Figure 6. Cooperative inhibition of HCV entry by IFITM proteins and neutralizing antibodies can be attenuated by treatment with amphotericin B.
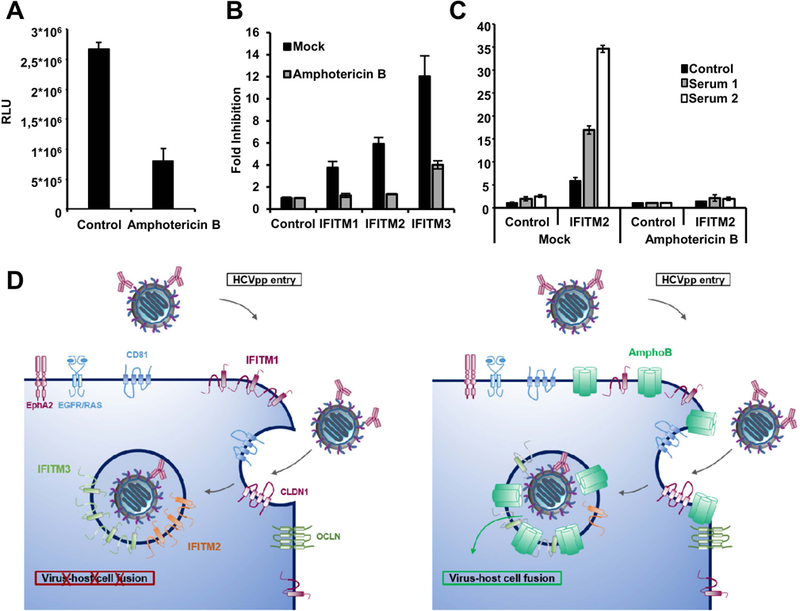
Huh7.5.1 cells were transduced by retroviral vectors coding for an empty control vector (A), with vectors coding for IFITM1, 2 and 3 and empty vector as control (B) or only IFITM2 plus control (C) Forty-eight h after transduction cells were treated with vehicle control or 5 µg/ml amphotericin B for 1 h. Afterwards, the cells were infected with HCVpp expressing the envelope of a variant not associated with viral escape and sensitive to antibody-mediated neutralization (P1VA). (C) Cells were infected with HCVpp pretreated with serum derived from chronically HCV infected patients or with control serum at 37 °C for 1 h. Entry of HCVpp was assessed 72 h post infection by measuring luciferase activity. (A) Results are shown in RLU. (B,C) Results were normalized for the vector control and are shown as fold inhibition compared to the respective controls. Shown are the means of representative experiments performed in triplicates (n=6) ± SD. (D) Model of cooperative inhibition of HCV entry by IFITMs and neutralizing antibodies and the antagonistic effect of amphotericin B. The interaction between infectious particles and cell surface receptors triggers endocytosis. Entry is blocked by IFITM proteins and neutralizing antibodies. Amphotericin B (AmphoB) is believed to rescue virus entry by antagonizing the IFITM-mediated increase of membrane rigidity and curvature.
