Abstract
Reconstruction of massive bone defects is challenging for orthopaedic clinicians, especially in cases of severe trauma and resection of tumors in various locales. Autologous iliac crest bone graft (ICBG) is the “gold standard” for bone grafting. However, the limited availability and complications at donor sites resulted in seeking other options like allografts and bone graft substitutes. Demineralized bone matrix (DBM) is a form of allograft using acidic solution to remove mineral components, while leaving much of the proteinaceous components native to bone, with small amounts of calcium‐based solids, inorganic phosphates, and some trace cell debris. It is an osteoconductive and osteoinductive biomaterial and is approved as a medical device for use in bone defects and spinal fusion. To pack consistently into the defect sites and stay firmly in the filling parts, DBM products have various forms combined with biocompatible viscous carriers, including sponges, strips, injectable putty, paste, and paste infused with chips. The present review aims to summarize the properties of various kind of viscous carriers and their clinical use combined with DBM in commercially available products. Given DBM'mercially available products. Given DBM;s long clinical track record and commercial accessibility in standard forms, opportunities to further develop and validate DBM as a versatile bone biomaterial in orthopaedic repair and regenerative medicine contexts are attractive.
Keywords: Bone graft, Bone regeneration, Demineralized bone matrix, Viscous carriers
Introduction
Reconstruction of massive bone defects is challenging for orthopaedic clinicians, especially in cases of severe trauma and resection of tumors in various locales. Many bone graft materials have been applied to accomplish this procedure, including autogenous bone, allogeneic bone, xenogenic bone, and other non‐bone derived substances. In general, an ideal bone substitute material should be equipped with the following three elements: (i) “osteoconductivity,” acting as the “soil,” the three‐dimensional process of ingrowth of sprouting capillaries, perivascular tissue, and osteoprogenitor cells from the recipient bed into the structure of an implant or bone graft1; (ii) “osteoinductivity,” acting as the “fertilizer,” the process of differentiation of pluripotential mesenchymal cells into osteoprogenitor cells and ultimately into osteoblasts that form bone as a consequence of a stimulating agent (i.e. bone morphogenetic protein)2; (iii) “osteogenesis,” acting as the “seed,” the ability to reconstruct tissue to form new bone tissue, which refers to the presence of osteoprogenitor cells that directly promote new bone growth at the transplant site. Among the multiple reconstruction substitutes, bone autograft, mainly referring to the iliac crest bone graft (ICBG), has been universally recognized as the “gold standard,” because it possesses the aforementioned elements simultaneously3. Despite this, a notable concern about autografts is the invasive “donor” procedure required to harvest the graft, which tends to result in more postoperative complications, such as severe pain and infection at the donor site4. Moreover, elevated time on the operating room table, increased patient care cost, inadequate donor bone, and mismatched bone shape with the recipient site also reveal that autografts are not an absolutely perfect transplant material5. Therefore, clinicians have been devoted to exploring alternatives to autografting, to overcome the drawbacks of autografts.
Demineralized bone matrix (DBM) has been developed as an allogeneic alternative to autografting, which is an important therapeutic option for appendicular, axial, and craniofacial bone defects. It is a decalcified product using acidic solution to remove mineral components, while leaving behind collagen (mainly type I with some types IV and X), non‐collagen proteins, some osteoinductive growth factors (e.g. bone morphogenic proteins, BMP), variable percentages of residual calcium phosphate mineral (1%–6%), and some small percentages of cellular debris6. After decalcification, BMP could be released from the surrounding mineral components and fully exert its osteoinductive potential7. The remaining collagen proteins in DBM could provide a 3D configuration for ingrowth of host capillaries, perivascular tissue, and osteoprogenitor cells into the graft. Thus, the DBM has been demonstrated to be an osteoconductive and osteoinductive substitute. In the meantime, the original cells and possible bacteria in the allogeneic bone are killed, which could reduce the risks of immune rejection and infection. DBM used for bone reconstruction has many advantages: (i) it is not limited by graft amount, as the donor source is abundant; (ii) it could reduce complications of autograft harvest at the donor site; and (iii) it could shorten the operation and recovery time.
The use of DBM can be traced back to 1889 when Sen8. first used DBM derived from oxen tibiae to repair the skull and long bone defects in humans. Then, the human‐derived DBM was successfully transplanted for human long bone defects and lumbar spine by Urist et al.9 in 1965, for the first time. Therefrom, DBM has been used more frequently in orthopaedic surgeries, and plenty of research has been carried out to explore the bone regeneration capacity of DBM as a bone substitute material. Although DBM has been widely proved to have attractive osteoinductive and osteoconductive potential, but the end products, of various forms, are not easy for clinicians to manage: (i) the powder or particles of DBM can be loose in structure and may not stay firmly in the filling site, and could be easily dispersed by irrigation and blood flow during surgery; and (ii) the application of pre‐formed materials for filling may leave a dead space due to the inconsistency of the repair material and the shape of the defect, resulting in non‐union or delayed healing6. Hence, currently the most popular DBM product is a moldable bone paste or putty, which can be consistently packed into defect sites and remain firm. A moldable DBM product could be made of the composite of DBM powder/particles combined with biocompatible viscous carriers, which provides a stable suspension of DBM powder/particles. In general, according to their molecular weight, the viscous carriers could be divided into the following two classifications: (i) polymer materials, such as collagen, chitosan, hyaluronic acid (HA), carboxymethylcellulose (CMC), and poloxamer 407; and (ii) low molecular materials, such as glycerol, calcium sulfate, and bioactive glass (Fig. 1).
Figure 1.

The common viscous carriers could be divided into two classifications according to molecular weight.
This review aims to summarize the properties of various viscous carriers and their applications combined with DBM in commercially available products.
Study Searching and Selecting
To identify studies concerning DBM carriers and their clinical applications, a systematic literature search was performed. We searched electronic platforms including PubMed, Embase, and Cochrane Library from inception to 25 March 2018, and the language was restricted to English. Search terms included “carrier name” and “demineralized bone matrix.” A combined search using the subject terms (Medical Subject Heading, MeSH) and free terms was carried out in every database. Reference lists of the included studies were also viewed for any additional papers. In addition, we consulted related companies for additional published or unpublished studies.
Two authors independently selected studies following the predetermined selection criteria; any disagreement was resolved by discussion. EndNote X8 (Clarivate Analytics, Philadelphia, USA) was used to detect and merge the duplicates, and then titles and abstracts were evaluated to identify the ones that met the criteria. Finally, full texts were reviewed for inclusion.
A total of 3458 potentially relevant articles were identified. After removing duplicates (1476 articles) by using EndNote X8 and screening all titles and abstracts, 897 articles were excluded. Then full texts were read carefully, and, finally, 97 articles were included. The literature search process is presented in Fig. 2.
Figure 2.
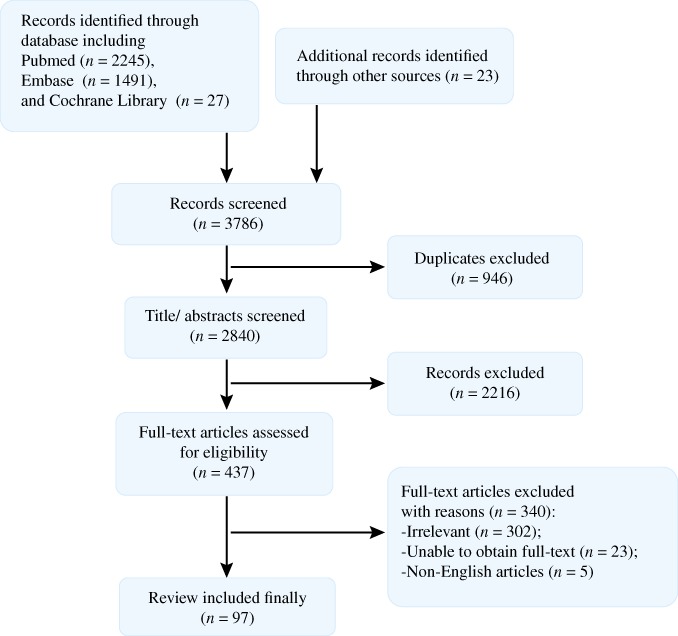
Literature searching process. A total of 3786 articles are identified from databases, including PubMed, Embase, and Cochrane Library. After removing duplicates and screening all titles and abstracts, 437 articles were excluded. Then full texts were read carefully, and, finally, 97 articles were included.
Demineralized Bone Matrix Carriers
Generally, a viscous carrier should be equipped with the following characteristics: (i) Good biocompatibility with the tissues: nontoxicity, nonimmunogenicity, nonteratogenicity, and noncarcinogenicity; (ii) Proper biodegradation rate: corresponding to the osteogenesis procedure, to ensure the successful repairing of the bony defect; (iii) Viscidity and plasticity: assuring graft could be shaped as necessary and stay firmly in filling site; (iv) Osteoconductive three‐dimensional microstructure: promoting ingrowth of host capillaries, perivascular tissue, and osteoprogenitor cells into the graft; and (v) Optimal mechanical property 10. Satisfying the above conditions, a variety of carrier materials have been selected to compound the DBM to deliver a plastic decalcified bone paste, and these carriers include the polymer materials and the low molecular materials according to their molecular weight.
Polymer Carriers Materials
The polymer carriers mainly include the natural polymer materials (e.g. chitosan, HA, sodium alginate, CMC, and type I collagen) and synthetic polymer materials (e.g. poloxamer 407). They are all demonstrated to be biocompatible and biodegradable after implantation in vivo, and have attractive viscidity to cohere with DBM powder particles.
Chitosan
The history of chitosan could be retrospected to 1859 when Rouget first heated chitin to the boiling point in a concentrated KOH solution11. Chitin, the source material for chitosan, is the major component of the exoskeleton of invertebrates, crustaceans and insects, and the cell wall of fungi and yeast, in which it acts as a supportive and protective structure12. Chitosan is a linear polysaccharide and is composed of glucosamine and N‐acetyl‐glucosamine units linked by β (1‐4) glycosidic bonds10. In general, when the percent of N‐acetyl‐glucosamine units is higher than 50%, the biopolymer should be termed chitin, and vice versa. As represented in Fig. 3, the chitosan is a product obtained from the de‐N‐acetylation of chitin, in the presence of hot alkali. The content of glucosamine in chitosan molecules is defined as the degree of deacetylation (DD). The DD and source of chitin may obviously influence the molecular weight of chitosan, which ranges from 300 to over 1000 kDa, with a DD of 30%–95%13.
Figure 3.

Chemical structure of chitin and chitosan, and the de‐N‐acetylation process from chitin to chitosan.
Chitosan could not be dissolved in neutral or alkaline solution but could be easily dissolved in dilute acid solution (pH < 6, such as dilute acetic acid), where the free amino groups are protonated and the molecule becomes soluble14. Porous chitosan structures can be formed by freezing and lyophilizing chitosan acetic acid solutions in suitable molds. Porous materials play a significant role in the bone implantation process.
It has been widely recognized that the chitosan is biocompatible and biodegradable in vivo, and its degradation products are non‐toxic, non‐immunogenic, and non‐carcinogenic15. Lysozyme is the primary enzyme that is responsible for degradation of chitosan in vivo, which appears to target acetylated residues16. The final degradation products are biocompatible chitosan oligosaccharides with variable length. The biodegradation rate of chitosan may be diverse among biopolymers with different DD, as it would influence the degree of crystallinity. It has been reported that highly deacetylated forms of chitosan (>85%) exhibit a relatively slow degradation rate that may last several months, while the forms with lower DD degrade more rapidly17.
Chitosan is a unique natural animal cellulin with positively charged cation. The cationic nature of chitosan is responsible for attracting various negatively charged proteoglycans, which helps the osteoblasts’ adhesion to it. In addition, Chitosan has antibacterial activity18, and antifungal19, mucoadhesive20, analgesic19, and hemostatic properties21, and the ability to promote osteogenic progenitor cell recruitment and attachment, thus facilitating bone formation22. It can be produced in various forms, including hydrogel, powder, small sphere, tablet, capsule, microbead, particulate, sponge, nano‐fiber, and textile fiber. Therefore, this unique biopolymer is an outstanding candidate for biomedical applications, especially for tissue engineering.
Hyaluronic Acid
Hyaluronic acid (HA) is a unique linear macromolecule acidic mucopolysaccharide composed of N‐acetyl‐D glucosamine and D‐glucuronic acid. D‐glucuronic acid and N‐acetylglucosamine are linked by a β‐1,3‐glycosidic bond, and the disaccharide units are linked by a β‐1,4‐glycosidic bond. The two monosaccharides in the molecule are composed in a molar ratio of 1:1. The chemical structural of HA is shown in Fig. 4. HA molecules contain a large amount of carboxyl groups and hydroxyl groups, forming intramolecular and intermolecular hydrogen bonds in aqueous solution, so they have strong water retention, and can bind more than 400 times of water at higher concentrations, because the complex three‐stage network structure formed by its intermolecular action has an aqueous solution with remarkable viscoelasticity.
Figure 4.

Chemical structure of hyaluronic acid, which is composed of N‐acetyl‐D glucosamine and D‐glucuronic acid. D‐glucuronic acid and N‐acetylglucosamine are linked by a β‐1,3‐glycosidic bond, and the disaccharide units are linked by a β‐1,4‐glycosidic bond.
The polysaccharide chains of HA are linear and unbranched and roll up into a coil conformation. The irregular crimping state and hydrodynamics of HA in solution give it many important physical properties, such as high degree of viscoelasticity, moldability, degradability, permeability, and good biocompatibility23.
The presence of HA in an aqueous solution shows a complex rheological behavior, including this system into the pseudoplastic fluids,which is very important for its applications. The rheological characterization of HA aqueous solutions has been carried out byGarcia24, determining the value of the intrinsic viscosity and the average molecular weight. Both the increase of temperature and the presence of an electrolyte produce an important decrease in the viscosity magnitude, as well as an approximation to the Newtonian behavior related to its rheology. Procedures for introducing covalent cross‐links in hyaluronan matrices have been developed to create stable networks and semisolid materials exhibiting pronounced viscoelastic properties25.
Hyaluronic acid widely exists in human tissues such as joints, vitreous bodies, synovial fluid, cartilage, skin, and other tissues and organs as a major constituent of the extracellular matrix (ECM). It has been reported to play an important role in tissue repair and regeneration26. Moreover, it has been demonstrated that HA hydrogels could retain BMP‐227. HA has also been found to affect the interplay between osteoclasts and osteoblasts that is important in bone remodeling and fracture healing28.
Most of the HA absorbed by the human body enters the lymph nodes and degrades into monosaccharides in the lymph nodes, and a small part enters the blood circulation. Approximately 80% of HA in the blood circulation is quickly ingested by the liver, and the rest is handled by the spleen and other organs. The final product of HA metabolism in the body is carbon dioxide and water, which has no toxic side effects on the body.
Sodium Alginate
Alginate is the most abundant marine biopolymer, which comprises a rather broad family of polysaccharides found in brown seaweeds (Laminariasp., Macrocystis sp., Lessonia sp., and others). The major source of alginate is found in the cell walls and in the intracellular spaces of brown seaweed. The alginate molecules provide the plant with both flexibility and strength, which are necessary for plant growth in the sea. The first scientific studies on the extraction of alginates from brown seaweed were made by the British chemist E.C. Stanford who found that the extracted substance, which he named algin, possessed several interesting properties29, including the ability to thicken solutions, to make gels, and to form films. Sodium alginate is the main form of alginate. Other types of alginate include alginic acid, calcium, ammonium and potassium salts, and propylene glycol alginate, an ester of alginic acid.
Alginates are linear unbranched polymers containing β‐(1 → 4)‐linked D‐mannuronic acid (M) and α‐(1 → 4)‐linked L‐guluronic acid (G) residues. The blocks are composed of consecutive G residues (GGGGGG), consecutive M residues (MMMMMM), and alternating M and G residues (GMGMGM). Only the G‐blocks of alginate are believed to participate in intermolecular cross‐linking with divalent cations (e.g. Ca2+) to form hydrogels. The composition (i.e. M/G ratio), sequence, G‐block length, and molecular weight are critical factors affecting the physical properties of alginate and its resultant hydrogels30. The ability of alginates to form soft hydrogels with calcium ions forms the basis for a wide variety of applications. The structure of sodium alginate is shown in Fig. 5.
Figure 5.

Chemical structure of sodium alginate, which is composed of consecutive G residues (GGGGGG), consecutive M residues (MMMMMM), and alternating M and G residues (GMGMGM).
Sodium alginate slowly dissolves in cold water, forming a viscous, colloidal solution. It is insoluble in alcohol and hydroalcoholic solutions in which alcohol content is greater than 30% by weight. It is also insoluble in other organic solvents such as chloroform and ether and in acids where the pH of the resulting solution falls below 3.0.
Although the biocompatibility of alginates has been extensively evaluated in vitro as well as in vivo, there is still some debate regarding the impact of the alginate composition. Much of this confusion is likely related to various levels of purity in the alginates studied in various reports. One study suggests that as long as the alginate is purified, it is biocompatible31. However, the study of Gomez et al.32 indicates that the following two factors were more important for biocompatibility: M/G content and molar mass, probably via their influence on the physical properties (stiffness and swell ability) of the resulting gels.
Carboxymethylcellulose
Carboxymethylcellulose (CMC) is widely used in the pharmaceutical industry. It is a vegetable cellulose derivative obtained by the action of chloroacetic acid on cellulose in an alkaline medium, whereby hydroxyl functions are substituted by carboxymethyl groups. The basic structure is a (1–4) D glucopyranosyl polymer. The chemical structural of CMC is shown in Fig. 6.
Figure 6.

Chemical structure of carboxymethylcellulose (CMC) and its basic structure is a (1–4) d glucopyranosyl polymer.
Carboxymethylcellulose is a white to off‐white, odorless, and slightly hygroscopic powder with physiological inertia. It is soluble in water at all temperatures but practically insoluble in organic solvents. In addition, CMC is a stabilizing, emulsifying, thickening, binding, hydrophilic agent that can retain water and form a protective film. When dissolved or dispersed in water, it could increase its viscosity and contribute to form suspensions (from fluids to gels). Because CMC has good tissue compatibility and no toxic side effects on the body, it can be used as a biological material for implantation in vivo 33.
The role of CMC anti‐adhesion has been affirmed. The most important and widely recognized mechanism for its anti‐adhesion is the biomechanical isolation. CMC can also weaken the activity or proliferation of fibroblasts, prevent fibrin deposition on the damaged serosal surface34, prevent the removal of plasminogen from the wound surface, and increase its activation effectiveness35. Adanali et al.36 believe that the mechanism of CMC prevention of joint adhesion are exerting physical barriers, mitigating different inflammatory changes by exerting potential anti‐inflammatory effects after bone injury, including reducing joint capsule thickening and loss of adjacent tissue extensibility. The CMC prepared by Bae et al.37, which dissolves pure a‐cellulose in an alkaline solution, has a hemostatic effect, and it is believed that CMC can reduce adhesion by regulating the urokinase‐type plasminogen activator and its cellular receptor.
Type I Collagen
Collagen is a type of fibrous, macromolecular protein found in all mammals. It is secreted by connective tissue cells and other types of cells (such as liver, lung, spleen, and brain tissue cells) in mammals. As a major component of bones and skin, they are the most abundant protein in mammalian cells, accounting for approximately one‐quarter of total cellular proteins38. At present, 27 kinds of collagen have been found. The different collagen types are characterized by considerable complexity and diversity in their structure, splice variants, the presence of additional, non‐helical domains, assembly, and function. Despite the rather high structural diversity among the different collagen types, all members of the collagen family have one characteristic feature: a right‐handed triple helix composed of three a‐chains39. The type I collagen triple helix is usually formed as a heterotrimer by two identical a1(I)‐chains and one a2(I)‐chain40, whose structure is shown in Fig. 7.
Figure 7.

Structure of type I collagen, a right‐handed triple helix composed of two identical a1(I)‐chains and one a2(I)‐chain.
Type I collagen is the most abundant collagen and is reported in many studies. It forms more than 90% of the organic mass of bone and is the major collagen of tendons, skin, ligaments, cornea, and many interstitial connective tissues, with the exception of very few tissues, such as hyaline cartilage, brain, and vitreous body. In most organs and notably in tendons and fascia, type I collagen provides tensile stiffness, and in bone, it defines considerable biomechanical properties concerning load bearing, tensile strength, and torsional stiffness in particular after calcification41.
Type I collagen contributes to the entrapment, local storage, and delivery of growth factors and cytokines, and plays an important role during organ development, wound healing, and tissue repair42. Furthermore, some additional features of collagens, such as biodegradability, low immunogenicity, inducing expression of BMP‐2 receptor43, and the possibilities for large‐scale isolation make them interesting compounds for medical application.
Poloxamer 407
Poloxamer copolymer is a tri‐block copolymer consisting of a central hydrophobic polyoxypropylene (PPO) block flanked by two hydrophilic polyoxyethylene (PEO) blocks (PEOx–PPOy–PEOx), whose chemical formula is HO[CH2‐CH2O]x[CH(CH3)‐CH2O]y[CH2‐CH2O]xOH, and y is higher than 14. It has a molecular weight of 1000 to more than 16 000, and is soluble in aromatic solvents and insoluble in ethylene glycol, kerosene, and mineral oil. Moreover, it is stable to acid, alkali, and metal ions44.
Poloxamer 407 is composed of approximately 70% ethylene oxide and 30% polypropylene oxide. Its aqueous solution with a concentration of 20%–30% has the property of reverse thermal gelation45. That is to say, it exists as a liquid at refrigerated temperature (4–5°C) but gels at physiological temperature. Bohorquez et al. indicate that when the critical micelle temperature is reached, the hydrophobic PPO block on the polymer chain is dehydrated and the poloxamer molecules aggregate in aqueous solution to form spherical micelles with dehydrated PPO chains as the core and hydrated expanded PEO chains as the outer shell46. With the increasing of temperature, gelation occurs due to the aggravation of entanglement and stacking between micelles. The temperature of solution–gel conversion is affected by the ratio of PEO/PPO, polymer concentration, and electrolytes in the solution. Some small molecules can change the phase transition temperature and gel strength of Poloxham 407 solution. The study by Yong demonstrated that diclofenac sodium significantly increased the gelation temperature and weakened the gel strength and bioadhesive force of Poloxamer 407, while sodium chloride did the opposite47.
Poloxamer 407 is a polymer material that cannot be excreted by the kidneys, so it is preferentially taken up by liver tissue, which may be one of the reasons for changing lipid metabolism. Poloxamer 407 interferes with the catalytic activity of 3‐hydroxy‐3methylglutaryl coenzyme A reductase required for cholesterol biosynthesis, while altering the release of heparin and intracellular lipoproteinase. Hyperlipidemia is caused by intraperitoneal administration of poloxamer 407 0.5–1 g·kg−1. Changes in lipid metabolism are still controversial and further research is required. Long‐term (1 year) administration of mouse poloxamer 407 did not reveal an effect on total cholesterol or alanine/aspartate activity. Poloxamer 407 did not cause an increase in morbidity or mortality compared to control mice.
Low Molecular Carriers Materials
The low molecular carriers mainly include glycerol, calcium sulfate, and bioactive glass. They all have good plasticity, could be arbitrarily shaped mixed with DBM, and could help with osteogenesis due to their unique characteristics.
Glycerol
Glycerol was initially discovered by Schell in 1779. Glycerol is a colorless, transparent, odorless, sweet organic compound with a chemical structure, which is shown in Figure 8. It is a clear and viscous liquid, mixed with water and alcohol, amines, and phenols in any proportion, and the aqueous solution is neutral. It has good biocompatibility and viscosity and is one of the commonly used viscous carriers for DBM.
Figure 8.

Chemical structure of glycerol.
Calcium Sulfate
Calcium sulfate (CS) is commonly referred to as gypsum and is a common mineral consisting of calcium sulfate dihydrate (CaSO4 • 2 H2O). CS and its products differ in purity and form, but a major feature is that water can be removed under controlled heat to form calcium sulfate hemihydrate, also known as plaster of Paris. This process is referred to as calcinatio48.:
| (1) |
Calcium sulfate hemihydrate has plasticity and in‐situ self‐curing properties. It can be made into various shapes according to the filling part, which is very suitable for filling and repairing bone defects. The setting of calcium sulfate hemihydrate is influenced by the milieu where it occurs. It is commonly reported that the addition of inorganic salts to CS, such as sodium chloride and potassium sulfate, could accelerate the setting reaction by increasing the density of the seed crystals49.
When the hemihydrate is mixed with water, Equation 1 is reversed in a mild exothermic reaction:
| (2) |
Because the hemihydrate is insoluble in water, a suspension is initially formed. As it slowly dissolves, a two‐phase suspension of hemihydrate particles in saturated aqueous solution exists. When the hemihydrate solution becomes supersaturated with dihydrate, dihydrate crystals nucleate in the suspension. Nucleation and crystal growth continue until the solution is no longer saturated, leading to further dissolution of the hemihydrate. Alternating dissolution and precipitation continues, with growth of existing crystals or nucleation of new crystals.
Calcium sulfate offers many advantages as it presents a structure similar to bone. It is osteoconductive50, inexpensive, and available in different forms (hard pellets and injectable fluids)51. The exact mechanisms through which calcium sulfate may enhance osteogenesis are unknown. It is possible that calcium ions are released during dissolution of calcium sulfate. Local increases in calcium ion concentration may affect osteoblast genesis and function, and they may act as the stimulus to osteoblast differentiation52. Other studies report that calcium sulfate has a crystalline structure that is osteoconductive, onto which bone capillaries and perivascular mesenchymal tissue can invade53. Walsh et al.54 report that locally altered pH may also play a role in osteogenesis around calcium sulfate implants. This increased acidity as the material dissolves can demineralize adjacent bone and release matrix‐bound bone growth factors that stimulate bone formation.
Biocompatibility is a sine qua non for implantable materials and is the result of complex interactions at the host–material interface55. The lack of significant host responses subsequent to implantation is an important characteristic of biocompatible materials. Many investigators have observed minimal inflammatory responses subsequent to implantation of CS56, 57, 58, 59.
Bioactive Glass
Bioactive glass is defined as a glass designed to elicit specific physiological responses60. In 1969, the first bioactive glass (Bioglass 45S5) was developed by L. Hench61, and it has been in clinical use since 1985. Many bioactive glasses consist of the same components, in slightly different concentrations (some representative bioactive glass compositions are shown in Table 1).
Table 1.
Compositions of various bioactive glasses (wt%)
| SiO2 | CaO | Na2O | K2O | MgO | P2O5 | B2O3 | |
|---|---|---|---|---|---|---|---|
| 45S5 | 45.0 | 24.5 | 24.5 | — | — | 6.0 | — |
| 13–93 | 53.0 | 20.0 | 6.0 | 12.0 | 5.0 | 4.0 | — |
| 13‐93B1 | 34.4 | 19.5 | 5.8 | 11.7 | 4.9 | 3.8 | 19.9 |
| 13‐93B3 | ‐ | 18.5 | 5.5 | 11.1 | 4.6 | 3.7 | 56.6 |
| 6P53B | 52.7 | 18.0 | 10.3 | 2.8 | 10.2 | 6.0 | — |
| 58S | 58.2 | 32.6 | — | — | — | 9.2 | — |
| 70S30C | 71.4 | 28.6 | — | — | — | — | — |
| P50C35N15 | — | 19.7 | 9.3 | — | — | 71.0 | — |
Bioactive glasses are extremely biocompatible. They do not evoke an inflammatory response when implanted into human or animal models. Injecting large doses of bioactive glass intramuscularly or subcutaneously had no adverse effect in a murine model62.
Bioactive glass is osteoconductive and osteoinductive and it can form a tight chemical bond with bone63. When exposed to real or simulated body fluids, dissolution of the bioactive glass surface is seen releasing Ca, P and Si ions because of its own special chemical composition. Dissolution and repolymerization of silica occur to form a silica gel on the surface. Amorphous calcium phosphate nucleates and grows in and on the SiO2‐rich layer. With time, the CaO‐P2O5 mineral incorporates carbonate and hydroxyl species from the ambient fluid, and hydroxycarbonate apatite (HCA) crystallizes. This layer is necessary for bone bonding64. This is similar to the process seen in hydroxyapatite.
It has been reported that bioactive glass can upregulate some essential genes for new bone formation such as insulin‐like growth factor (IGF‐II) and vascular endothelial growth factor (VEGF), in which IGF‐II could induce osteoblast proliferation, and VEGF could promote angiogenesis, which is required for new bone formation65.
Commercially Available Demineralized Bone Matrix Products
There are many commercially available DBM products that have been used as a bone graft extender or as a bone graft substitute for a wide range of trauma‐related and orthopaedic‐related indications. The carriers and processing methods of different DBM products are different, which may have a certain impact on the osteogenic ability of the products. DBM products come in various forms, including sponges, strips, injectable putty, paste, and paste infused with chips66. These various forms also affect the products’ ability to serve as graft extenders, enhancers, or substitutes. Product names of common commercially available DBM products are shown in Table 2.
Table 2.
Commercially available demineralized bone matrix (DBM)
| Product | Source company | DBM(%) | Carrier | Form | Indication |
|---|---|---|---|---|---|
| Accell Connexus | Integra | 70 | Poloxamer reverse phase medium | Putty | Bone void filler/bone graft extender |
| Accell Evo3 | Integra | 70 | Poloxamer reverse phase medium and cancellous bone chips | Putty | Bone void filler |
| Accell TBM | Integra | 100 | No carrier | Strip | Bone void filler/bone graft extender |
| AlloCraft | Stryker | 80 | Acellular matrix | Paste | Bone void filler |
| AlloFuse | Allosource | 36 (putty), 29 (gel) | Reverse phase medium | Putty, gel | Bone void filler/bone graft extender |
| Allomatrix | Wright Medical | 40 to 86 | Calcium sulphate | Paste | Bone void filler/bone graft extender |
| AlphaGRAFT | Alphatech | 80 | Acellular matrix | Paste | Bone void filler |
| Altiva | Exactech | ND | Gelatin | Paste | Bone void filler |
| BioSet | Penta Biomedical | 24 | Porcine gelatin | Paste, strip, disc, with or without cancellous bone chips | Bone void filler |
| DBX | Medtronic | 31 (putty), 26 (paste), 35 (mix), 45 (strip) | Hyaluronic acid | Putty, paste, mix, strip | Bone void filler |
| DynaGraft III | Integra | ND | Poloxamer reverse phase medium | Putty, gel | Bone void filler/bone graft extender |
| Grafton | Osteotech | 17 to 31 | Glycerol | Paste, strip | Bone void filler/bone graft extender/bone graft substitute |
| InterGro | Zimmer Biomet | 40 (putty), 35 (paste) | Lecithin | Putty, paste | Bone void filler/bone graft extender |
| NanoFUSE | Amend Surgical | ND | 45S5 bioactive glass | Putty | Bone void filler |
| Optefil | Exactech | 24 | Gelatin | Paste | Bone void filler |
| Opteform | Exactech | ND | cortical and cancellous bone chips suspended in collagen‐gelatin | Paste | Bone void filler |
| Optium | LifeNet Health | ND | Glycerol | Putty, gel | Bone void filler |
| OrthoBlast | Integra | ND | Poloxamer reverse phase medium | Paste | Bone void filler/bone graft extender |
| OrthoBlast II | Integra | ND | Poloxamer reverse phase medium | Putty, paste | Bone void filler/bone graft extender |
| Osteofil | Medtronic | 24 | Collagen | Paste, strip | Bone void filler |
| OsteoSelect | Bactarin International | 74 | Carboxymethylcellulose, phosphate buffered saline | Putty | Bone void filler |
| Progenix Plus | Medtronic | 60 | Type‐1 bovinecollagen and sodiumalginate | Putty | Bone void filler/bone graft extender/bone graft substitute |
| Progenix Putty | Medtronic | 70 | Type‐1 bovinecollagen and sodiumalginate | Putty | Bone void filler/bone graft extender/bone graft substitute |
| PRO‐STIM | Wright Medical | 40 | Calcium sulfate and calcium phosphate | Paste, putty | Bone void filler |
| VIAGRAF | Medtronic | ND | Glycerol | Paste, strip | Bone void filler |
ND, no data available
Grafton
Osteotech, in the United States, initially used glycerol as a carrier mixed with DBM to make Grafton, which has a DBM content of 17%–31%. It has been experimentally and clinically proven that Grafton has good biodegradability and arbitrary plasticity, and can be combined with growth factors or bone marrow stem cells.
Grafton was studied as a bone graft extender for posterolateral spinal fusion in a randomized controlled trial (RCT) by Cammisa et al. in 200467. In 120 patients, posterolateral lumbar fusions were carried out with pedicle screw fixation and one side of the spine was grafted with autograft (17.2 standard deviation [SD] 9.7 mL), while the contralateral side was grafted with autograft and Grafton (17.2 SD 9.7 mL, mixed 1:2). Two years later, autograft with Grafton resulted in fusion in 42 cases (52%) and autograft alone resulted in fusion in 44 cases (54%). Kang et al.68 performed an RCT of 46 patients undergoing lumbar fusion surgery. Grafton was mixed with local bone or autogenous iliac bone at a ratio of 2:1 as bone graft material and followed up for 2 years. There was no significant difference in fusion rate and spinal function between the two groups. The authors concluded that Grafton is an effective bone graft extender and could be used in combination with local bone as a safe and effective spinal fusion method. In another prospective cohort study69, patients undergoing instrumented posterolateral lumbosacral spinal fusion were grafted with Grafton and aspiration of bone marrow (19 cases), Grafton and autologous bone (27 cases), or autologous bone alone (27 cases). All groups showed similar fusion rates after 2 years’ follow‐up (63%, 70%, and 67%, respectively). These studies provide evidence that Grafton can be used as a bone graft extender for lumbar spinal fusion.
Grafton was used in an RCT by An et al.70, which included 77 patients who underwent anterior cervical fusion. Grafton was combined with allografts and compared with autografts alone. Nonunion occurred in 46% of the patients who were grafted with Grafton and allografts, while in only 26% of patients who received an autograft (P = 0.11), suggesting that the combination of Grafton and allograft resulted in a higher rate of nonunion. Elsawaf et al.71 described completely filling the polyether ether ketone (PEEK) cage with Grafton in anterior cervical discectomy and fusion in a case series of 20 patients. The mean Cobb angle improved (3.4° pre‐operatively vs. 14.5° postoperatively) and Japanese Orthopaedic Association (JOA) myelopathy scores and neck disability index also subsequently improved after surgery. Park et al.72 used PEEK cages containing autologous bone chips and Grafton for cervical fusion in 31 patients. One year later, the overall fusion rate was 97%. Both the visual analogue scale (score for neck and arm pain) and the modified JOA scoring system for myelopathy were significantly improved. These studies indicate that the role of Grafton is uncertain as a bone graft extender for cervical spinal fusion.
Grafton was the only DBM product used for thoracic fusions. In a retrospective cohort, Park et al.72 used Grafton in patients who underwent anterior thoracic discectomies and compared their results with using morselized cancellous allografts. On the final radiographs, the allograft group fusion rate was 82% and the Grafton group fusion rate was 92%. There was no significant difference between the two groups. This study provides evidence that Grafton could be used as a bone graft substitute for thoracic spinal fusion.
Cheung et al.73 used Grafton as a bone graft extender, which was mixed with cancellous allografts to filled bone defects encountered in periarticular fractures of the tibia, fibula, femur, humerus, forearm, and acetabulum. Fracture healing occurred in 69% of the patients who received Grafton (n = 13). Grafton was also used to enhance cancellous allografts in two tibial stress fractures treated by drilling and bone grafting74, and for reconstructing large segmental bone defects of the tibia (n = 275. and humerus (n = 1)76, using a titanium mesh cage filled with Grafton and cancellous allograft chips. These studies provide evidence that Grafton could be used in combination with allograft as a bone graft extender or enhancer to treat bone defects during fracture surgery.
Hierholzer et al.77 retrospectively analyzed 78 patients with nonunion of the tibia, of which 45 patients were treated with autogenous iliac bone transplantation and 33 patients were treated with Grafton. The healing rates of the two groups reached 100% and 97%, respectively. It is believed that Grafton can effectively promote bone healing, and the postoperative complications are lower than those in the ICBG group, which can be used as a treatment standard. In a case report78, Grafton was used to treat a non‐displaced coracoid fracture. After screw fixation, the nonunion site was debrided and successfully grafted with Grafton. These studies provide evidence that Grafton could be used as a bone graft substitute or bone graft extender to treat nonunion.
Furthermore, solitary bone cysts in children could be treated with Grafton. After filling the defects with Grafton in 7 cases, a continuous decrease in radiographic bone transparency was observed over a period of 2 years79. This study indicates that Grafton could be used as a bone graft substitute to treat solitary bone cysts.
Although Grafton has been widely used clinically, glycerol is water‐soluble, unstable, and potentially toxic in large‐scale use, and there is still some controversy about its use. To test the toxicity of Grafton, Wang et al.80 performed in vivo experiments on mice, and the results showed that the median lethal dose was 0.004 69 mL/g. It is recommended that the clinical application dose should not exceed 2 mL/kg. However, no serious adverse reactions related to glycerol have been found in clinical studies, adverse event reports, and published literature on Grafton. Therefore, from the previous clinical experience, glycerol toxicity should be considered unlikely81. In addition, Ziran et al.82 treated 25 patients who were smokers with fractures with Grafton, and found that the fracture healing rate was only 52% in the later follow‐up. Therefore, Grafton should be used carefully in the treatment of smokers with bone graft.
Allomatrix
Allomatrix is manufactured by Wright Medical of the UK with calcium sulfate as a carrier. It has been widely used in clinical applications such as spinal fusion and trauma surgery because of its excellent osteoconductivity and degradability; it provides stable structural support for the growth of new bone.
Allomatrix has been used in posterolateral lumbar fusions. A case‐control study by Fu et al. showed that Allomatrix and autologous bone resulted in comparable fusion rates when used with hydroxyapatite/tricalcium phosphate granules: 81% and 86%, respectively83. Sapkas et al.84 described a retrospective case series following 32 patients who underwent posterior lumbar interbody fusion with Allomatrix, and clinical and radiological scores improved significantly with the mean follow‐up of 36 months (range, 18 to 42 months). At the latest follow‐up, the mean Oswestry Disability Index improved from 52% to 22%. The mean Roland–Morris Disability Questionnaire improved from 52% to 29%, while >90% of the operated levels were fused. In another retrospective case serie85. of 65 patients who underwent lumbar fusion by using Allomatrix mixed (1:1) with iliac crest bone, radiological follow‐up showed an improvement in the Lenke scores: 3.7 after 1 month to 1.6 after 12 months. These studies provide evidence that Allomatrix may be used as a bone graft extender for lumbar spinal fusion.
Allomatrix has also been used to treat distal radial fractures. Agostino et al.86 performed an RCT of 50 patients, in which unstable distal radial fractures were treated by operative fixation with Kirschner wires, with (n = 24) or without (n = 26) augmentation of the fracture site with Allomatrix. The physical and radiological outcomes did not show any significant difference in wrist function, speed to recovery, union rate, and complication rate after 1‐year follow‐up. In another RCT of 44 patients with femoral fractures who were treated with open reduction and internal fixation with (n = 33) or without (n = 11) Allomatrix, after 22 months of follow‐up, there was no significant difference in healing rate between the two groups. In the Allomatrix group, there were 5 cases of wound nonunion after surgery. Allomatrix has been used for primary treatment of fresh bone defects caused by small‐caliber gunshot wounds in the hand. In a retrospective case series of 12 patients, 11 bone defects healed without further intervention and 1 defect required a second bone grafting procedure87. These studies provide evidence that Allomatrix is not an ideal bone graft substitute to treat unstable fractures that have already been treated with internal fixation. It may even lead to complications such as wound nonunion.
Allomatrix, mixed with cancellous allograft chips, was used to treat 41 atrophic or avascular nonunions, which were located in the femur, radius, tibia, and humerus88. It was found that the secondary infection rate from drainage tubes was 51%; the deep infection rate was 34%, and the treatment failure rate was 34%. Therefore, the incidence of complications of Allomatrix was too high for it to be recommended for bone nonunion treatment, especially when there is a large volume defect or any previous infection of the focus. In addition, Allomatrix has been used to graft bone defects resulting after nonunion (n = 35)89. Allomatrix was mixed (1:3) with calcium sulphate pellets, and after 7 months, 85% of the grafted nonunions were healed. The abovementioned studies provide evidence that Allomatrix should not be used in the treatment of nonunion of bone, especially when there is a large volume defect or any previous infection of the focus.
Allomatrix has been used to treat benign bone tumors in the tibia (n = 17), humerus (n = 11), fibula (n = 3), and radius (n = 2)89. Allomatrix was mixed (1:3) with a calcium sulphate bone substitute(Osteoset) to fill defects. After 7 months, 93% of the bone defects were healed. Tumor recurrence was seen in 3 cases, and 1 wound infection required antibiotic treatment. In addition, in a study that investigated the treatment of 98 benign bone tumors located in the tibia, humerus, femur, and pelvis with various bone grafts, Allomatrix was used in 34 of the grafting procedures but no Allomatrix‐specific outcomes were reported90. These studies identify that Allomatrix could be used as a bone graft substitute with or without a calcium sulphate bone substitute to treat benign bone tumors.
Osteofil
Osteofil is an injectable DBM product with porcine collagen as a carrier, in which DBM content is 24%. The common form of Osteofil is injectable paste and moldable strips, which can be arbitrarily changed in shape to fill bone defects. Due to its excellent osteoconductivity, osteoinductivity, and degradability, it has been widely used in the clinic.
Epstein et al.91 used a 1:1 mixture of Osteofil and autologous lamina for lumbar fusion, including 95 cases of primary fusion and 45 cases of secondary fusion. After 3 years of follow‐up, 93 (97.9%) of the patients with primary fusion had stable spinal fusion, and only 2 (2.1%) had unstable or pseudo articular joints on average 8 months after surgery. Of the patients with secondary fusion, 43 (95.6%) had stable spinal fusion, and 2 (4.4%) had instability on average 10 months after surgery. It could be concluded that Osteofil was an effective bone graft expander with autologous bone for lumbar spinal fusion.
Osteofil was mixed with autologous bone (n = 11) in a study by Epstein92, and this study also included 24 patients in which Vitoss (β‐tricalcium phosphate) was mixed with autologous bone. Radiological follow‐up showed that all levels were fused after an average of 5.2 months. Less than 50% of the original fusion mass remained visible on 2D‐CT scans after 6 months in 64% of fusions grafted with Osteofil, compared with 21% of fusions grafted with Vitoss, which suggested a quicker resorption rate of Vitoss. This study provides evidence that Osteofil may be used as a bone graft extender with autologous bone for cervical spinal fusion.
DBX
DBX manufactured by Medtronic is a DBM product with HA as a viscous carrier. DBX can be used to promote osteogenesis and repair bone defects, but it can only be used for stable bone defects due to lack of structural strength for load‐bearing applications.
DBX has been used to treat sternal segment dislocations by Divisi et al.93 Eight patients with sternal segment dislocations were treated with titanium screws and DBX. Use of titanium crews and DBX reduced the length of hospitalization, and led to rapid functional recovery and excellent aesthetic results according to the authors. This study provides evidence that DBX could be used as a bone graft substitute to treat fractures of the sternum.
DBX has also been described in a case report, showing the successful treatment for subtrochanteric nonunion of an 11‐year‐old patient with an adult proximal humeral locking plate and additional grafting with DBX94. This study provides evidence that DBX could be used as a bone graft substitute to treat nonunion.
DBX was used as a graft in the treatment of enchondromas. Kwok et al.95 and Dietz et al.96 reported small case series of five and two patients, respectively. No recurrence or pathological fractures were reported. These studies provide evidence that DBX could be used as a bone graft substitute to treat hand enchondromas.
Accell Connexus
Accell connexus is an injectable DBM product with Poloxamer as a carrier, in which DBM content is 70%. Provided in a syringe, the putty is moldable and resists irrigation. The putty may be implanted directly from the syringe. Accell connexus has been used as a bone graft extender in lumbar fusions.
Schizas et al.97 used a mixture of Accell Connexus and iliac crest bone for lumbar fusion in 33 patients. Compared with 26 patients who used iliac crest bone alone, there was no significant difference between the fusion rate and postoperative function. This study provides evidence that Accell connexus could be used as a bone graft extender of iliac crest bone for lumbar spinal fusion.
Summary and Outlook
After many years of research, DBM products have been increasingly used in clinical applications, and some therapeutic effects have been achieved. However, there are still several problems that need to be studied and discussed: (i) there is currently no DBM product that can combine the various conditions of ideal bone graft materials; and (ii) different materials, different methods, different reagents, and even the same conditions of batch processing of DBM products have differences in osteogenic activity. At present, there is no determination of the conditions under which the products have the best osteogenic capacity. Therefore, research efforts with DBM must be continued to expand clinical applications, produce validated utility, and demonstrate new options and opportunities to enhance clinical outcomes in bone repair. In the future, the combined use of DBM with other bone‐promoting substances such as autologous stem cells from bone marrow aspirates, seed cells, and growth factors may be an important research direction.
Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Acknowledgements
The authors are grateful for the support from the Library of Tianjin Medical University. We would also like to thank the friends who gave us help in the creation and revision of the article.
References
- 1. Cohen JMD. Fundamental and clinical bone physiology. J Bone Joint Surg Am, 1981, 63: 1198. [Google Scholar]
- 2. Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res, 1971, 50: 1392–1406. [DOI] [PubMed] [Google Scholar]
- 3. Finkemeier CG. Bone‐grafting and bone‐graft substitutes. J Bone Joint Surg Am, 2002, 84‐A: 454–464. [DOI] [PubMed] [Google Scholar]
- 4. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res, 1996, 329: 300–309. [DOI] [PubMed] [Google Scholar]
- 5. Russell JL, Block JE. Surgical harvesting of bone graft from the ilium: point of view. Med Hypotheses, 2000, 55: 474–479. [DOI] [PubMed] [Google Scholar]
- 6. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev, 2012, 64: 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urist MR, Dowell TA. Inductive substratum for osteogenesis in pellets of particulate bone matrix. Clin Orthop Relat Res, 1968, 61: 61–78. [PubMed] [Google Scholar]
- 8. Listed NA. Senn on the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Ann Surg, 1889, 10: 352–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urist MR. Bone: formation by autoinduction. Science, 1965, 150: 893–899. [DOI] [PubMed] [Google Scholar]
- 10. Kim IY, Seo SJ, Moon HS, et al Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv, 2008, 26: 1–21. [DOI] [PubMed] [Google Scholar]
- 11. Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharmscitechnol, 1998, 1: 246–253. [Google Scholar]
- 12. Su CT, Tan TK, Wong SM, Khor E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr Polym, 1996, 30: 239–242. [Google Scholar]
- 13. Dornish M, Kaplan D, Skaugrud O. Standards and guidelines for biopolymers in tissue‐engineered medical products: ASTM alginate and chitosan standard guides. American Society for Testing and Materials. Ann N Y Acad Sci, 2010, 944: 388–397. [DOI] [PubMed] [Google Scholar]
- 14. Madihally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials, 1999, 20: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 15. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Re, 2005, 104: 6017–6084. [DOI] [PubMed] [Google Scholar]
- 16. Hirano S, Tsuchida H, Nagao N. N ‐acetylation in chitosan and the rate of its enzymic hydrolysis. Biomaterials, 1989, 10: 574–576. [DOI] [PubMed] [Google Scholar]
- 17. Lee KY, Ha WS, Park WH. Blood compatibility and biodegradability of partially N ‐acylated chitosan derivatives. Biomaterials, 1995, 16: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 18. Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan‐based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials, 2008, 29: 4323–4332. [DOI] [PubMed] [Google Scholar]
- 19. Aranaz I, Mengibar M, Harris R, Panos I. Functional characterization of chitin and chitosan. Curr Chem Biol, 2009, 3: 203–230. [Google Scholar]
- 20. Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm, 1992, 78: 43–48. [Google Scholar]
- 21. Yang J, Tian F, Wang Z, Wang Q, Zeng YJ, Chen SQ. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B Appl Biomater, 2008, 84: 131–137. [DOI] [PubMed] [Google Scholar]
- 22. Kim IS, Park JW, Kwon IC, Baik BS, Cho BC. Role of BMP, betaig‐h3, and chitosan in early bony consolidation in distraction osteogenesis in a dog model. Plast Reconstr Surg, 2002, 109: 1966–1977. [DOI] [PubMed] [Google Scholar]
- 23. Tsai SW, Fang JC, Chen JH, Su LT, Jan SH. Preparation and evaluation of a hyaluronate‐collagen film for preventing post‐surgical adhesion. J Int Med Res, 2005, 33: 68–76. [DOI] [PubMed] [Google Scholar]
- 24. García‐Abuín A, Gómez‐Díaz D, Navaza JM, Regueiro L, Vidal‐Tato I. Viscosimetric behaviour of hyaluronic acid in different aqueous solutions. Carbohydr Polym, 2011, 85: 500–505. [Google Scholar]
- 25. Stern R. The Chemistry, Biology and Medical Applications of Hyaluronan and its Derivatives by T. C. Laurent. Quarterly Review of Biology, Vol. 75. Chicago, IL: The University of Chicago Press, 2008; 362. [Google Scholar]
- 26. Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen, 2014, 22: 579–593. [DOI] [PubMed] [Google Scholar]
- 27. Piskounova S, Gedda L, Hulsart‐Billström G, Hilborn J, Bowden T. Characterization of recombinant human bone morphogenetic protein‐2 delivery from injectable hyaluronan‐based hydrogels by means of 125I‐radiolabelling. J Tissue Eng Regen Med, 2014, 8: 821–830. [DOI] [PubMed] [Google Scholar]
- 28. Spessotto P, Rossi FM, Degan M, et al Hyaluronan‐CD44 interaction hampers migration of osteoclast‐like cells by down‐regulating MMP‐9. J Cell Biol, 2002, 158: 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venkatesan J, Nithya R, Sudha PN, Kim SK. Chapter four–role of alginate in bone tissue engineering. Adv Food Nutr Res, 2014, 73: 45–57. [DOI] [PubMed] [Google Scholar]
- 30. George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan — a review. J Control Release, 2006, 114: 1–14. [DOI] [PubMed] [Google Scholar]
- 31. De Vos P, De Haan B, Van Schilfgaarde R. Effect of the alginate composition on the biocompatibility of alginate‐polylysine microcapsules. Biomaterials, 1997, 18: 273–278. [DOI] [PubMed] [Google Scholar]
- 32. Gomez CG, Lambrecht MV, Pérez LJE, Rinaudo M, Villar MA. Influence of the extraction‐purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int J Biol Macromol, 2009, 44: 365–371. [DOI] [PubMed] [Google Scholar]
- 33. Cicchetti S, Leone MS, Franchelli S, Santi PL. Evaluation of the tolerability of hydrogel breast implants: a pilot study. Minerva Chir, 2002, 57: 53–57. [PubMed] [Google Scholar]
- 34. Ryan CK, Sax HC. Evaluation of a carboxymethylcellulose sponge for prevention of postoperative adhesions. Am J Surg, 1995, 169: 154–159. [DOI] [PubMed] [Google Scholar]
- 35. Mayer M, Yedgar S, Hurwitz A, Palti Z, Finzi Z, Milwidsky A. Effect of viscous macromolecules on peritoneal plasminogen activator activity: a potential mechanism for their ability to reduce postoperative adhesion formation. Am J Obstet Gynecol, 1988, 159: 957–963. [DOI] [PubMed] [Google Scholar]
- 36. Adanali G, Verdi M, Tuncel A, Erdogan B, Kargi E. Effects of hyaluronic acid‐carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg, 2003, 19: 29–36. [DOI] [PubMed] [Google Scholar]
- 37. Bae JS, Jin HK, Jang KH. The effect of polysaccharides and carboxymethylcellulose combination to prevent intraperitoneal adhesion and abscess formation in a rat peritonitis model. J Vet Med Sci, 2004, 66: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 38. Fraser RD, Macrae TP, Suzuki E. Chain conformation in the collagen molecule. J Mol Biol, 1979, 129: 463–481. [DOI] [PubMed] [Google Scholar]
- 39. Birk D, Silver F. Molecular structure of collagen in solution: comparison of types I, II, III and V. Int J Biol Macromol, 1984, 6: 125–132. [Google Scholar]
- 40. Fleischmajer R, Macdonald ED, Perlish JS, Burgeson RE, Fisher LW. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J Struct Biol, 1990, 105: 162–169. [DOI] [PubMed] [Google Scholar]
- 41. Bruckner P, van der Rest M. Structure and function of cartilage collagens. Microsc Res Tech, 1994, 28: 378–284. [DOI] [PubMed] [Google Scholar]
- 42. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor‐beta by the proteoglycan decorin. Nature, 1990, 346: 281–284. [DOI] [PubMed] [Google Scholar]
- 43. Regazzoni C, Winterhalter KH, Rohrer L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem Biophys Res Commun, 2001, 283: 316–322. [DOI] [PubMed] [Google Scholar]
- 44. Schmolka IR. Artificial skin. I. Preparation and properties of pluronic F‐127 gels for treatment of burns. J Biomed Mater Res, 1972, 6: 571–582. [DOI] [PubMed] [Google Scholar]
- 45. Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an insitu gel for ophthalmic use. Eur J Pharm Sci, 1998, 6: 105–112. [DOI] [PubMed] [Google Scholar]
- 46. Bohorquez M, Koch C, Trygstad T, Pandit N. A study of the temperature‐dependent Micellization of Pluronic F127. J Colloid Interface Sci, 1999, 216: 34–40. [DOI] [PubMed] [Google Scholar]
- 47. Yong CS, Jin SC, Quan QZ, et al Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm, 2001, 226: 195–205. [DOI] [PubMed] [Google Scholar]
- 48. Ware AL, McLaverty VG. Gypsum products. Aust Dent J, 1960, 5: 273–279. [Google Scholar]
- 49. Gao C, Huo S, Li X, You X, Zhang Y, Gao J. Characteristics of calcium sulfate/gelatin composite biomaterials for bone repair. J Biomater Sci Polym Ed, 2007, 18: 799–824. [DOI] [PubMed] [Google Scholar]
- 50. Nathan E, Victoria T, Naveen P, et al Use of a calcium sulfate‐calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics, 2013, 36: E216–E222. [DOI] [PubMed] [Google Scholar]
- 51. Liodaki E, Kraemer R, Mailaender P, Stang F. The use of bone graft substitute in hand surgery: a prospective observational study. Medicine (Baltimore), 2016, 95: e3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomas MV, Puleo DA, Al‐Sabbagh M. Calcium sulfate: a review. J Long Term Eff Med Implants, 2005, 15: 599–607. [DOI] [PubMed] [Google Scholar]
- 53. Campana V, Milano G, Pagano E, et al Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med, 2014, 25: 2445–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walsh WR, Morberg P, Yu Y, et al Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res, 2003, 406: 228–236. [DOI] [PubMed] [Google Scholar]
- 55. Schmalz G, Arenholt‐Bindslev D. Biocompatibility of Dental Materials. Berlin, Heidelberg: Springer, 2009; 1–379. [Google Scholar]
- 56. Alderman NE. Sterile plaster of Paris as an implant in the infrabony environment: a preliminary study. J Periodontol, 1969, 40: 11–13. [DOI] [PubMed] [Google Scholar]
- 57. Peltier LF, Bickel EY, Lillo R, Thein MS. The use of plaster of Paris to fill defects in bone. Ann Surg, 1957, 146: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peltier LF, Orn D. The effect of the addition of plaster of Paris to autogenous and homogenous bone graft in dogs. Surg Forum, 1957, 8: 571–574. [PubMed] [Google Scholar]
- 59. Peltier LF. The use of plaster of Paris to fill large defects in bone: a preliminary report. Clin Orthop Relat Res, 1959, 2001: 3–5. [DOI] [PubMed] [Google Scholar]
- 60. Clark AE, Hench LL, Paschall HA. The influence of surface chemistry on implant interface histology: a theoretical basis for implant materials selection. J Biomed Mater Res, 1976, 10: 161–174. [DOI] [PubMed] [Google Scholar]
- 61. Hench LL, Splinter RJM, Allen WC, Greenlee TKJ. Bonding mechanism at Interface of ceramic prosthetic materials. J Biomed Mater Res, 1971, 5: 117–141. [Google Scholar]
- 62. Kawanabe K, Yamamuro T, Nakamura T, Kotani S. Effects of injecting massive amounts of bioactive ceramics in mice. J Biomed Mater Res, 1991, 25: 117–128. [DOI] [PubMed] [Google Scholar]
- 63. Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin‐like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun, 2000, 276: 461–465. [DOI] [PubMed] [Google Scholar]
- 64. Thomas MV, Puleo DA, Al‐Sabbagh M. Bioactive glass three decades on. J Long Term Eff Med Implants, 2005, 15: 585–597. [DOI] [PubMed] [Google Scholar]
- 65. Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Gene‐expression profiling of human osteoblasts following treatment with the ionic products of bioglass 45S5 dissolution. J Biomed Mater Res, 2001, 55: 151–157. [DOI] [PubMed] [Google Scholar]
- 66. van der Stok J, Hartholt KA, Schoenmakers DAL, Arts JJC. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: a systematic review. Bone Joint Res, 2017, 6: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cammisa FP Jr, Lowery G, Garfin SR, et al Two‐year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side‐by‐side comparison in the same patient. Spine (Phila Pa 1976), 2004, 29: 660–666. [DOI] [PubMed] [Google Scholar]
- 68. Kang J, An H, Hilibrand A, Yoon ST, Kavanagh E, Boden S. Grafton and local bone have comparable outcomes to iliac crest bone in instrumented single‐level lumbar fusions. Spine (Phila Pa 1976), 2012, 37: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 69. Vaccaro AR, Stubbs HA, Block JE. Demineralized bone matrix composite grafting for posterolateral spinal fusion. Orthopedics, 2007, 30: 567–570. [DOI] [PubMed] [Google Scholar]
- 70. An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976), 1995, 20: 2211–2216. [PubMed] [Google Scholar]
- 71. Elsawaf A, Mastronardi L, Roperto R, Bozzao A, Caroli M, Ferrante L. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev, 2009, 32: 215–224. [DOI] [PubMed] [Google Scholar]
- 72. Park HW, Lee JK, Moon SJ, Seo SK, Lee JH, Kim SH. The efficacy of the synthetic interbody cage and Grafton for anterior cervical fusion. Spine (Phila Pa 1976), 2009, 34: E591–E595. [DOI] [PubMed] [Google Scholar]
- 73. Cheung S, Westerheide K, Ziran B. Efficacy of contained metaphyseal and periarticular defects treated with two different demineralized bone matrix allografts. Int Orthop, 2003, 27: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miyamoto RG, Dhotar HS, Rose DJ, Egol K. Surgical treatment of refractory tibial stress fractures in elite dancers: a case series. Am J Sports Med, 2009, 37: 1150–1154. [DOI] [PubMed] [Google Scholar]
- 75. Cobos JA, Lindsey RW, Gugala Z. The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma, 2000, 14: 54–59. [DOI] [PubMed] [Google Scholar]
- 76. Attias N, Lehman RE, Bodell LS, Lindsey RW. Surgical management of a long segmental defect of the humerus using a cylindrical titanium mesh cage and plates: a case report. J Orthop Trauma, 2005, 19: 211–216. [DOI] [PubMed] [Google Scholar]
- 77. Hierholzer C, Sama D, Toro JB, Peterson M, Helfet DL. Plate fixation of ununited humeral shaft fractures: effect of type of bone graft on healing. J Bone Joint Surg Am, 2006, 88: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 78. Skedros JG, Mears CS, Phippen CM. Glenohumeral instability and coracoid fracture nonunion corrected without coracoid transfer or nonunion takedown. J Shoulder Elbow Surg, 2014, 23: e166–e169. [DOI] [PubMed] [Google Scholar]
- 79. Hass HJ, Krause H, Kroker S, Wagemann W, Meyer F. Bone formation using human demineralised bone matrix (Grafton®) for the treatment of bone cysts in children. Eur J Pediatr Surg, 2007, 17: 45–49. [DOI] [PubMed] [Google Scholar]
- 80. Wang JC, Kanim LE, Nagakawa IS, Yamane BH, Vinters HV, Dawson EG. Dose‐dependent toxicity of a commercially available demineralized bone matrix material. Spine (Phila Pa 1976), 2001, 26: 1435–1436. [DOI] [PubMed] [Google Scholar]
- 81. Dinopoulos HT, Giannoudis PV. Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expert Opin Drug Saf, 2006, 5: 847–866. [DOI] [PubMed] [Google Scholar]
- 82. Ziran B, Cheung S, Smith W, Westerheide K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long‐bone nonunions in heavy tobacco smokers. Am J Orthop, 2005, 34: 329–332. [PubMed] [Google Scholar]
- 83. Fu TS, Wang IC, Lu ML, Hsieh MK, Chen LH, Chen WJ. The fusion rate of demineralized bone matrix compared with autogenous iliac bone graft for long multi‐segment posterolateral spinal fusion. BMC Musculoskelet Disord, 2016, 17: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sapkas GS, Mavrogenis AF, Themistocleous GS, Zachos VC, Kelalis G, Papagelopoulos PJ. Posterior lumbar interbody fusion versus circumferential fusion using the B‐Twin expandable spinal system. J Long Term Eff Med Implants, 2007, 17: 217–227. [DOI] [PubMed] [Google Scholar]
- 85. Girardi FP, Cammisa FP Jr. The effect of bone graft extenders to enhance the performance of iliac crest bone grafts in instrumented lumbar spine fusion. Orthopedics, 2003, 26: s545–s548. [DOI] [PubMed] [Google Scholar]
- 86. D'Agostino P, Barbier O. An investigation of the effect of AlloMatrix bone graft in distal radial fracture: a prospective randomised controlled clinical trial. Bone Joint J, 2013, 95‐B: 1514–1520. [DOI] [PubMed] [Google Scholar]
- 87. Nguyen V, Wollstein R. Civilian gunshot wounds to the fingers treated with primary bone grafting. J Plast Reconstr Aesthet Surg, 2009, 62: e551–e555. [DOI] [PubMed] [Google Scholar]
- 88. Ziran BH, Smith WR, Morgan SJ. Use of calcium‐based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: preliminary results and complications. J Trauma, 2007, 63: 1324–1328. [DOI] [PubMed] [Google Scholar]
- 89. Wilkins RM, Kelly CM. The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics, 2003, 26: s567–s570. [DOI] [PubMed] [Google Scholar]
- 90. Gitelis S, Virkus W, Anderson D, Piasecki P, Yao TK. Functional outcomes of bone graft substitutes for benign bone tumors. Orthopedics, 2004, 27: s141–s144. [DOI] [PubMed] [Google Scholar]
- 91. Epstein NE, Epstein JA. SF‐36 outcomes and fusion rates after multilevel laminectomies and 1 and 2‐level instrumented posterolateral fusions using lamina autograft and demineralized bone matrix. J Spinal Disord Tech, 2007, 20: 139–145. [DOI] [PubMed] [Google Scholar]
- 92. Epstein NE. An argument for traditional posterior cervical fusion techniques: evidence from 35 cases. Surg Neurol, 2008, 70: 45–51. [DOI] [PubMed] [Google Scholar]
- 93. Divisi D, Crisci R. Use of demineralized bone matrix and plate for sternal stabilization after traumatic dislocation. Gen Thorac Cardiovasc Surg, 2011, 59: 52–56. [DOI] [PubMed] [Google Scholar]
- 94. Cortes LE, Miguel T, Francisco V, Slongo TF, Streubel PN. Adult proximal humerus locking plate for the treatment of a pediatric subtrochanteric femoral nonunion: a case report. J Orthop Trauma, 2011, 25: 63–67. [DOI] [PubMed] [Google Scholar]
- 95. Kwok TY, Wong HK. Evolving treatment modality of hand Enchondroma in a local hospital: from autograft to artificial bone substitutes. J Orthop Trauma Rehabil, 2016, 20: 19–23. [Google Scholar]
- 96. Dietz JF, Kachar SM, Nagle DJ. Endoscopically assisted excision of digital enchondroma. Arthroscopy, 2007, 23: 678.e1–e4. [DOI] [PubMed] [Google Scholar]
- 97. Schizas C, Triantafyllopoulos D, Kosmopoulos V, Tzinieris N, Stafylas K. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: a controlled case pilot study. Arch Orthop Trauma Surg, 2008, 128: 621–625. [DOI] [PubMed] [Google Scholar]


