Clinical Trial Registration:
TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial); ClinicalTrials.gov Identifier:
Keywords: clinical decision tool, clinical trial, heart failure with preserved ejection fraction, natriuretic peptides
Diagnostic uncertainties and lack of standardized strategies to enrich baseline risk have posed significant challenges to the effective conduct of global trials of heart failure with preserved ejection fraction (HFpEF). Differences in event rates across regions in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial) trial underscore the importance of consistent standards for HFpEF diagnosis (1). The recent H2FPEF-score, which uses 6 routinely available clinical and echocardiographic variables, is the first validated diagnostic algorithm for identification of HFpEF in patients with unexplained dyspnea (2) and offers promise as a screening measure in clinical trials. The TOPCAT trial presents a unique opportunity to evaluate the application of this score in a trial population with known background heterogeneity, and to understand its relationship with risk of clinical events.
TOPCAT was a global, phase-3, double-blind, placebo-controlled randomized clinical trial of spironolactone in HFpEF enrolling patients from the Americas (United States, Canada, Brazil, Argentina), Russia, and the Republic of Georgia (3). Eligible patients were those ≥50 years with symptomatic HF and left ventricular (LV) ejection fraction ≥45%, well-controlled blood pressure, and a serum potassium<5.0mmol/L as well as a recent HF hospitalization within 12 months or elevated natriuretic peptide (NP) concentrations within 60 days. In a subset, pre-randomization echocardiograms were submitted to a central core laboratory (4). The primary outcome was time to composite hospitalization for HF, cardiovascular death, or aborted cardiac arrest.
The H2FPEF-score was developed from a retrospective analysis of patients referred for invasive hemodynamic exercise testing for the evaluation of unexplained dyspnea at a tertiary care center (2). Analyses in this TOPCAT substudy were performed in 362 patients with available data necessary to calculate the H2FPEF-score: age, body mass index, hypertension medication use, history of atrial fibrillation, pulmonary artery systolic pressure (PASP, estimated from the modified Bernoulli equation of the peak tricuspid valve regurgitation velocity + 5mmHg as a surrogate of right atrial pressure) and E/e’.
We estimated diagnostic probabilities of HFpEF reported in the original derivation report of the H2FPEF-score (2). We assessed the association between H2FPEF-score and baseline NP levels (either B-type NP or N-terminal pro-B-type NP), which were log-transformed and standardized (expressed per 1 standard deviation; Z-score). Multivariable Cox proportional hazards models were used to assess the association between H2FPEF-score and the primary composite outcome. Restricted cubic splines models with the number of knots selected based on the lowest Akaike Information Criterion were used to flexibly model the relationship between H2FPEF-score and standardized NPs and the incidence of the primary endpoint. All patients provided written informed consent, and the study was approved by institutional review board or ethics committees at each participating institution. All statistical analyses were performed using STATA 14.1 (College Station, TX, USA).
Of the 313 (18%) patients from the Americas and 49 (3%) from Russia/Georgia with available data, the median H2FPEF-score was 6 (interquartile range [IQR] 4–7) and 5 (IQR 3–6), respectively; P=0.01 for difference between regions (Figure, Panel A), and there were no differences between patients enrolled by hospitalization- or NP-strata (P=0.83). Of the total 362 patients, 216 (60%) had BMI>30 kg/m2 (2-points), 344 (95%) used ≥2 antihypertensive drugs (1-point), 177 (49%) had a history of atrial fibrillation (paroxysmal or persistent, 3-points), 171 (47%) had PASP>35 mmHg (1-point), 315 (87%) were older than 60 years (1-point), and 307 (85%) had E/e’>9 (1-point). Overall, 74% and 59% of patients had H2FPEF-scores ≥5 (corresponding to HFpEF diagnostic probabilities of >80%) in the Americas and Russia/Georgia, respectively (P=0.026). Patients with higher scores were more likely to be enrolled in the Americas region, male, carry a history of diabetes mellitus, have lower estimated glomerular filtration rate and greater left atrial and left ventricular volumes, in addition to parameters included in the H2FPEF-score; all P<0.01 (Table). There was a trend for greater concentrations of NPs with higher H2FPEF-score, although this did not reach statistical significance (P=0.07, Figure, Panel B).
Figure. Distribution of the H2FPEF–score in the global TOPCAT trial, and its association with concentrations of natriuretic peptides and the primary composite outcome.
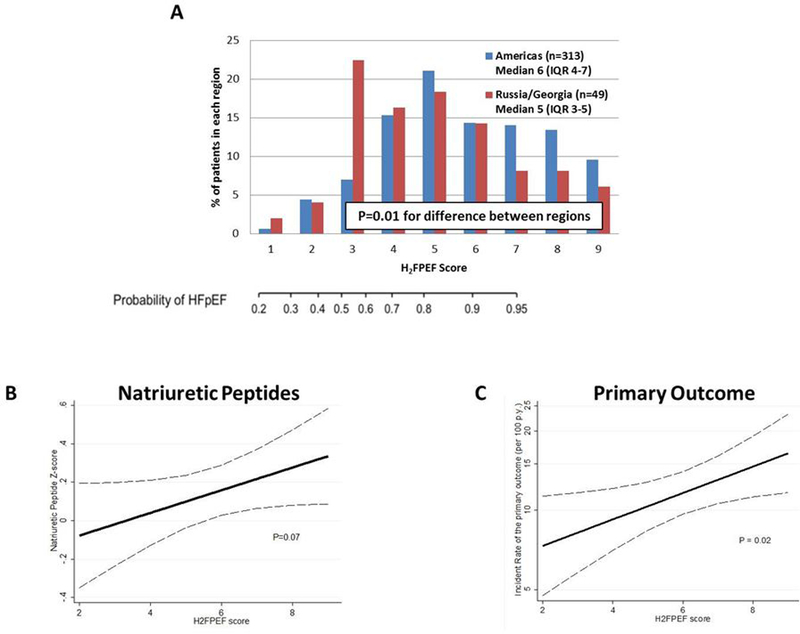
Overall, 362 patients had available data to calculate the H2FPEF-score. Distribution of scores in patients enrolled in the Americas and Russia/Georgia are presented in Panel A. The association between the H2FPEF-score and log-transformed, standardized natriuretic peptide concentrations as a continuous variable is presented in Panel B, and association between the H2FPEF-score and incidence of the primary composite endpoint in Panel C. Natriuretic peptide levels (either B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide) were log-transformed and standardized (expressed per 1 standard deviation; Z-score). The primary outcome for the TOPCAT trial and for this analysis was time to composite hospitalization for heart failure, cardiovascular death, or aborted cardiac arrest. The dotted lines reflect the 95% confidence interval.
Table.
Baseline characteristics of patients in TOPCAT by categories of H2FPEF-score (n=362)
| H2FPEF score | H2FPEF score | H2FPEF score | ||
|---|---|---|---|---|
| ≤3 | >3 to <6 | ≥6 | ||
| n=100 (28%) | n=130 (36%) | n=132 (36%) | P | |
| Age (years) | 69.9 ± 11.2 | 70.7 ± 9.4 | 74.7 ± 8.7 | <0.001 |
| Male | 34 (34.0%) | 61 (46.9%) | 68 (51.5%) | 0.009 |
| Black race | 19 (19.0%) | 33 (25.4%) | 15 (11.4%) | 0.09 |
| Region | 0.017 | |||
| Russia and Georgia | 20 (20.0%) | 17 (13.1%) | 12 (9.1 %) | |
| The Americas | 80 (80.0%) | 113 (86.9%) | 120 (90.9%) | |
| Eligibility Criteria | 0.64 | |||
| Prior HF Hospitalization in 12mo | 61 (61.0%) | 82 (63.1%) | 77 (58.3%) | |
| Elevated NP in 60 days | 39 (39.0%) | 48 (36.9%) | 65 (41.7%) | |
| New York Heart Association Class III or IV | 41 (41.4%) | 56 (43.4%) | 63 (47.7%) | 0.33 |
| Hypertension | 94 (94.0%) | 118 (90.8%) | 122 (92.4%) | 0.71 |
| Diabetes Mellitus | 35 (35.0%) | 59 (45.4%) | 59 (44.7%) | 0.16 |
| Previous Myocardial Infarction | 22 (22.0%) | 29 (22.3%) | 31 (23.5%) | 0.78 |
| Previous Cerebrovascular Accident | 12 (12.0%) | 9 (6.9 %) | 17 (12.9%) | 0.72 |
| Peripheral Artery Disease | 10 (10.0%) | 19 (14.6%) | 9 (6.8 %) | 0.35 |
| History of Atrial Fibrillation | 1 (1.0 %) | 44 (33.8%) | 132 (100.0%) | <0.001 |
| Body Mass Index (kg/m2) | 29.5 ± 8.2 | 34.0 ± 8.2 | 34.4 ± 7.4 | <0.001 |
| Waist Circumference (cm) | 98.7 ± 16.1 | 107.0 ± 16.0 | 110.9 ± 16.1 | <0.001 |
| Systolic Blood Pressure (mmHg) | 128.5 ± 16.6 | 125.4 ± 16.3 | 123.7 ± 15.0 | 0.025 |
| eGFR (ml/min/1.73 m2) | 65.8 ± 21.3 | 64.4 ± 20.9 | 59.7 ± 18.1 | 0.018 |
| N-terminal pro-B-type NP (ng/L) | 791 [437 , 2054] | 1068 [475 , 1615] | 1354 [751 , 2023] | 0.32 |
| B-type NP (ng/L) | 234 [155 , 698 ] | 208 [130 , 375 ] | 329 [165 , 568 ] | 0.21 |
| Baseline Medication Use | ||||
| β-blocker | 76 (76.0%) | 106 (81.5%) | 102 (77.3%) | 0.89 |
| Calcium Channel Blocker | 38 (38.0%) | 63 (48.5%) | 55 (41.7%) | 0.67 |
| Diuretic | 81 (81.0%) | 113 (86.9%) | 123 (93.2%) | 0.005 |
| ACEi/ARB | 72 (72.0%) | 107 (82.3%) | 109 (82.6%) | 0.06 |
| Aspirin | 72 (72.0%) | 82 (63.1%) | 70 (53.0%) | 0.003 |
| Statin | 66 (66.0%) | 86 (66.2%) | 89 (67.4%) | 0.81 |
| Baseline Echocardiography | ||||
| LV ejection fraction (%) | 60.6 ± 8.1 | 60.1 ± 7.4 | 58.8 ± 7.9 | 0.07 |
| LV mass index (g/ m2) | 104.8 ± 32.1 | 109.4 ± 26.7 | 109.5 ± 33.2 | 0.28 |
| LV end diastolic volume index (ml/m2) | 49.8 ± 15.9 | 49.3 ± 15.1 | 43.8 ± 12.9 | 0.002 |
| LA volume index (ml/m2) | 29.1 ± 10.9 | 31.4 ± 9.6 | 36.5 ± 18.7 | <0.001 |
| E/e’ lateral ratio | 12.4 ± 5.4 | 12.4 ± 6.6 | 13.1 ± 7.0 | 0.41 |
| Pulmonary Artery Systolic Pressure (mmHg) | 35.7 ± 10.8 | 35.0 ± 11.0 | 40.1 ± 11.4 | 0.002 |
Data reported as n (%), mean ± standard deviation or median (quartile 1 to quartile 3)
Abbreviations: ACEi/ARB = angiotensin-converting enzyme inhibitor / angiotensin II receptor blocker; eGFR = estimated glomerular filtration rate; HF=heart failure; NP = natriuretic peptide; LV, left ventricular; LA, left atrial
Over 2.7 years mean follow-up, 112 primary outcome events occurred. Higher H2FPEF-scores (per point) were associated with increased risk of the primary outcome: hazard ratio [HR] 1.12, 95% confidence interval [CI] 1.02–1.23;P=0.02 (Figure, Panel C). Similar, yet not statistically significant, associations between the H2FPEF-score and the primary endpoint were found in analysis restricted to patients with left ventricular EF ≥50% (n=319): HR 1.09 (95% CI 0.98–1.21);P=0.12. The incidence rate of the primary outcome in patients with H2FPEF-score≤4, 5–6, and ≥7 was 8.3 (95% CI 5.6–12.4), 11.8 (95% CI 8.7–16.0), and 13.7 (10.2–18.3), respectively. The association between the H2FPEF-score and the primary endpoint did not differ by enrollment strata (recent hospitalization or elevated NP); Pinteraction=0.57. Higher H2FPEF-scores (per point) were also associated with increased risk of HF hospitalization (HR 1.14 [95% CI 1.01–1.27], P=0.03) and cardiovascular death (HR 1.15 [95% CI 0.98–1.34], P=0.10) separately, although the latter association did not reach statistical significance.
HFpEF is a syndrome that is challenging to differentiate from non-cardiac sources of dyspnea based on clinical examination alone. Although invasive and/or exercise hemodynamic assessments are available to affirm the HFpEF diagnosis, cost, complexity, procedural risk, and limited availability preclude their use in large clinical trials. In addition, risk enrichment strategies applied in HFpEF trials are subject to important limitations. Thresholds for hospitalization for HF may vary globally and across health systems (5). NP concentrations have traditionally been used to identify HFpEF patients with greater certainty and to enrich risk, however these vary substantially and may be systematically lower in select populations (including black and obese patients) (6).
In this study we demonstrate that the H2FPEF-score correlates with increased risk of adverse cardiovascular events in the TOPCAT trial. Despite variation in analytic approaches, another group recently independently supported the prognostic value of the H2FPEF-score in TOPCAT (7). We further demonstrate that the H2FPEF-score was only partially and non-significantly associated with NPs, suggesting that these 2 parameters may provide orthogonal and incremental information.
Among patients determined eligible for enrollment in a large HFpEF trial, we observed that <20% of patients in TOPCAT Americas had diagnostic probabilities of HFpEF <55% as estimated by the H2FPEF-score, while 40% of patients enrolled in TOPCAT Russia/Georgia fell into this lower diagnostic probability. These findings are in keeping with regional differences in event rates suggesting a 4-fold lower risk of the primary outcome in Russia/Georgia as compared with the Americas (1). When applied to a referral cohort from Alberta, Canada, the discriminatory value of the H2FPEF-score was lower than that observed in the original derivation and internal validation cohorts (8). Taken together, these data emphasize the ongoing need to understand the variability in distribution and performance of the H2FPEF-score across global, heterogenous populations.
The study is subject to certain limitations, including the restricted number of patients with available data for H2FPEF-score calculation which may introduce selection bias. The H2FPEF-score was derived from a population of patients with unexplained dyspnea, while we applied it retrospectively to patients deemed to have symptomatic HFpEF by a site investigator in a randomized clinical trial. TOPCAT enrolled patients with left ventricular EF≥45%, which is below accepted diagnostic cut-offs for HFpEF; sensitivity analysis restricted to patients with left ventricular EF≥50% yield directionally consistent, but non-significant findings. Finally, it is uncertain whether its prognostic value can be attributed to the composite score or to individual component elements.
Disease heterogeneity and diagnostic uncertainty have long been concerns in explaining the failures of previous HFpEF trials. This simple score based on 6 routinely collected clinical and echocardiographic variables represents an attractive option as a risk enrichment strategy in enrollment for future global clinical trials of HFpEF. However, future prospective studies are needed to externally validate this diagnostic algorithm in larger samples, determine the scope of its applicability in a broad range of patients with dyspnea syndromes, and test its utility as a metric of clinical trial eligibility and risk enrichment in HFpEF against current strategies (prior hospitalization for HF & NPs).
ACKNOWLEDGEMENTS
We would like to acknowledge the contributions of the TOPCAT participants, site investigators, and leadership, including the clinical events committee (Brigham and Women’s Hospital, Boston, MA, USA).
FUNDING
TOPCAT was supported by the NHLBI (HHSN268200425207C).
Dr. Myhre has received consulting fees from Novartis and is supported by a postdoctoral research grant from the South-Eastern Norway Regional Health Authority, the Norwegian Medical Association and the Unger Vetlesen Medical Fund. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), and serves on advisory boards for Amgen, AstraZeneca, Bayer AG, and Baxter Healthcare. Dr. Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; and has served on the Advisory Board/ Steering Committee/ Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, and Darma. Dr. Sweitzer has received research funding from the NIH and Merck. Dr. O’Meara has received subventions/research support and consulting fees from Novartis, Bayer, AstraZeneca, Servier, and Amgen. Dr. S. Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, and United Therapeutics. Dr A. Shah has received research support from Novartis and consulting fees from Philips Ultrasound and Bellerophon. The work for this manuscript was also supported by NIH/NHLBI grants K08HL116792, R01HL135008, and R01HL143224 (Dr. A. Shah). Dr. Desai has received research grant support from Novartis and consulting fees from Novartis, AstraZeneca, Abbott, Relypsa, and DalCor Pharma. Dr. Lewis has received research grants from NHLBI, Novartis, and Sanofi; and consults for Novartis. Dr. Pitt reports receiving consulting fees from Amorcyte, AstraZeneca, Aurasense, Bayer, BG Medicine, Gambro, Johnson & Johnson, Mesoblast, Novartis, Pfizer, Relypsa, and Takeda; receiving research grant support from Forest Laboratories; and holding stock in Aurasense, Relypsa, BG Medicine, and Aurasense. Dr. Pitt reports a pending patent related to site specific delivery of eplerenone to the myocardium. Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Celladon, Gilead, GSK, Ionis Pharmaceutics, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Alnylam, Amgen, AstraZeneca, Bayer, BMS, Corvia, Gilead, GSK, Ironwood, Merck, Novartis, Pfizer, Takeda, and Theracos.
Footnotes
CONFLICT OF INTEREST
All other authors report no other relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42. [DOI] [PubMed] [Google Scholar]
- 2.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation. 2018;138(9):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. [DOI] [PubMed] [Google Scholar]
- 4.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7(1):104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene SJ, Hernandez AF, Sun JL, Butler J, Armstrong PW, Ezekowitz JA, Zannad F, Ferreira JP, Coles A, Metra M, Voors AA, Califf RM, O’Connor CM, Mentz RJ. Relationship Between Enrolling Country Income Level and Patient Profile, Protocol Completion, and Trial End Points. Circ Cardiovasc Qual Outcomes. 2018;11(10):e004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O’Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of Natriuretic Peptides and Cardiovascular Prognosis in Heart Failure with Preserved Fraction: Secondary Analysis of the TOPCAT Randomized Clinical Trial. JAMA Cardiol. 2018;3(10):1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and Implications of the H2FPEF Score in a Cohort of Patients With Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139(15):1851–1853. [DOI] [PubMed] [Google Scholar]
- 8.Sepehrvand N, Alemayehu W, Dyck GJB, Dyck JRB, Anderson T, Howlett J, Paterson I, McAlister FA, Ezekowitz JA. External Validation of the H2F-PEF Model in Diagnosing Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2019;139(20):2377–2379. [DOI] [PubMed] [Google Scholar]


