Abstract
Background:
The long-term trajectory of and factors affecting lean mass in people living with HIV (PLWH) are incompletely described.
Methods:
PLWH in the Modena HIV Metabolic Cohort underwent dual-energy X-ray absorptiometry (DXA) scans every 6–12 months for up to 10 years (median 4.6 scans). Mixed effect regression modeling in combined and sex-stratified models determined annual rates of and clinical factors significantly associated with appendicular lean mass (ALM).
Results:
839 women and 1759 men contributing ≥2 DXA scans had baseline median age 44 years and 14 years since HIV diagnosis; 76% were virologically suppressed on antiretroviral therapy (ART). Baseline median ALM was 16.9 kg for women and 24.8 kg for men. ALM decreased during the study period, with mean yearly ALM loss of −231 g in women and −322 g in men. Less ALM was associated with female sex, age >50 years, detectable HIV-1 RNA, and tenofovir and integrase inhibitor use. Greater ALM was associated with longer ART duration. In sex-stratified models, relationships between ALM and total ART duration and integrase inhibitor use were not significant for women, but the relationship with tenofovir use persisted. For men, AIDS wasting and CD4+ T lymphocyte nadir <200 cells/μL were independently associated with lower ALM.
Conclusions:
ALM steadily declined over time in this cohort of PLWH on ART that included a large number of women. HIV-and ART-specific risk factors emerged that varied by sex. The observed associations between tenofovir or integrase inhibitor use and lower ALM particularly warrant further study.
Introduction
Age-related loss of lean mass (LM) is central to the etiology of frailty, which is notable among middle-aged persons living with HIV (PLWH). [1,2] Lower LM is associated with functional dependence and increased mortality in PLWH. [3,4] Dual-energy X-ray absorptiometry (DXA) is a widely accepted, non-invasive method that can precisely quantify fat free mass (FFM; sum of lean and bone masses) and provide absolute quantities of total LM and appendicular lean mass (ALM; sum of lean mass in the arms and legs). [5]
Although initiation of antiretroviral therapy (ART) is associated with an increase in LM in the first 2 years, [6–9] only a few longitudinal studies of changes of LM in PLWH on long-term ART have been performed, with conflicting results. [2,6,10] We aimed to understand the longitudinal ALM trajectory of and factors associated with ALM quantity in HIV-infected adults on long-term ART.
Methods
We conducted a longitudinal secondary analysis of data from participants enrolled in the cohort of the multidisciplinary Modena HIV Metabolic Clinic (MHMC), at the University of Modena and Reggio Emilia, Italy. PLWH who receive care in the MHMC underwent DXA scans approximately every 6 to 12 months, beginning in 2004. We included all participants who were on ART and who had at least 2 DXA scans over up to 10 years, beginning in 2004. All procedures were in accordance with the ethical standards of Comitato Etico Provinciale di Modena and with the Helsinki Declaration of 1975, as revised in 2000; all participants provided written, informed consent.
Demographic characteristics, HIV infection history (history of AIDS wasting, nadir CD4+ T lymphocyte (CD4) count, ART duration, cumulative ART use by class and agent), smoking (number of cigarettes/day), and amount of physical activity (none, low [<4 hours weekly], high [≥4 hours weekly]) were collected at enrollment. Past medical history, including hypogonadism (defined as post-menopausal in women and serum total testosterone <300 ng/dL in men), [11] metabolic syndrome (NCEP-Adult Treatment Panel III criteria), [12] vitamin D insufficiency (25-OH vitamin D <30 ng/mL), and hepatitis C virus (HCV) serostatus were recorded from chart review. Body weight was measured using a digital scale to the nearest 0.1 kg, with participants wearing light clothes without shoes. Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm. Body mass index (BMI) was defined as weight in kilograms divided by height in meters squared.
Laboratory analysis included the following: HIV-1 RNA (Abbott RealTime™ HIV-1 assay; Abbott Laboratories, Chicago, IL; lower limit of detection: 50 copies/mL), CD4 count by flow cytometry, HCV antibody testing (anti-HCV; Abbott HCV EIA 3.0 enzyme immunoassay; Abbott Laboratories, Chicago, IL), and 25-hydroxy vitamin D (DiaSorin 25-hydroxyvitamin D chemiluminescence immunoassay; Stillwater, MN). Changes in ALM were determined overall and by sex. All participants were scanned using the same densitometer (Hologic Discovery W, Inc., Waltham, MA). The instrument was calibrated daily with a hydroxyapatite phantom.
Descriptive statistics were used to characterize the sample. To account for correlation within patients in outcome measures, mixed effect regression models were created assuming compound symmetry variance-covariance structure to determine the sex-adjusted effect, and then in sex-stratified models to determine whether factors associated with changes in ALM differ between men and women. A significant sex*year interaction in the combined model further supported the use of sex-stratified models. Mixed effect regression models determined variables significantly (p<0.05) associated with ALM quantity, after exclusion of variables not statistically significant in the univariate analyses. In combined models, estimates reported include the sex*year interaction.
Regression models with random intercept and slope were adjusted for sex (for combined model), and the following variables: time on study, BMI, total ART duration, age group by five-year increments, self-reported physical activity level, metabolic syndrome, HCV seropositivity, hypogonadism, history of AIDS wasting, per pack year tobacco use, and vitamin D insufficiency. Additional variables were considered for inclusion, but either excluded due to extent of missing data or lack of significance in univariable models (p>0.10). Stepwise forward model selection methods with backward elimination were used in building the final models. The covariates identified in the combined model were used in the sex-stratified models to examine their associations with the outcomes in men and women separately. Annual change rates were calculated by applying simple regression to mean estimates derived from the mixed effects models. A p-value <0.05 was considered statistically significant. All analysis were conducted using SAS 9.4 (Cary, NC).
Results
Persons who contributed ≥2 DXA scans included 839 women and 1759 men (median [interquartile range, IQR] 5 scans [IQR 3,7]), with a median follow-up of 4.6 years [IQR 2.1, 7.7]). Baseline characteristics at the initial assessment are shown in (Table 1). All participants were Caucasian. The median age was 44 years. The median CD4 count was 528 cells/µL; 76% had undetectable HIV-1 RNA; the median duration of ART was 9.6 years in women and 8.3 in men. Median BMI was 21.6 kg/m2 for women and 23.5 kg/m2 for men. At baseline, 7% of men had hypogonadism and 15% of women were post-menopausal, with 24% of women categorized as post-menopausal at some point after study entry; 42% of men with hypogonadism were on testosterone supplementation; no women were on hormone replacement therapy.
Table 1.
Baseline Demographic & Clinical Characteristics at Time of First Lean Mass Assessment
| Characteristics | Women (N=839) | Men (N=1759) |
|---|---|---|
| Age (%) | ||
| >55 years | 37 (4) | 166 (9) |
| 51–55 years | 71 (9) | 197 (11) |
| 46–50 years | 183 (22) | 450 (26) |
| 41–45 years | 303 (36) | 536 (31) |
| 35–40 years | 182 (22) | 265 (15) |
| <35 years | 63 (8) | 145 (8) |
| Body mass index (kg/m2, IQR) | 21.6 (20.0–24.1) | 23.5 (21.6–25.5) |
| Smoker (%) | 349 (41.6) | 740 (42.1) |
| Smoking (pack years, IQR) | 10.0 (1.1–20.0) | 12.5 (0–25.0) |
| Physical activity [n (%)] | ||
| None | 577 (69) | 1020 (58) |
| Moderate | 184 (22) | 438 (25) |
| Vigorous | 44 (5) | 224 (13) |
| Hypogonadism1 (%) | 124 (15) | 124 (7) |
| Metabolic Syndrome (%) | 84 (10) | 144 (8) |
| HCV seropositivity2 (%) | 250 (30) | 468 (27) |
| Vitamin D Insufficiency (%) | 414 (49) | 831 (47) |
| History of AIDS Wasting (%) | 113 (13) | 81 (5) |
| CD4 Nadir <200 cells/µL (%) | 448 (53) | 856 (49) |
| HIV-1 VL ≤50 (%) | 646 (77) | 1319 (75) |
| ART duration (years, IQR) | 9.6 (5.6–13.1) | 8.3 (3.4–12.0) |
| TDF use (%) | 538 (64) | 1144 (65) |
| INSTI use (%) | 70 (8) | 129 (7) |
| ALM (kg, IQR) | 16.9 (15.1–18.9) | 24.8 (22.2–27.3) |
Defined as post-menopausal female or male hypogonadism
Defined as presence of hepatitis C virus antibody. IQR= interquartile range, HCV=hepatitis C virus, TDF=tenofovir disoproxil fumarate, INSTI=integrase strand transfer inhibitor ALM=appendicular lean mass
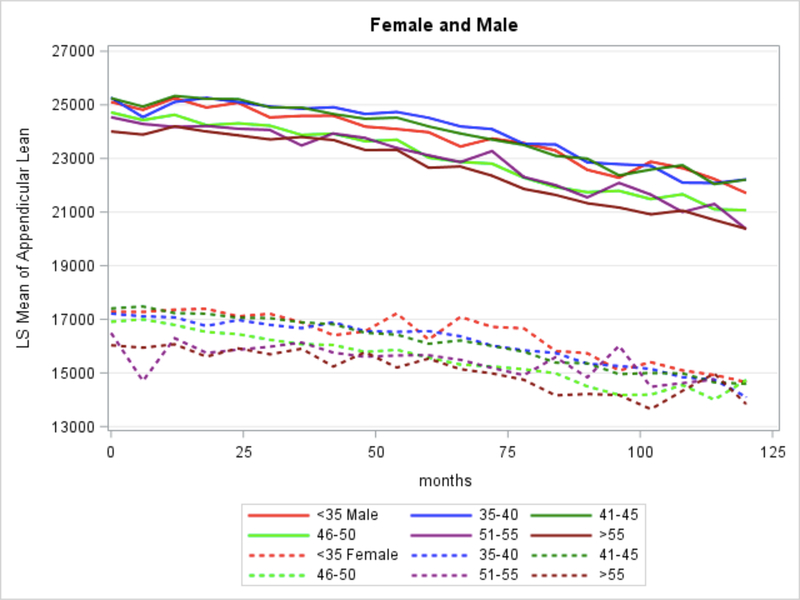
Baseline ALM was 16.9 kg for women and 24.8 kg for men. For both sexes, ALM consistently declined over the study period (Figure 1), with a mean (standard deviation, SD) yearly ALM decrease of −231 g (640g) in women and −322 g (906g) in men. The interaction sex*year was significant for ALM changes over time (p<0.001). In the combined-sex model (Table 2), female sex was most strongly associated with ALM quantity, with women having 4.5 kg less ALM over the study period than men. Other factors significantly associated with less ALM were: age >50 years, less than intense regular physical activity, HIV-1 RNA >50 copies/mL, and current tobacco use. Although longer duration of any ART was associated with greater ALM, per year TDF and INSTI use were associated with less ALM. Greater ALM was also seen in persons with higher (per unit increase) BMI, vitamin D insufficiency, metabolic syndrome, and hypogonadism, regardless of testosterone use (data not shown).
Figure 1.
Decreases in appendicular lean mass over time.
Table 2.
Combined and Sex-Stratified Model for Appendicular Lean Massa
| Variable | Combined | Women | Men | |||
|---|---|---|---|---|---|---|
| Model Estimate (SE) | P value | Model Estimate (SE) | P value | Model Estimate (SE) | P value | |
| Female Sex | − 4178.68 (289.33) | <0.0001 | N/A | N/A | ||
| Body mass index (kg/m2)1 | 519.41 (9.2) | <0.0001 | 359.5 (11.9) | <0.0001 | 602 (11.1) | <0.0001 |
| Age >55 years2 | −1035.56 (260.32) | <0.0001 | −936.9 (468.6) | 0.0461 | −1225.8 (265.3) | <0.0001 |
| Age 51–55 years2 | −1039.19 (247.08) | <0.0001 | −901.5 (373.0) | 0.016 | −996.5 (256.8) | 0.0001 |
| Age 46–50 years2 | NS | NS | NS | NS | −509.2 (228.8) | 0.0262 |
| Age 41–45 years2 | NS | NS | NS | NS | NS | NS |
| Age 35–40 years2 | NS | NS | NS | NS | NS | NS |
| No regular physical activity3 | −510.8 (56.4) | <0.0001 | −212.9 (82.4) | 0.0102 | −513.6 (66.3) | <0.0001 |
| Low physical activity3 | −410.98 (53.9) | <0.0001 | −155.5 (81.3) | 0.0567 | −540.0 (62.9) | <0.0001 |
| Metabolic syndrome | −165.48 (61.3) | <0.0076 | 239.3 (88.5) | 0.0097 | 163.3 (69.8) | <0.0204 |
| CD4 nadir <200 cells/µL | NS | NS | NS | NS | −269.5 (108.3) | 0.0129 |
| Per year ART use | 20.0 (9.3) | 0.0242 | NS | NS | 29.9 (11.1) | 0.0035 |
| Per year INSTI use | −0.5 (0.1) | <0.0001 | NS | NS | −0.6 (0.1) | <0.0001 |
| Per year TDF use | −31.2 (9.1) | <0.0001 | −28.8 (12.0) | 0.0017 | −41.9 (10.9) | 0.0001 |
| Vitamin D insufficiency | 291.5 (49.9) | <0.0001 | 275.1 (69.4) | 0.0001 | 250.5 (57.2) | <0.0001 |
| Hypogonadism | 232.4 (71.1) | 0.0011 | NS | NS | 381.7 (101.9) | 0.0002 |
| HCV seropositivity | NS | NS | NS | NS | NS | NS |
| History of AIDS wasting | NS | NS | 224.5 (93.9) | 0.0207 | −534.6 (133.4) | 0.0002 |
| HIV-1 RNA >50 copies/mL4 | −266.8 (39.1) | <0.0001 | −150.8 (51.4) | 0.0035 | −286.1 (45.8) | <0.0001 |
| Per year pack years | −6.5 (3.0) | 0.0324 | −12.9 (5.1) | 0.0108 | NS | NS |
All model estimates are presented in grams per year.
Per unit increase
Reference group <35 years of age
Reference intense regular physical activity
Reference >50 copies/mL, NS= not significant, TDF=tenofovir disoproxil fumarate, INSTI=integrase strand transfer inhibitor, HCV=hepatitis C virus
In sex-stratified models, differences emerged: Among women, the association between per year TDF use and lower ALM persisted, but per year any ART and INSTI use were no longer significant. Smoking was associated with less ALM in women but not men. Among men, age >45 years, history of AIDS wasting, and CD4 nadir <200 cells/µL were significantly associated with less ALM.
Discussion
In this large cohort of PLWH on long-term ART, ALM steadily declined over a median of 4.6 years follow-up, despite persistent virologic suppression. Consistent with reports in the general population, older age and female sex were associated with less ALM. [5,13] However, in our cohort, ALM loss occurred in all age groups an both sexes (Figure 1), a concerning finding given the majority of our cohort was <50 years old at enrollment.
Reported LM decline rates in the general population after age 50 vary from 0.16–2% per year, [14–16] with yearly total LM declines of only 183 g and 149 g in men and women, respectively. [15] Earlier studies of LM changes in PLWH reported an initial increase by DXA consistent with a “return to health” effect, but follow up was limited to <96 weeks. [7,8] Although an analysis comparing MRI-quantified LM changes between PLWH and HIV-uninfected controls did not show any difference after 5 years of follow-up, the control population was 33–45 years of age, and PLWH were at various stages of ART use, limiting comparability. [10] A recent analysis comparing PLWH with HIV-uninfected individuals showed a higher rate of LM loss in PLWH after 96 weeks of treatment, but with few women included and no sex-specific or HIV-related factors significantly associated with this decline. [6]
In a novel finding, we found a significant association between per year INSTI (in men) and TDF (in both men and women) use and less ALM. Of note, we did not look at the potential effects of all individual ART agents, previously published data on body composition changes with specific ART agents vary, and the currently available analyses of longer-term effects of INSTIs on body composition are limited to weight and fat gain analyses, with LM changes not documented beyond 96 weeks, again limiting comparison. [9,17] Thus, we provide the first report of longer-term changes in LM in PLWH on INSTIs.
There are several important strengths to our study. Foremost, the extent of serial DXA data, with a high number of DXAs per participant, allowed us to trend LM closely over time. The large proportion of women included in the analysis allowed exploration of factors associated with ALM by sex. However, the lack of a significant association between less ALM and INSTI exposure in women might reflect the lack of power to detect it given the larger proportion of men included in the study. Additional limitations include that our data reflects the ethnic background of Italy and may not be generalizable to other races/ethnicities[6] and an HIV-uninfected Italian control population was not available for comparison.
In conclusion, PLWH on long term-ART have continued loss of ALM related to both traditional and HIV-/ART-related factors that vary by sex. The early identification of these factors creates an important target for therapy to prevent the adverse effects of ALM mass loss. Further studies are needed to understand how cumulative TDF and INSTI use will impact LM in PLWH.
Acknowledgements
The authors would like to thank the participants and staff of the Modena HIV Metabolic Clinic Cohort for their participation and hard work. Specifically, we would like to thank Andrea Malagoli, Stefano Zona, Marianna Menozzi, Valentina Masi, Maria Mancini, Agnese Caselgrandi, and Maria Giulia Corni for their time and dedication to the Cohort’s daily operations and data management.
Conflicts of Interest
TTB has served as a consultant to Gilead Sciences, Merck, BMS, Theratechnologies, and EMD-Serono. JEL has served as a consultant to and receives research funding from Merck and Gilead Sciences. GG has served as a consultant to Gilead Sciences, Merck, and ViiV. KME has received research funding (paid to the University of Colorado) from Gilead Sciences. JF has served as consultant to Theratechnologies and EMD-Serono.
Sources of Funding
This work was supported by an unrestricted education grant from ViiV to GG, the National Institute of Aging on the National Institutes of Health (K23 AG050260 and R01 AG054366 to KME), and the National Institute of Allergy and Infectious Diseases (K24 AI120834 to TTB and K23 AI110532 to JEL).
References
- 1.Kooij KW, Wit FWNM, Schouten J, Van Der Valk M, Godfried MH, Stolte IG, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. Aids 2016;30(2):241–50. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman P, Segal-Maurer S, Rubin DS. High prevalence of low skeletal muscle mass associated with male gender in midlife and older HIV-infected persons despite cd4 cell reconstitution and viral suppression. J Int Assoc Provid AIDS Care 2014;13(2):145–52. [DOI] [PubMed] [Google Scholar]
- 3.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013;63(2):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. Aids 2011;25(11):1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinton BJ, Fan B, Ng BK, Shepherd JA. Dual energy X-ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999–2004 with additional visualization methods. PLoS One 2017;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016;30(18):2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolland MJ, Grey AB, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, et al. Bone mineral density remains stable in HAART-treated HIV-infected men over 2 years. Clin Endocrinol (Oxf) 2007;67(2):270–5. [DOI] [PubMed] [Google Scholar]
- 8.McDermott AY, Terrin N, Wanke C, Skinner S, Tchetgen E, Shevitz AH. CD4+ Cell Count, Viral Load, and Highly Active Antiretroviral Therapy Use Are Independent Predictors of Body Composition Alterations in HIV-Infected Adults: A Longitudinal Study. Clin Infect Dis 2005;41(11):1662–70. [DOI] [PubMed] [Google Scholar]
- 9.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dubé MP, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016;62(7):853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarasheski KE, Scherzer R, Kotier DP, Dobs AS, Tien PC, Lewis CE, et al. Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. Journals Gerontol - Ser A Biol Sci Med Sci 2011;66 A(3):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochira V, Guaraldi G. Hypogonadism in the HIV-Infected Man. Endocrinol Metab Clin North Am 2014. September;43(3):709–30. [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation and T of HBC in A. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J Am Med Assoc 2001. May 16;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 13.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MAF. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 2002;76(2):473–81. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr 2009;90(6):1457–65. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. Journals Gerontol Ser A Biol Sci Med Sci 2006;61(10):1059–64. [DOI] [PubMed] [Google Scholar]
- 16.Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: The prospective MINOS study. Am J Clin Nutr 2010. May 1;91(5):1227–36. [DOI] [PubMed] [Google Scholar]
- 17.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Weight Gain in Persons with HIV Switched from Efavirenz-based to Integrase Strand Transfer Inhibitor-based Regimens. JAIDS J Acquir Immune Defic Syndr 2017. December;Publish Ah(5):527–31. [DOI] [PMC free article] [PubMed]