Abstract
Objective
Limb salvage in pediatric patients remains a challenge. We describe a staged strategy. The procedure includes: (i) tumor removal and non‐hinged static endoprosthesis reconstruction; (ii) leg length discrepancy (LLD) correction by shoe lift or distraction osteogenesis; and (iii) maturity reconstruction by regular endoprosthesis. The aim of the study was to investigate the results of non‐hinged static megaprosthesis reconstruction and staged LLD correction in the treatment of malignant tumors in the distal femur in children.
Methods
Non‐hinged megaprostheses were implanted in 12 pediatric patients with osteosarcoma in the distal femur. The prosthesis consists of a femoral component with constrained condylar knee (CCK) design, and a tibial component with a small‐diameter press‐fit stem and derotation fins. A posterior stabilizing polyethylene component is fixed on the tibial component. The cases were prospectively followed up with focus on the growth rate of adjacent uninvolved bone in the salvaged limb, joint stability, knee stability, function outcome, length discrepancy, and surgery‐related complications.
Results
There were five girls and seven boys included in the study, with an average age at the time of primary surgery of 10.0 years (range, 8–12 years). All the tumors were located in the distal femur. The average follow up was 76.3 months (range, 24–139 months). The Ligament Augmentation and Reconstruction System (LARS) ligament was used in two patients to enhance the soft tissue reattachment and reconstruct medial collateral ligament (MCL). Ten patients were alive at the final follow‐up and two had died of lung metastases. Expected LLD was 6.7 cm (range, 3.0–13.2 cm) at initial surgery. At the final follow‐up, nine patients reached skeletal maturity and the actual LLD at the femur was 5.3 cm (range, 3.0–10.1 cm), excluding 1 cm correction at initial surgery by endoprosthesis. The proximal tibia physis showed an average of 86.7% (range, 56.5%–100%) growth of the contralateral side. The mean reduction in tibial length was 1.2 cm (range, 0.5–4.7 cm). Six patients received distraction osteogenesis at a mean length of 5.4 cm (range, 3.0–9.1 cm). Range of knee movement was between 85° and 125°, with an average of 102.5°. The Musculoskeletal Tumor Society 93 score of patients alive was 80.6 (range, 60–90).
Conclusion
Non‐hinged static megaprosthesis followed by LLD correction with shoe lift or staged distraction osteogenesis appears to be an alternative option to treat children with malignant bone tumors around the knee.
Keywords: Distraction osteogenesis, Limb length discrepancy, Limb salvage, Osteosarcoma, Skeletally immature
Introduction
The distal end of the femur is the most common site for primary malignant tumors of bone in children, with osteosarcoma being the most common. The choice of limb salvage procedure depends on the location and extent of the tumor, psychosocial considerations, and the age of the patient. Endoprosthetic and biological reconstruction are two major types of limb salvage surgeries. There is additional challenge in the bony reconstruction in children, due to their continuing growth. The potential for further limb growth is affected when tumor removal necessitates resection of one or more growth plates. Because the epiphysis of both sides of the knee joint account for more than two‐thirds of longitudinal lower limb growth, resection of osteosarcoma around the knee in skeletally immature patients presents unique reconstructive challenges. Maintaining equal limb lengths at the completion of the growth period is the desired result. Surgical options for such patients include amputation, rotationplasty, hemiarthroplasty with fixed‐length endoprosthesis, invasive or non‐invasive extendible megaprosthesis, and distraction osteogenesis. However, the optimal choice for young patients remains controversial.
Among biological reconstruction methods, allograft, vascularized graft (or combined with allograft), and devitalization and re‐implantation of tumor bone are often used. Both free and vascularized fibular grafts are commonly used in the reconstruction of bone defects after tumor resection in children. The fibula provides well‐perfused bone and the capability of osteogenesis, but it often lacks the structural strength of allografts for lower limb reconstruction. To achieve adequate strength early after surgery, a vascularized fibular graft has been used in combination with a large structural allograft to reconstruct the bony defects. Stress fractures and nonunion are two major complications after fibular reconstruction. Another autograft option is to use the diseased bone after devitalization. The patients’ own tumor‐bearing bone is sterilized by irradiation, microwave, pasteurization, or autoclave; however, this technique is useful only for diaphysis or for flat bones in children.
Non‐biological reconstruction using a prosthesis has the advantages of allowing early weight‐bearing and having predictable function and low risks of early complications. An expandable endoprosthesis can be lengthened as the child grows, similar to the standard endoprosthetic reconstruction. It allows immediate weight‐bearing with early rehabilitation and return to function. The design of expandable endoprostheses has significantly evolved over the past 30 years. In the past, all the lengthening mechanisms required a formal surgical procedure, which resulted in a high incidence of infection. The younger the child, the more operations were needed not only for lengthening but also for management of complications such as stiffness, infection, wear, and loosening. Expandable metallic endoprosthesis can achieve meaningful growth, resulting in little to no limb‐length discrepancy (LLD) for skeletally immature patients by the end of their growth. However, the high complication rate is not comparable with that associated with a static prosthesis. An average of 3.2 complications per patient has been reported in extendible prostheses1. Besides the high complication rate, 8%–50% of patients who receive an extendible prosthesis do not undergo a lengthening procedure due to tumor relapse2, 3, 4, 5. The cost of non‐invasive expandable endoprostheses has been reported to be as high as US$379 000 in 4‐year‐old patients6. Considering complication rates and the high cost of an extendible endoprosthesis, some authors advocate temporary hemiarthroplasty and staged lengthening, with advantages including the simple technique and the low cost7, 8. At the authors’ institution, hemiarthroplasty with fixed length megaprosthesis has been used for pediatric limb salvage with preservation of growth plates in the adjacent bone (Fig. 1A). In our patients, staged distraction osteogenesis was performed when LLD reached 4 cm and above. Due to loss of major ligaments of the knee joint in hemiarthroplasty, instability or dislocation were the most common complications after hemiarthroplasty reconstruction even when the children had adequate adaption ability. To improve the stability of the knee joint, the constrained condylar knee (CCK) design was introduced (Fig. 1B–D). The CCK prosthesis is usually used in situations requiring the use of increased constrained in total knee arthroplasty. A sliding prosthetic stem design was used across the remaining open physis, also with the purpose of decreasing stress at the bone–implant interface3, 9.
Figure 1.

(A) A hemiarthroplasty megaprosthesis was used to preserve the growth plate in the proximal tibia before 2010. (B) To improve the stability of the knee joint, a non‐hinged CCK design was used in the static megaprosthesis design.
We therefore asked the following questions:
i) Can the non‐hinged distal femoral endoprosthesis provide a stable knee joint? ii) To what extent of growth potential can the adjacent open physis be preserved? iii) What is the functional outcome and complication rate after non‐hinged distal femoral endoprosthesis reconstruction?
Overall, the aim of the current study was to investigate the results of static non‐hinged megaprosthesis reconstruction and staged LLD correction by shoe lift or distraction osteogenesis in the treatment of malignant tumors in the distal femur in children.
Materials and Methods
Patients
We retrospectively reviewed a consecutive series of 15 skeletally immature patients with osteosarcoma in distal femurs reconstructed by non‐hinged endoprostheses following tumor resection between May 2010 and May 2015. Use of the endoprostheses was approved by the hospital's institutional review board, and informed consent was obtained from patients' parents. There were seven boys and five girls included in the study, with an average age of 10.1 years (range, 8–12 years) at the time of diagnosis (Table 1). All the lesions were located in the distal femur.
Table 1.
Demographics and oncological outcomes
| Number | Gender/ Age at resection (years) | Diagnosis | Length of resection (cm) | Prediction of LLD (cm) | Reconstruction length (cm) | Longitudinal growth of tibia (cm) | Definitive lengthening of DO (cm) | LLD at final follow up (cm) (Femur/Tibia)* | Procedures | MSTS Score | Complications | Follow up (months) | Survival status | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salvaged (% of NO) | Non‐operated | |||||||||||||
| 1 | M/11 | OS | 21 | 6.4 | 22 | 4.0 (66.7) | 6.0 | 4.0 | 5.0/2.0 | HA → LL | 90.0 | Lung metastases | 69 | DOD |
| 2 | M/8 | OS | 20 | 9.7 | 20 | 6.1 (56.5) | 10.8 | 9.1 | 9.0/−5.0 | HA → LL | 80.0 | Malalignment of tibia stem | 101 | NED |
| 3 | M/9 | OS | 15 | 10.5 | 16 | 9.3 (100) | 9.3 | 6.0 | 9.1/−6.0 | HA → DFP → LL | 60.0 | Local recurrence | 94 | NED |
| 4 | M/12 | OS | 16 | 5.0 | 17 | 11.0 (91.6) | 12.0 | 4.0 | 4.0/−3.0 | HA → DFP → LL | 73.3 | Dislocation | 139 | NED |
| 5 | F/12 | OS | 15 | 3.0 | 16 | 3.4 (100) | 3.4 | ‐ | 2.0/0 | HA → SL | 90.0 | ‐ | 91 | NED |
| 6 | F/10 | OS | 16 | 3.5 | 17 | 3.5 (87.5) | 4.0 | ‐ | 2.0/0 | HA → SL | 86.7 | malalignment of tibia stem | 81 | NED |
| 7 | F/10 | OS | 20 | 3.2 | 20 | 5.1 (91.1) | 5.6 | ‐ | 2.0/0.5 | HA → SL | 90.0 | ‐ | 80 | NED |
| 8 | M/10 | ES | 17 | 4.1 | 18 | 4.5 (90.0) | 5.0 | ‐ | 3.0/0.5 | HA → SL | 86.7 | Lung metastases | 60 | AWD |
| 9 | F/8 | OS | 16 | 8.8 | 17 | 4.0 (81.6) | 5.0 | 6.0 | 5.0/−5.0 | HA → LL | 80.0 | Pin site infection | 103 | NED |
| 10 | M/9 | ES | 13 | 9.0 | 14 | 0.7 (100) | 0.7 | ‐ | 3.0/0 | HA → SL | 66.7 | Lung metastases | 24 | DOD |
| 11 | M/11 | OS | 12.5 | 5.1 | 13.5 | 4.5 (90) | 5.0 | 3.0 | 4.0/−2.5 | HA → LL | 83.3 | ‐ | 37 | NED |
| 12 | F/8 | OS | 14 | 13.2 | 14 | 3.5 (87.5) | 4.0 | ‐ | 3.0/0.5 | HA → SL | 80.0 | ‐ | 36 | NED |
DFP, distal femur prosthesis; DO, distraction osteogenesis; ES, Ewing's sarcoma; F, female; HA, hemiarthroplasty; LL, limb lengthening; LLD, limb length discrepancy; M, male; MSTS, Musculoskeletal Tumor Society Scoring System 1993; NO, non‐operated side; OS, osteosarcoma; SL, shoe lift.
Minus indicates lengthening length.
Inclusion and Exclusion Criteria
The inclusion criteria were: (i) patient aged between 8 and 13 years old with expected LLD ≥3 cm; (ii) stage IIB malignant bone tumor; (iii) the patients underwent the non‐hinged distal femoral endoprosthesis reconstruction after tumor resection; (iv) postoperative follow‐up for at least 2 years, with clinical and radiological assessments performed; and (v) retrospective study.
The exclusion criteria were: (i) patients with distal metastasis at diagnosis; (ii) pathological fracture presented at initial diagnosis; (iii) incomplete medical records and radiographic data; and (iv) lost to follow‐up.
Treatment Strategy
Treatment included preoperative chemotherapy, surgery, and postoperative chemotherapy. Our strategy for pediatric patients is composed of three stages: tumor control period, LLD period, and skeletal maturity period (Fig. 2).
Figure 2.
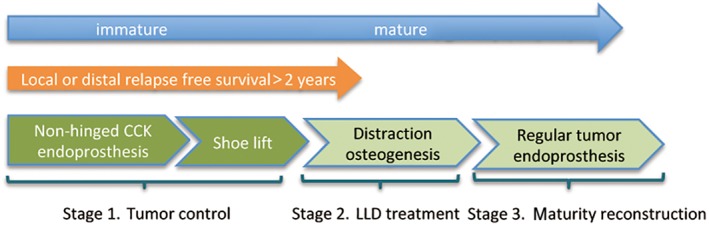
The flow chart showing the principal of staged limb salvage in skeletally immature patients.
For the tumor control period, a non‐hinged static megaprosthesis was initially used to reconstruct bone defects after tumor resection. The mean resection length was 17.6 cm (range, 12.5–21 cm) in the 12 patients. Based on Paley's multiplier tables10, the potential for residual growth and predicted LLD was 3.2–13.2 cm at the time of primary tumor resection surgery. As patients grew, we recommended an insole or shoe lift to correct LLD of 2 cm or more. After a follow‐up of 2–3 years of relapse‐free survival, the focus of treatment shifted from tumor local control to LLD correction. An OrthoFix uniaxial external fixator was used to correct LLD during this period. In stage 3, the endoprosthesis was converted to an adult‐type rotating hinged (regular) tumor prosthesis once skeletal maturity was reached or the non‐hinged endoprosthesis was kept for use after discussing with the patients.
Implant Design Rationale
The prosthesis consists of a femoral component, including fixed length of segmental defect body and cemented stem, and a tibial component, with a design of a non‐hinged base plate, small‐diameter press‐fit stem, and derotation fins. A posterior stabilizing polyethylene component is fixed on the tibial component (Fig. 3). Anchorage of the tibial component requires penetration of adjacent uninvolved bone, through the growth plate by an intramedullary stem with a smooth surface which prevents bone on growth which might cause growth plate arrest. The anchorage stem has a diameter of 8, 9 or 10 mm, with a length of 10 mm.
Figure 3.
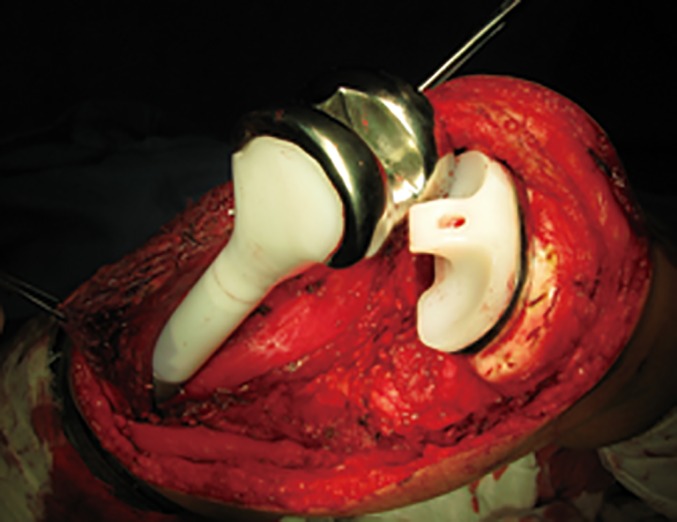
An intraoperative photo showing the non‐hinged joint with a posterior stabilizing polyethylene component fixed on the tibial component.
Surgical Technique
During preparation of the tibia, care was taken to remove only the tibial eminence, preserving tibia plateau cartilage, physis, and its blood supply through the ring of LaCroix, which might also prevent the tibia component from sinking. Then tibial canal was opened using a 6‐mm awl, followed by minimal reaming and tibia component fixation. In nine patients, reconstruction was performed allowing 1 cm longer than the actual defect to compensate contralateral growth potential. An artificial ligament was used as previously reported11 to provide extra stability for the knee joint. Postoperatively, a long‐leg splint with 10° knee flexion was used for 6–8 weeks to stabilize the soft‐tissues.
Oncological Outcome
Survival status was evaluated according to both local and distant tumor control. The patients were evaluated at 3‐month intervals by chest CT and X‐rays of the operative site. Clinical and radiological assessments were performed at each visit to determine local recurrence or distal metastasis.
Musculoskeletal Tumor Society 93
Functional outcomes were evaluated using the Musculoskeletal Tumor Society (MSTS) 93 scoring system for the lower extremity. This system includes numerical values from 0 to 5 points assigned to each of the following six categories: pain, level of activity and restriction, emotional acceptance, use of orthopaedic supports, walking ability, and gait. The final MSTS score is calculated as a percentage of the maximum possible score; the higher the percentage, the better the functional outcome. The range of motion of the knee joint was used to describe the active range of knee flexion and extension postoperative periods. In addition, knee stability was recorded by drawer test.
Leg‐Length Discrepancy
The LLD is the difference in the length of one leg compared to the other. Regular radiography of bilateral lower limbs at 3‐month intervals was used to measure LLD and the growth of the ipsilateral tibia. The cases were prospectively followed up for growth of adjacent uninvolved bone in the salvaged limb and LLD. A shoe lift or distraction osteogenesis plan was made based on the LLD.
Complications
The clinical complications, such as infection, aseptic loosening, dislocation, and deformity, were recorded. Oncological failure was not recorded as a complication.
Statistical Analysis
SPSS Statistics 20.0 software (IBM SPSS, Chicago, IL, USA) was used for the statistical analyses. The descriptive statistics were used to determine ranges, means, and standard deviations. A P‐value <0.05 was considered significant.
Results
Oncological Outcome
The mean follow‐up time for all patients was 76.3 months (range, 24–139 months). All living patients were followed for longer than 2 years. Ten patients were alive at the follow‐up. Lung metastasis occurred in three patients and two of them died of the disease. Two lung metastases occurred before limb lengthening (case 8 and case 10). One patient with parosteal osteosarcoma presented with local recurrence during the follow‐up period. Resection of recurrent tumors was performed and rotating hinged endoprosthesis was used to replace the primary reconstruction and the patient was alive without disease.
Leg‐Length Discrepancy After Surgery
At the time of primary surgery, expected LLD was 6.7 cm (range, 3.0–13.2 cm). Of the 12 patients, 9 had 1 cm longer reconstruction during the initial procedure. No transient nerve palsy occurred. At the final follow‐up, 9 patients reached skeletal maturity (from case 1 to case 9) and the actual LLD at the femur was 5.3 cm (range, 3.0–10.1 cm), excluding 1 cm correction at the initial surgery by endoprosthesis. The observed LLD was 88.5% (range, 62.5%–100%) of the predicted value. Growth at the proximal tibia physis after surgery was observed in all the patients during follow‐up, with an average of 86.7% (range, 56.5%–100%) growth of the contralateral proximal tibial physis. At the last follow‐up, nine (75%) of the patients exhibited tibia length shortening. The mean reduction in tibia length was 1.2 cm (range, 0.5–4.7 cm).
Staged Lengthening
Six patients underwent distraction osteogenesis limb lengthening (Fig. 4) at a mean length of 5.4 cm (range, 3.0–9.1 cm). The average rate of elongation per day was 1 mm and the average external fixation index of the six patients was 2.2 months/cm. In six cases, shoe lift was used to correct mild LLD ranging from 2.0 to 3.5 cm. Two patients received distal femoral rotating hinged knee replacement before limb lengthening because of resection of local recurrence (case 3) and recurrent dislocation (case 4) during follow up of tumor control period.
Figure 4.
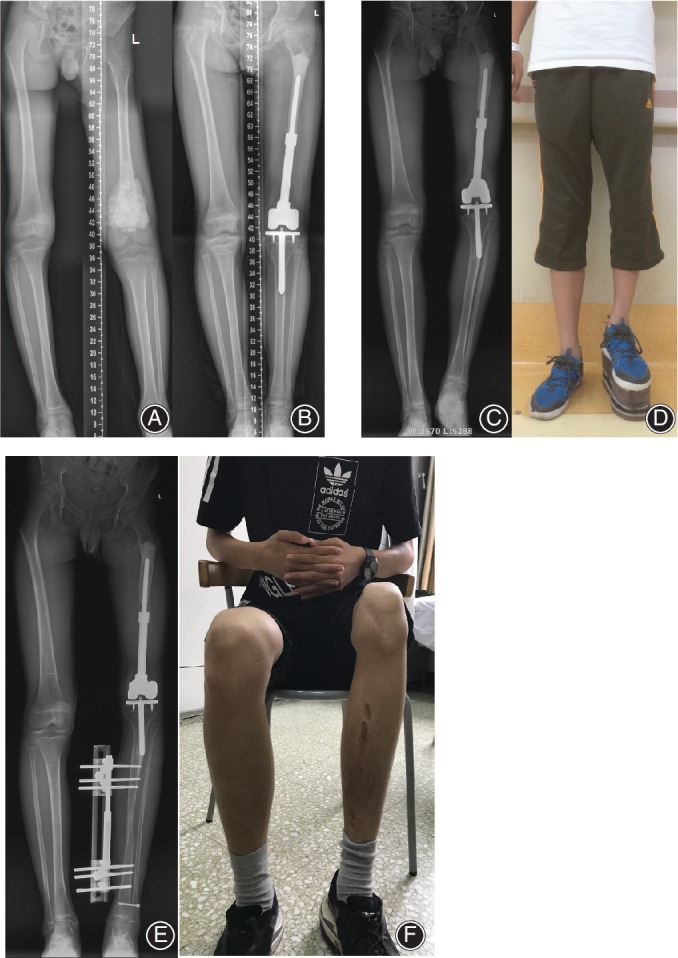
An illustrative case (case 2). (A) The patient was an 8‐year‐old boy with an osteosarcoma of the left distal femur; (B) radiograph 2.5 years after surgery and (C) X‐ray taken 5 years after surgery showing cortical atrophy at host bone–prostheses junction with femoral shortening of 9 cm; (D) shoe lift was used to correct the limb length discrepancy; limb lengthening of 5 cm by OrthoFix apparatus (E) and final tibia length (F).
Functional Outcome
Range of knee movement was between 85° and 125°, with an average of 102.5°. The average functional results of patients alive was 80.6 (range, 60–90) according to MSTS 93. Among them, 5 patients with limb‐length inequality of 2 cm or less had an average MSTS score of 82.7 (range, 73.3–90), whereas five patients with a discrepancy greater than 2 cm had a score of 79.2 (range, 60–90). No knee instability was found during the last follow‐up.
Complications
Two patients developed angular deformity at the proximal tibia. Progressive stem valgus developed in one patient (case 2); however, the patient declined to receive tibia component exchange to an adult‐type stem and deformity correction at skeletal maturity. Radiographs revealed no evidence of loosening of tibia stems. On the femoral side, distinct stress‐shielding was observed in five patients with pencil‐tip shaped cortical bone atrophy at the host bone–prosthesis junction. One pin site infection occurred during limb lengthening by external fixation and was treated conservatively.
Discussion
Limb salvage in skeletally immature children is challenging, with the greatest difficulty associated with resection of the open physis3, 4, 12. Sacrifice of major growth plates during resection and fixed‐length reconstruction of a limb in a skeletally immature child with osteosarcoma usually result in significant limb‐length inequality as growth progress. Surgical options have shifted from amputation to limb salvage. Reconstruction strategies for this specific population include rotationplasty, megaprosthesis implantation with expandable mechanism or fixed length, allograft, and staged distraction osteogenesis12, 13, 14, 15, 16, 17. Rotationplasty and expandable endoprosthesis have been successfully used to treat skeletally immature patients with osteosarcoma of the distal femur. Anticipating the remaining growth potential is essential for decision‐making for treatment plans, as is age, prognosis, treatment features, and patient expectations13.
The optimal reconstruction choice in skeletally immature patients with malignant bone tumors around metaphysis remains controversial. The vast majority of the published data are derived from small case series with pool data from a wide range of ages across anatomical sites. To address LLD following limb salvage surgery, expandable prostheses have been developed4, 5, 18. However, besides the high complication rate, up to 50% of patients who have expandable prosthesis reconstruction do not undergo further lengthening procedures due to oncological failures or overestimation of expected LLD7, 12, 16, 19. In addition, small degrees of LLD up to 2.2 cm can be well compensated by mechanism of pelvic obliquity20, and the children usually have a normal gait. Considering the factors mentioned above, a staged limb salvage strategy was proposed at the authors’ institution. The procedure includes limb salvage with static megaprosthesis and staged LLD correction. Distraction osteogenesis is only performed for patients with a minimum of 2 years of recurrence‐free survival because the recurrence risk significantly decreased after this period of time. During the first 2 years after tumor removal surgery, shoe lift was the first choice for LLD correction. However, the distraction was usually performed at the tibia side, which resulted in a higher knee level compared with that of the contralateral side.
Before year 2010, hemiarthroplasty was routinely used to preserve the ipsilateral uninvolved proximal tibia growth plate at the authors’ institution. Due to loss of the main ligaments of the knee, stability was jeopardized and compensated by scar tissue and high adaptability in children21. To improve the stability of hemiarthroplasty in limb salvage for skeletally immature patients, a tibial component was introduced. The design of a CCK along with a thin polished stem was used. Most extendable tumor prostheses for children had a polished, press‐fit stem passing through the central portion of the uninvolved adjacent physis4, 5, 9, 22. Neel et al.22 reported that the stem did not result in growth retardation or arrest in a series of six patients. However, another study found that 65% of the immature patients experienced less proximal tibia growth in the operative limb23. In the current study, an average of 86.7% growth potential preservation of the proximal tibial physis was observed. Only three (25%) patients had exactly the same length of longitudinal growth in the salvaged limb compared with that of the contralateral side.
Increasing constraint in TKA typically involves a thicker and wider post on the polyethylene insert that conforms more intimately to the femoral box. This creates increased constraint in the varus‐valgus coronal plane as well as more rotational constraint. Recurrent dislocation occurred in patient number 4 and the reconstruction was revised to a rotating hinged distal femoral prosthesis. Usually, a 1‐cm longer reconstruction was performed to compensate partial LLD afterward and this also facilitated immediate postoperative mobilization and avoided problems such as ischemia or nerve palsies due to overstretching of the soft tissue.
Chung et al.7 reported a similar principal staged limb salvage approach. A synthetic device was reconstructed for the bone defect, followed by soft‐tissue distraction using an external device. A regular endoprosthesis was used when skeletal maturity was reached. Subluxation occurred in 20% of the patients and 12% of the hemiarthroplasties experienced failure. Subluxation occurred in one patient in the current series because of the CCK design of the prosthesis, which showed adequate stability of the reconstruction. In another study reported from the same group14, resection arthrodesis was used for the first stage but may have a negative influence on functional results over an endoprosthesis due to the prolonged arthrodesis period. The non‐hinged endoprosthesis used in the current study can provide both stable and functional reconstruction during the first stage.
A high complication rate of the extendable prosthesis has been highlighted in some studies1, 2, 6, 12, 15, 19, 24. An average 50% revision rate is reported for non‐invasive expandable endoprostheses25, 26. In light of the high cost of expandable endoprostheses and only half of patients actually undergoing lengthening14 in expandable prosthesis reconstruction, the non‐hinged static megaprostheis and staged LLD correction described here present an alternative option for skeletally immature patients.
Disclosure: Beijing Municipal Science and Technology Project (Z181100001918025); The Capital Health Research and Development of Special (2018‐2‐4088).
References
- 1. Schinhan M, Tiefenboeck T, Funovics P, Sevelda F, Kotz R, Windhager R. Extendible prostheses for children after resection of primary malignant bone tumor: twenty‐seven years of experience. J Bone Joint Surg Am, 2015, 97: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 2. Cipriano CA, Gruzinova IS, Frank RM, Gitelis S, Virkus WW. Frequent complications and severe bone loss associated with the repiphysis expandable distal femoral prosthesis. Clin Orthop Relat Res, 2015, 473: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cool WP, Carter SR, Grimer RJ, Tillman RM, Walker PS. Growth after extendible endoprosthetic replacement of the distal femur. J Bone Joint Surg Br, 1997, 79: 938–942. [DOI] [PubMed] [Google Scholar]
- 4. Eckardt JJ, Kabo JM, Kelley CM, et al Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res, 2000, 373: 51–61. [DOI] [PubMed] [Google Scholar]
- 5. Grimer RJ, Belthur M, Carter SR, Tillman RM, Cool P. Extendible replacements of the proximal tibia for bone tumours. J Bone Joint Surg Br, 2000, 82: 255–260. [PubMed] [Google Scholar]
- 6. Henderson ER, Pepper AM, Letson GD. What are estimated reimbursements for lower extremity prostheses capable of surgical and nonsurgical lengthening?. Clin Orthop Relat Res, 2011, 470: 1194–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung SH, Jeon DG, Cho WH, et al Temporary hemiarthroplasty with a synthetic device in children with osteosarcoma around the knee as a bridging procedure until skeletal maturity. J Surg Oncol, 2015, 112: 107–114. [DOI] [PubMed] [Google Scholar]
- 8. Kong CB, Lee SY, Jeon DG. Staged lengthening arthroplasty for pediatric osteosarcoma around the knee. Clin Orthop Relat Res, 2010, 468: 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abudu A, Grimer R, Tillman R, Carter S. The use of prostheses in skeletally immature patients. Orthop Clin North Am, 2006, 37: 75–84. [DOI] [PubMed] [Google Scholar]
- 10. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb‐length discrepancy. J Bone Joint Surg Am, 2000, 82: 1432–1446. [DOI] [PubMed] [Google Scholar]
- 11. Ji T, Tang X, Guo W. The use of Ligament Advanced Reinforcement System (LARS) in limb salvage surgery: a pilot clinical study. J Arthroplasty, 2013, 28: 892–894. [DOI] [PubMed] [Google Scholar]
- 12. Groundland JS, Binitie O. Reconstruction after tumor resection in the growing child. Orthop Clin North Am, 2016, 47: 265–281. [DOI] [PubMed] [Google Scholar]
- 13. Levin AS, Arkader A, Morris CD. Reconstruction following tumor resections in skeletally immature patients. J Am Acad Orthop Surg, 2017, 25: 204–213. [DOI] [PubMed] [Google Scholar]
- 14. Kang S, Lee JS, Park J, Park SS. Staged lengthening and reconstruction for children with a leg‐length discrepancy after excision of an osteosarcoma around the knee. Bone Joint J, 2017, 99: 401–408. [DOI] [PubMed] [Google Scholar]
- 15. Torner F, Segur JM, Ullot R, et al Non‐invasive expandable prosthesis in musculoskeletal oncology paediatric patients for the distal and proximal femur. First results. Int Orthop, 2016, 40: 1683–1638. [DOI] [PubMed] [Google Scholar]
- 16. Groundland JS, Ambler SB, Houskamp LD, Orriola JJ, Binitie OT, Letson GD. Surgical and functional outcomes after limb‐preservation surgery for tumor in pediatric patients: a systematic review. JBJS Rev, 2016, 4: 1–13. pii: 01874474‐201602000‐00002. [DOI] [PubMed] [Google Scholar]
- 17. Aponte‐Tinao LA, Albergo JI, Ayerza MA, Muscolo DL, Ing FM, Farfalli GL. What are the complications of allograft reconstructions for sarcoma resection in children younger than 10 years at long‐term followup?. Clin Orthop Relat Res, 2018, 476: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arkader A, Viola DC, Morris CD, Boland PJ, Healey JH. Coaxial extendible knee equalizes limb length in children with osteogenic sarcoma. Clin Orthop Relat Res, 2007, 459: 60–65. [DOI] [PubMed] [Google Scholar]
- 19. Staals EL, Colangeli M, Ali N, Casanova JM, Donati DM, Manfrini M. Are complications associated with the Repiphysis(®) expandable distal femoral prosthesis acceptable for its continued use?. Clin Orthop Relat Res, 2015, 473: 3003–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh M, Connolly P, Jenkinson A, O'Brien T. Leg length discrepancy‐‐an experimental study of compensatory changes in three dimensions using gait analysis. Gait Posture, 2000, 12: 156–161. [DOI] [PubMed] [Google Scholar]
- 21. Tang XD, Guo W, Yang RL, Yang Y, Ji T. Limb salvage surgery for osteosarcoma around the knee in children and adolescent patients. Zhonghua Wai Ke Za Zhi, 2007, 45: 669–672. [PubMed] [Google Scholar]
- 22. Neel MD, Heck R, Britton L, Daw N, Rao BN. Use of a smooth press‐fit stem preserves physeal growth after tumor resection. Clin Orthop Relat Res, 2004, 426: 125–128. [DOI] [PubMed] [Google Scholar]
- 23. Arteau A, Lewis VO, Moon BS, Satcher RL, Bird JE, Lin PP. Tibial growth disturbance following distal femoral resection and expandable endoprosthetic reconstruction. J Bone Joint Surg Am, 2015, 97: e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiller C, Windhager R, Fellinger EJ, Salzer‐Kuntschik M, Kaider A, Kotz R. Extendable tumour endoprostheses for the leg in children. J Bone Joint Surg Br, 1995, 77: 608–614. [PubMed] [Google Scholar]
- 25. Picardo NE, Blunn GW, Shekkeris AS, et al The medium‐term results of the Stanmore non‐invasive extendible endoprosthesis in the treatment of paediatric bone tumours. J Bone Joint Surg Br, 2012, 94: 425–430. [DOI] [PubMed] [Google Scholar]
- 26. Hwang N, Grimer RJ, Carter SR, Tillman RM, Abudu A, Jeys LM. Early results of a non‐invasive extendible prosthesis for limb‐salvage surgery in children with bone tumours. J Bone Joint Surg Br, 2012, 94: 265–269. [DOI] [PubMed] [Google Scholar]


