Abstract
Purpose
To determine if variation in anterior lamina cribrosa surface depth (ALCSD) differs between glaucoma patients of African (AD) and European descent (ED).
Methods
A total of 178 eyes from 123 glaucoma patients in the African Descent and Glaucoma Evaluation Study (ADAGES) and Diagnostic Innovations in Glaucoma Study (DIGS) were included. ALCSD and choroidal thickness were measured using the San Diego Automated Layer Segmentation Algorithm (SALSA). ALCSD was defined by both Bruch's membrane opening (BMO)-based (ALCSD-BMO) and scleral-based (ALCSD-Scl) reference planes. Racial differences in ALCSD were evaluated using cross-sectional univariate and multivariable models.
Results
A deeper ALCSD-Scl was found in males (52.4 μm, P = 0.0401), AD individuals (78.6 μm, P = 0.0004), younger individuals (−3.1 μm/year, P < 0.0213), and eyes with larger discs (81.0 μm/mm2, P = 0.024), increased visual field loss (mean defect, MD: −6.4 μm/dB [decibel], P = 0.0106), and higher intraocular pressure (IOP: 14.1 μm/mm Hg, P = 0.0256). Significant deepening of ALSCD was observed with increasing IOP and visual field severity only in the AD group. Race modified the relationship between ALCSD-Scl and age (P = 0.0145) with ALCSD-Scl in AD individuals becoming more shallow with increasing age (−3.1 μm/year, P = 0.0213), while there was no significant association in the ED group (2.1 μm/mm Hg, P < 0.2026).
Conclusions
This study demonstrates that a deeper ALCSD, regardless of the ALCSD reference plane used, is associated with more severe glaucoma and higher IOP in the ADAGES cohort, particularly in individuals of AD. These results suggest that characterizing ALCSD morphology and its relationships to IOP, aging, and glaucoma progression may help explain racial differences in disease susceptibility.
Keywords: optic nerve, glaucoma, lamina cribrosa
Spectral-domain optical coherence tomographic (SDOCT) imaging of the optic nerve head (ONH) has provided unprecedented insight into the histopathologic changes seen in the ONH due to aging and with disease.1 The ability to image deep within the ONH allows for the objective quantitative assessment of the morphology of the load-bearing connective tissues of the lamina cribrosa distinct from the overlying neurovascular tissues within the nerve head. This provides the opportunity to observe the effect of remodeling of the lamina cribrosa due to aging or pathologic processes directly in vivo. Since the mechanical behavior of any solid structure is determined by its material properties and morphology,2 alterations in the morphology of the lamina cribrosa due to age-related and/or glaucomatous remodeling in addition to individual variation in ONH morphology likely alter the biomechanical response of these tissues. Thus, the development of automated in vivo quantitative measurements of the morphometry of lamina cribrosa may provide mechanistically relevant biomarkers for glaucomatous optic neuropathy.
Remodeling of the lamina cribrosa is a well-described component of the glaucomatous process and has been studied in primate models,3–10 human donor tissues,11–14 and more recently via in vivo imaging studies.15–21 A significant posterior shift in the position of the lamina cribrosa has been demonstrated at a very early stage of glaucoma (preceding retinal nerve fiber layer loss) in the chronically elevated intraocular pressure (IOP) nonhuman primate model.6 In vivo imaging in humans has shown that the anterior lamina cribrosa surface depth (ALCSD) can be affected by the stage of the disease and may potentially move either anteriorly or posteriorly within the neural canal.3,17 A few recent cross-sectional studies have been published that suggest that the relationship between laminar remodeling and the development of glaucoma is significant and suggest that ALCSD may be an important biomarker for progression, which may be modulated by age.15–17
While changes in ALCSD have been demonstrated in glaucoma and with aging, no prior study has included individuals of African descent (AD) with glaucoma to determine if the association between ALCSD and disease severity or IOP exposure differs across racial groups with differential susceptibility to glaucoma. The AD population is at greater risk of disease progression22 and blindness from glaucoma.23–25 Our group has previously found significant differences in the association with ALCSD and normal aging26 and in the mechanical responses of the lamina cribrosa between AD and ED groups27 that suggest there are differences in age-related remodeling and biomechanical behavior of the ONH that could explain in part the differential susceptibility to glaucomatous injury across these racial groups. This study employs a recently developed automated segmentation algorithm28 with data collected as part of the African Descent and Glaucoma Evaluation Study (ADAGES)29 to determine if the variation in the depth of the anterior lamina cribrosa surface seen with age- and glaucoma-related remodeling of the optic nerve differs across individuals of African (AD) and European descent (ED).
Methods
The current study utilized SDOCT imaging data from participants in the National Eye Institute–funded multicenter ADAGES. ADAGES is conducted at three sites: the University of California at San Diego (UCSD), Columbia University (previously at New York Eye and Ear Infirmary), and the University of Alabama at Birmingham and is registered at clinicaltrials.gov under NCT00221897. Participants from the UCSD-based Diagnostic Innovations in Glaucoma Study (DIGS) were also included. The current study examined data from 178 eyes from 123 subjects with glaucoma (54 ED, 69 AD) that met all image quality and other study criteria. All participants gave written informed consent. The institutional review boards at all three sites approved the study methods. All methods adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act.
Details of the ADAGES cohort are described in detail elsewhere.29 Briefly, ADAGES and DIGS utilize identical methods and are conducted in accordance with manuals of procedures developed in parallel. While initial enrollment for ADAGES began in 2002, additional imaging studies have been added as new OCT instruments and scan protocols became available. Patients have been followed with imaging and visual field testing every 6 months since SDOCT imaging with the Spectralis (Heidelberg Engineering, Heidelberg, Germany) was added in 2010. The data used for the current study were obtained at this baseline Spectralis visit.
All participants underwent an ophthalmologic exam that included medical history, Snellen best-corrected visual acuity, Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, color vision, slit-lamp biomicroscopy, gonioscopy, applanation tonometry, central corneal thickness (CCT) measurement, lens opacity estimation, keratometry (K), axial length measurement, dilated funduscopy, stereoscopic ophthalmoscopy of the optic disc with a 78-diopter (D) lens, and simultaneous stereoscopic fundus photography. Standard perimetry tests were performed. Information regarding systemic conditions, medications, and several risk factors associated with glaucoma were also obtained, including measured blood pressure, family history, highest known IOP, age, history of diabetes, heart disease, and vascular disease.
At baseline, all eligible participants were over 18 years old, had open angles on gonioscopy, a best-corrected acuity of 20/40 or better, and refractive error up to 5.0 D sphere and 3.0 D cylinder. Diabetic participants with no evidence of retinal involvement were not excluded. Diagnosis was defined by two reliable tests on 24-2 standard automated perimetry (SAP) (Carl Zeiss Meditec, Dublin, CA, USA) using the Swedish Interactive Thresholding Strategy (SITA) at baseline for inclusion in the study. Reliability was defined as <33% false positives, false negatives, and fixation losses. All fields were reviewed by the UCSD Visual Field Assessment Center (VisFACT; see previous publication for description).29 Participants with a history of retinal pathologies, nonglaucomatous optic neuropathy, uveitis, ocular trauma, neurologic disease that may impact visual function (Parkinson's disease, Alzheimer's disease, dementia, or a history of stroke) or intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery) were excluded from the study. Participants with primary open-angle glaucoma were defined by two consecutive and reliable SAP examinations (SITA Standard) with either a pattern standard deviation outside 95% confidence limits or a glaucoma hemifield test result outside the normal limits and pattern of visual field damage consistent with glaucoma.
Image Acquisition and Processing
All subjects underwent imaging with Spectralis SDOCT (software version 5.6.4.0). The details of SDOCT imaging have been described elsewhere. For this study, a radial scan of the optic nerve consisting of 48 radial enhanced depth imaging (EDI) scans with 15 scan averaging (ART) per B-scan was used to assess ALSCD, and a high-density retinal nerve fiber layer circle scan was used to assess choroidal thickness. The San Diego Automated Layer Segmentation Algorithm (SALSA) was used to define the Bruch's membrane opening (BMO) reference plane, anterior laminar surface depth, and choroidal thickness in each B-scan.28 The mean of ALCSD measurements from all 48 B-scans within 1.8 μm of the center of the BMO from each individual eye was used for the analysis after qualitative review of the accuracy of the BMO and ALCSD segmentation. This approach has been described previously30 and employs a shape-constrained surface evolution method where the surface is refined iteratively using a nonlocal Markov random field-based segmentation. In particular, the method utilizes both two- and three-dimensional information of the lamina to overcome the problem of low signal in some areas. SALSA segmentation was also used to determine choroidal thickness from high-density retinal nerve fiber layer circle scans centered on the ONH.31 Similar to prior approaches with manual delineation,26,32 two measurements of ALCSD were used in the separate multivariable models: (1) ALCSD-BMO is defined from a BMO reference plane as described above; (2) ALCSD-Scl is defined from a sclera reference plane computed as the distance between the scleral and BMO plane at the 1.73-mm scan radius from the center of the optic disc (i.e., ALCSD-Scl = ALCSD-BMO − mean choroidal thickness).
Statistical Analysis
Associations between ALCSD-BMO and ALCSD-Scl and ocular and demographic characteristics were determined using univariable and separate multivariable linear regression models. In each model, generalized estimating equations (GEE) were used to account for correlations between both eyes of the same individual. An independent correlation structure with robust sandwich variance estimator was used. Independent variables in the model defining ALCSD-BMO included age, race, IOP, CCT, BMO area, choroidal thickness, and axial length. Independent variables in the model defining ALCSD-Scl included age, race, IOP, CCT, BMO area, and axial length, but not choroidal thickness as it was used in the calculation of ALCSD-Scl. All statistical analysis was performed in R Statistics (Foundation for Statistical Computing, Vienna, Austria) and a P value of <0.05 was considered statistically significant.
Results
The ocular and demographic characteristics are summarized in Table 1. The AD glaucoma patients were younger than the ED glaucoma patients (68.0 vs. 73.2 years; P = 0.0007). Mean IOP was higher in the AD group compared to the ED group (14.8 vs.13.7 mm Hg, respectively; P = 0.053), and CCT was thinner (527 vs. 537.7 μm, respectively; P = 0.109). All multivariable models were adjusted for age, axial length, sex, IOP, mean defect (MD), BMO area, and CCT. Choroidal thickness was also included in a covariate for ALCSD-BMO. Overall, mean ALCSD-BMO was 98 μm deeper and ALCSD-Scl was 86 μm deeper in the AD group compared to the ED group (Table 1; Fig. 1). After adjustment for covariates the differences in the AD compared to the ED group were 79.6 μm for ALCSD-BMO and 78.6 μm deeper for ALCSD-Scl.
Table 1.
Ocular and Demographic Characteristics of the African Descent and European Descent Groups*
|
European Descent |
African Descent |
P
Value |
|
| By patient | n = 54 | n = 69 | |
| Age | 73.2 (8.7) y | 68.0 (12.4) y | <0.001 |
| Sex | 40 F/41 M | 58 F/39 M | <0.001 |
| By eye | n = 79 | n = 97 | |
| Intraocular pressure | 13.7 (3.8) mm Hg | 14.8 (3.7) mm Hg | 0.053 |
| Central corneal thickness | 538 (43.0) μm | 527 (38) μm | 0.109 |
| Visual field mean defect | −7.1 (7.0) dB | −7.8 (7.4) dB | 0.499 |
| Axial length | 23.7 (2.8) mm | 23.9 (0.8) mm | 0.478 |
| BMO area | 2.08 (0.48) mm2 | 2.12 (0.5) mm2 | 0.574 |
| Choroidal thickness | 132 (68.0) μm | 145 (62.1) μm | 0.178 |
| ALCSD-BMO | 442 (127) μm | 540 (184) μm | <0.001 |
| ALCSD-Scl | 309 (119) μm | 395 (167) μm | <0.001 |
Significant P values are shown in bold.
Means and standard deviations unless otherwise noted.
Figure 1.
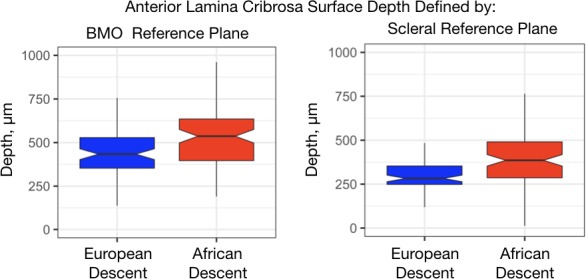
Notched box plots of the anterior lamina cribrosa surface depth by race defined by Bruch's membrane opening (left) and scleral reference planes (right). Central line, median; notch, 95th percentile confidence interval for the median; box, interquartile range, whisker 1.5*interquartile range above and below the 75th and 25th percentile, respectively.
The results of the GEE models for ALCSD-Scl and ALCSD-BMO are shown in Table 2 for the overall group without race as covariate. Central corneal thickness (P = 0.7293) and axial length (P = 0.6230) were not associated with ALCSD-Scl or ALCSD-BMO and therefore not included in the final models. Overall, the models based on ALCSD-Scl provide similar results to ALCSD-BMO models. For both models, a deeper ALCSD was associated with increasing BMO area, worse visual field MD, higher IOP, male sex, and younger age. Choroidal thickness was significantly and positively associated with ALCSD-BMO (0.6 μm/μm, P value = 0.0002), and including choroidal thickness in the multivariate model improved the model goodness-of-fit (χ2 < 0.001).
Table 2.
Results of Multivariable Models for Anterior Laminar Cribrosa Surface Depth for the Full Cohort, Excluding Race
|
Parameter |
Defined by Scleral Reference Plane |
Defined by Bruch's Membrane Opening Reference Plane |
||
|
Estimate (SE) |
P
Value |
Estimate (SE) |
P
Value |
|
| Intercept | 382.6 (19.6) μm | <0.001 | 521.6 (19.6) μm | <0.0001 |
| BMO area | 58.8 (26.1) μm/mm2 | 0.0241 | 62.8 (24.4) μm/mm2 | 0.0101 |
| Visual field mean defect | −6.42 (2.07) μm/dB | 0.0019 | −5.8 (2.0) μm/dB | 0.0033 |
| IOP | 12.8 (4.6) μm/mm Hg | 0.0057 | 10.5 (4.8) μm/mm Hg | 0.0289 |
| Age | −4.7 (1.1) μm/y | <0.0001 | −3.39 (1.13) μm/y | 0.0027 |
| Sex, male reference | −61.2 (25.3) | 0.0155 | −47.49 (25.4) | 0.0614 |
| Choroidal thickness | Not included | – | 0.6 (0.2) μm/μm | 0.0002 |
Significant P values are shown in bold.
Table 3 provides the estimates of the associations with ALCSD in the multivariable models across racial groups. These estimates were obtained by alternating ED and AD as reference in multivariable models adjusted for age, IOP, MD, sex, BMO area, and choroidal thickness (ALCSD-BMO only). Axial length and corneal thickness were nonsignificant and not included in the final models. The unadjusted associations between ALCSD-Scl and visual field MD, IOP, BMO area, and age are illustrated in the scatterplots in Figure 2. A significantly deeper ALCSD-Scl and ALCSD-BMO was associated with increasing BMO area, higher IOP, and increasing visual field severity, and with younger age in both models only in the AD group. The association with choroidal thickness and ALCSD-BMO was significant only in the ED group.
Table 3.
Estimates of Associations With Anterior Lamina Cribrosa Depth Across Racial Groups From Full Multivariable Models
|
Parameter |
Race |
Defined by Scleral Reference Plane |
Defined by Bruch's Membrane Opening Reference Plane |
||
|
Estimate (SE) |
P
Value |
Estimate (SE) |
P
Value |
||
| BMO area | ED | 32.7 (33.7) μm/mm2 | 0.3316 | 30.0 (33.5) μm/mm2 | 0.3698 |
| AD | 81.0 (26.7) μm/mm2 | 0.0024 | 80.6 (26.5) μm/mm2 | 0.0024 | |
| Visual field mean defect | ED | −3.5 (2.5) μm/1 dB | 0.1599 | −3.1 (2.5) μm/1 dB | 0.2231 |
| AD | −6.4 (2.5) μm/1 dB | 0.0106 | −7.6 (2.5) μm/1 dB | 0.0024 | |
| Intraocular pressure | ED | −2.4 (5.1) μm/mm Hg | 0.6426 | −0.2 (5.3) μm/mm Hg | 0.6969 |
| AD | 14.1 (6.3) μm/mm Hg | 0.0256 | 15.0 (6.0) μm/mm Hg | 0.0119 | |
| Age | ED | 2.1 (1.7) μm/y | 0.2026 | 2.0 (1.6) μm/y | 0.2182 |
| AD | −3.1 (1.4) μm/y | 0.0213 | −4.6 (1.5) μm/y | 0.0027 | |
| Choroidal thickness | ED | Not included | – | 0.6 (0.2) μm/μm | 0.0002 |
| AD | Not included | – | 0.5 (0.3) μm/μm | 0.0990 | |
All estimates of association were obtained by alternating ED and AD as reference in multivariable models adjusted for all significant covariates and racial interactions. Significant P values are shown in bold.
Figure 2.
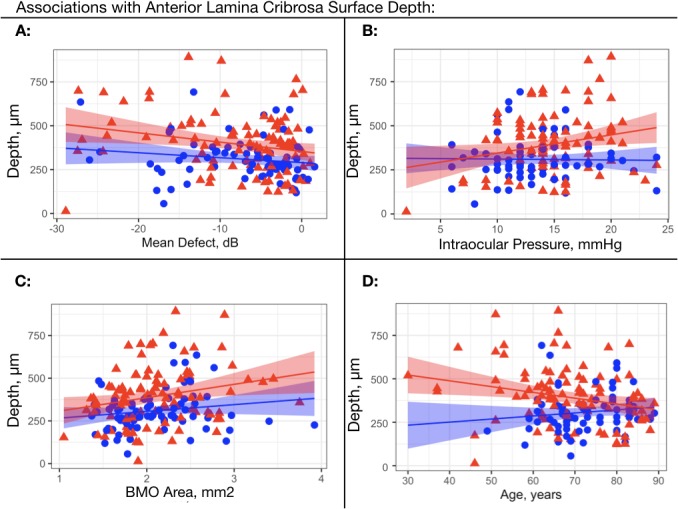
Scatterplots for the association of ALCSD with a sclera reference plane with visual field mean defect, intraocular pressure, Bruch's membrane opening, and age across racial groups. (A) Anterior lamina cribrosa surface depth defined from a scleral reference plane (ALCSD-Scl) plotted against visual field mean deviation. (B) ALCSD-Scl plotted against intraocular pressure. (C) ALCSD-Scl plotted against BMO area. (D) ALCSD-Scl plotted against age. Red triangles: African descent; blue triangles: European descent; regression line with 95% confidence intervals shown for AD (red band) ED (blue band).
The results of multivariable models for ALCSD-Scl and ALCSD-BMO are shown in Table 4 including racial interactions with age, IOP, MD, and BMO area. While the significant associations between visual field severity and ALCSD were only seen in the AD group, the interactions between race and MD were not significant in either model (ALCSD-Scl, P value = 0.4260; ALCSD-BMO, P value = 0.2205). The association between ALCSD-Scl and IOP (Fig. 2) was statistically significant in the AD group (Table 2, P value = 0.0119) but not the ED group (Table 2, P value = 0.9689) and the interaction with race was also significant (Table 3, P value = 0.045). For ALCSD-BMO, the interaction between race and IOP was weaker with this less parsimonious approach (Table 4; P value = 0.059). The association of ALCSD with age was also found to vary by race with greater shallowing of the lamina cribrosa with age in the AD compared to the ED group in both models (ALCSD-Scl: AD: −3.1 μm/year, ED: 2.1 μm/year, P value = 0.0145; ALCSD-BMO: AD: −4.6 μm/year, ED: 2.0 μm/year, P value = 0.0031). Given the narrower age range in the ED group, we performed an additional truncated analysis excluding AD subjects younger than the youngest ED subject (49 years old), and the results were similar to the results without excluding these younger ED subjects (see Table A1).
Table 4.
Results of Multivariable Models for Anterior Laminar Cribrosa Surface Depth Including Race and Racial Interactions
|
Parameter |
Defined by Scleral Reference Plane |
Defined by Bruch's Membrane Opening Reference Plane |
||
|
Estimate (SE) |
P
Value |
Estimate (SE) |
P
Value |
|
| Intercept | 408.1 (20.5) μm | <0.001 | 469.5 (21.8) μm | <0.001 |
| BMO area | 81.0 (26.7) μm/mm2 | 0.0024 | 80.6 (26.5) μm/mm2 | 0.0024 |
| Visual field mean defect | −6.4 (2.5) μm/dB | 0.0106 | −7.6 (2.5) μm/dB | 0.0024 |
| Intraocular pressure | 14.1 (6.3) μm/mm Hg | 0.0256 | 15.0 (6.0) μm/mm Hg | 0.0119 |
| Age | −3.1 (1.4) μm/y | 0.0213 | −4.6 (1.5) μm/y | 0.0027 |
| Sex, male reference | −52.4 (25.6) μm | 0.0401 | −57.7 25.3 μm | 0.0226 |
| Race, AD reference | −78.6 (22.1) μm | 0.0004 | −79.6 (22.0) μm | 0.0003 |
| Race*IOP, AD reference | −16.5 (8.2) μm/mm Hg | 0.0450 | −15.2 (8.0) μm/mm Hg | 0.0590 |
| Race*age | 5.2 (2.1) μm/y | 0.0145 | 6.6 (2.2) μm/y | 0.0031 |
| Race*visual field | 2.9 (3.7) μm/dB | 0.4260 | 4.5 (3.7) μm/dB | 0.2205 |
| Race*BMO area | −48.3 (42.9) μm/mm2 | 0.2602 | 50.6 (42.7) μm/mm2 | 0.2359 |
| Choroidal thickness | Not included | – | 0.46 (0.28) μm/μm | 0.0990 |
| Race*choroidal thickness | Not included | – | 0.16 (0.32) μm/μm | 0.6295 |
Significant P values are shown in bold.
Discussion
The current study is the first to evaluate the racial differences in the association between ALCSD and disease severity and ocular and demographic parameters in glaucomatous eyes of AD and ED. Overall, a deeper lamina cribrosa surface depth was independently associated with higher IOP, more severe visual field loss, thicker peripapillary choroid, male sex, AD, and a larger BMO area. A shallower ALCSD was associated with advancing age. The multivariable models produced similar results when ALCSD was defined from a scleral reference plane or by Bruch's membrane with choroidal thickness included in the model, suggesting these associations are not due to age- and disease-related changes in the choroid.
Moreover, there were several interactions between race, age, and IOP in the association with ALCSD. Specifically, there was greater shallowing in ALCSD in the AD group compared to the ED group with increasing age, regardless of which reference plane was used. There was less deepening of ALCSD with increased IOP in the AD group compared to the ED group in the measurement of ALCSD-Scl (Table 3). For both ALCSD-BMO and ALCSD-Scl, a significantly deeper lamina cribrosa was observed with increased visual field loss only in the AD group, but the interaction between with race and field severity was nonsignificant (Tables 3, 4).
Our results are consistent with several recent studies examining the association between laminar depth and glaucoma severity suggesting that the relationship between laminar remodeling and the development of glaucoma is significant and that anterior laminar morphology may be an important biomarker for progression.15–17 These studies have demonstrated that the anterior laminar surface can migrate both anteriorly and posteriorly within the neural canal as glaucoma progresses and that this migration is modulated by age.15–17 Ren and colleagues3 demonstrated an age-dependent effect on the remodeling of the lamina cribrosa in an ED population showing an increase in ALCSD depth in younger eyes; in contrast, in older eyes the anterior lamina became shallower when controlling for disease severity. Wu et al.17 followed 88 glaucoma patients and 20 healthy controls from a Chinese population with a mean follow-up of 5.3 years and reported that 12.3% of the glaucoma subjects demonstrated posterior displacement and 12.3% showed anterior displacement. Consistent with the findings of the current study in the AD population, posterior shifts in the lamina in the Chinese population were significantly associated with higher mean follow-up IOP (2 μm/mm Hg) and inversely related to age with less posterior displacement. Lee et al.15 studied 53 glaucoma patients and 53 age-matched controls using swept-source OCT and found that ALCSD was significantly correlated with disease severity. Park et al.16 also recently demonstrated that the stage of glaucoma was significantly associated with laminar depth. While these prior studies demonstrate that the complex association with ALCSD and glaucoma severity is modulated by age and IOP, none of these studies examined these associations in individuals of AD, who have a greater susceptibility to glaucomatous injury and clear evidence of morphologic differences in the load-bearing connective tissues of the ONH that likely affect the remodeling of the lamina cribrosa.
Vianna and colleagues32 have recently reported the results of ALCSD based on manually delineated scleral and Bruch's membrane-based reference planes in a longitudinal dataset. Their scleral reference plane is calculated by subtracting the mean choroidal thickness within radial scans at 1.7 to 1.8 mm from the measurement of ALCSD taken from a Bruch's-based reference plane. This is similar to the methods we used to define a scleral reference plane from our early work also using manual segmentation first demonstrating the association between BMO and choroidal thickness in SDOCT.33 The automated methods used in the current paper are based on choroidal thickness measurement taken from a similarly located circle scan with a radius of 1.73 mm. We approached the analysis in two ways for this study. First we included choroidal thickness as a covariate in a multivariable model defining ALSCO based on a BMO reference plane. This allows for the observation of the relative impact of choroidal thickness within the model. The second analysis subtracted choroidal thickness from the BMO-based ALCSD measurements to develop a “scleral”-based reference plane similar to the approach performed by Vianna and colleagues.32 As expected, both approaches provide very similar results, with the scleral-based approach being more efficient from a statistical modeling perspective. Using similar reference planes, our results are consistent with those of Vianna and colleagues, who reported a positive association between ALCSD and IOP and a strong association between ALCSD and choroidal thickness. These associations were lessened but not removed when using a scleral reference plane to define ALCSD.
The current study paradoxically found anterior migration of the ALCSD associated with age in the AD group. The negative association between ALCSD with age observed within the AD glaucoma group is in contrast to positive associations with aging reported in our prior studies using SDOCT in normal eyes26 within an AD cohort and in human donor studies examining the three-dimensional histomorphology of the lamina cribrosa and ONH.34 Given that the analysis in the current study provides similar results when adjusting for choroidal thickness within the multivariable model (ALCSD-BMO, Table 4) or accounting for choroidal thickness variation within the measurement of ALCSD (ALCSD-Scl, Table 2), it is unlikely that these results are driven by differences in reference plane and measurement approach. There are several possible explanations for the differences in the age-related changes between our current study and our prior publications. Prior publications from our group using SDOCT26,27,35 and histology34 included only younger, normal individuals. It is possible that the associations with aging are indeed different in glaucoma patients and/or are stronger in older individuals.
There are several limitations to the current study. The study sample size is relatively small and may in part explain why racial differences in the association with visual field loss that were suggested in the stratified analysis did not reach statistical significance based on testing of the interaction term that included race. The age range in ADAGES subjects was less broad in the ED group compared to the AD group. This could potentially have made it more difficult to find a statistically significant association between age and ALCSD in the ED group compared to the AD group. However, when analysis excluded AD patients younger than the youngest ED (49 years old), no meaningful differences in the estimated parameters were observed (Table A1).
The study is also limited due to categorization using self-described race, a term that represents an amalgam of cultural, geographic, socioeconomic, and biologic characteristics, which cannot be interpreted in the strict biologic sense.36 However, self-described race has demonstrated associations in several diseases37 and correlates will with more sophisticated measures of genetic admixture.38 When we examined the distribution of biogeographic ancestral (BGA) testing in our baseline normal sample from a portion of the ADAGES cohort, we demonstrated that self-described race strongly correlated to genetically defined race.39 Moreover, BGA testing added little additional information regarding racial differences in ocular structures known to sizably differ across AD and ED groups (corneal thickness and optic disc area) beyond self-described race alone.39
In summary, this study has demonstrated significant racial differences in the association of the morphology of the anterior load-bearing surfaces of the ONH and peripapillary choroid with aging and variation in IOP. ALCSD was associated with disease severity only in the AD group. Longitudinal studies involving racially diverse cohorts are needed to characterize variation in ALCSD morphology and its influence on glaucoma development and progression in this at-risk minority population.
Acknowledgments
Supported by National Eye Institute R01 EY028284 (CAG, MAF), R01 EY026574 (MAF, CAG, LMZ), R01 EY 029058 (RNW), EY11008 (LMZ), EY19869 (LMZ), EY14267 (LMZ), EY027510 (LMZ), P30EY022589 (LMZ), EyeSight Foundation of Alabama (CAG, MAF), and Research to Prevent Blindness (CAG, MAF, RNW).
Disclosure: C.A. Girkin, Heidelberg Engineering, GmbH (F); M.A. Fazio, Heidelberg Engineering, GmbH (F), Equinox Ophthalmic (F), Vasoptic Medical, Inc. (F); C. Bowd, None; F.A. Medeiros, Alcon (C), Allergan (C, F), Ametek (C, F), Bausch & Lomb (F), Carl-Zeiss Meditec (C, F, R), Heidelberg Engineering (C, F), Sensimed (F), Topcon (F); R.N. Weinreb, Alcon (C), Aerie Pharmaceuticals (C, F), Allergan (C), Bausch & Lomb (C), Carl Zeiss Meditec (C, F), Centervue (F), Eyenovia (C), Genentech (F), Heidelberg Engineering (F), Implandata (C), Meditec-Zeiss (F), Optovue (F), Topcon (C, F); J.M. Liebmann, Alcon (C), Allergan (C), Bausch & Lomb (C, F), Carl Zeiss Meditec (C, F), Heidelberg Engineering (C, F), National Eye Institute (F), Optovue (F), Reichert (C, F), Topcon (F), Valeant Pharmaceuticals (C); J. Proudfoot, None; L.M. Zangwill, Carl Zeiss Meditec (F), Heidelberg Engineering (F, R), National Eye Institute (F), Optovue, Inc. (F), Topcon Medical System, Inc. (F); A. Belghith, None
Table A1.
Age-Truncated Results of Multivariable Models for Anterior Laminar Cribrosa Surface Depth Including Race and Racial Interactions
|
Parameter |
Defined by Scleral Reference Plane |
Defined by Bruch's Membrane Opening Reference Plane |
||
|
Estimate (SE) |
P
Value |
Estimate (SE) |
P
Value |
|
| Intercept | 405.2 (21.3) μm | <0.001 | 467.7 (21.1) μm | <0.001 |
| BMO area | 74.4 (26.5) μm/mm2 | 0.0050 | 74.3 (26.2) μm/mm2 | 0.0046 |
| Visual field mean defect | −8.1 (2.4) μm/dB | 0.0006 | −8.5 (2.5) μm/dB | 0.0006 |
| Intraocular pressure | 9.7 (5.7) μm/mm Hg | 0.0888 | 10.7 (5.7) μm/mm Hg | 0.0594 |
| Age | −3.8 (1.7) μm/y | 0.0263 | −4.5 (4.7) μm/y | 0.0305 |
| Sex, male reference | −43.4 (25.4) μm | 0.0873 | −50.5 (25.8) μm | 0.0504 |
| Race, AD reference | −78.0 (21.8) μm | 0.0004 | −76.0 (22.0) μm | 0.0005 |
| Race*IOP, AD reference | −11.8 (7.7) μm/mm Hg | 0.1256 | −10.5 (7.9) μm/mm Hg | 0.1826 |
| Race*age | 5.9 (2.4) μm/y | 0.0139 | 6.2 (2.8) μm/y | 0.0235 |
| Race*visual field | 4.7 (3.6) μm/dB | 0.1886 | 5.7 (3.6) μm/dB | 0.1201 |
| Race*BMO area | −40.8 (42.3) μm/mm2 | 0.3351 | −44.8 (42.4) μm/mm2 | 0.1201 |
| Choroidal thickness | Not included | – | 0.71 (0.27) μm/μm | 0.0930 |
| Race*choroidal thickness | Not included | – | −0.08 (0.31) μm/μm | 0.9020 |
Analysis performed eliminating AD individuals below the lowest age of the ED group (49 years). Mean ages of truncated groups were similar across race, P = 0.4566.
References
- 1.Tan NY, Koh V, Girard MJ, Cheng CY. Imaging of the lamina cribrosa and its role in glaucoma: a review. Clin Exp Ophthalmol. 2018;46:177–188. doi: 10.1111/ceo.13126. [DOI] [PubMed] [Google Scholar]
- 2.Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93:120–132. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren R, Yang H, Gardiner SK, et al. Anterior lamina cribrosa surface depth, age, and visual field sensitivity in the Portland Progression Project. Invest Ophthalmol Vis Sci. 2014;55:1531–1539. doi: 10.1167/iovs.13-13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockwood H, Reynaud J, Gardiner S, et al. Lamina cribrosa microarchitecture in normal monkey eyes part 1: methods and initial results. Invest Ophthalmol Vis Sci. 2015;56:1618–1637. doi: 10.1167/iovs.14-15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Williams G, Downs JC, et al. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52:7109–7121. doi: 10.1167/iovs.11-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF. Longitudinal change detected by spectral domain optical coherence tomography in the optic nerve head and peripapillary retina in experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52:1206–1219. doi: 10.1167/iovs.10-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Thompson H, Roberts MD, Sigal IA, Downs JC, Burgoyne CF. Deformation of the early glaucomatous monkey optic nerve head connective tissue after acute IOP elevation in 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2011;52:345–363. doi: 10.1167/iovs.09-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts MD, Grau V, Grimm J, et al. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Downs JC, Girkin C, et al. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48:4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgoyne CF, Downs JC, Bellezza AJ, Hart RT. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest Ophthalmol Vis Sci. 2004;45:4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- 11.Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. [PubMed] [Google Scholar]
- 12.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- 13.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 14.Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95:673–691. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee KM, Kim TW, Weinreb RN, Lee EJ, Girard MJ, Mari JM. Anterior lamina cribrosa insertion in primary open-angle glaucoma patients and healthy subjects. PLoS One. 2014;9:e114935. doi: 10.1371/journal.pone.0114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SC, Brumm J, Furlanetto RL, et al. Lamina cribrosa depth in different stages of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:2059–2064. doi: 10.1167/iovs.14-15540. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Xu G, Weinreb RN, Yu M, Leung CK. Optic nerve head deformation in glaucoma: a prospective analysis of optic nerve head surface and lamina cribrosa surface displacement. Ophthalmology. 2015;122:1317–1329. doi: 10.1016/j.ophtha.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Lee KM, Choung HK, Kim M, Oh S, Kim SH. Positional change of optic nerve head vasculature during axial elongation as evidence of lamina cribrosa shifting: Boramae Myopia Cohort Study Report 2. Ophthalmology. 2018;125:1224–1233. doi: 10.1016/j.ophtha.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Ha A, Kim TJ, Girard MJA, et al. Baseline lamina cribrosa curvature and subsequent visual field progression rate in primary open-angle glaucoma. Ophthalmology. 2018;125:1898–1906. doi: 10.1016/j.ophtha.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Gizzi C, Cellini M, Campos EC. In vivo assessment of changes in corneal hysteresis and lamina cribrosa position during acute intraocular pressure elevation in eyes with markedly asymmetrical glaucoma. Clin Ophthalmol. 2018;12:481–492. doi: 10.2147/OPTH.S151532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ersoz MG, Kunak Mart D, Hazar L, Ayintap E, Botan Gunes I, Konya HO. Evaluation of prelaminar region and lamina cribrosa with enhanced depth imaging optical coherence tomography in pseudoexfoliation glaucoma. Turk J Ophthalmol. 2018;48:109–114. doi: 10.4274/tjo.05882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 23.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996;103:1721–1726. doi: 10.1016/s0161-6420(96)30435-1. [DOI] [PubMed] [Google Scholar]
- 24.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 25.Drance S, Anderson DR, Schulzer M;, Collaborative Normal-Tension Glaucoma Study Group Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes LA, Huisingh C, Johnstone J, et al. Variation of laminar depth in normal eyes with age and race. Invest Ophthalmol Vis Sci. 2014;55:8123–8133. doi: 10.1167/iovs.14-15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazio M, Johnstone J, Smith B, Wang L, Girkin C. Displacement of the lamina cribrosa in response to acute intraocular pressure elevation in normal individuals of African and European descent. Invest Ophthalmol Vis Sci. 2016;57:3331–3339. doi: 10.1167/iovs.15-17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belghith A, Bowd C, Medeiros FA, Weinreb RN, Zangwill LM. Automated segmentation of anterior lamina cribrosa surface: how the lamina cribrosa responds to intraocular pressure change in glaucoma eyes. Proc IEEE Int Symp Biomed Imaging. 2015. pp. 222–225.
- 29.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belghith A, Bowd C, Medeiros FA, et al. Does the location of Bruch's membrane opening change over time? Longitudinal analysis using San Diego Automated Layer Segmentation Algorithm (SALSA) Invest Ophthalmol Vis Sci. 2016;57:675–682. doi: 10.1167/iovs.15-17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mundae RS, Zangwill LM, Kabbara SW, et al. A longitudinal analysis of peripapillary choroidal thinning in healthy and glaucoma subjects. Am J Ophthalmol. 2018;186:89–95. doi: 10.1016/j.ajo.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vianna JR, Lanoe VR, Quach J, et al. Serial changes in lamina cribrosa depth and neuroretinal parameters in glaucoma: impact of choroidal thickness. Ophthalmology. 2017;124:1392–1402. doi: 10.1016/j.ophtha.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone J, Fazio M, Rojananuangnit K, et al. Variation of the axial location of Bruch's membrane opening with age, choroidal thickness, and race. Invest Ophthalmol Vis Sci. 2014;55:2004–2009. doi: 10.1167/iovs.13-12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girkin CA, Fazio MA, Yang H, et al. Variation in the three-dimensional histomorphometry of the normal human optic nerve head with age and race: lamina cribrosa and peripapillary scleral thickness and position. Invest Ophthalmol Vis Sci. 2017;58:3759–3769. doi: 10.1167/iovs.17-21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhodes LA, Huisingh C, Johnstone J, et al. Peripapillary choroidal thickness variation with age and race in normal eyes. Invest Ophthalmol Vis Sci. 2014;56:1872–1879. doi: 10.1167/iovs.14-16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommer A. Epidemiology, ethnicity, race, and risk. Arch Ophthalmol. 2003;121:1194. doi: 10.1001/archopht.121.8.1194. [DOI] [PubMed] [Google Scholar]
- 37.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 38.Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 39.Girkin CA, Nievergelt CM, Kuo JZ, et al. Biogeographic Ancestry in the African Descent and Glaucoma Evaluation Study (ADAGES): association with corneal and optic nerve structure. Invest Ophthalmol Vis Sci. 2015;56:2043–2049. doi: 10.1167/iovs.14-15719. [DOI] [PMC free article] [PubMed] [Google Scholar]


