This continues to be an exciting time for pediatric heart transplantation. Since the first orthotopic heart transplantation a little over 50 years ago, the field has grown tremendously, including many advancements in the application of this therapy to children. Today, heart transplantation is performed routinely in many pediatric centers throughout the world with over 100 centers reporting to the International Thoracic Transplant Registry (Figure 1, eSlide H(p) 4). The Registry is the largest source of worldwide data on pediatric heart transplantation with over 14,000 transplants reported in children. The focus theme for this year’s report is donor and recipient size match.
Figure 1.
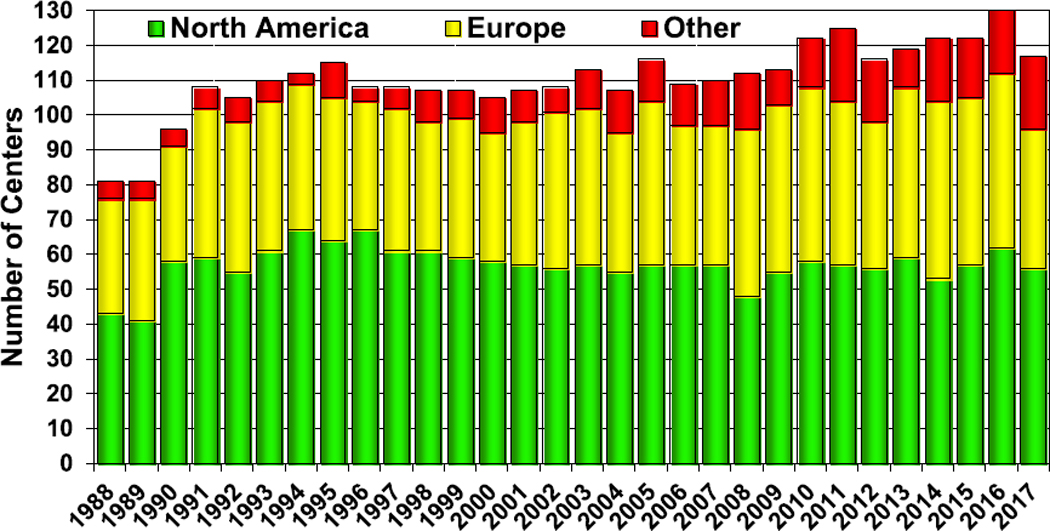
Number of centers reporting pediatric heart transplants.
Statistical methods
Data collection, conventions, and statistical methods
National and multinational organ and data exchange organizations and individual centers submit data to the International Society for Heart and Lung Transplantation (ISHLT) International Thoracic Organ Transplant Registry. Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 heart-lung transplant centers have reported data to the Registry. We estimate that data submitted to the Registry represent approximately 80% of worldwide heart transplant activity.
An overview of donor and recipient characteristics and outcomes is presented throughout the report. The data are supplemented with additional and extended analyses presented in the online slide sets (3 separate slide sets, named “Introduction/ General Statistics”, “Overall Heart Transplantation Statistics”, and “Pediatric Heart Transplantation Statistics”, https://ishltregistries.org/registries/slides.asp). Slide sets for previous annual reports are also available on this site. This report refers to specific online e-slides when particular data are discussed but not shown because of space limitations; e-slide numbers refer to the online pediatric heart transplant slide set (eSlide H(p)).
The ISHLT Registry website (http://ishlt.org/registries/ttxregistry) provides detailed spreadsheets of data elements collected in the Registry. The ISHLT Registry requires submission of core donor, recipient, and transplant procedure variables at baseline (before and at time of transplantation) and at yearly follow-up, and these variables therefore have low rates of missingness. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for ISHLT Registry variables that depend on voluntary reporting. The ISHLT Registry uses various quality control measures to ensure acceptable data quality and completeness before including data from individual centers and regions for analyses.
Analytic conventions
Unless otherwise specified, heart-lung transplants are not included in analyses of heart transplants or lung transplants. Retransplant includes those with a previously reported transplant of the same organ type, same organ type in combination, or with a retransplant diagnosis. Because identification of all transplants for an individual may not be complete, the number of retransplant events may be slightly underestimated. The ISHLT Registry does not capture the exact occurrence date for most secondary outcomes (e.g., coronary artery disease), but it does capture the window of occurrence (i.e., the event occurred between the first and the second annual follow-up visits). For the annual report, the midpoint between annual follow-ups is used as a proxy for the event date. There are specific conventions in reporting secondary outcomes and other follow-up information where a recipient has died. To reduce the possibility of underestimating event rates or other outcomes, some analyses are limited to surviving patients. For time-to-event rates and cumulative morbidity rates, follow-up of recipients not experiencing the event of interest is censored at the last time the recipient was reported not to have had the event, either the most recent annual follow-up or the time of retransplantation. Time-to-event graphs (survival graphs) are truncated when the number of individuals still at risk is <10. Additional information regarding the general statistical methods used for analyses and data interpretation is included in the Supplementary Material available online (www.jhltonline.org).
Focus theme methods: Donor-recipient size match
The ISHLT Registry Steering Committee selected donor-recipient size match as the theme topic for the 2019 annual report given recent interest in identifying the optimal metric for matching donor and recipient size and in studying the short- and long-term clinical consequences of size mismatch. Body weight has been the traditional metric for matching donor and recipient size1–3 as suggested by the ISHLT guidelines, which state that “As a general rule, the use of hearts from donors whose body weight is no greater than 30% below that of the recipient is uniformly safe, though greater size mismatches have been successfully used in pediatric heart transplantation.”4,5 Some transplant centers, however, prefer using height as a metric to match donor and recipient size,6,7 whereas body mass index and body surface area have been suggested as other suitable measures. Recently, predicted heart mass (PHM), an estimate of heart size that incorporates height, weight, age, and sex, has been proposed as the optimal metric for size matching in adult heart transplantation.8,9 However, given the lack of validation of PHM calculation in children, PHM was not used for the pediatric analysis in the annual ISHLT Registry report.
Pediatric heart transplant—overview of donor and recipient demographics, survival, and morbidity outcomes
Centers and activity
Data were reported to the Registry from 117 centers in 2017, a slight decrease from the previous year (Figure 1, eSlide H(p) 4). The majority of the centers continue to be from North America and Europe. There are 21 centers from other parts of the world, representing a substantial increase from the 13 centers that reported data on pediatric heart transplantation in 2007. Among pediatric transplants, most (73%) are performed at small-volume centers, averaging 1 to 4 transplants per year. Only 10% are large-volume centers, averaging 10 or more transplants per year (Figure 2, eSlide H(p) 5). These large centers performed 46% of all transplants since 2010 (eSlide H(p) 6). Medium-volume centers (centers averaging 5–9 transplants per year) performed 29% of all transplants since 2010, an increase from 17% from 2005 to 2009. More transplants are performed at small- and medium-volume centers in Europe and in other parts of the world than in North America (eSlide H(p) 8).
Figure 2.
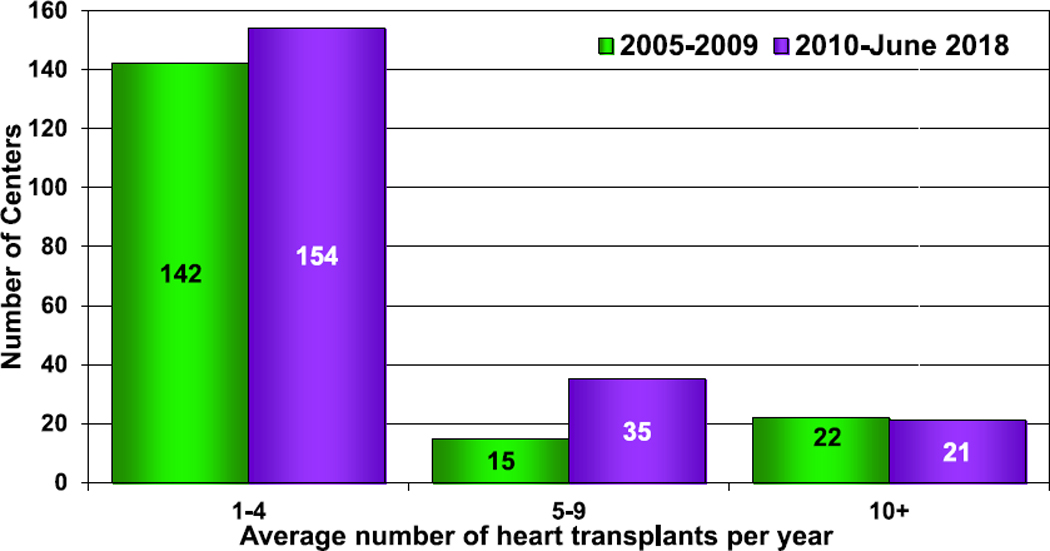
Number of centers by center volume (transplants: January 2005−June 2018).
Donor and recipient demographics and characteristics
The age distribution of pediatric recipients is similar to prior reports. Infants (age <1 year at transplant) continue to account for the greatest number of transplants per 1 year of life with nearly 1,800 transplants from January 2005 to June 2018 (eSlide H(p) 9, 10). The primary indication for transplant varies by age, with congenital heart disease (CHD) being the most common indication in infants (57%) and cardiomyopathy being the most common indication in older children (43% in children aged 1–10 years and 53% in children aged 11–17 years) (eSlides H(p) 16–19). The indication for transplant and the age at transplant varies by geographic region, with more patients with CHD undergoing transplants in North America than in Europe and other parts of the world (Figure 3, eSlide H(p) 20).
Figure 3.
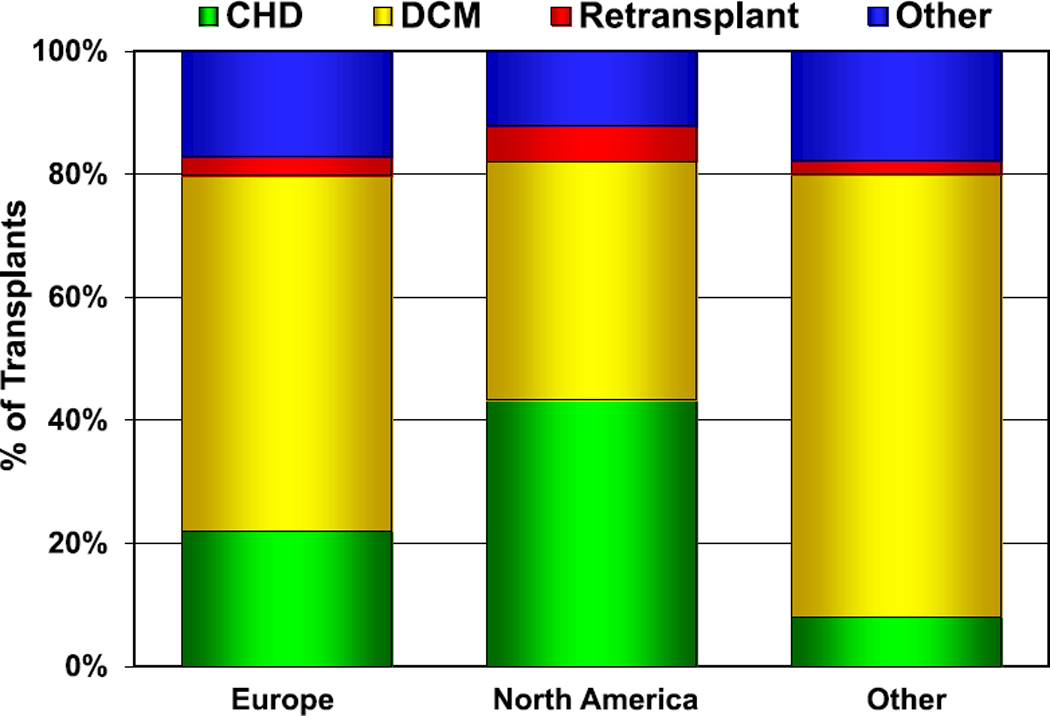
Diagnosis distribution by location (transplants: January 2005–June 2018). CHD, congenital heart disease; DCM, dilated cardiomyopathy.
Mechanical circulatory support (MCS) as a bridge to heart transplantation is being increasingly utilized in children, as it is in adults. In 2017, 37% of children were supported to transplant on some form of MCS, with the vast majority supported on a ventricular assist device (VAD) (eSlides H(p) 21, 22). Fewer patients with CHD were supported with VADs than were children with dilated cardiomyopathy (DCM) (Figure 4, eSlides H(p) 23–25). Only 12% of infants with CHD were bridged to transplant using some form of MCS, with extracorporeal membrane oxygenation (ECMO) used as commonly as a VAD. This is in contrast to over 50% of older children with DCM supported on MCS before transplant, with ECMO as a bridge in <3% of patients.
Figure 4.
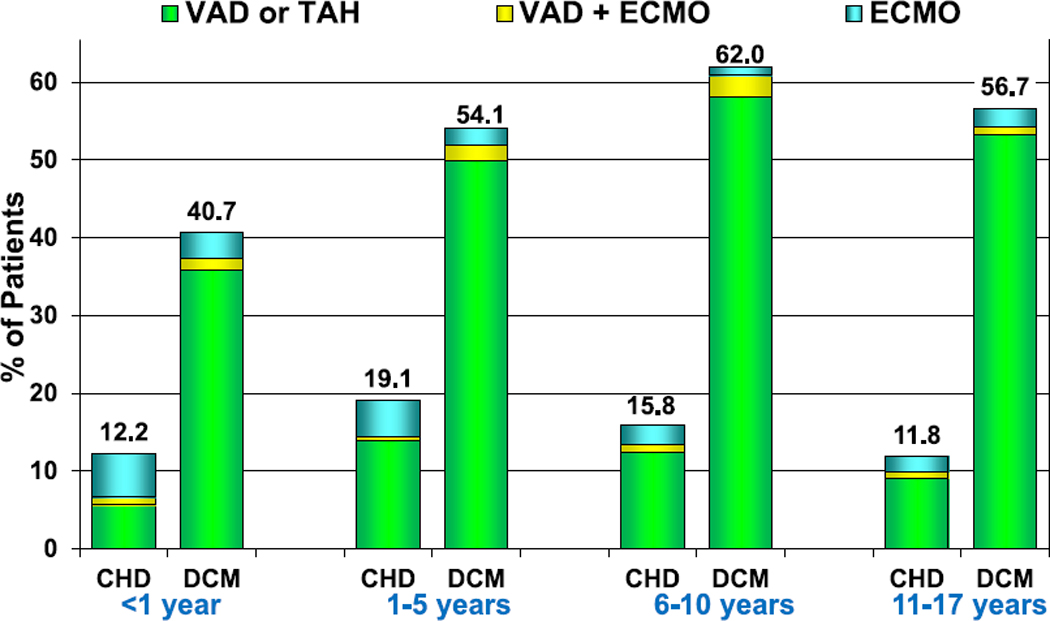
Percentage of patients bridged with mechanical circulatory support by age group and diagnosis (transplants: January 2010–June 2018). CHD, congenital heart disease; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; TAH, total artificial heart; VAD, ventricular assist device.
Pediatric heart transplant recipients are at risk for allosensitization from a variety of factors including prior surgeries, blood product exposure, and the use of homografts for the repair of CHD. Allosensitization, defined as a panel reactive antibody (PRA) level ≥ 10%, continues to increase among pediatric heart transplant recipients. In 2007, only 14% of recipients had allosensitization compared with 32% in 2017 (eSlide H(p) 26). Allosensitization is more common in older children than infants and among patients with CHD and retransplant than DCM or other types of heart disease (Figure 5, eSlides H(p) 27–29).
Figure 5.
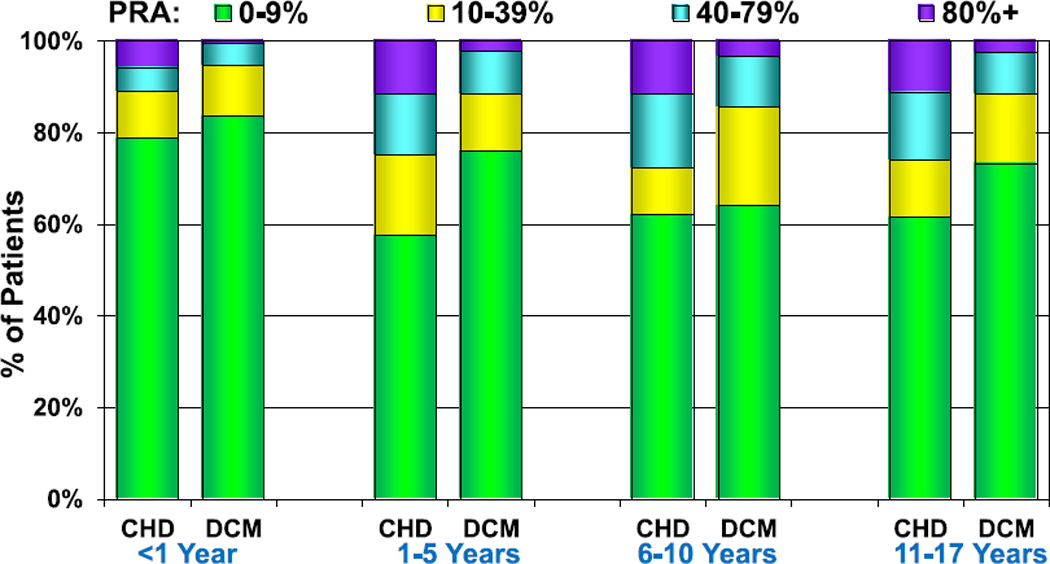
PRA distribution by age group and diagnosis (transplants: January 2010–June 2018). CHD, congenital heart disease; DCM, dilated cardiomyopathy; PRA, panel reactive antibody.
There are geographic differences in donor characteristics that parallel the geographic differences in recipient characteristics. Young donors, especially 5 years or younger, are more common in North America whereas older donors, especially those aged 35 years and older, are more common in Europe and other parts of the world (eSlides H (p) 12–15). As donor-recipient size match is the focus theme for this report, it is described in greater detail below.
Survival
Given the long duration of follow-up available in the ISHLT Registry, survival rates up to 25 years post-transplant can be determined. The overall median survival is more than 18 years, with the longest survival among children undergoing their transplant in infancy (median survival 24.5 years) and the shortest survival among children undergoing transplant at 11 to 17 years of age (median survival 14.3 years) (Figure 6, eSlide H(p) 31). As prior reports have indicated, the highest risk of death is during the early post-transplant period.10 Recipients that survive to 1-year post-transplant have excellent long-term survival. More than 60% of those who underwent transplant as infants and survived past 1 year after transplant are alive 25 years later (and thus median conditional survival cannot be estimated in this cohort). Median conditional survival ranges from 16.7 years to 21.9 years in recipients undergoing transplant as older children (Figure 7, eSlide H(p) 32). There continues to be an era effect with improved survival in recent eras compared with earlier eras (Figure 8, eSlide H(p) 35). Although the improved survival between eras is statistically significant, it is not seen in all age groups, and the magnitude of improvement appears to be decreasing (eSlides H(p) 37–40).
Figure 6.
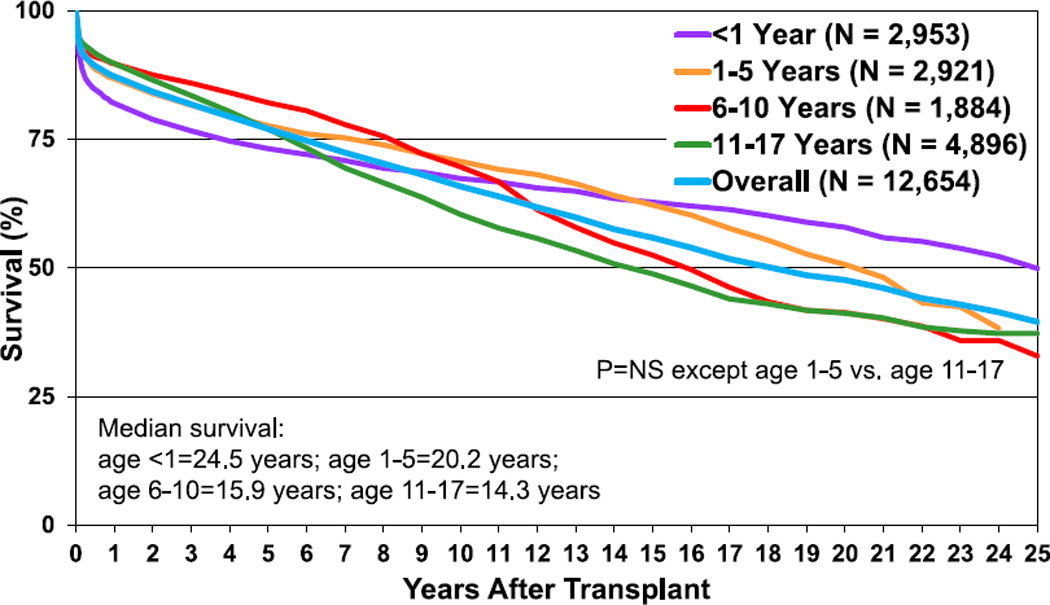
Kaplan-Meier survival by recipient age at transplant (transplants: January 1992–June 2017). NS, not significant.
Figure 7.
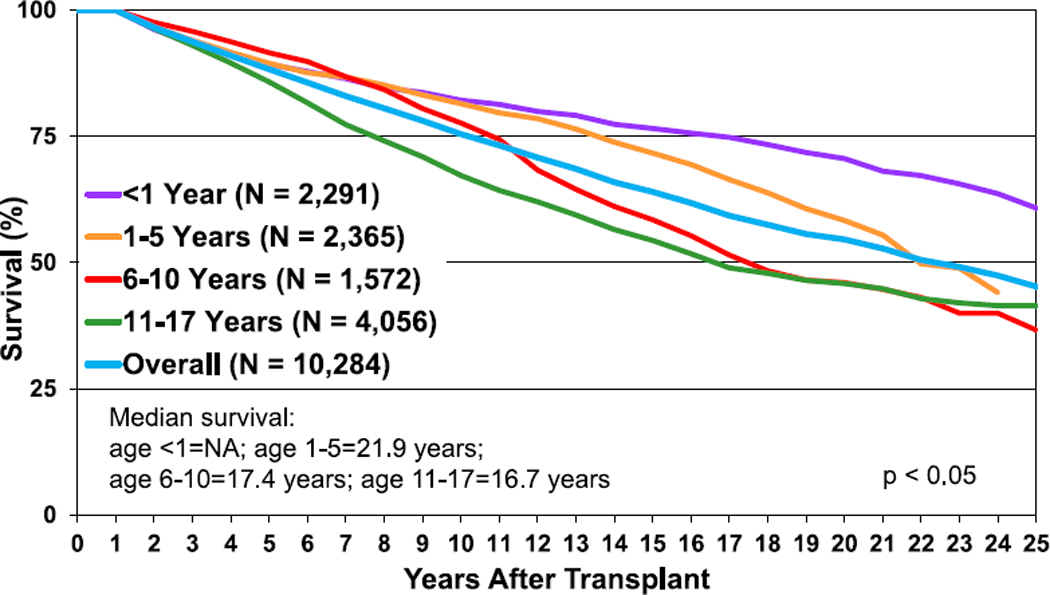
Kaplan-Meier survival conditional to survival to 1 year after transplant, by recipient age at transplant (transplants: January 1992–June 2017). NA, not applicable.
Figure 8.
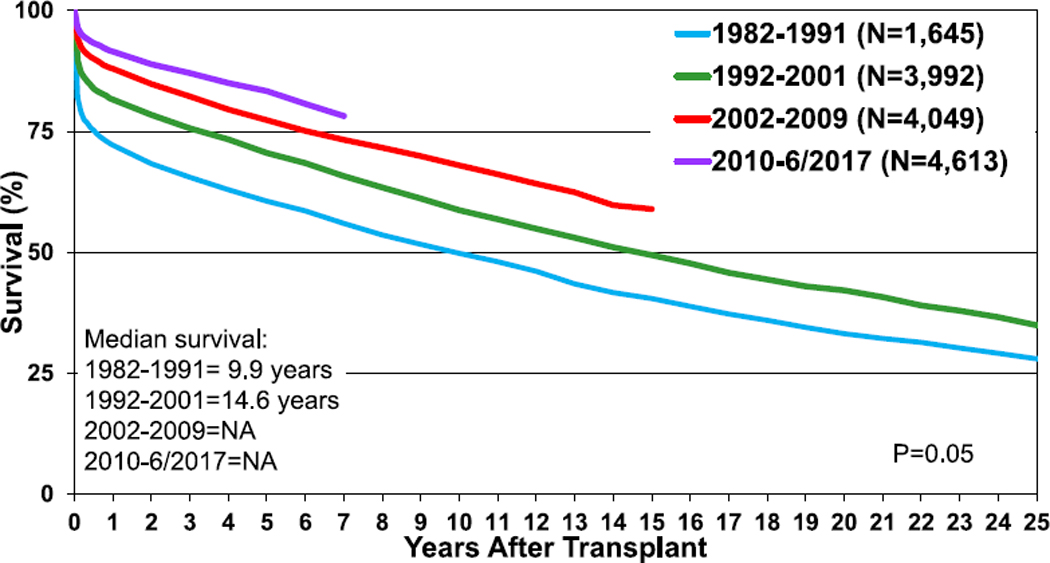
Kaplan-Meier survival by era (transplants: January 1982–June 2017). NA, not applicable.
Survival does not differ by sex or by sex match or mismatch on univariable analyses (eSlides H(p) 41, 42). Survival differences do exist by indication for transplant, and the magnitude of these differences varies by the age at transplant (Figure 9, eSlides H(p) 43–46). Overall, recipients with DCM have superior survival than those with CHD or those undergoing retransplantation. Importantly, recipients supported with a VAD or total artificial heart had similar survival to those without MCS. The use of ECMO as a bridge to transplant continues to be associated with reduced survival (eSlide H(p) 51).
Figure 9.
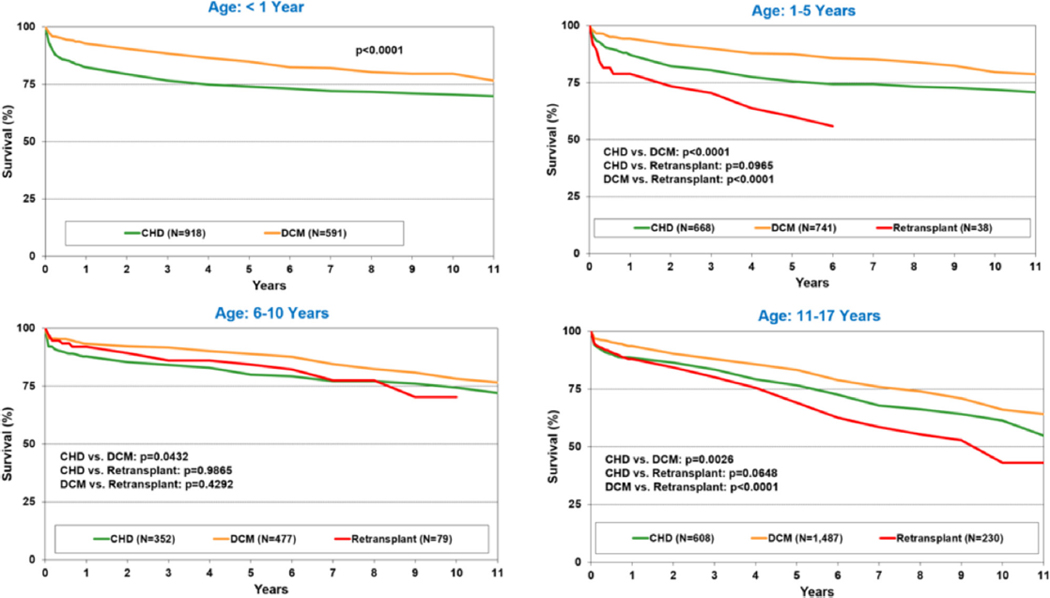
Kaplan-Meier survival by recipient age group and diagnosis (transplants: January 2005–June 2017). CHD, congenital heart disease; DCM, dilated cardiomyopathy.
Immunosuppression
Induction therapy continues to be commonly and increasingly utilized (Figure 10, eSlides H(p) 55, 56). Nearly 75% of recipients received induction therapy from January 2010 to June 2018, a significant increase from 64% in 2005 to 2009. Polyclonal anti-lymphocyte or anti-thymocyte globulin was given to the majority of recipients, with interleukin2 receptor antagonists used in 18% of recipients. As in previous reports, induction therapy did not affect survival among those alive at least 14 days after transplantation (eSlide H(p) 57).
Figure 10.
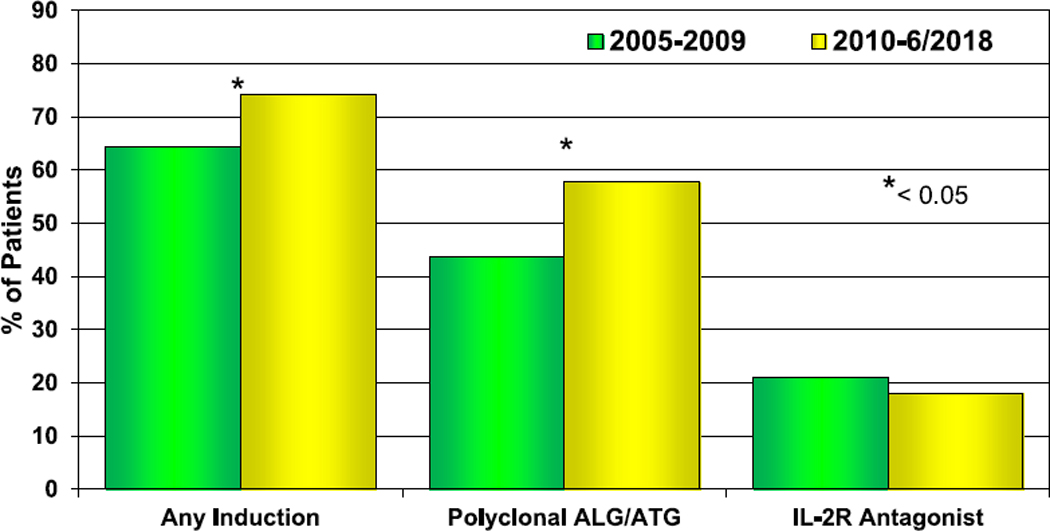
Induction immunosuppression use by era (transplants: January 2005–June 2018). ALG, anti-lymphocyte globulin; ATG, anti-thymocyte globulin; IL-2R, interleukin-2 receptor.
Tacrolimus and mycophenolate mofetil (MMF) or mycophenolic acid (MPA) remain the most common medications for maintenance immunosuppression at the time of hospital discharge (eSlide H(p) 58). In the most recent era (January 2010–June 2018), 89% of recipients were discharged on tacrolimus and 94% were discharged on MMF or MPA. Prednisone use is decreasing, with 66% of recipients discharged on prednisone in the most recent era compared with 74% in the previous era (January 2005–December 2009). At 1-year post-transplant, tacrolimus and MMF or MPA are used in 84% and 81% of recipients, respectively, representing the most common maintenance immunosuppressive regimen. The proliferation signal inhibitors sirolimus and everolimus are used in 11% of patients overall, generally combined with tacrolimus with or without MMF or MPA (Figure 11, eSlide H(p) 59, 60).
Figure 11.
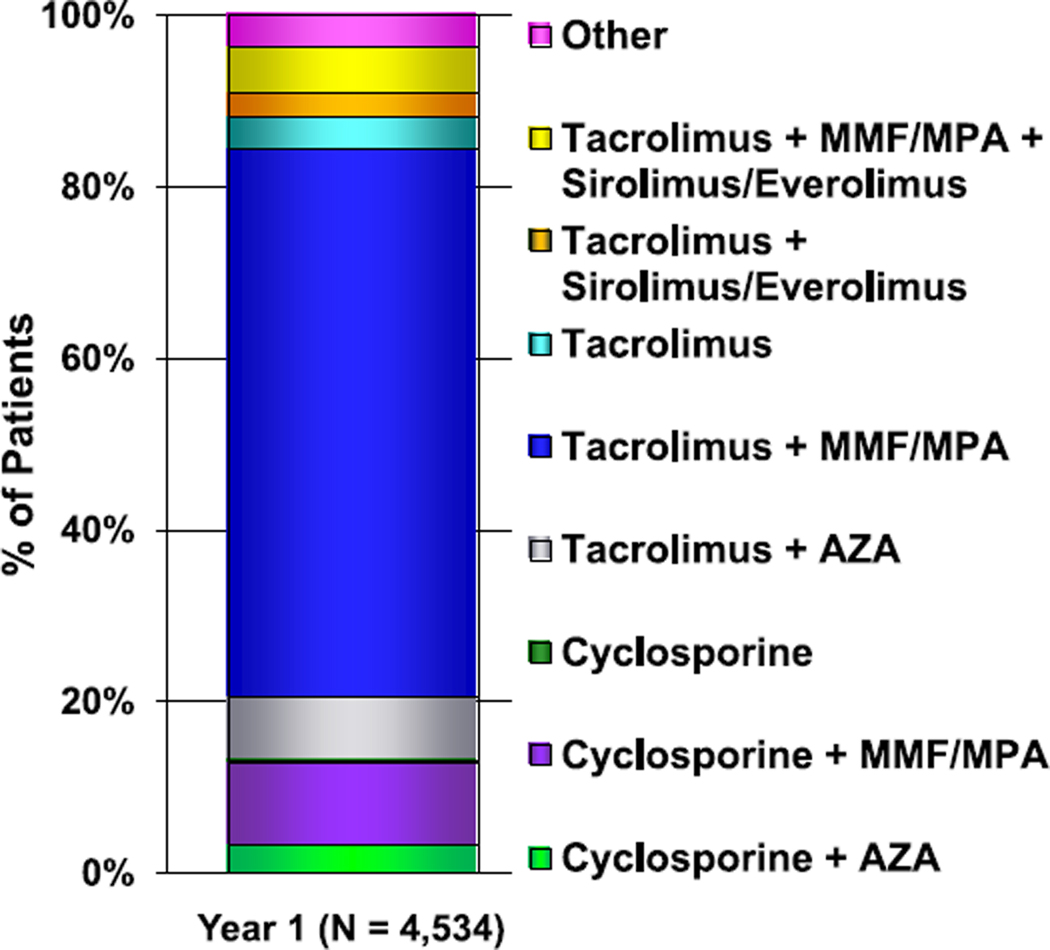
Maintenance immunosuppression drug combinations at 1-year follow-up (follow-ups: January 2005–June 2018). AZA, azathioprine; MMF, mycophenolate mofetil; MPA, mycophenolic acid.
Survival is not associated with the type of calcineurin inhibitor used at discharge or 1-year post–heart transplantation. However, among recipients who survived to 1 year, those treated with prednisone had worse survival than those without the use of prednisone at discharge or at 1-year post-transplantation (eSlides H(p) 61–64). This association is likely confounded by the indication for continued use of prednisone, such as episodes of acute rejection.
Post-transplant morbidity
Rejection
The overall incidence of treated rejection from hospital discharge to 1-year post-transplant continues to decrease (eSlide H(p) 72). In the most recent era (January 2010–June 2018), 13% of recipients were treated for rejection between hospital discharge and 1-year post-transplant compared with 24% in the prior era (January 2005–December 2009). This finding was observed in all age groups and was similar in males and females. This is important, as an episode of treated rejection within the first year post-transplant is associated with worse survival—a finding observed in all age groups whether tacrolimus or cyclosporine was used at the time of hospital discharge (eSlides H(p) 66–71). Induction therapy was not associated with risk of treated rejection between hospital discharge and 1-year post-transplant (Figure 12, eSlide H(p) 73). Additionally, the type of induction therapy used was not associated with an increased risk of rejection (eSlides H(p) 74). Cyclosporine use at discharge was associated with a higher risk of rejection compared with tacrolimus, and this was observed regardless of the use of induction therapy and other maintenance immunosuppressive medications (eSlides H(p) 75–77).
Figure 12.
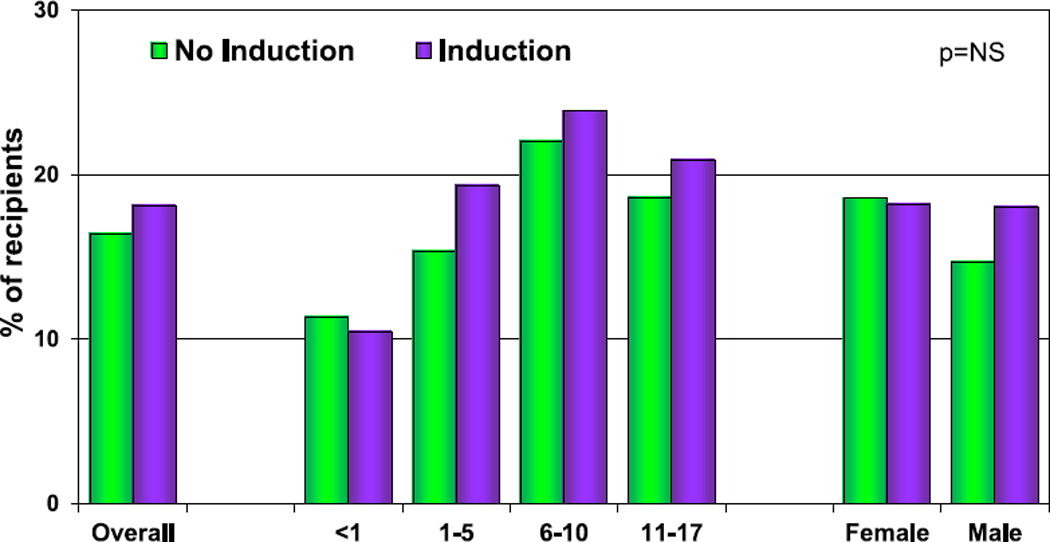
Percentage of recipients experiencing treated rejection between discharge and 1-year follow-up by use of induction therapy (follow-ups: January 2005–June 2018). NS, not significant.
Cardiac allograft vasculopathy
Cardiac allograft vasculopathy (CAV) continues to be a major morbidity and cause of graft loss in pediatric heart transplant recipients. Although nearly 50% of all transplant recipients have developed CAV by 15 years post-transplant, freedom from CAV has improved in the recent era (eSlides H(p) 78,79). Interestingly, on univariable analysis, the use of induction therapy is associated with increased freedom from CAV (Figure 13, eSlide 80). CAV is not associated with graft ischemic time or type of calcineurin inhibitor used at discharge among those patients who survived beyond 1 year (eSlides H(p) 81, 83, 84). Age is an important risk factor, with risk of CAV increasing with age (eSlide H(p) 82). There is a high mortality rate in all age groups within 5 years of the CAV diagnosis with no significant difference between age groups (Figure 14, eSlide H(p) 85). Consistent with prior reports, a rejection episode in the first year post-transplant is associated with a higher frequency of CAV by 3 years post-transplant (eSlide H(p) 93).
Figure 13.
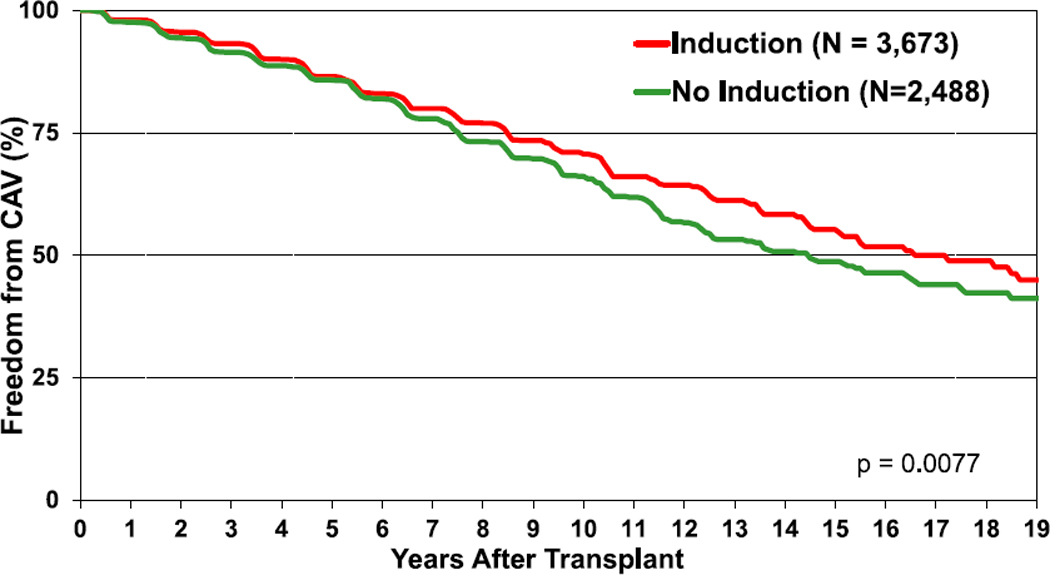
Freedom from CAV by use of induction therapy (transplants: January 1995–June 2017). CAV, cardiac allograft vasculopathy.
Figure 14.
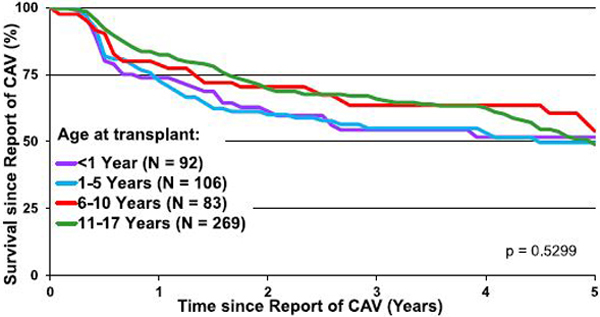
Survival following report of CAV (transplants: January 2005–June 2017). CAV, cardiac allograft vasculopathy.
Renal dysfunction
Severe renal dysfunction, defined as a serum creatinine level > 2.5 mg/dl, dialysis, or renal transplant, is uncommon in pediatric heart transplant recipients but increases over time post-transplant (eSlide H(p) 86). The risk of developing severe renal dysfunction differs by age at transplant. By 10 years post-transplant, 2.4% of recipients who received their transplant in infancy, 4% of those transplanted at age 1 to 5 years, 11% of those transplanted at age 6 to 10, and 14% of recipients transplanted at age 11 to 17 years have developed severe renal dysfunction. A similar age-related finding is observed in freedom from renal replacement therapy (eSlide 88). Calcineurin inhibitor type is not associated with the risk of developing severe renal dysfunction (eSlide H(p) 87).
Malignancy
The risk of developing malignancy increases over time post-transplant with 16% of survivors developing a malignancy by 15 years post-transplant (Figure 15, eSlides 89–91). The vast majority of malignancies are lymphomas and, in contrast to adult heart transplant recipients, skin cancers are exceptionally rare.11
Figure 15.
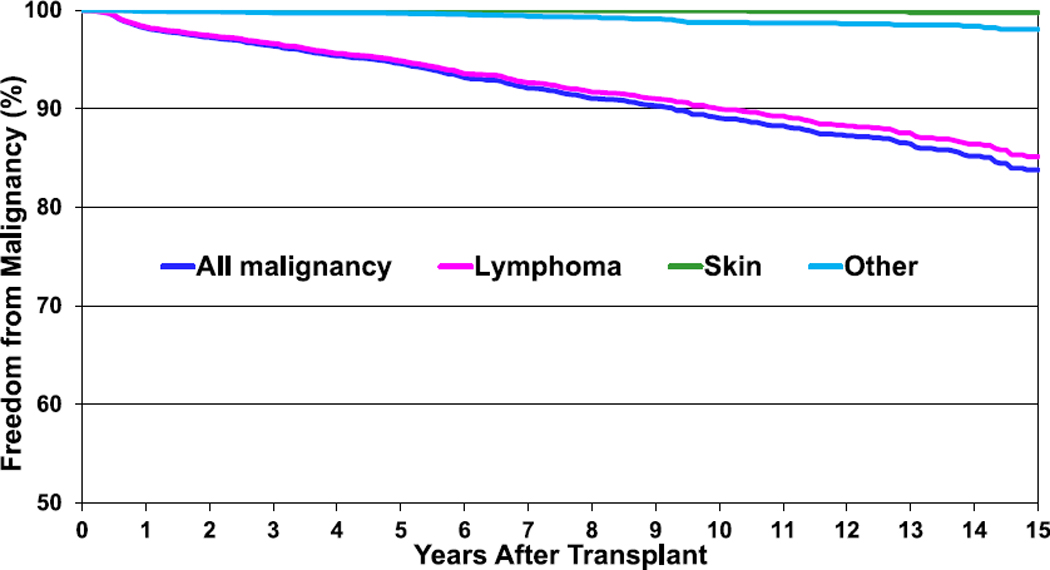
Freedom from malignancy (transplants: January 1995–June 2017).
Functional status
The functional status of heart transplant recipients remains good, with over 80% of survivors reporting either none or minor restrictions in physically strenuous activity (eSlide H (p) 52). Rehospitalizations, however, remain common, with infections and episodes of rejection being the most frequent reasons for hospital admission (eSlide H(p) 53).
Cause of death
The leading causes of death vary over time post-transplant and by recipient age at the time of transplant (Figure 16, eSlides H(p) 94–99). Graft failure is a common cause of death at all time periods post-transplant, though the etiology of the graft failure likely differs. The greatest risk of death from infections occurs in the first year post-transplant, and acute rejection remains a cause of death in >10% of patients up to 10 years post-transplant. CAV accounts for >20% of deaths at 10 or more years post-transplant.
Figure 16.
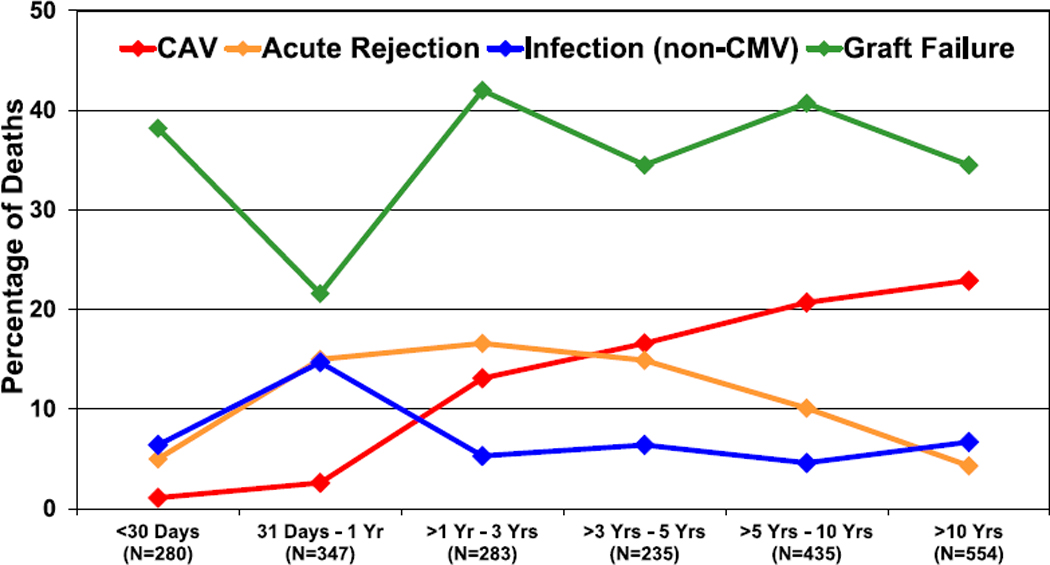
Relative incidence of leading causes of death (deaths: January 2005–June 2018). CAV, cardiac allograft vasculopathy; CMV, cytomegalovirus.
Retransplantation
Retransplants make up a small percentage of all pediatric heart transplants, representing <3% of transplants in 2017 (eSlide H(p) 101). Most retransplants occur with an intertransplant interval of > 60 months. A shorter intertransplant interval is associated with worse survival (Figure 17, eSlide H(p) 104), which is particularly poor if the intertransplant interval
Figure 17.
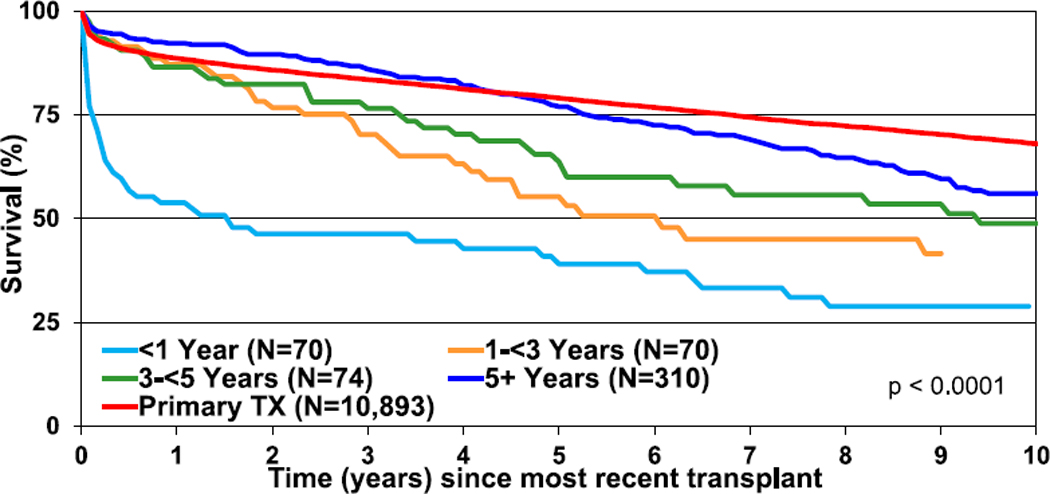
Kaplan-Meier survival rates after retransplant by intertransplant interval (transplants: January 1995–June 2017). TX, transplantation.
is < 1 year. Retransplant is associated with lower overall survival than primary transplants (eSlides 103, 106). Survival after retransplant is higher when indicated for CAV and lower when the indication is primary graft failure.
Multivariable analyses
Several multivariable analyses were performed to assess factors independently associated with mortality and major morbidities after transplant. The models for 1-year and 5-year mortality are reviewed in the focus theme section. Factors independently associated with 5-year mortality (conditional on survival to 1-year post-transplant) are depicted in eSlides H(p) 108 to 120. These include transplant in an earlier era, female sex, retransplant, and treated rejection between hospital discharge and 1-year post-transplant. Continuous risk factors include recipient age and weight percentile. The risk factors for 10-year mortality were similar to those for 5-year mortality, with additional factors including pre-transplant renal dysfunction, ECMO support, PRA, donor age, and transplant center volume. Risk factors for the development of CAV within 5 years post-transplant were identified and include retransplant diagnosis, cyclosporine-based immune suppression (vs. tacrolimus), and higher PRA, whereas induction therapy was associated with lower risk of CAV development (eSlides H(p) 121–125). Risk factors for the development of severe renal dysfunction within 10 years of transplant include cardiac reoperation before discharge, dialysis before discharge, retransplant diagnosis, older recipient age, lower estimated glomerular filtration rate pre-transplant, PRA, and center volume (eSlides H(p) 126–131).
Focus theme: Donor and recipient size match
The aim of the focus theme was to describe the range of donor and recipient size match and assess the impact of size match on outcomes after pediatric heart transplantation. As noted above, calculations of PHM have not been validated in children and are therefore not included in these analyses.
Characteristics by donor and recipient size match
During normal childhood growth, both weight and height increase with age and thus, not surprisingly, there is a significant correlation between donor-recipient height match and donor-recipient weight match (Figure 18, eSlide H(p) 134). Overall, the use of a donor that is larger than the recipient is much more common than the use of a donor that is smaller, and this has been consistent over time (eSlides H(p) 135–138). More than 50% of transplants have a donor-recipient weight match of >20%, whereas fewer than 10% have a donor-recipient weight match < −20%. A greater donor-recipient weight difference is seen among recipients <11 years of age compared with recipients aged 11 to 17 years (Figure 19, eSlide H(p) 136). A greater donor-recipient weight difference was also observed among transplants from Europe and other parts of the world, compared with North America (Figure 20, eSlide H (p) 141). The donor-recipient weight match was similar in groups stratified by sex, pulmonary vascular resistance, and ischemic time (eSlides H(p) 137, 139–140).
Figure 18.
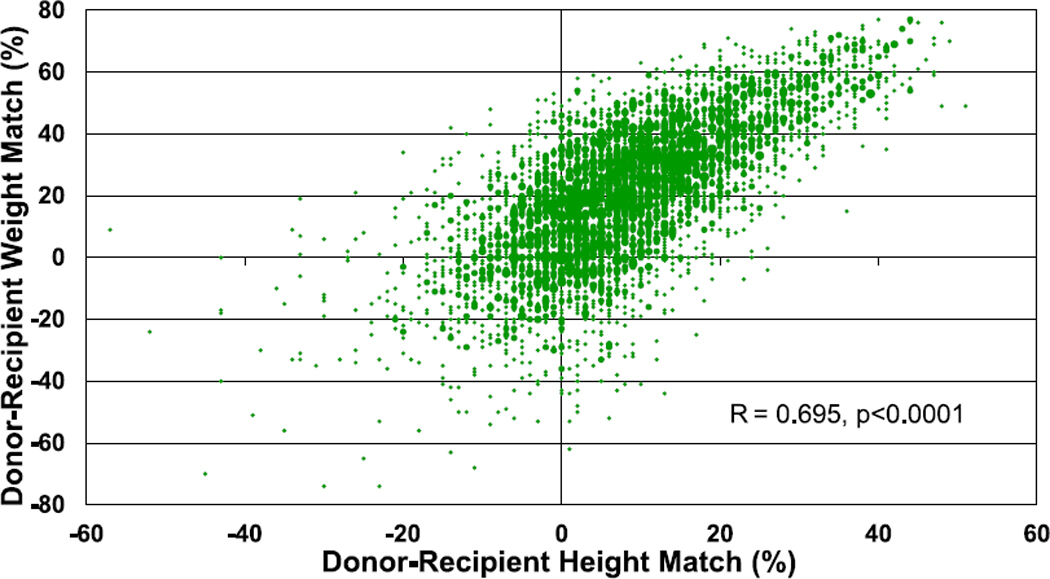
Donor-recipient height and weight match (transplants: January 2010–June 2018).
Figure 19.
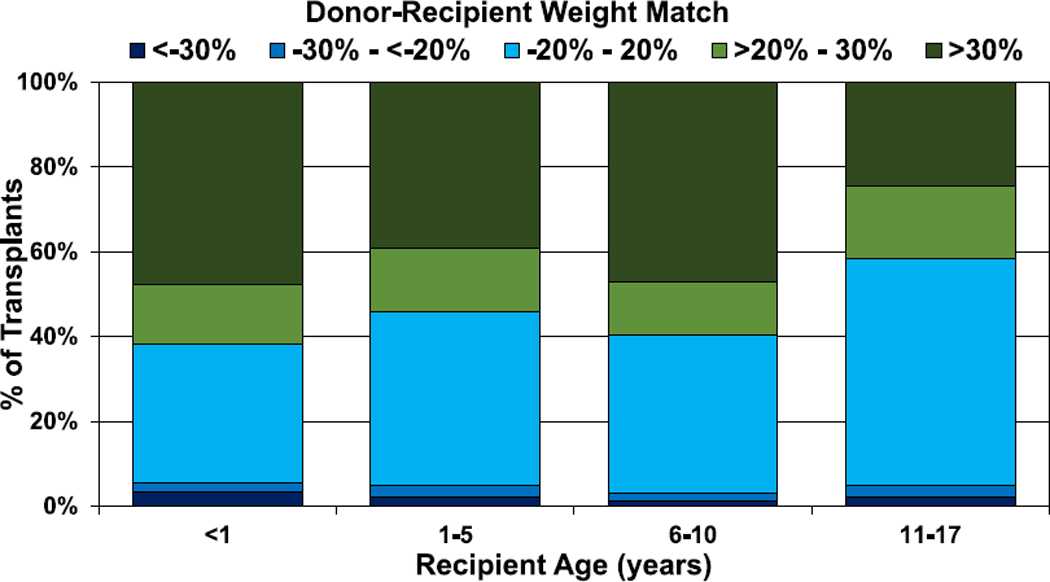
Distribution of donor-recipient weight match by recipient age (transplants: January 2010–June 2018).
Figure 20.
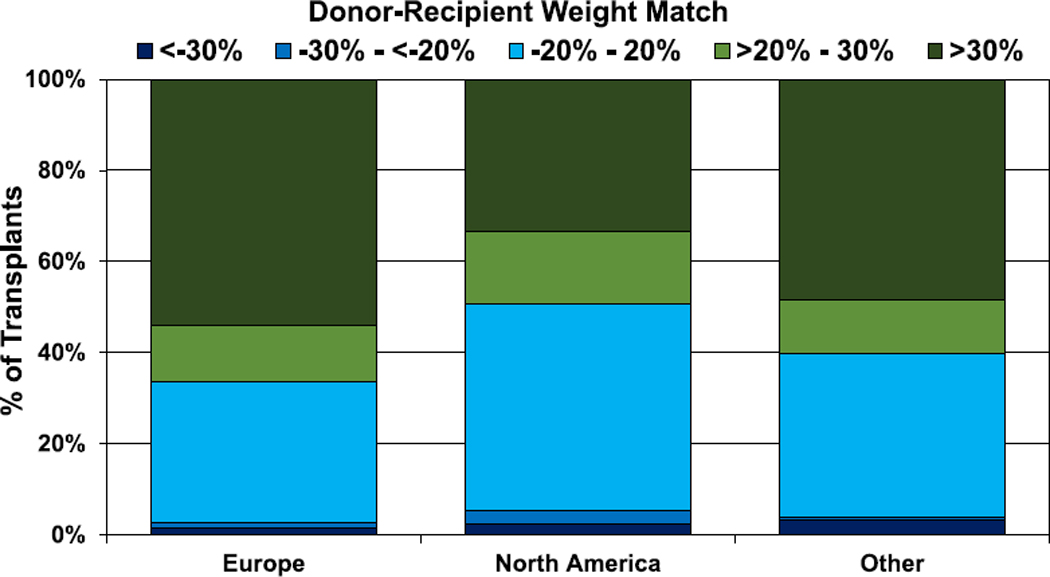
Distribution of donor-recipient weight match by geographic location (transplants: January 2010–June 2018).
Donor and recipient size match: Post-transplant outcomes
When assessing mismatch by height, there was no difference in 1-year and 5-year survival by donor-recipient height match in the overall cohort or by age groups (eSlides H(p) 146–150, 156–160). Likewise, there was no difference in 1-year survival by donor-recipient weight mismatch in the overall cohort or for most age groups (Figure 21, eSlides H(p) 151–155, 161–165). Among recipients aged 1–5 years at transplant, a donor-recipient weight difference > 30% was associated with better 1-year survival compared with a difference <−30%.
Figure 21.
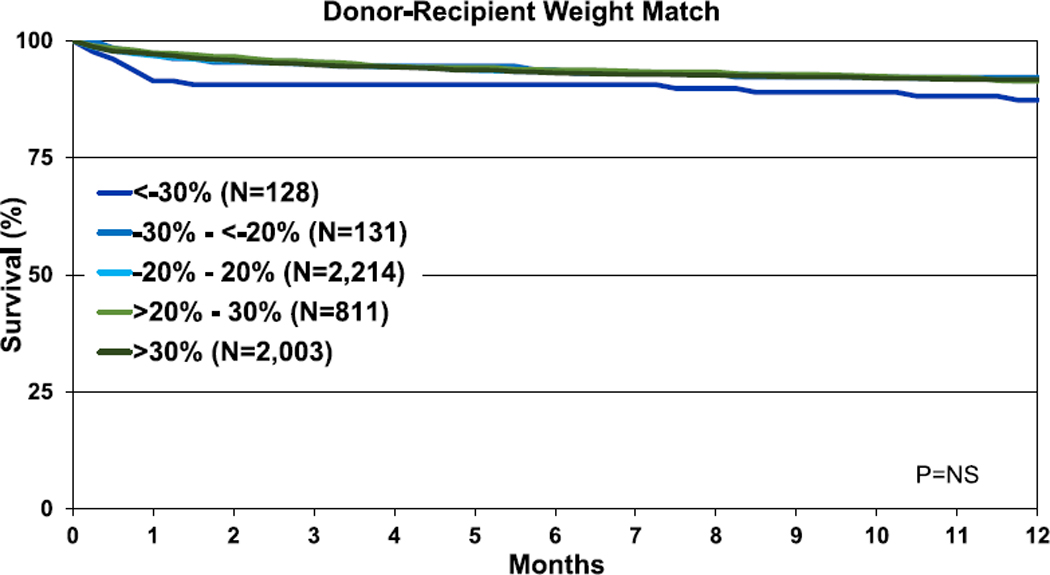
Kaplan-Meier survival within 1 year of transplant by donor-recipient weight match (transplants: January 2006–June 2017). NS, not significant.
Multivariable analyses were performed to assess for factors independently associated with 1- and 5-year mortality. Factors associated with 1-year mortality included sex mismatch, MCS, diagnosis, ischemic time, renal function, and bilirubin. A donor-recipient weight mismatch was also associated with 1-year mortality with a greater risk observed with smaller donor relative to the recipient (Figures 22 and 23, eSlides 167–172, Table 1). Similar factors were associated with 5-year mortality (Figure 24, eSlides H(p) 173–179). However, for 5-year mortality, donor-recipient height mismatch was a significant risk factor and donorrecipient weight mismatch was not. Interestingly, a greater risk of 5-year mortality was observed with larger donors relative to recipients. The explanation for this is not clear. It is possible that our models do not fully account for all important confounders, such as the risk profile associated with a clinical decision to oversize the recipient, and further study is needed to understand this relationship.
Figure 22.
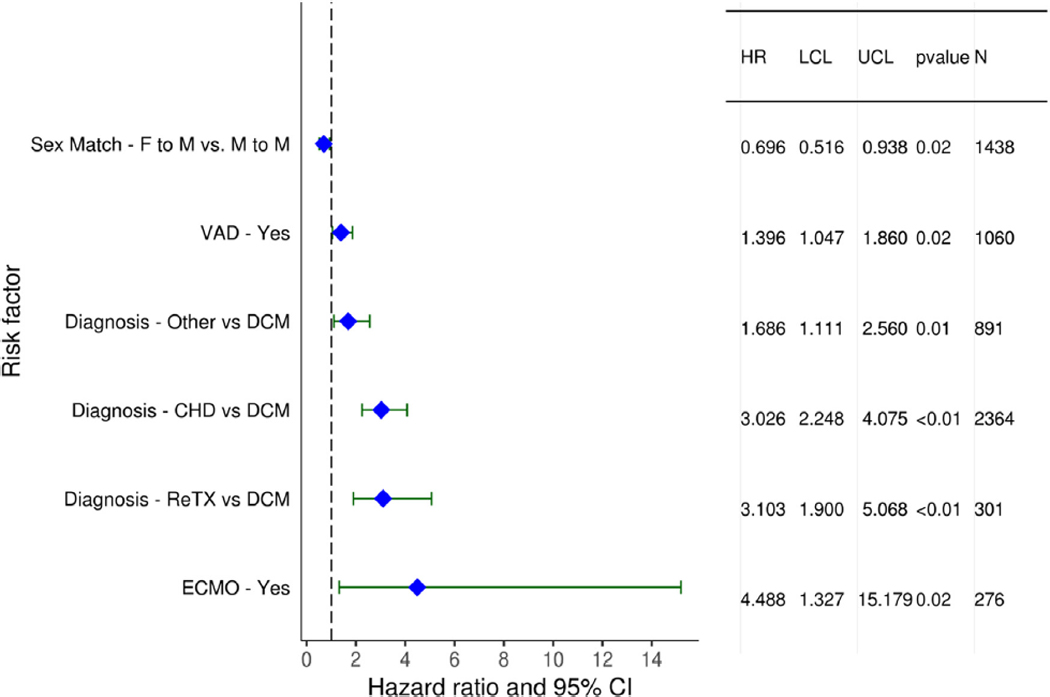
Statistically significant categorical risk factors for 1-year mortality (transplants: January 2006–June 2017). CHD, congenital heart disease; CI, confidence interval; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; F, female; HR, hazard ratio; LCL, lower control limit; M, male; ReTX, retransplantation; UCL, upper control limit; VAD, ventricular assist device.
Figure 23.
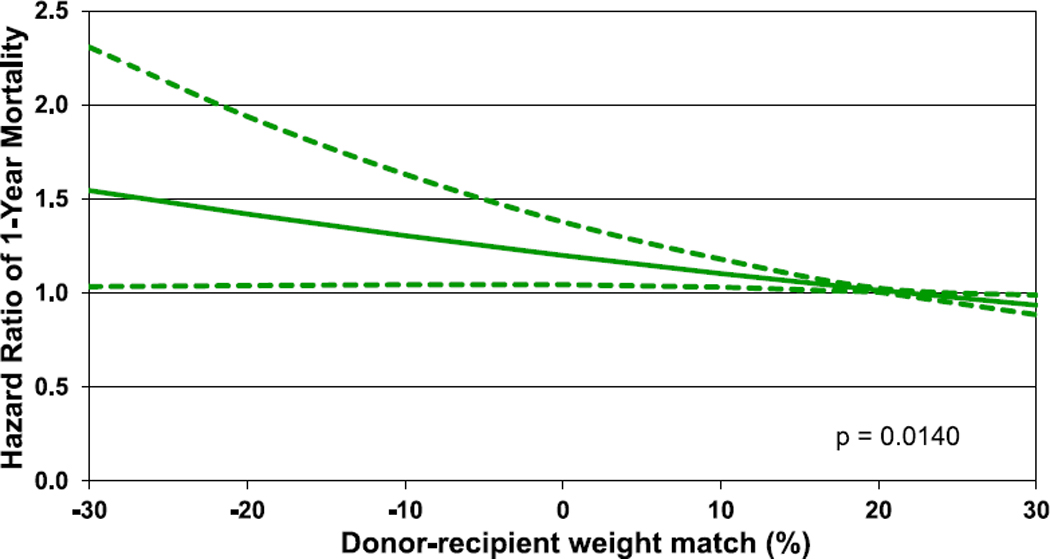
Hazard ratio of 1-year mortality by donor-recipient weight match (transplants: January 2006–June 2017).
Table 1.
Statistically significant continuous risk factors for 1-year mortality (Transplants: 1/2006–6/2017)
|
Statistically significant continuous risk factors for 1-year mortality | |
| Ischemic time (hours) | Recipient eGFR (mL/min/1.73 m2) |
| Recipient bilirubin (mg/dl) | Donor-recipient weight match (%) |
Figure 24.
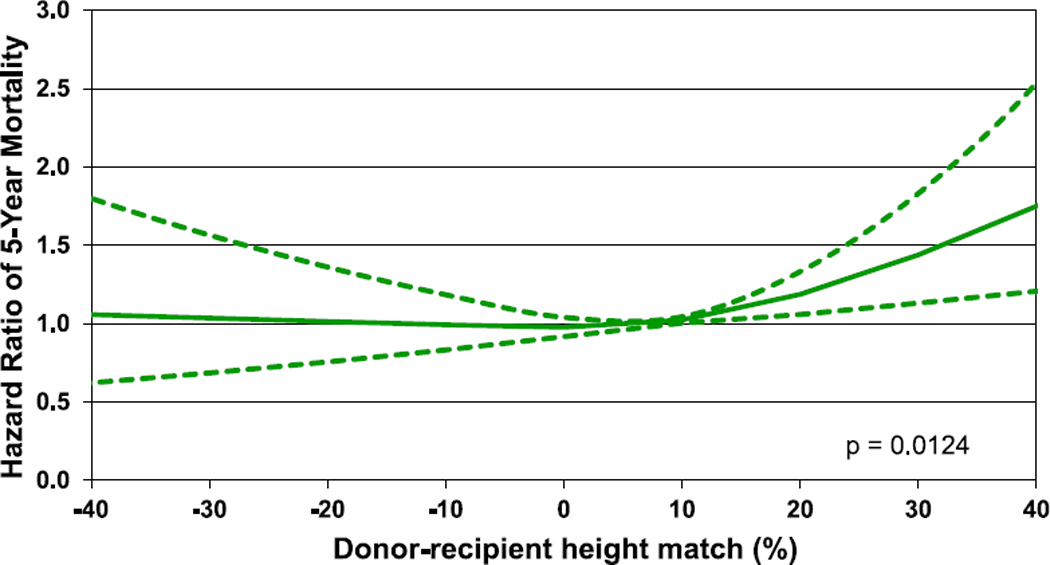
Hazard ratio of 5-year mortality by donor-recipient height match (transplants: January 2003–June 2013).
Conclusion
This annual report of the ISHLT Transplant Registry high-lights important findings in the field of pediatric heart transplantation, including the increased use of induction therapy, the potential protective effect of induction therapy on sub-sequent development of CAV, and the increased use of VADs, although infants and recipients with CHD continue to be infrequently bridged with VAD support. The focus theme, donor-recipient size match, demonstrates that donor-recipient weight match was independently associated with 1-year survival with undersized donors associated with lower survival, and donor-recipient height match was independently associated with 5-year survival, with over-sized donors associated with lower survival. The Registry continues to provide worldwide data on pediatric heart transplantation and allows for analyses that would not otherwise be possible given the relatively small sample size at individual centers.
Supplementary Material
Footnotes
Disclosure statement
M.H, D.H, T.S, E.H, B.M, W.C, A.R, and A.S have no conflicts to report. K.K serves as scientific adviser and speaker for CareDx, Inc; L.P serves as a speaker for Thermofisher, Sandoz, One Lambda, and Novartis, and is advisory board member for Qiagen and Novartis; J.S serves as consultant for Medtronic and Abbott; J.R serves as consultant for Bayer, Novartis, Amgen, and CSL Behring; A.Z serves on the speakers bureau of Paragonix, Novartis, Mallincrodt, Sanofi-Genzyme, and Franz Köhler Chemie and on the advisory board for Chiesi. D.C received research funding from Astellas and Boeringher-Ingelheim.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2019.08.002.
References
- 1.Sethi GK, Lanauze P, Rosado LJ, et al. Clinical significance of weight difference between donor and recipient in heart transplantation. J Thorac Cardiovasc Surg 1993;106:444–8. [PubMed] [Google Scholar]
- 2.Taghavi S, Wilson LM, Brann SH, Gaughan J, Mangi AA. Cardiac transplantation can be safely performed with low donor-to-recipient body weight ratios. J Card Fail 2012;18:688–93. [DOI] [PubMed] [Google Scholar]
- 3.Tamisier D, Vouhé P, Le Bidois J, Mauriat P, Khoury W, Leca F. Donorrecipient size matching in pediatric heart transplantation: a word of caution about small grafts. J Heart Lung Transplant 1996;15:190–5. [PubMed] [Google Scholar]
- 4.Kanani M, Hoskote A, Carter C, Burch M, Tsang V, Kostolny M. Increasing donor-recipient weight mismatch in pediatric orthotopic heart transplantation does not adversely affect outcome. Eur J Cardiothorac Surg 2012;41:427–34. [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Du W, Delius RE, L’Ecuyer TJ, Zilberman MV. Low donorto-recipient weight ratio does not negatively impact survival of pediatric heart transplant patients. Pediatr Transplant 2010;14:741–5. [DOI] [PubMed] [Google Scholar]
- 6.Kobashigawa J, Khush K, Colvin M, et al. Report From the American Society of Transplantation conference on donor heart selection in adult cardiac transplantation in the United States. Am J Transplant2017;17:2559–66. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Bock MJ, Wollstein A, Nguyen K, Malerba S, Lytrivi ID. Donor-recipient height ratio and outcomes in pediatric heart transplantation. Pediatr Transplant 2016;20:652–7. [DOI] [PubMed] [Google Scholar]
- 8.Kransdorf EP, Kittleson MM, Benck LR, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant 2019;38:156–65. [DOI] [PubMed] [Google Scholar]
- 9.Gong TA, Joseph SM, Lima B, et al. Donor predicted heart mass as predictor of primary graft dysfunction. J Heart Lung Transplant 2018;37:826–35. [DOI] [PubMed] [Google Scholar]
- 10.Rossano JW, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; focus theme: Multiorgan transplantation. J Heart Lung Transplant 2018;37:1184–95. [DOI] [PubMed] [Google Scholar]
- 11.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult heart transplantation Report-2018; focus theme: Multiorgan transplantation. J Heart Lung Transplant 2018;37:1155–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


