Abstract
Chronic kidney disease (CKD) is a global public health problem, affecting over 10% of the world’s population and more than half of the population aged over 70 years, imposing major costs on healthcare systems. Although the primary causes of CKD include various diseases such as diabetes, glomerulonephritis, and acute kidney injury (AKI), the progression of CKD is mediated by a common pathological pathway, which is mainly characterized by fibrosis and chronic inflammation. In this process, resident fibroblasts in the kidney play crucial roles. Accumulating evidence highlights the existence of functional heterogeneity and plasticity of fibroblasts and their diverse roles in kidney disease progression and resolution. In addition to renal fibrosis, renal anemia and peritubular capillary loss, two major complications of progressive CKD, are also caused by dysfunction of resident fibroblasts. Furthermore, age-dependent alterations in fibroblast behavior also contribute to age-dependent unique pathological conditions. In this article, we describe the current understanding regarding the behaviors of fibroblasts in the kidney in health, disease, and aging.
Keywords: chronic kidney disease, fibroblast, renal anemia, tertiary lymphoid tissue, acute kidney injury
Introduction
Chronic kidney disease (CKD) is recognized as a global public health concern because of its high prevalence and mortality.1) CKD affects more than 10% of the world’s population and more than half of the population aged over 70 years. In addition to progression to end-stage kidney disease requiring transplantation or dialysis, CKD is a strong and independent risk factor for cardiovascular diseases (CVD), which occur before progression to end-stage kidney disease.2,3) Despite its clinical relevance, however, current treatment options for CKD are limited, and the development of novel therapies for CKD has been the subject of intense research in recent years.
Although CKD results from multiple diseases, such as diabetes, hypertension, and glomerulonephritis, the progression of CKD is mediated by a common pathological pathway irrespective of etiology.4) Although multiple types of cells are involved in common pathological conditions, resident fibroblasts play crucial roles in this process. In response to injury, fibroblasts in the kidney not only transdifferentiate into myofibroblasts and execute fibrosis, but also drive inflammation-associated pathology in some cases, and can play beneficial roles in regeneration. In this article, we describe the current understanding of the pathophysiology of kidney diseases, with a focus on resident fibroblasts.
1. Resident fibroblasts in the kidney
Fibroblasts are spindle-shaped cells with multiple projections residing in the interstitial space that are essential for organ architecture and homeostasis under physiological conditions. Although commonly used markers for resident fibroblasts in the kidney include platelet-derived growth factor receptor β (PDGFRβ) and CD73, these markers are not homogeneously expressed by resident fibroblasts in the kidney. Therefore, resident fibroblasts are detected by their anatomical localization in the interstitial space, expression of the above markers, and the absence of markers for other cell lineages, such as pan-white blood cell marker CD45.5) Although fibroblasts are present in essentially all tissues and their basic functions such as regulation of extracellular matrix (ECM) are conserved across tissues, they are highly adapted to the tissue they reside in and they fulfill tissue-specific functions.6) In the kidney, for instance, a subpopulation of fibroblasts produces erythropoietin (EPO), a glycoprotein hormone that is essential for erythropoiesis, in a hypoxia-dependent manner, playing a crucial role in maintaining homeostasis.7) Unlike most hematopoietic growth factors, which are produced by bone marrow cells in the vicinity of their target cells, EPO is produced mainly in the kidney during adulthood. One of the reasons for this unique localization of EPO-producing cells is that, although the kidneys receive 25% of cardiac output, the oxygen tension in the kidney tissue is low, which renders the kidney more sensitive to changes in oxygen delivery compared with other tissues and is a suitable site for EPO production.7)
Although the essential role of EPO has been well established, the behavior and developmental origin of EPO-producing cells and other fibroblasts in the kidney have not been fully elucidated. Based on two notable observations that EPO-producing cells express neural markers8) and that neural crest-derived cells contribute to the interstitium of the developing kidneys,9) we conducted a lineage-tracing study utilizing myelin protein zero (P0)-Cre mice, which labels migrating neural crest.10) P0-Cre lineage-labeled cells were detectable in the renal interstitial spaces and were positive for fibroblast markers, PDGFRβ and CD73, and more than 98% of interstitial fibroblasts in the renal cortex and outer medulla, including EPO-producing cells, were lineage labeled with P0-Cre.11) This indicates that most of the resident fibroblasts in the renal cortex and outer medulla are P0-Cre lineage-labeled cells. Among P0-Cre lineage-labeled resident fibroblasts in the kidney, up to 10% produced EPO in severe anemic conditions. A subsequent study utilizing EPO-Cre mice also revealed that most resident fibroblasts in the kidney had the potential to produce EPO.12) However, resident fibroblasts in the kidney are not lineage-labeled with Wnt1-Cre, another Cre mouse line labeling neural crest, and the kidney in Splotch (Sp2H/Sp2H) embryo, which has a neural crest defect in the lumbo-sacral region, develops normally.13) The reasons for these discrepancies remain elusive, although a difference in the efficiency and timing of labeling neural-crest derived cells between both Cre lines has been suggested.11) A subsequent study revealed EPO production by certain subpopulations of neural crest cells,14) suggesting a possible link between the kidney and neural crest. Further studies will be required to address these points.
2. Renal fibrosis as a common pathological feature of CKD
Renal fibrosis is the most common and significant pathological finding in all forms of CKD, which is defined as the accumulation of ECM proteins in the interstitial space (Fig. 1).5,15) Abnormal ECM expansion in the renal interstitium expands the space between the tubular basement membrane and peritubular capillaries, and distorts organ architecture. These alterations disturb normal blood supply and decrease renal oxygenation, which in turn promote fibrosis through various cytokine signaling pathways, forming a vicious cycle and leading to renal function decline.4) Indeed, renal fibrosis in the cortex has been recognized as the best histological predictor of renal dysfunction in CKD.16)
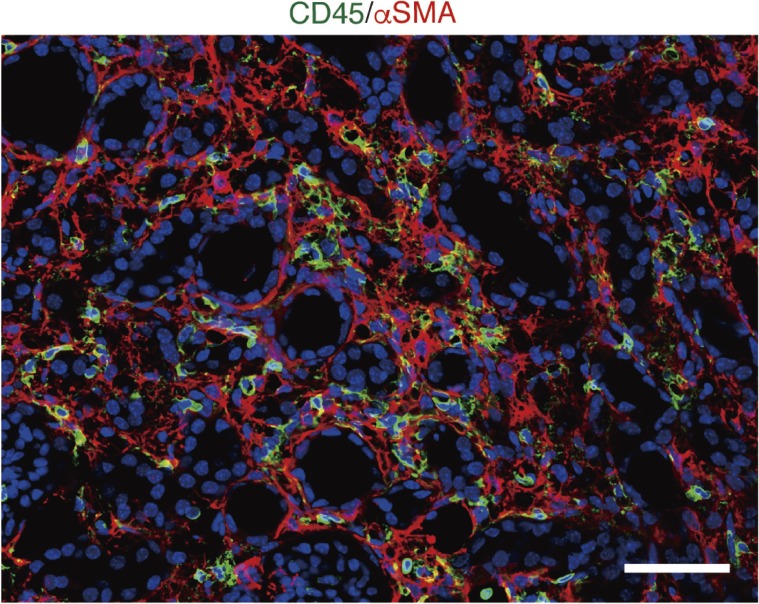
Figure 1.
Renal fibrosis and inflammatory cell infiltration are common pathological features in injured kidney. α-Smooth muscle actin (αSMA)-positive myofibroblasts and inflammatory cell infiltration are commonly detectable in injured kidneys in mice. Immunofluorescence of CD45 and αSMA 10 days after unilateral ureteral obstruction (UUO). Sections were counterstained with DAPI. Scale bar: 50 µm.
Although multiple cell types participate in fibrosis development and progression, the development of myofibroblasts is a critical step for renal fibrosis irrespective of the underlying etiology.15,17) Myofibroblasts are the cell types that secrete greater levels of interstitial ECM and express contractile genes such as α-smooth muscle actin (αSMA), which enable them to contract and enclose an injured area. Although the origin of myofibroblasts in the kidney has been controversial over past decades,5) recent evidence from genetic lineage-tracing analysis revealed that resident fibroblasts and pericytes represent the primary precursors of myofibroblasts,11,18) although mesenchymal stem cells19) and fibrocytes,20) circulating fibroblast progenitor cells, also play a role.5) In the kidney, fibroblasts and pericytes are highly overlapped, as demonstrated by lineage-tracing experiments utilizing P0-Cre and Foxd1-Cre mouse lines, which label fibroblasts and pericytes, respectively.5) In our previous study, we demonstrated that, in response to injury, P0-Cre lineage-labeled resident fibroblasts in the kidney transdifferentiated into αSMA-positive myofibroblasts and executed fibrosis in several kidney injury models.11) Indeed, most myofibroblasts were lineage-labeled with P0-Cre, indicating the major contribution of this population. On the other hand, in bone marrow transplantation experiments utilizing αSMA-RFP mice, LeBleu et al. reported that 35% of αSMA-positive myofibroblasts were bone marrow derived,21) though the activity of short transgenic reporters utilized in the study has been questioned.22) Although a contribution of fibrocytes to αSMA-positive myofibroblasts in renal fibrosis has been controversial, a recent study utilizing single cell RNA sequencing (scRNA-seq) and parabiosis models have demonstrated that fibrocytes contribute a small fraction of myofibroblasts in renal fibrosis, whereas most myofibroblasts are derived from resident fibroblasts.23)
Of note, renal anemia and peritubular capillary loss, which are common complications of CKD, are also caused by dysfunction of fibroblasts. Renal anemia is caused mainly by the relative deficiency in EPO production, and exogeneous recombinant human EPO has been widely used as a treatment for patients with renal anemia.6) In parallel to CKD progression, relative EPO deficiency becomes more common, and this deficiency becomes almost universal in patients with end-stage kidney disease.24) Although it has been debated for a long time whether the relative EPO deficiency in patients with CKD is derived from an absolute loss or a functional disturbance of EPO-producing cells, we demonstrated that EPO-producing cells also transdifferentiated into myofibroblasts in response to injury at the cost of EPO production.11) Notably, the phenotypic changes were reversed by the administration of a selective estrogen receptor modulator.11) Furthermore, we also demonstrated that the administration of neuroprotective reagents such as neurotrophins or renoprotective reagent HGF restored the ability to produce EPO in myofibroblasts, but these did not attenuate the production of ECM, suggesting that the acquisition of pathological matrix gene expression and suppression of EPO expression in myofibroblasts can be dissociated and regulated independently. In a later study, Souma and colleagues demonstrated that short-term NFκB activation suppressed EPO expression but did not induce αSMA and collagen expression in myofibroblasts, whereas TGFβ induced αSMA and collagen expression but failed to suppress EPO expression in myofibroblasts.12)
Destruction of peritubular capillaries is detectable in all types of CKD and also becomes more frequent in parallel with CKD progression. Peritubular capillary loss has been considered as a strong driving force of CKD progression, because it leads to chronic hypoxia, the final common pathway by which CKD progresses to end-stage kidney disease.4) In physiological conditions, fibroblasts wrap peritubular capillaries with their multiple processes and contribute to vascular stabilization. In response to injury, however, fibroblasts detach themselves from capillaries, migrate to the site of injury, and wrap adjacent injured tubules,25,26) which makes the peritubular capillary structurally unstable, leading to capillary regression and rarefaction.
Collectively, dysfunction of resident fibroblasts results in a series of clinically relevant pathological conditions common in CKD, indicating the importance of maintaining fibroblasts in healthy states.
3. Beneficial roles of fibroblasts
Myofibroblasts are widely accepted as the cells executing fibrosis. However, the view that the fibroblast is simply a fibrosis driver was recently challenged by several observations that suggest possible beneficial roles of myofibroblasts. We and other groups have demonstrated that, utilizing murine cell-type specific genetic ablation models, proximal tubule-specific injury alone caused fibroblast-to-myofibroblast transdifferentiation, suggesting that injured proximal tubules trigger fibrosis.27,28) We also showed that proximal tubule injury alone caused a reduction in EPO production, which was also caused by fibroblast-to-myofibroblast transdifferentiation. A recent study utilizing an in vivo imaging technique showed that in response to laser-induced tubular cell ablation, remaining proximal tubular cells migrated to the injury site and regenerated damaged proximal tubules.25) Consistent with this, by employing a genetic fate mapping technique several groups, including ours, have also demonstrated that with injury lost epithelial cells are replaced by proliferation of epithelial cells that have survived the injury.29–31) Of note, during this process, resident fibroblasts also migrated to the site of injury and enclosed the injured tubule via PDGFRβ signaling,25) providing a structural support for the injury site. Administration of trapidil to inhibit PDGFRβ signaling hinders fibroblast migration and tubular regeneration, suggesting the supportive roles of fibroblasts for tubular regeneration.25)
Consistent with this, we also demonstrated that genetic depletion of fibroblasts inhibited tubular cell proliferation and hindered normal intrinsic repair in the acute phase of kidney injury.32) After injury, fibroblasts acquired strong expression of retinaldehyde dehydrogenase 2 (Raldh2, also known as Aldh1a2), a gene that encodes a retinoic acid (RA) synthesizing enzyme,33) during the transition to myofibroblasts in several kidney injury models. Although RA is an essential molecule for embryogenesis and tissue development including kidney,33,34) during kidney injury, RA derived from fibroblasts may support tubular regeneration, because the RA receptor γ (RARγ) and its target gene, αB-crystalin, were strongly expressed in proximal tubules after injury and an inverse agonist of RARs significantly attenuated tubular proliferation in vitro.32) These observations suggested the existence of “regulatory fibroblasts” supporting tubular regeneration and the notion that the development of myofibroblasts is, at least in early phase of injury, an adaptive and transient process of regeneration. They provide structural support and maintain the integrity of the damaged tubules, and enhance proliferation as well as regeneration of the damaged tubules. These studies also suggested a need for better understanding of how myofibroblasts influence other key aspects of pathophysiology of kidney diseases, which will help in further interpreting the meaning of fibrosis and provide a clue to the development of novel therapeutic strategies for kidney diseases.
4. Resolution of renal fibrosis
Although fibrosis has long been viewed as an irreversible process, accumulating evidence has shown that fibrosis can regress after withdrawal of underlying etiological agents.17) For instance, in the liver, even in patients with hepatitis B virus (HBV)-related cirrhosis, long-term suppression of HBV can result in significant regression of fibrosis and reversal of cirrhosis.35) Experimental models of fibrosis resolution in the liver have shown that elimination of myofibroblasts is a key step for the onset of fibrosis resolution, as elimination of myofibroblasts precedes degradation of fibrotic ECM and regression of fibrosis.36,37) Currently, in several tissues, elimination of myofibroblasts is mediated by at least three distinct mechanisms: myofibroblast senescence,38) apoptosis,39) and dedifferentiation.36,37) During normal repair after liver injury, some myofibroblasts undergo senescence or apoptosis and are removed by immune cells, whereas other myofibroblasts escape immune-mediated clearance and dedifferentiate into a less active state. Cell senescence is defined as the irreversible form of cell cycle arrest that can be induced by p53/p21 or p16INK4a/pRb tumor suppressor pathway activation.40) Senescent cells exhibit a senescence-associated secretory phenotype (SASP) and excrete various chemokines and ECM-degrading enzymes such as matrix metalloproteinases.38) Although myofibroblasts are cells with high proliferative and ECM-producing capacity, senescent myofibroblasts are in irreversible cell cycle arrest and degrade ECM via SASP. Additionally, senescent myofibroblasts excrete multiple cytokines and chemokines, which recruit immune cells such as natural killer cells and confer susceptibility to immune-cell-mediated clearance of senescent cells.38) Apoptosis of myofibroblasts is also induced and promoted mainly by immune cells, particularly cytotoxic T cells expressing FasL.41) On the other hand, surviving myofibroblasts, which escape from immune surveillance, as described above, can dedifferentiate and acquire another inactive phenotype. In liver fibrosis models, deactivated myofibroblasts do not completely suppress their pro-fibrotic gene expression and remain in an intermediately primed state, which is able to respond more vigorously than the original quiescent state in response to further episodes of liver injury.36,37) This suggested that recovered livers are likely more susceptible to further injury.
The reversibility of renal fibrosis has also been reported in several experimental models. For instance, bone morphogenic proteins (BMP) 7 can reverse renal fibrosis in several experimental models by antagonizing TGF-β1, a master regulator of myofibroblast differentiation.42) The local activity of endogenous BMP is regulated by several classes of binding molecules that control BMP signaling activity positively or negatively.43) We previously identified USAG-1, a kidney specific BMP antagonist, which is abundantly expressed in distal tubules colocalizing with BMP7 in the kidneys.44,45) We also demonstrated that USAG-1-deficient mice were resistant to not only tubular injury but also glomerular injury,46,47) although its expression is confined to distal tubules. These results suggested that USAG-1 secreted from distal tubules might affect extraglomerular mesangial cells through the macula densa, a unique anatomical structure equipped with each nephron, which bridges extraglomerular cells and distal tubular cells. Treatment with a neutralizing antibody against USAG-1 also improved proteinuria and graft function in a rat renal transplantation model.48) Although receptors for BMP7 are widely expressed throughout the body and unfavorable extra-renal side effects of the administration of BMP7 can be induced, USAG-1 is a kidney-specific gene, and a therapeutic option targeting USAG-1 enables more specific targeting and minimizes adverse effects. However, evidence of the mechanisms for the resolution of renal fibrosis and the fate of myofibroblasts after kidney regeneration are quite limited, and further research will be required.
5. Age dependent alterations in fibroblast activation
Aging seems to confer a profibrotic and/or pro-inflammatory phenotype on fibroblasts, which may have a profound impact on disease outcomes.49,50) Indeed, aged animals have a lower capacity for regeneration and are more susceptible to fibrosis after injury in several tissues. Hecker and colleagues examined the lung injury response of young and aged mice and have found that the lungs of aged mice failed to resolve fibrosis, whereas the lungs of young mice exhibited significant resolution of fibrosis.49) Myofibroblasts in aged lungs had acquired a apoptosis-resistant phenotype, which was mediated by a redox imbalance resulting from sustained activation of NADPH oxidase 4 (Nox4), an enzyme that generates reactive oxygen species, and impaired capacity to induce Nrf2 anti-oxidant responses. Notably, pharmacological inhibition of Nox4 reset the redox balance and reversed the myofibroblast phenotype, promoting the susceptibility to apoptosis and prolonging survival in aged mice after lung injury.49)
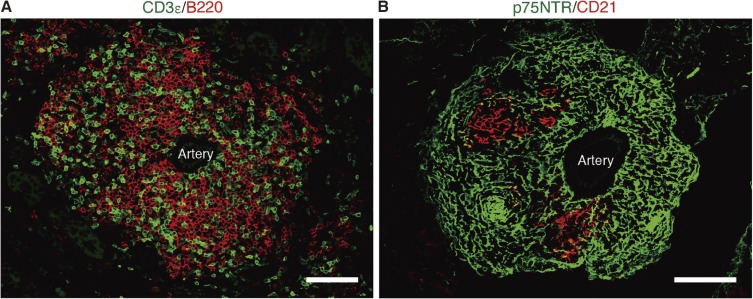
We have also compared kidney injury responses between young and aged mice in three different kidney injury models, and we found that although young mouse kidneys recovered from injury, aged mouse kidneys exhibited fibrosis, tubular injury, and persistent inflammation,50) which were consistent with the results of clinical studies that showed worse prognosis after kidney injury in the elderly.51,52) Unexpectedly, we also found that aged mice, but not young mice, developed multiple tertiary lymphoid tissues (TLTs) in the kidney long after injury (Fig. 2).50) TLTs are lymphocyte aggregates that are induced at sites of chronic inflammation, and they support T and B lymphocyte proliferation as well as germinal center responses.53) Of note, the whole architecture of TLTs is supported structurally and functionally by fibroblasts within TLTs.54) For instance, in aged injured kidney, resident fibroblasts transdifferentiated into several distinct phenotypic fibroblasts, which orchestrated TLT formation (Fig. 3). In the initiation of TLT formation in mice, fibroblasts positive for Raldh2 surrounded TLTs and seemed to foster TLT growth. On the other hand, fibroblasts within TLTs were negative for Raldh2 and instead strongly positive for p75 neurotrophin receptor (p75NTR), a marker of neural crest. Some of the p75NTR+ fibroblasts inside TLTs acquired the ability to produce the homeostatic chemokines CXCL13 and CCL19, both of which are powerful driving forces for recruiting lymphocytes and are sufficient to drive TLTs.55,56) In addition to the reciprocal expression pattern of Raldh2 and p75NTR, in vitro experiments revealed that RA induced p75NTR expression in fibroblasts, indicating the possibility that RA derived from fibroblasts induced p75NTR expression in fibroblasts inside TLTs to become the producer of CXCL13 and CCL19.50) Subsequently, as the TLTs matured, CD21+/p75NTR− follicular dendritic cells (FDCs) emerged as an integral part of the fibroblast network within TLTs. FDC is a conventional stromal cell found in secondary lymphoid organs, which forms B cell area by producing CXCL13 and provides cytokines essential for germinal center reaction, such as B cell-activating factor (BAFF) and IL6.57) Notably, all these TLT-associated fibroblasts were also lineage labeled in P0-Cre, suggesting that resident fibroblasts in the kidney acquire various phenotypes depending on their microenvironment.50) Of clinical importance, depletion of TLTs using an anti-CD4 monoclonal antibody GK1.5 resulted in the resolution of inflammation and fibrosis.50) Additionally, the late administration of dexamethasone, a common immunosuppressive agent, also halted TLT formation and attenuated renal inflammation and fibrosis. These results suggested potential of TLT as a novel therapeutic target of kidney disease in the elderly. Notably, age-dependent TLT formation was also confirmed in human kidneys, and the components are closely similar to those in mice, indicating this phenomenon is conserved across species.50) One remaining important question to be addressed is why the kidney becomes prone to TLT formation with aging, and further studies will be required.
Figure 2.
Tertiary lymphoid tissue (TLT) in aged injured kidney. TLT is mainly composed of T and B lymphocytes and is structurally and functionally supported by a fibroblastic network. Immunofluorescence of (A) CD3ε (T cell marker) and B220 (B cell marker); (B) p75 neurotrophin receptor (p75NTR) and CD21. CD21 is a marker for follicular dendritic cells (FDCs). Scale bars: 50 µm.
Figure 3.
A model for stepwise formation of tertiary lymphoid tissue (TLT) in the kidney. In the initiation of TLT formation in mice, fibroblasts positive for Raldh2 surround TLTs. On the other hand, fibroblasts within TLTs are negative for retinaldehyde dehydrogenase 2 (RALDH2), and instead are strongly positive for p75 neurotrophin receptor (p75NTR), a marker of neural crest. Some of the p75NTR+ fibroblasts inside TLTs acquire the ability to produce homeostatic chemokines CXCL13 and CCL19, and these drive and maintain TLTs. Subsequently, as TLTs mature, CD21+/p75NTR− follicular dendritic cells (FDCs) emerge as an integral part of the fibroblast-network within the TLT, and peripheral lymph node addressin-positive (PNAd+) high endothelial venules (HEVs) also develop within TLTs in this phase. Although major cellular and molecular components of TLTs in human kidneys are similar to those in murine TLT, p75NTR colocalize with CD21, and RALDH2+ stromal cells surround CD21+ FDC.
Conclusion and perspectives
Accumulating evidence has led to the identification of resident fibroblasts in the kidney as a plastic population that can adopt a wide range of phenotypes, from fibrosis-driving myofibroblasts and inflammatory fibroblasts to “regulatory fibroblasts”. Given the central roles of myofibroblasts as a driver of CKD progression, as described above, targeting myofibroblasts or their differentiation may hold clinical potential to halt renal fibrosis and its associated pathological conditions. Additionally, accumulating evidence has shown that the same resident fibroblasts in the kidney can acquire distinctive phenotypes depending on their local microenvironmental signals (Fig. 4).6,50,51) Therefore, to achieve a deeper understanding of fibroblast-related pathophysiology in kidney diseases, the different impacts of the microenvironment should be carefully ascertained for each fibroblast type with a distinct phenotype.
Figure 4.
Functional heterogeneity of resident fibroblasts in the kidney. Resident fibroblasts in the kidney are a plastic population that can adopt a wide range of phenotypes dependent on their surrounding microenvironment.6,11,32,50,51,59) (A) In response to injury, fibroblasts transdifferentiate into myofibroblasts, some of which acquire the ability to produce retinoic acid (RA). Although RA derived from myofibroblasts can support the regeneration of tubular cells,32) RA in aged injured kidneys additionally promotes the transdifferentiation of fibroblasts into tertiary lymphoid tissue (TLT)-associated fibroblasts,6,50,51) suggesting that RA signaling in the injured kidney appears to be context dependent. Resident fibroblasts within the TLT produce CXCL13 and CCL19, playing a central role in TLT formation and maintenance. In IgG4-related disease, fibroblasts express strong expression of glucocorticoid receptor. (B) Multiple roles of resident fibroblasts in healthy and diseased kidney. αSMA: α-smooth muscle actin, ECM: extracellular matrix, EPO: erythropoietin, GR: glucocorticoid receptor, IgG4-RD: IgG4-related disease, p75NTR: p75 neurotrophin receptor, RALDH: retinaldehyde dehydrogenase.
Notably, we examined renal fibroblasts in patients with IgG4-related disease, which is characterized by unique storiform fibrosis,58) and found that fibroblasts and myofibroblasts in this rare disease exhibited strong glucocorticoid receptor expression (Fig. 4).59) Given that patients with IgG4-related disease respond well to glucocorticoid therapy, it is tempting to speculate that glucocorticoids directly affect fibroblasts and attenuate fibrosis.
Recently, in the field of cancer research, the phenotypic diversity of fibroblasts has been highlighted by scRNA-seq analysis. These studies revealed novel fibroblast populations and prompted a reevaluation of fibroblast identities and functions.60,61) Likewise, these novel technologies will reveal further heterogeneity among fibroblasts in the kidney and be crucial to facilitate better understanding of the pathophysiology of kidney diseases, and may provide a rational strategy for the treatment of kidney diseases.
Acknowledgments
This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP18gm5010002 and JP18gm0610011; partially by grants from the TMK Project, KAKENHI Grant-in-Aids for Scientific Research B (17H04187), Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid on Innovative Areas (17H05642, 18H04673), Grant-in-Aid for Exploratory Research (17K19677), the Translational Research Program, and the Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) from AMED; and by grants from the Uehara Memorial Foundation, Takeda Science Foundation, Yukiko Ishibashi Foundation, and the Sumitomo Foundation. This work was partly supported by World Premier International Research Center Initiative (WPI), MEXT, Japan.
Abbreviations
- αSMA
α-smooth muscle actin
- CKD
chronic kidney disease
- ECM
extracellular matrix
- EPO
erythropoietin
- p75NTR
p75 neurotrophin receptor
- PDGFR
platelet-derived growth factor receptor
- RA
retinoic acid
- Raldh2
retinaldehyde dehydrogenase 2
- TLT
tertiary lymphoid tissue
Profile
Motoko Yanagita was born in Kobe in 1969, and graduated from Kyoto University School of Medicine in 1994. After an internship, she majored in Nephrology, and received her M.D. and Ph.D. at Kyoto University. After postdoctoral training in the ERATO project headed by Prof. Masashi Yanagisawa, she has been a first Professor of the Department of Nephrology in Kyoto University since 2011. She is a physician scientist conducting basic and clinical research. Her basic research area is the pathophysiology of kidney injury and repair processes, and her current research focuses on identification of the cellular origins of kidney regeneration and fibrosis as well as the crosstalk among various types of cells in the progression of kidney disease. Her clinical research area is Onconephrology, a new area connecting oncology and nephrology. She is an elected member of American Society of Clinical Investigation, Councilor of the Japanese Society of Nephrology, Program Committee member of Kidney Week 2018 of the American Society of Nephrology, and on the editorial boards of the Journal of the American Society of Nephrology and American Journal of Physiology-Renal Physiology.
References
- 1).Coresh J., Selvin E., Stevens L.A., Manzi J., Kusek J.W., Eggers P., et al. (2007) Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047. [DOI] [PubMed] [Google Scholar]
- 2).Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. [DOI] [PubMed] [Google Scholar]
- 3).National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 39, S1-S266. [PubMed] [Google Scholar]
- 4).Nangaku M. (2006) Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25. [DOI] [PubMed] [Google Scholar]
- 5).Mack M., Yanagita M. (2015) Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 87, 297–307. [DOI] [PubMed] [Google Scholar]
- 6).Sato Y., Yanagita M. (2017) Resident fibroblasts in the kidney: A major driver of fibrosis and inflammation. Inflamm. Regen. 37, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sato Y., Yanagita M. (2013) Renal anemia: From incurable to curable. Am. J. Physiol. Renal Physiol. 305, F1239–F1248. [DOI] [PubMed] [Google Scholar]
- 8).Obara N., Suzuki N., Kim K., Nagasawa T., Imagawa S., Yamamoto M. (2008) Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 111, 5223–5232. [DOI] [PubMed] [Google Scholar]
- 9).Le Douarin N.M., Teillet M.A. (1974) Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 41, 162–184. [DOI] [PubMed] [Google Scholar]
- 10).Yamauchi Y., Abe K., Mantani A., Hitoshi Y., Suzuki M., Osuzu F., et al. (1999) A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev. Biol. 212, 191–203. [DOI] [PubMed] [Google Scholar]
- 11).Asada N., Takase M., Nakamura J., Oguchi A., Asada M., Suzuki N., et al. (2011) Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J. Clin. Invest. 121, 3981–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Souma T., Yamazaki S., Moriguchi T., Suzuki N., Hirano I., Pan X., et al. (2013) Plasticity of renal erythropoietin-producing cells governs fibrosis. J. Am. Soc. Nephrol. 24, 1599–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Itaranta P., Viiri K., Kaartinen V., Vainio S. (2009) Lumbo-sacral neural crest derivatives fate mapped with the aid of Wnt-1 promoter integrate but are not essential to kidney development. Differentiation 77, 199–208. [DOI] [PubMed] [Google Scholar]
- 14).Suzuki N., Hirano I., Pan X., Minegishi N., Yamamoto M. (2013) Erythropoietin production in neuroepithelial and neural crest cells during primitive erythropoiesis. Nat. Commun. 4, 2902. [DOI] [PubMed] [Google Scholar]
- 15).Humphreys B.D. (2018) Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326. [DOI] [PubMed] [Google Scholar]
- 16).Nath K.A. (1992) Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17. [DOI] [PubMed] [Google Scholar]
- 17).Jun J.I., Lau L.F. (2018) Resolution of organ fibrosis. J. Clin. Invest. 128, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., et al. (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kramann R., Schneider R.K., DiRocco D.P., Machado F., Fleig S., Bondzie P.A., et al. (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Buchtler S., Grill A., Hofmarksrichter S., Stockert P., Schiechl-Brachner G., Rodriguez Gomez M., et al. (2018) Cellular origin and functional relevance of collagen I production in the kidney. J. Am. Soc. Nephrol. 29, 1859–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).LeBleu V.S., Taduri G., O’Connell J., Teng Y., Cooke V.G., Woda C., et al. (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Duffield J.S. (2014) Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 124, 2299–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Kramann R., Machado F., Wu H., Kusaba T., Hoeft K., Schneider R.K., et al. (2018) Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight 3, e99561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Fehr T., Ammann P., Garzoni D., Korte W., Fierz W., Rickli H., et al. (2004) Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 66, 1206–1211. [DOI] [PubMed] [Google Scholar]
- 25).Schiessl I.M., Grill A., Fremter K., Steppan D., Hellmuth M.K., Castrop H. (2018) Renal interstitial platelet-derived growth factor receptor-β cells support proximal tubular regeneration. J. Am. Soc. Nephrol. 29, 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Souma T., Nezu M., Nakano D., Yamazaki S., Hirano I., Sekine H., et al. (2016) Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. J. Am. Soc. Nephrol. 27, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Grgic I., Campanholle G., Bijol V., Wang C., Sabbisetti V.S., Ichimura T., et al. (2012) Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 82, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Takaori K., Nakamura J., Yamamoto S., Nakata H., Sato Y., Takase M., et al. (2016) Severity and frequency of proximal tubule injury determines renal prognosis. J. Am. Soc. Nephrol. 27, 2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Endo T., Nakamura J., Sato Y., Asada M., Yamada R., Takase M., et al. (2015) Exploring the origin and limitations of kidney regeneration. J. Pathol. 236, 251–263. [DOI] [PubMed] [Google Scholar]
- 30).Kusaba T., Lalli M., Kramann R., Kobayashi A., Humphreys B.D. (2014) Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. U.S.A. 111, 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Humphreys B.D., Valerius M.T., Kobayashi A., Mugford J.W., Soeung S., Duffield J.S., et al. (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291. [DOI] [PubMed] [Google Scholar]
- 32).Nakamura J., Sato Y., Kitai Y., Wajima S., Yamamoto S., Oguchi A., et al. (2019) Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition in kidney disease. Kidney Int. 95, 526–539. [DOI] [PubMed] [Google Scholar]
- 33).Rhinn M., Dolle P. (2012) Retinoic acid signalling during development. Development 139, 843–858. [DOI] [PubMed] [Google Scholar]
- 34).Cullen-McEwen L.A., Caruana G., Bertram J.F. (2005) The where, what and why of the developing renal stroma. Nephron, Exp. Nephrol. 99, e1–e8. [DOI] [PubMed] [Google Scholar]
- 35).Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., et al. (2013) Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 381, 468–475. [DOI] [PubMed] [Google Scholar]
- 36).Troeger J.S., Mederacke I., Gwak G.Y., Dapito D.H., Mu X., Hsu C.C., et al. (2012) Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., et al. (2012) Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. U.S.A. 109, 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., et al. (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Iredale J.P., Benyon R.C., Pickering J., McCullen M., Northrop M., Pawley S., et al. (1998) Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Campisi J. (2013) Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Wallach-Dayan S.B., Golan-Gerstl R., Breuer R. (2007) Evasion of myofibroblasts from immune surveillance: A mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. U.S.A. 104, 20460–20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., et al. (2003) BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968. [DOI] [PubMed] [Google Scholar]
- 43).Yanagita M. (2006) Modulator of bone morphogenetic protein activity in the progression of kidney diseases. Kidney Int. 70, 989–993. [DOI] [PubMed] [Google Scholar]
- 44).Tanaka M., Endo S., Okuda T., Economides A.N., Valenzuela D.M., Murphy A.J., et al. (2008) Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int. 73, 181–191. [DOI] [PubMed] [Google Scholar]
- 45).Yanagita M., Oka M., Watabe T., Iguchi H., Niida A., Takahashi S., et al. (2004) USAG-1: A bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem. Biophys. Res. Commun. 316, 490–500. [DOI] [PubMed] [Google Scholar]
- 46).Yanagita M., Okuda T., Endo S., Tanaka M., Takahashi K., Sugiyama F., et al. (2006) Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J. Clin. Invest. 116, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Tanaka M., Asada M., Higashi A.Y., Nakamura J., Oguchi A., Tomita M., et al. (2010) Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. J. Clin. Invest. 120, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Qian X., Yuan X., Vonderfecht S., Ge X., Lee J., Jurisch A., et al. (2013) Inhibition of WISE preserves renal allograft function. J. Am. Soc. Nephrol. 24, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., et al. (2014) Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 6, 231ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Sato Y., Mii A., Hamazaki Y., Fujita H., Nakata H., Masuda K., et al. (2016) Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1, e87680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Sato Y., Yanagita M. (2018) Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Renal Physiol. 315, F1501–F1512. [DOI] [PubMed] [Google Scholar]
- 52).Ishani A., Xue J.L., Himmelfarb J., Eggers P.W., Kimmel P.L., Molitoris B.A., et al. (2009) Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Jones G.W., Hill D.G., Jones S.A. (2016) Understanding immune cells in tertiary lymphoid organ development: It is all starting to come together. Front. Immunol. 7, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Barone F., Gardner D.H., Nayar S., Steinthal N., Buckley C.D., Luther S.A. (2016) Stromal fibroblasts in tertiary lymphoid structures: A novel target in chronic inflammation. Front. Immunol. 7, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Luther S.A., Lopez T., Bai W., Hanahan D., Cyster J.G. (2000) BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481. [DOI] [PubMed] [Google Scholar]
- 56).Luther S.A., Bidgol A., Hargreaves D.C., Schmidt A., Xu Y., Paniyadi J., et al. (2002) Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433. [DOI] [PubMed] [Google Scholar]
- 57).Heesters B.A., Myers R.C., Carroll M.C. (2014) Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol. 14, 495–504. [DOI] [PubMed] [Google Scholar]
- 58).Stone J.H., Zen Y., Deshpande V. (2012) IgG4-related disease. N. Engl. J. Med. 366, 539–551. [DOI] [PubMed] [Google Scholar]
- 59).Iguchi T., Takaori K., Mii A., Sato Y., Suzuki Y., Yoshifuji H., et al. (2018) Glucocorticoid receptor expression in resident and hematopoietic cells in IgG4-related disease. Mod. Pathol. 31, 890–899. [DOI] [PubMed] [Google Scholar]
- 60).Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., et al. (2018) Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24, 1277–1289. [DOI] [PubMed] [Google Scholar]
- 61).Li H., Courtois E.T., Sengupta D., Tan Y., Chen K.H., Goh J.J.L., et al. (2017) Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 49, 708–718. [DOI] [PubMed] [Google Scholar]