Enzymatic Dissociation of Stromal Vascular Fraction: GID Stromal Vascular Fraction Protocol
Stromal vascular fraction isolation was performed in 20 healthy patients as described by Dos-Anjos Vilaboa et al.24 Briefly, fat tissue was harvested from the abdominal area and transferred into a GID SVF-1 (GID Europe, London, United Kingdom). Lipoaspirate was washed three times with lactated Ringer solution and incubated at 37°C for 40 minutes under continuous agitation with an equal volume of digestion buffer containing 200 collagen digestion units/ml dissociation enzyme (GIDzyme-2; GID Europe). Enzymatic activity was stopped with human albumin (2.5% final concentration), and the GID SVF-1 device was centrifuged at 800 g for 10 minutes. Supernatant was discarded and the resulting cell pellet (stromal vascular fraction) was resuspended in a suitable volume of lactated Ringer solution.
Mechanical Disaggregation of Nanofat: Tulip NanoTransfer Protocol
Lipoaspirate was obtained from six healthy patients. Tumescent fluid (INIBSACAIN Plus 0.05%; Inibsa Hospital, Barcelona, Spain) was infiltrated at the abdominal area using a Tumescent Infiltrator cannula (Tulip Medical). Forty cubic centimeters of lipoaspirate was harvested using a Carraway Harvester cannula (Tulip Medical) into 20-cc syringes with a Johnnie Lock (Tulip Medical) installed to hold suction. Lipoaspirate was transferred into a Fat Press (Tulip Medical), and washed with an equal volume of saline solution by hand rocking eight to 10 times. Fluids were expelled by manual pressure, and dry lipoaspirate was collected into 20-cc syringes. The fat-containing syringe was sequentially passed 30 times through a 2.4-mm and 1.2-mm Luer-to-Luer transfer (Tulip Medical) device connected to another empty 20-cc syringe. Preemulsified lipoaspirate was then connected in a sterile NanoTransfer device (Tulip Medical) and transferred by a single pass through a 0.6- to 0.4-mm mesh screen into an empty 20-cc syringe. [See Video (online), which demonstrates mechanical disaggregation of nanofat following Tulip NanoTransfer protocol. For harvest, the abdominal region was infiltrated with tumescent fluid and adipose tissue was subsequently harvested. Aspirated adipose tissue (lipoaspirate) was washed with an equal volume of saline solution by hand rocking and removing the excess fluid. Dry lipoaspirate was mechanically disaggregated by a shearing process of transferring fat 30 times through 2.4-mm and 1.2-mm Luer-to-Luer devices, causing stroma fragmentation and a major adipocyte breakage. As a result, intracellular triglycerides released from broken adipocytes together with residual fluid present in lipoaspirate formed an emulsion containing stromal cells as aggregates. Mechanically disaggregated lipoaspirate was transferred through the NanoTransfer device to select for 0.6- to 0.4-mm particles, generating nanofat as a final product.]
Video. This video demonstrates mechanical disaggregation of nanofat following the Tulip NanoTransfer protocol. For harvest, the abdominal region was infiltrated with tumescent fluid and adipose tissue was subsequently harvested. Aspirated adipose tissue (lipoaspirate) was washed with an equal volume of saline solution by hand rocking and removing the excess fluid. Dry lipoaspirate was mechanically disaggregated by a shearing process of transferring fat 30 times through 2.4-mm and 1.2-mm Luer-to-Luer devices, causing stroma fragmentation and a major adipocyte breakage. As a result, intracellular triglycerides released from broken adipocytes together with residual fluid present in lipoaspirate formed an emulsion containing stromal cells as aggregates. Mechanically disaggregated lipoaspirate was transferred through the NanoTransfer device to select for 0.6- to 0.4-mm size particles, generating nanofat as a final product.
Excised Adipose Tissue Harvesting and Sampling
Excised adipose tissue was harvested from the abdomen of one healthy patient who underwent abdominoplasty. Five different samples (n = 5) were excised from the abdomen using dissecting scissors.
Cell Number and Viability Quantification by Image Cytometry
Cell count was performed on stromal vascular fraction cell suspensions by an automated nucleocounter (NC-3000; ChemoMetec, Lillerød, Denmark). Cell viability was measured on stromal vascular fraction isolated cells from each specimen (lipoaspirate and nanofat). Stromal vascular fraction pellets were resuspended in a suitable volume of phosphate-buffered saline and filtered using a 100-µm cell strainer. Cell number and viability were determined automatically in 30 to 40 seconds by means of the NC-3000 device.
Cell Number Quantification by DNA Analysis
Fifty to 100 mg of lipoaspirate, nanofat, and excised adipose tissue specimens were placed into a sterile 1.5-ml Eppendorf tube. In addition, a known stromal vascular fraction pellet (previously quantified by the NC-3000 device) was used as a reference control. DNA extraction was performed following the manufacturer’s instructions from E.Z.N.A. tissue DNA Kit (Promega, Madison, Wis.). DNA content was measured by NanoDrop (ThermoFisher Scientific, Waltham, Mass.) quantification. Cell numbers from test specimens were interpolated from DNA-cell ratio obtained from the stromal vascular fraction reference control sample.
Histologic Analysis
Excised adipose tissue, unprocessed lipoaspirate, and postdigested lipoaspirate were placed directly into plastic cassettes. Nanofat was previously centrifuged at 800 g for 10 minutes, the aqueous phase was discarded, and the resulting condensate nanofat was placed into the cassette. All samples were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Paraffin blocks were sent to the histopathology facility at the Institute for Research in Biomedicine in Barcelona sectioned at 3-μm thickness, and stained with hematoxylin and eosin standard protocols. Bright-field images were acquired with a NanoZoomer-2.0 HT C9600 digital scanner (Hamamatsu Photonics, Hamamatsu, Japan).
Statistical Analysis
Bar graphs are represented as mean ± SEM and box plots represent the median, mean, and the minimum and maximum values. Statistical analyses were performed using GraphPad Prism software version 7.0a (GraphPad Software, Inc., San Jose, Calif.). Paired t tests were used to compare lipoaspirate versus nanofat samples (Figs. 1 through 3). Unpaired t tests were used to compare excised adipose tissue versus lipoaspirate and nanofat (Fig. 2) and stromal vascular fraction versus nanofat samples (Fig. 4 and Table 1). Values of p < 0.05 were considered significant.
Fig. 1.
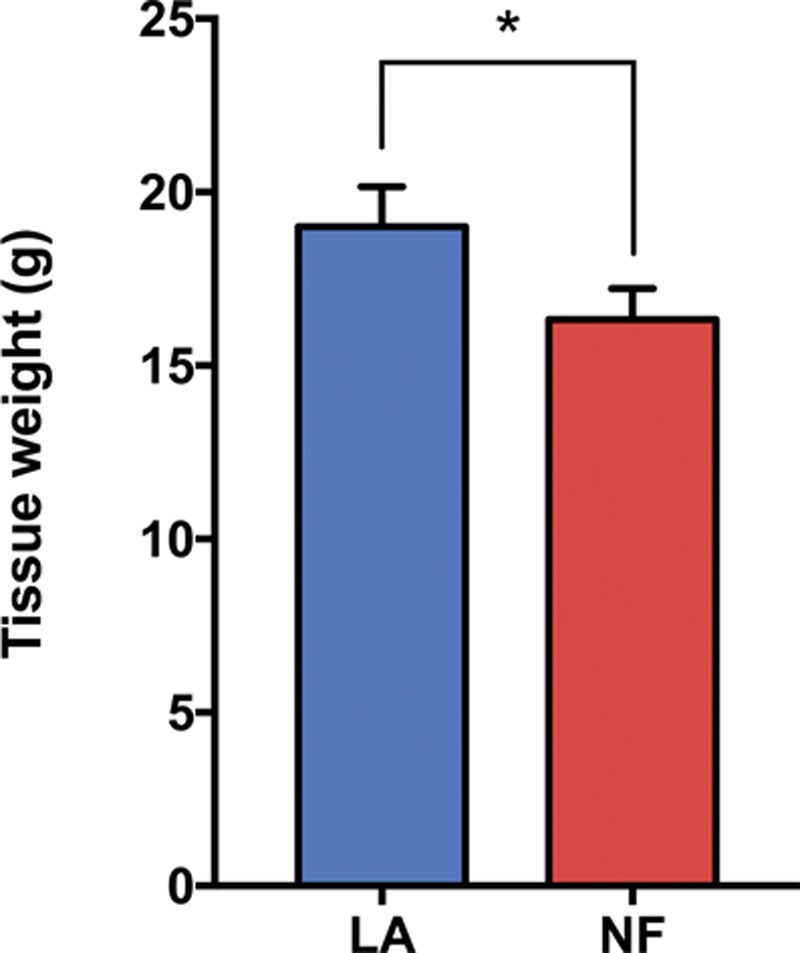
Tissue fraction of nanofat preparations. Bar graphs show tissue weight before (lipoaspirate) and after (nanofat) mechanical processing (n = 3). Values are represented as mean ± SEM and statistical analysis was performed using the paired t test (*p < 0.05). LA, lipoaspirate; NF, nanofat.
Fig. 3.
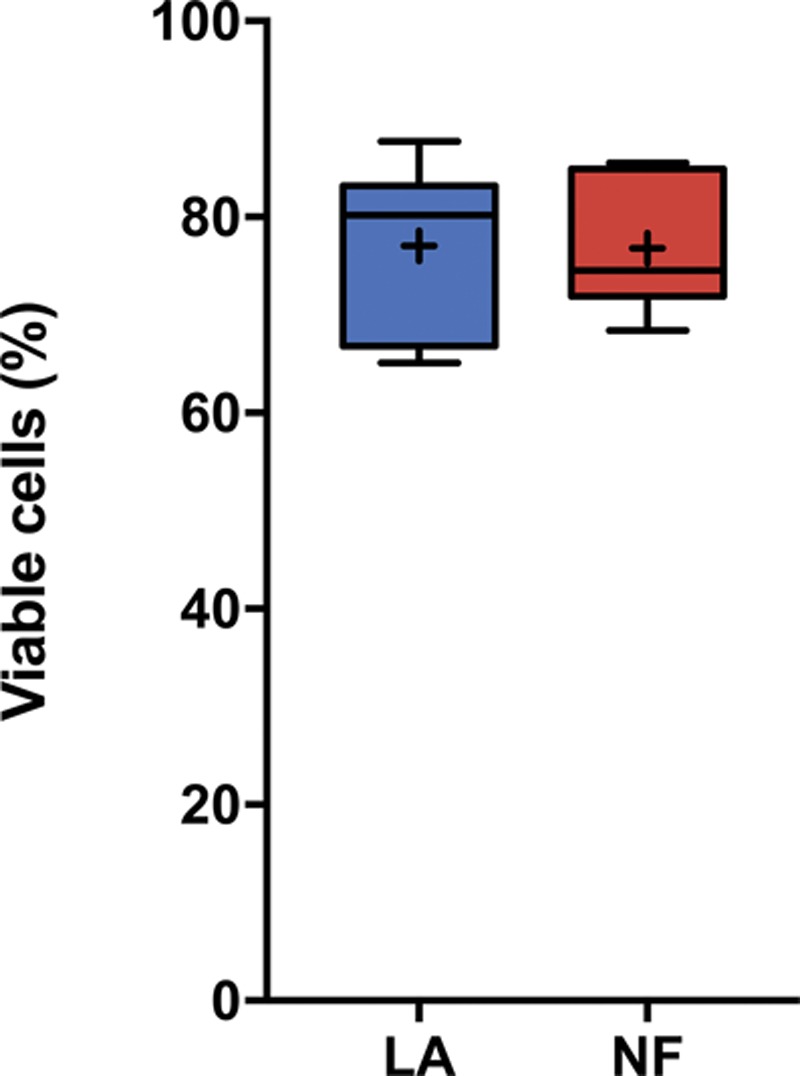
Effect of mechanical disaggregation on nanofat cell viability. Both lipoaspirate and nanofat samples were enzymatically dissociated to analyze cell viability in stromal vascular fraction isolated cells. Values are represented as the percentage of viable cells in isolated stromal vascular fraction from each sample (n = 6). The boxes show the interquartile range (from lower to upper quartile) of viable cells, including the median (middle quartile) and mean. The whiskers show the location of the minimum and maximum values of all the data. Statistical analysis was performed using the paired t test (p = not significant). LA, lipoaspirate; NF, nanofat; SVF, stromal vascular fraction.
Fig. 2.
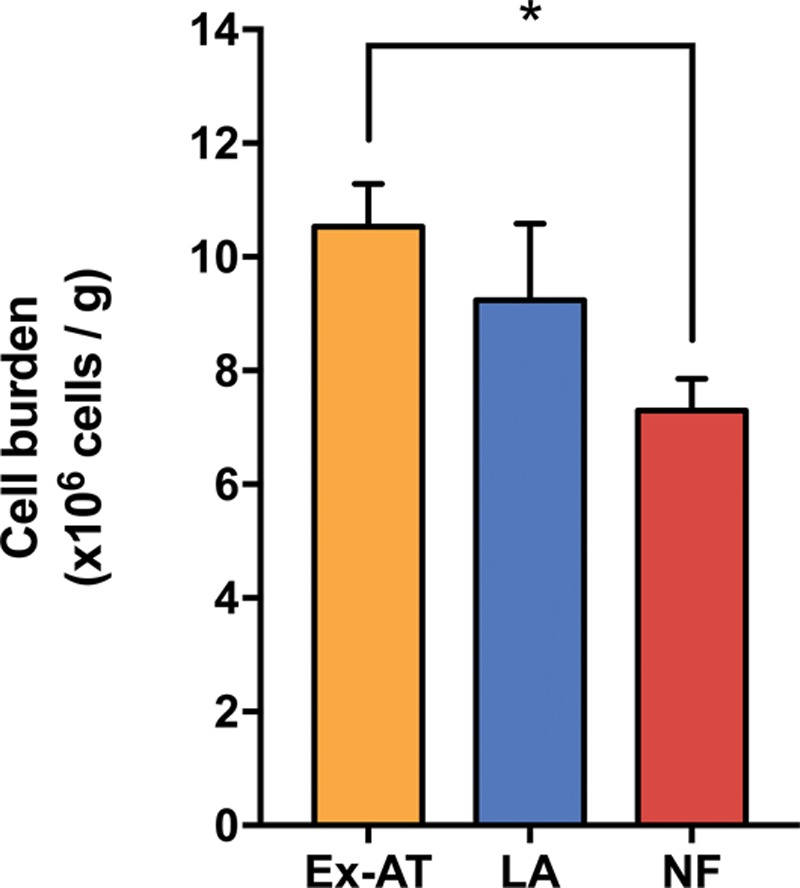
Cell content of excised adipose tissue, lipoaspirate, and nanofat preparations. Bar graphs show the cell burden of different specimens as the number of cells × 106 per gram of sample (n = 5 in excised adipose tissue; n = 4 in lipoaspirate and nanofat). Values are represented as mean ± SEM and statistical analysis was performed using the paired t test between lipoaspirate and nanofat (p = not significant), and unpaired t test between excised adipose tissue and lipoaspirate (p = not significant) and excised adipose tissue and nanofat (*p < 0.05). Ex-AT, excised adipose tissue; LA, lipoaspirate; NF, nanofat.
Fig. 4.
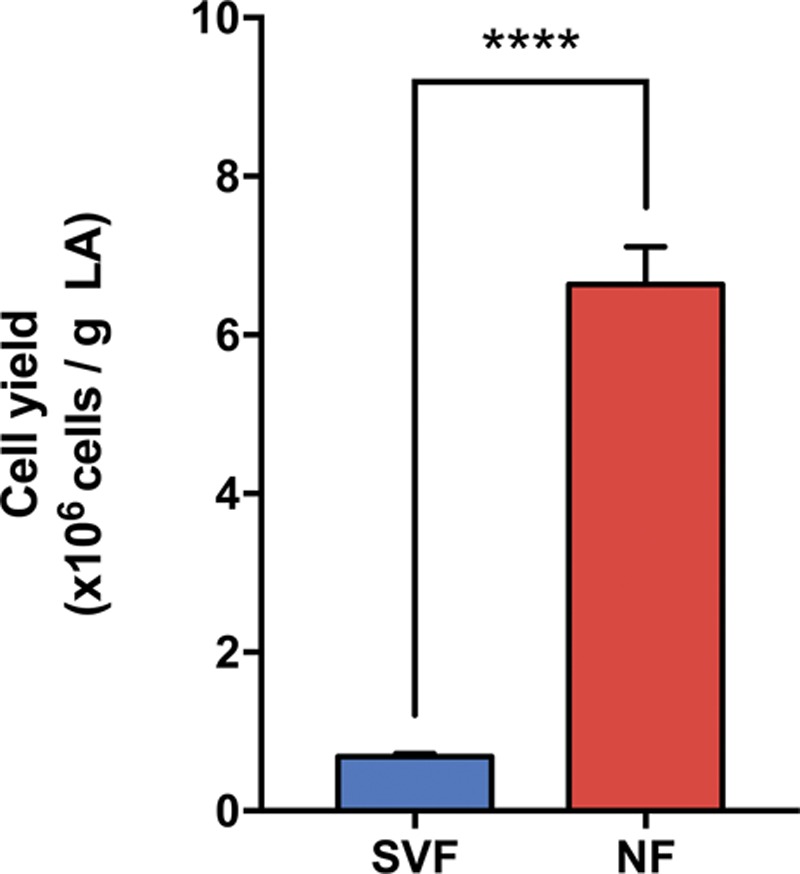
Cell yield comparison between enzymatic (stromal vascular fraction) and mechanical (nanofat) procedures. Bar graphs show the number of cells × 106 recovered per gram of dry lipoaspirate processed through enzymatic dissociation (stromal vascular fraction, n = 20) or mechanical disaggregation (nanofat, n = 3) Values are represented as mean ± SEM, and statistical analysis was performed using the unpaired t test (****p < 0.0001). SVF, stromal vascular fraction; NF, nanofat.
Table 1.
Summary Table of Final Cell Inoculum Obtained from Enzymatic and Mechanical Procedures*
| Processing Method (Product) | Starting Material (cc LA) | Cell Yield (× 106 cells/g dry LA) |
Tissue Weight (g dry LA) |
Final Cell Inoculum (× 106 cells) |
|---|---|---|---|---|
| Enzymatic (SVF) | >200 | 0.68 ± 0.04 | 175.26 ± 4.43 | 120.07 ± 7.21 |
| Mechanical (NF) | 20 | 6.63 ± 0.47 | 19.26 ± 1.15 | 125.81 ± 5.86 |
LA, lipoaspirate; NF, nanofat; SVF, stromal vascular fraction.
*Starting material represents the amount of raw lipoaspirate used in each protocol. Cell yield indicates the number of recovered cells per gram of dry lipoaspirate processed, as shown in Figure 4. Tissue weight shows the grams of dry lipoaspirate per GID (SVF) and Tulip NanoTransfer (NF) protocols. Final cell inoculum represents the total number of cells obtained in each procedure. Values are represented as mean ± SEM (SVF, n = 20; NF, n = 3).
RESULTS
According to our hypothesis, our results show that (1) nanofat preparation consists of an 85 percent volume of processed dry lipoaspirate; (2) nanofat contains 70 percent of native adipose tissue (excised adipose tissue) cell mass; (3) mechanical disaggregation does not affect cell viability; (4) mechanical disaggregation yields 10 times more cells per gram of dry lipoaspirate compared with enzymatic dissociation methods; and (5) mechanically disaggregated nanofat exhibits some clinical advantages over stromal vascular fraction isolation protocols.
Tissue Fraction of Nanofat Preparations
After mechanical disaggregation of lipoaspirate, we determined the tissue fraction of nanofat compared with unprocessed dry lipoaspirate. For accurate measurements, we compared both specimens based on tissue weight and determined the percentage of tissue loss in nanofat preparations. According to the Tulip protocol, unprocessed dry lipoaspirate had a tissue weight of 19.2 g, and mechanically disaggregated nanofat resulted in 16.3 g (Fig. 1), presenting a 15 percent tissue loss.
Quantification of Cell Content in Nanofat Preparations
The total nucleated cells contained in adipose tissue and derivate preparations can be determined by DNA tissue content. DNA extraction and quantification were performed in nanofat, unprocessed dry lipoaspirate as the control, and native excised adipose tissue samples to determine the constitutive cell burden contained in fat tissue. To estimate the cell content, we used a known stromal vascular fraction cell suspension to interpolate the cell number from DNA content. Based on DNA analysis, excised adipose tissue and unprocessed dry lipoaspirate cell burden was 10.5 and 9.2 million cells/g, respectively, whereas nanofat resulted in 7.3 million cells/g (Fig. 2).
Evaluation of Cell Viability in Nanofat Preparations
To evaluate the impact of mechanical disaggregation in cell membrane integrity, we analyzed the viability of enzymatically dissociated stromal vascular fraction cells from nanofat and lipoaspirate preparations following a stromal vascular fraction isolation protocol. Stromal vascular fraction viability was used as a fair estimate of the entire cell population of a given tissue sample. Based on image cytometry, the viability of the enzymatically released stromal vascular fraction cells was 77.1 percent for unprocessed dry lipoaspirate and 76.8 percent for nanofat samples (Fig. 3).
Cell Yield Comparison between Enzymatic Dissociation (Stromal Vascular Fraction) and Mechanical Disaggregation (Nanofat) Procedures
Cell yield was represented as the number of cells obtained per 1 g of dry lipoaspirate (Fig. 4). Regarding enzymatic dissociation, stromal vascular fraction data were obtained from 20 different patients that underwent liposuction surgery between 2012 and 2015 following the GID stromal vascular fraction protocol.24 For mechanical disaggregation, nanofat data were obtained from the total number of cells in nanofat (cell burden in Fig. 2 times nanofat weight in Fig. 1) relative to dry lipoaspirate weight (Fig. 1). Enzymatically dissociated stromal vascular fraction resulted in 0.68 million cells/g lipoaspirate, whereas mechanically disaggregated nanofat resulted in 6.63 million cells/g lipoaspirate. Thus, enzymatic dissociation results in only 7.3 percent of nucleated cells contained in dry lipoaspirate samples, whereas nanofat retained more than 70 percent of cells after mechanical disaggregation.
Moreover, cell yield data were verified by visualization of the three-dimensional structure of excised adipose tissue, unprocessed dry lipoaspirate, nanofat, and enzymatically postdigested lipoaspirate samples in hematoxylin and eosin–stained sections (Fig. 5). After enzymatic digestion and subsequent centrifugation, stromal vascular fraction cells were pelleted at the bottom and postdigested lipoaspirate fraction remained floating above the infranatant buffer phase. Histologic analysis of nanofat samples revealed that mechanical disaggregation caused the rupture of mature adipocyte membranes and retained a high density of stromal cells compared with excised adipose tissue and unprocessed dry lipoaspirate where adipocytes stood packed and organized. In accordance with low cell yield observed in enzymatic stromal vascular fraction isolation (7.3 percent), postdigested lipoaspirate fraction exhibited a high concentration of cells that had not been enzymatically dissociated from lipoaspirate.
Fig. 5.
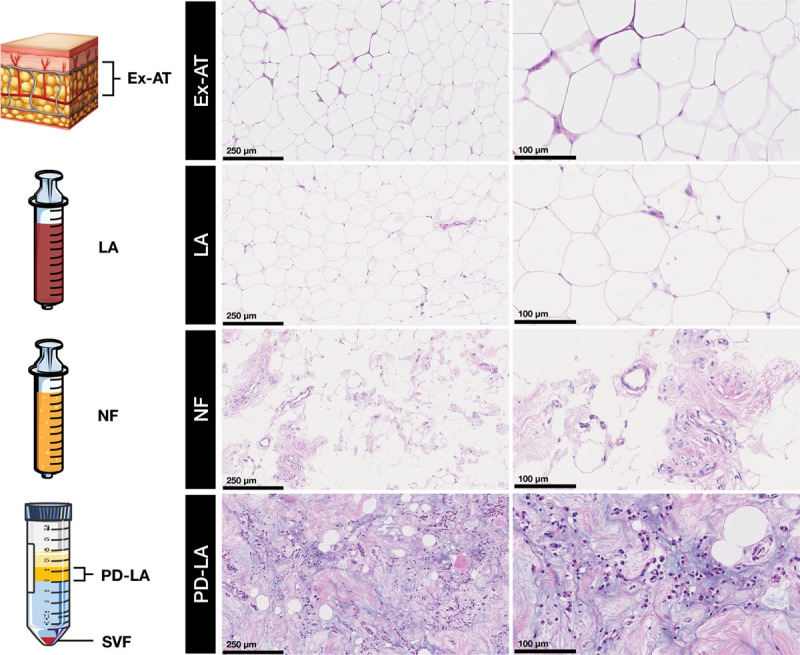
Histologic analysis of excised adipose tissue, lipoaspirate, nanofat, and postdigested lipoaspirate specimens by hematoxylin and eosin staining. A schematic representation of each specimen is shown: excised adipose tissue as a block, lipoaspirate and nanofat in syringes, and enzymatically digested lipoaspirate after centrifugation in a 50-cc Falcon tube showing a floating postdigested lipoaspirate fraction and pelleted stromal vascular fraction. Histologic sections are shown at two different magnifications. Scale bars = 250 µm (left) and 100 µm (right). Ex-AT, excised adipose tissue; LA, lipoaspirate; NF, nanofat; SVF, stromal vascular fraction; PD-LA, postdigested lipoaspirate.
Clinical Relevance of Enzymatic Dissociation versus Mechanical Disaggregation Protocols: Final Cell Inoculum
We evaluated the final cell inoculum as the total number of cells to be delivered into the patient. Table 1 summarizes all the data obtained from enzymatic (stromal vascular fraction) and mechanical (nanofat) methods together with their respective protocol specifications. Following protocol instructions, starting material for stromal vascular fraction isolation was between 200 and 250 cc of raw lipoaspirate, whereas starting material for nanofat preparations was only 20 cc. Final cell inoculum was measured by cell yield data and the amount of dry lipoaspirate. Based on cell yield performance, stromal vascular fraction isolation resulted in a final cell inoculum of 120 million cells, and nanofat preparations resulted in 125 million cells per procedure.
DISCUSSION
The present study aimed to explore the process of mechanical disaggregation of adipose tissue in comparison with more traditional methods involving enzymatic dissociation. To compare both methods, we focused on cellular content as the final outcome given that stromal cells act as the biological agent involved in repair and regeneration.25–27 As the use of image cytometry is limited to single-cell suspensions, we have used an alternative DNA analysis approach to quantify the total nucleated cells present in a given tissue or derivate specimens such as cell aggregates from nanofat preparations. By definition, a human nondividing somatic cell contains approximately 6 pg of DNA.28,29 Thus, the number of cells is directly proportional to its DNA content. [See Figure, Supplemental Digital Content 1, where two independent standard curves demonstrate a strong linearity between DNA content and cell number, presenting an R2 > 0.99. The figure shows linear regression analysis of DNA content (micrograms of DNA, y axis) against cell number (× 106 cells, x axis). SC-T represents the definition of 6 pg of DNA contained in each human diploid, nondividing cell (R2 = 1). Two independent standard curves (SC1 and SC2) were obtained from serial stromal vascular fraction cell pellets (10, 5, 2.5, 1.25, 0.625, and 0.312 × 106 stromal vascular fraction cells). SC-1 and SC-2 showed a strong linearity (R2 = 0.9958 and R2 = 0.9968, respectively). SC-T, theoretical standard curve; SC-1, standard curve 1; SC-2, standard curve 2, http://links.lww.com/PRS/D741.] A known stromal vascular fraction suspension was used as a reference to determine the DNA-to-cell ratio to interpolate the cell number from DNA content of tested samples. Our DNA analysis allowed us to further quantify the overall constitutive cell burden of native adipose tissue (excised adipose tissue) and lipoaspirated adipose fragments (lipoaspirate), including live and dead cells, resulting in 10.5 and 9.2 million cells per gram, respectively (Fig. 2). Eto et al. also addressed this question by using whole-mount staining analysis of excised and aspirated adipose tissue, which resulted in a total of 5.9 and 6 million cells per cubic centimeter in both excised adipose tissue and lipoaspirate samples.30 The difference in cell number estimates might be caused by inherent sample variability, or because cell staining analysis could present some limitations for reagents to access and penetrate into cellular components because of tissue thickness.
Nanofat can be defined as an injectable product composed of cell aggregates which, based on tissue weight measurements in Figure 1, consist of 85 percent in volume of initial dry lipoaspirate. The tissue loss observed in nanofat could be mainly caused by tissue fibers retained inside the NanoTransfer device. Nanofat was characterized based on cell content and viability to identify the impact of physical forces applied on cells within the adipose tissue compared with unprocessed dry lipoaspirate. Cell content analysis showed no statistical differences between nanofat and lipoaspirate cell burden, which suggests that nanofat comprises a stromal cell population equivalent to the aspirated adipose tissue. Most importantly, nanofat specimens represent an injectable product that approaches the constitutive cell burden present in excised adipose tissue by containing 70 percent of its native cell mass (Fig. 2). It is worth mentioning that quantification of nanofat cell burden may include residual DNA released by the rupture of mature adipocytes during nanofat shearing, which can no longer be considered a part of nanofat cell mass. Although further experiments would be required to address this limitation, it has been reported that lipoaspirate contains 6.9 × 105 mature adipocytes per gram (<10 percent of total lipoaspirate cell mass), which may result in a minimal overestimation of nanofat cell burden data.31 As shown by hematoxylin and eosin staining, nanofat presents cell-enriched fat aggregates along with adipocytes ruptured because of shear force exposure (Fig. 5). Importantly, the fact that nanofat sections showed a higher density of cells compared with exogenous adipose tissue and lipoaspirate samples was attributable to an additional centrifugation step to condensate nanofat preparations to reduce their viscosity and facilitate paraffin embedding.
Cell viability tests were performed by enzymatic isolation and image cytometry of stromal vascular fraction from nanofat and unprocessed dry lipoaspirate samples, with no effect caused by mechanical disaggregation. This method, however, only measures the enzymatically isolated cell fraction, which is less than 10 percent (Fig. 4). Similarly to cell counting limitations in tissue samples, no current reliable method has been established to determine cell viability. Colorimetric assays based on tetrazolium compounds [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; and 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] are widely used to study proliferation and viability in cell culture but present some limitations when evaluating tissue samples.32,33 Thus, we can interpret the viability of stromal vascular fraction isolated cells as a representative population of the entire tissue sample. Overall, no statistical differences were observed in cell viability between nanofat and lipoaspirate samples. These data are consistent with previous characterization of nanofat preparations, where no differences in cell viability were observed as a result of the mechanical disaggregation process.15
Based on cell yield analysis obtained from enzymatic dissociation (stromal vascular fraction) and mechanical disaggregation (nanofat), our data indicate that only 7.3 percent of stromal vascular fraction cells were recovered by enzymatic dissociation, whereas mechanical disaggregated nanofat showed a 70 percent cell recovery rate from unprocessed dry lipoaspirate (Fig. 4). It is important to note that, despite having used different methods to quantify stromal vascular fraction and nanofat cell yield (image cytometry and DNA analysis, respectively), we have shown that both methods are comparable, which supports our DNA analysis as a reliable method of determining the total cell content of adipose tissue samples. [See Figure, Supplemental Digital Content 2, which shows no statistical significance between both methods in four independent stromal vascular fraction preparations. Comparison of cell quantification methods between image cytometry (NC-3000) and DNA analysis is shown. Four independent stromal vascular fraction cell pellets were split in two and analyzed following both methods. For DNA analysis, the reference sample was a known cell pellet obtained from stromal vascular fraction cell culture grown in monolayer at passage 3. SVF1–SVF4, stromal vascular fraction 1 to 4, http://links.lww.com/PRS/D742.] Based on low-rate cell recovery exhibited by stromal vascular fraction isolation, we hypothesized that 90 percent unsuccessfully recovered stromal cells must have been left behind and remained within lipoaspirate tissue following digestion. To validate this hypothesis, hematoxylin and eosin staining revealed a high density of cells in postdigestion lipoaspirate tissue (Fig. 5), suggesting that a vast majority of nonisolated cells remained within the extracellular matrix after enzymatic digestion. The increase in cell density compared with excised adipose tissue and unprocessed dry lipoaspirate can be explained by the dramatic volume decrease of postdigestion lipoaspirate as a result of the massive adipocyte release after enzymatic digestion. Nonetheless, as stromal vascular fraction isolation may be a more suitable approach for specific procedures such as intravenous delivery, alternative methods for enzymatic optimization are needed to improve and maximize the stromal vascular fraction cell yield.34,35
From a clinical standpoint, the number of transplanted cells is of vital importance and plays a critical role in cell-based therapy outcomes.36–38 Recently, total nucleated cell dose has been shown to act as a more relevant prognosis indicator than the commonly known CD34+ cell number in hematopoietic cell transplantation.39,40 However, there are still some discrepancies in establishing the optimal dosage for stromal cell administration in several cell therapies.38–41 Because the native cell population of a given tissue has the ability to initiate and perform a reparative response, we hypothesized that the therapeutic dose required to treat any damaged tissue must be at least near the constitutive cell burden present in the injury site. Following this rationale, we have named this new therapeutic concept “constitutive cell dose.” Conceiving stromal cells as the biological agent with regenerative and reparative properties, we compared the final cell inoculum as the total number of cells obtained from enzymatic dissociation (stromal vascular fraction) and mechanical disaggregation (nanofat) methodologies in a clinical context. As shown in Table 1, the final cell inoculum resulting from the different methods was 120 million cells as isolated stromal vascular fraction cells and 125 million cells as aggregated nanofat particles. Enzymatic stromal vascular fraction isolation procedures start with 200 to 250 cc of raw lipoaspirate (depending on body fat levels from the patient), whereas mechanically disaggregated nanofat requires only 20 cc of starting material. Therefore, mechanical disaggregation requires 10 times less fat tissue as starting material to provide a similar or even higher cell dose compared with conventional enzymatic stromal vascular fraction isolation. In addition to enhanced cell yield performance, mechanically disrupted cell aggregates (nanofat) remain attached to their natural matrix niche, which has been shown to promote cell viability, proliferation, and differentiation.16–18 Moreover, one would expect that cell aggregates also potentiate a reparative effect (extracellular matrix repair, angiogenesis, and immunoregulation) compared with isolated cells, although this last statement is yet to be proven. Overall, nanofat offers a nearly constitutive cell dose that can be easily implemented at the point of care, given its ease of use and cost-effective technology, encouraging the use of cell aggregates as a therapeutic agent over isolated cells for intralesional cell therapies.
CONCLUSIONS
Mechanical disaggregation of adipose tissue is becoming more popular than enzymatically dissociated stromal vascular fraction for its use in surgical treatments and clinical applications. Mechanically disrupted cell aggregates (nanofat) require 10 times less fat tissue as starting material compared with enzymatic isolation to approach a constitutive cell dose. Accordingly, mechanical disaggregation is shown to be a more cost-effective and easy-to-use process because of the substantial reduction in material harvesting and processing. Therefore, the use of stromal cell aggregates as an injectable product represents a promising treatment not only for aesthetic plastic surgery but also as an effective therapeutic tool for other fields, ranging from cardiovascular diseases to orthopedic medicine.
Supplementary Material
Footnotes
Disclosure: This article was partially self-financed (Cell Pro Tech Spain) together with Tulip Medical, Inc. All materials described in Tulip NanoTransfer Protocol were generously provided by Tulip Medical. The authors have no financial disclosures to report.
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
REFERENCES
- 1.Rué P, Martinez Arias A. Cell dynamics and gene expression control in tissue homeostasis and development. Mol Syst Biol. 2015;11:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wikipedia. Stroma (tissue). Available at: https://en.wikipedia.org/wiki/Stroma_(tissue). Accessed June 1, 2018.
- 3.Bluguermann C, Wu L, Petrigliano F, McAllister D, Miriuka S, Evseenko DA. Novel aspects of parenchymal-mesenchymal interactions: From cell types to molecules and beyond. Cell Biochem Funct. 2013;31:271–280. [DOI] [PubMed] [Google Scholar]
- 4.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. [DOI] [PubMed] [Google Scholar]
- 5.Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie 2013;95:2222–2228. [DOI] [PubMed] [Google Scholar]
- 6.Fu Y, Karbaat L, Wu L, Leijten J, Both SK, Karperien M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B Rev. 2017;23:515–528. [DOI] [PubMed] [Google Scholar]
- 7.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells 2011;3:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen A, Guo J, Banyard DA, et al. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J Plast Reconstr Aesthet Surg. 2016;69:170–179. [DOI] [PubMed] [Google Scholar]
- 9.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010;77:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res Ther. 2017;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015;4:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: Basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–1026. [DOI] [PubMed] [Google Scholar]
- 15.Alexander RW. Understanding mechanical emulsification (nanofat) versus enzymatic isolation of tissue stromal vascular fraction (tSVF) cells from adipose tissue: Potential uses in biocellular regenerative medicine. J Prolother. 2016;8:1–14. [Google Scholar]
- 16.Bayoussef Z, Dixon JE, Stolnik S, Shakesheff KM. Aggregation promotes cell viability, proliferation, and differentiation in an in vitro model of injection cell therapy. J Tissue Eng Regen Med. 2012;6:e61–e73. [DOI] [PubMed] [Google Scholar]
- 17.Lei X, Deng Z, Duan E. Uniform embryoid body production and enhanced mesendoderm differentiation with murine embryonic stem cells in a rotary suspension bioreactor. Methods Mol Biol. 2016;1502:63–75. [DOI] [PubMed] [Google Scholar]
- 18.Goh SK, Olsen P, Banerjee I. Extracellular matrix aggregates from differentiating embryoid bodies as a scaffold to support ESC proliferation and differentiation. PLoS One 2013;8:e61856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhooshan LS, Devi MG, Aniraj R, Binod P, Lekshmi M. Autologous emulsified fat injection for rejuvenation of scars: A prospective observational study. Indian J Plast Surg. 2018;51:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemaloğlu CA. Nanofat grafting under a split-thickness skin graft for problematic wound management. Springerplus 2016;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei H, Gu SX, Liang YD, et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget 2017;8:68542–68556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Dong Z, Liao Y, et al. Adipose extracellular matrix/stromal vascular fraction gel: A novel adipose tissue-derived injectable for stem cell therapy. Plast Reconstr Surg. 2017;139:867–879. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Feng J, Liao Y, et al. Ischemic flap survival improvement by composition-selective fat grafting with novel adipose tissue derived product: Stromal vascular fraction gel. Biochem Biophys Res Commun. 2018;495:2249–2256. [DOI] [PubMed] [Google Scholar]
- 24.Dos-Anjos Vilaboa S, Navarro-Palou M, Llull R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy 2014;16:1092–1097. [DOI] [PubMed] [Google Scholar]
- 25.Tögel F, Westenfelder C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis 2011;7:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal stromal cells and cutaneous wound healing: A comprehensive review of the background, role, and therapeutic potential. Stem Cells Int. 2018;2018:6901983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartczak A, McGilvray I, Keating A. Mesenchymal stromal cell therapy to promote cardiac tissue regeneration and repair. Curr Opin Organ Transplant. 2017;22:86–96. [DOI] [PubMed] [Google Scholar]
- 28.Dole el J, Barto J, Voglmayr H, Greilhuber J. Letter to the editor: Nuclear DNA content and genome size of trout and human. Cytometry A 2003;51:127–128; author reply 128. [DOI] [PubMed] [Google Scholar]
- 29.Greilhuber J, Dolezel J, Lysák MA, Bennett MD. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann Bot. 2005;95:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eto H, Suga H, Matsumoto D, et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. [DOI] [PubMed] [Google Scholar]
- 31.Suga H, Matsumoto D, Inoue K, et al. Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg. 2008;122:103–114. [DOI] [PubMed] [Google Scholar]
- 32.Riss TL. Is your MTT assay really the best choice? Available at: https://www.promega.es/resources/pubhub/is-your-mtt-assay-really-the-best-choice/. Accessed June 1, 2018.
- 33.Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA. Sittampalam GS, Grossman A, Brimacombe K, et al. Cell viability assays, 2013 May 1 [updated 2016 Jul 1]. In: Assay Guidance Manual [Internet]. 2004. Bethesda, Md: Eli Lilly & Company and the National Center for Advancing Translational Sciences; Available at: https://www.ncbi.nlm.nih.gov/books/NBK144065/. [Google Scholar]
- 34.Comella K, Blas JAP, Ichim T, Lopez J, Limon J, Moreno RC. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: A phase I trial of safety and tolerability. J Clin Med Res. 2017;9:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comella K, Parlo M, Daly R, Dominessy K. First-in-man intravenous implantation of stromal vascular fraction in psoriasis: A case study. Int Med Case Rep J. 2018;11:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florea V, Rieger AC, DiFede DL, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ Res. 2017;121:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galipeau J, Sensébé L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 2018;22:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu JY, Cai WY, Tian M, Liu D, Huang RC. Stem cell transplantation dose in patients with acute myocardial infarction: A meta-analysis. Chronic Dis Transl Med. 2016;2:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin PS, Li S, Nikiforow S, et al. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34+ cell number in reduced-intensity mobilized peripheral blood allogeneic hematopoietic cell transplantation. Haematologica 2016;101:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells JR, Sunkara A, Kang G, et al. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34+ cell dose for peripheral blood allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant. 2018;24:S423–S424. [Google Scholar]
- 41.Golpanian S, Schulman IH, Ebert RF, et al. ; Cardiovascular Cell Therapy Research Network. Concise review: Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016;5:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]


