Abstract
A patient with neurofibromatosis type 1 presented to the pain clinic with neuropathic pain. Thoracolumbar magnetic resonance imagining revealed meningocele T12–L2 with cauda equina distortion. After becoming pregnant, the patient interrupted opioid treatment, refusing pharmacological treatment until the pain became unbearable. Transcutaneous electrical nerve stimulation (TENS) was proposed. The patient used this treatment from the first trimester until month 6 postpartum, achieving good analgesia without any adverse effects for the mother or child. TENS may be a viable treatment for neuropathic pain (NP) during pregnancy. However, more data are needed due to the difficulty of conducting clinical trials in this population.
The use of opioids during pregnancy can cause symptoms characteristic of neonatal abstinence syndrome, including congenital heart defects, neural tube defects (spina bifida), glaucoma, gastroschisis, convulsions, and irritability.1 Opioid use can also negatively affect the mother, causing placental alterations, bleeding, premature birth, and pre-eclampsia. The available evidence regarding the risks of opioid use during pregnancy is contradictory because most studies did not adjust for confounding factors, such as socioeconomic status, stress, and alcohol use.1 The first trimester of pregnancy is a critical period for the development of organ systems, and any chemical or environmental aggression could cause severe congenital malformations. Consequently, clinical guidelines recommend that women planning to become pregnant avoid opioids or reduce the dose if discontinuation is not feasible.2
The present case report describes a patient diagnosed with neurofibromatosis type 1 (NF1) with neuropathic pain (NP) under treatment with opioids. After genetic counseling, the patient decided to become pregnant and, therefore, discontinued opioid therapy, refusing any other pharmacological treatment. Transcutaneous electrical nerve stimulation (TENS) was proposed as an alternative to pharmacotherapy.
TENS is a nonpharmacological therapy that uses adhesive electrodes applied to the skin surface to administer pulsed electrical stimulation that can be modified and is primarily used for pain control in a wide range of chronic pain conditions. It was developed as a result of the “pain gate theory,” which proposes that the stimulation of large-diameter afferent fibers inhibits nociceptive activity at the dorsal horn, with a resultant decrease in pain perception.3
The risks were explained in detail, particularly the limited evidence for TENS treatment. Although some studies have described TENS during labor,4,5 to our knowledge, no studies have evaluated TENS during the first and second trimesters of pregnancy. The patient provided signed informed consent, indicating that she understood the risks. This manuscript adheres to the applicable Case Report Guidelines (CARE) guidelines of the Enhancing the Quality and Transparency of Health Research (EQUATOR) network. After finishing follow-up, the patient provided written consent for publication.
CASE DESCRIPTION
A 33-year-old female patient with NF1 (exon-17 mutation: c.2978–2987) presented to the pain clinic at week 15 of pregnancy. At 26 years of age, she was diagnosed with T12–L2 meningocele, with cauda equina distortion, resulting in NP at the S1–S4 nerve roots. After becoming pregnant, a chorion biopsy showed that the embryo did not carry the NF1 gene.
The patient presented burning pain in the thoracolumbar region, with persistent left sciatica extending to the sole of the foot. The pain worsened at the start and end of the day. She was receiving treatment with a transdermal buprenorphine patch (35 µg/h), which she decided to discontinue, leading to withdrawal syndrome for which she was referred to the pain clinic. The patient’s pain level with the buprenorphine patch was 2 of 10 on the numerical pain rating scale (NPRS), worsening to 8.5 at rest and in movement, after patch removal. At week 15 of gestation (1 week after referral), she was given a TENS unit (DirectTENS; Empi Inc, St. Paul, MN) at a preset fixed frequency (125 Hz) and pulse duration (100 microseconds) for 30 minutes per session, 4 times a day. Table 1 shows the follow-up visits, treatments, and quality of analgesia. The patient did not experience any TENS-associated adverse events during pregnancy, and all ultrasound examinations were normal. The pregnancy was carried to full term, with a normal, spontaneous birth in July 2015. During labor, the TENS device was not used because she had left it at home. Peridural analgesia was contraindicated due to the meningocele location. Consequently, she received local anesthesia and intravenous remifentanil.
Table 1.
Treatment With TENS and Medication During Pregnancy Through Month 6 Postpartum
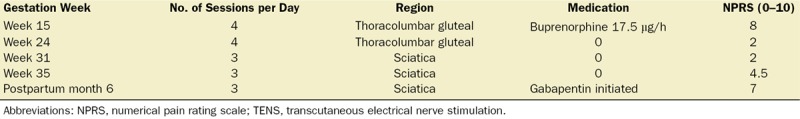
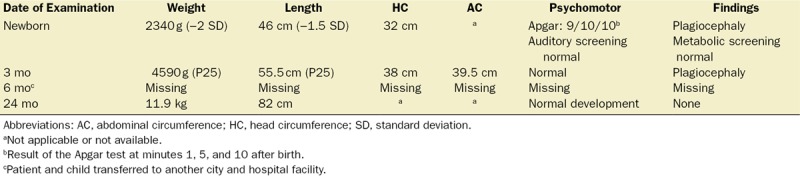
The patient continued to use TENS for analgesia while breastfeeding. At month 6 postpartum, she expressed a desire to restart pharmacotherapy due to an increase in end-of-day pain levels (NPRS, 7). Consequently, gabapentin was prescribed, and the patient discontinued TENS. At 24 months postpartum, the child’s development was normal, with no evidence of psychomotor impairment or neurological or congenital alterations (Table 2).
Table 2.
Postpartum Follow-Up of Daughter
DISCUSSION
The present case report describes the treatment of a pregnant patient with chronic NP due to NF1 who refused to continue opioid treatment. To our knowledge, there are no previous published data on NF1 and TENS.
Studies have shown that opioids can harm the fetus through direct effects on fetal development.1 Opioids can be detected in breast milk in concentrations directly related to plasma levels.6 During lactation, it may be necessary to evaluate the pharmacokinetics of the medications taken by the mother and the possibility of genetic or acquired impairments in the newborn’s metabolism. Mothers who report persistent perinatal pain are at increased risk of developing postpartum depression, and, therefore, pain management may be necessary.7 Recently, an association was found between birth defects and opioid usage before conception or during the first trimester.8 Consequently, physicians must carefully weigh the benefits and risks of prescribing opioids during pregnancy. In the final weeks of pregnancy, only the lowest effective dose should be used. Whenever possible, minor analgesics and complementary pain control methods should be considered to limit or reduce opioid use.2
Few studies have assessed the treatment of NP in pregnant patients. One study involving women in the third trimester of pregnancy found that TENS was more effective for the control of lumbar pain than either acetaminophen or exercise.4 The TENS group experienced a significantly (P < .001) greater degree of pain relief than the comparison groups, with no adverse effects associated. However, another study suggested that the use of TENS during pregnancy could harm intrauterine development.9 By contrast, a study conducted in an animal model found that the application of low- and high-frequency TENS to the abdomen of pregnant mice caused no deleterious teratogenic effects.10 In our case, the patient applied TENS only to the spinal column and sciatic regions. Due to anatomic differences, TENS is probably safer when applied to the spinal column rather than the abdomen due to the greater distance from the fetus. Nonetheless, to our knowledge, no studies have investigated the safety of abdominal TENS in humans during the first trimester of gestation.
Despite the limited evidence, some authors recommend TENS for obstetric and gynecological indications during the first or second trimesters due to its convenience, noninvasiveness, and efficiency.11 TENS can be used during labor, applied to both sides of the lower spine at a pulse duration of 200 microseconds at 100 Hz.12
Several case studies of pregnant patients treated with a neurostimulator have been reported. Although there are differences between TENS and neurostimulation, both of these 2 techniques apply an electrical current to reduce pain. One case report described a 24-year-old woman with a spinal cord stimulation (SCS) implant to control pain secondary to complex regional pain syndrome.13 After becoming pregnant, the patient continued to use the device intermittently. Those authors reviewed the published literature, finding that women who maintain the SCS activated during pregnancy have delivered healthy babies without life-threatening complications.
In our case, the patient had symptoms of withdrawal syndrome—attributed to the abrupt discontinuation of opioid therapy—when she presented to the pain clinic. Although she refused pharmacotherapy, she needed and requested treatment for the pain. This posed a serious care-related issue because nonpharmacological pain relief options are limited. After we explained the potential benefits and risks of TENS—emphasizing that only a few studies have evaluated TENS during pregnancy (especially in the first trimester)—the patient agreed to the proposed treatment. We monitored her closely to ensure safety and good analgesia. The pregnancy was carried to full term, and neither mother nor daughter experienced any treatment-related adverse effects. We followed the patient and child for 24 months after delivery to monitor for neurocognitive alterations.
At present, the evidence to support TENS for labor-related pain is limited. Evidence for treating NP with TENS is very low and heterogenic,3 being unable to state its effectiveness even in spinal pain, where evidence is inconclusive.14 Nevertheless, potential benefits may outweigh the risks, and the available data suggest that TENS does not appear to negatively impact either the mother or the baby. The use of TENS at home during the early stages of pregnancy has not been evaluated. Although the guidelines of the National Institute of Health and Clinical Excellence advise against TENS during labor, some women have chosen this treatment, with midwives’ support.12 Given the lack of adverse effects associated with TENS during labor, it seems unreasonable to deny women this option. However, more robust studies of effectiveness and safety are needed. Given the limited data and lack of high-quality scientific evidence, mainly due to the difficulty involved in conducting clinical trials in this patient population (the condition is relatively rare, and there are important ethical considerations in pregnant women), we believe that this case report may provide valuable data to add to the limited body of evidence, helping other clinicians to manage patients in similar circumstances. Clearly, given the limited evidence for TENS during pregnancy, both the mother and fetus should be closely monitored. A multidisciplinary approach involving specialists in obstetrics, neonatology, pain management, and anesthesiology should be applied in these cases to ensure good outcomes.
ACKNOWLEDGMENTS
The authors thank Carmen Caro of Bellvitge University Hospital for her help in reviewing the medical records. The authors especially thank the patient for her patience and help when we asked her for permission to publish her case report >2 years after the event. The authors also thank Bradley Londres for translating and editing the manuscript.
DISCLOSURES
Name: Victor Caño Silva, MD.
Contribution: This author helped treat the patient, write the discussion section, and review the complete manuscript.
Name: Ancor Serrano Afonso, MD.
Contribution: This author helped review the patient’s medical history and write the first draft of the full manuscript.
This manuscript was handled by: Mark C. Phillips, MD.
GLOSSARY
- CARE =
- Case Report Guidelines
- EQUATOR =
- Enhancing the Quality and Transparency of Health Research
- NF1 =
- neurofibromatosis type 1
- NP =
- neuropathic pain
- NPRS =
- numerical pain rating scale
- SCS =
- spinal cord stimulation
- TENS =
- transcutaneous electrical nerve stimulation
Funding: None.
The authors declare no conflicts of interest.
REFERENCES
- 1.Chan F, Koren G. Is periconceptional opioid use safe? Can Fam Physician. 2015;61:431–433. [PMC free article] [PubMed] [Google Scholar]
- 2.Kahan M, Wilson L, Mailis-Gagnon A, Srivastava A; National Opioid Use Guideline Group. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians: part 2–special populations. Can Fam Physician. 2011;57:1269–1276, e419e428. [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson W, Wand BM, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;9:CD011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keskin EA, Onur O, Keskin HL, Gumus II, Kafali H, Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecol Obstet Invest. 2012;74:76–83. [DOI] [PubMed] [Google Scholar]
- 5.Bedwell C, Dowswell T, Neilson JP, Lavender T. The use of Transcutaneous Electrical Nerve Stimulation (TENS) for pain relief in labour: a review of the evidence. Midwifery. 2011;27:e141–e148. [DOI] [PubMed] [Google Scholar]
- 6.Coluzzi F, Valensise H, Sacco M, Allegri M. Chronic pain management in pregnancy and lactation. Minerva Anestesiol. 2014;80:211–224. [PubMed] [Google Scholar]
- 7.Gaudet C, Wen SW, Walker MC. Chronic perinatal pain as a risk factor for postpartum depression symptoms in Canadian women. Can J Public Health. 2013;104:e375–e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritham UA, McKay L. Safe management of chronic pain in pregnancy in an era of opioid misuse and abuse. J Obstet Gynecol Neonatal Nurs. 2014;43:554–567. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira Guimarães CS, Santos Tavares FC, Santos MN, et al. Transcutaneous electrical nerve stimulation and placental vascularization in cases of uterine blood flow restriction. Fetal Pediatr Pathol. 2013;32:88–96. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama LM, Pires LA, Ferreira EA, Casarotto RA. Low- and high-frequency transcutaneous electrical nerve stimulation have no deleterious or teratogenic effects on pregnant mice. Physiotherapy. 2015;101:214–218. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan B, Rabinerson D, Pardo J, Krieser RU, Neri A. Transcutaneous Electrical Nerve Stimulation (TENS) as a pain-relief device in obstetrics and gynecology. Clin Exp Obstet Gynecol. 1997;24:123–126. [PubMed] [Google Scholar]
- 12.Francis R. TENS (Transcutaneous Electrical Nerve Stimulation) for labour pain. Pract Midwife. 2012;15:20–23. [PubMed] [Google Scholar]
- 13.Ahmed S, Lindsay JM, Snyder DI. Spinal cord stimulation for complex regional pain syndrome: a case study of a pregnant female. Pain Physician. 2016;19:E487–E493. [PubMed] [Google Scholar]
- 14.Resende L, Merriwether E, Rampazo ÉP, et al. Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain. 2018;22:663–678. [DOI] [PubMed] [Google Scholar]


