Abstract
Alcohol consumption is an established risk factor for colorectal cancer (CRC). However, while studies have consistently reported elevated risk of CRC among heavy drinkers, associations at moderate levels of alcohol consumption are less clear. We conducted a combined analysis of 16 studies of CRC to examine the shape of the alcohol-CRC association, investigate potential effect modifiers of the association, and examine differential effects of alcohol consumption by cancer anatomic site and stage. We collected information on alcohol consumption for 14,276 CRC cases and 15,802 controls from five case-control and 11 nested case-control studies of CRC. We compared adjusted logistic regression models with linear and restricted cubic splines to select a model that best fit the association between alcohol consumption and CRC. Study-specific results were pooled using fixed-effects meta-analysis. Compared to non-/occasional drinking (≤1 g/day), light/moderate drinking (up to 2 drinks/day) was associated with a decreased risk of CRC (OR: 0.92, 95% CI: 0.88–0.98, p=0.005), heavy drinking (2–3 drinks/day) was not significantly associated with CRC risk (OR: 1.11, 95% CI: 0.99–1.24, p=0.08), and very heavy drinking (more than 3 drinks/day) was associated with a significant increased risk (OR: 1.25, 95% CI: 1.11–1.40, p<0.001). We observed no evidence of interactions with lifestyle risk factors or of differences by cancer site or stage. These results provide further evidence that there is a J-shaped association between alcohol consumption and CRC risk. This overall pattern was not significantly modified by other CRC risk factors and there was no effect heterogeneity by tumor site or stage.
Keywords: Alcohol, Colorectal Cancer, Colon Cancer, Rectal Cancer
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide, and the second leading cause of cancer-related death in the United States (1,2). Alcohol consumption is an established risk factor for CRC (3–12). Several mechanisms have been suggested for the association between alcohol consumption and risk of developing CRC, including the metabolism of alcohol to acetaldehyde, the carcinogenic effect of nitrosamines, increased degradation of folate, modulation of folate uptake, mucosal and DNA damage, and modulation of gene expression (4,10,13–15).
While studies have consistently reported elevated risk of CRC among heavy drinkers, associations at light and moderate levels of alcohol consumption are less clear. Both cohort and case control studies have identified a J-shaped association between alcohol consumption and CRC, where moderate alcohol consumption is protective compared to no alcohol consumption (16–18). However, other studies have reported a positive dose-response relationship (5,9–11,19) or non-significant positive associations at moderate levels of alcohol consumption (3,6).
Several other risk factors for CRC have been identified, including smoking, obesity, high consumption of red meat, physical inactivity, and family history of CRC (12,20–23). A few studies have observed the association between alcohol consumption and CRC to differ by other factors including sex (7,12,17,18), family history of CRC (3), and obesity (6,24), but further research in statistically well-powered study settings is needed to explore potential interactions between alcohol consumption and other CRC risk factors.
We conducted a meta-analysis of 16 studies of CRC to examine the shape of the alcohol-CRC dose response association, identify potential effect modifiers of the association between alcohol consumption and CRC, and examine effects of alcohol consumption by CRC anatomic site and disease stage.
Materials and Methods
Study design and data collection
We conducted a meta-analysis of five case-control studies and 11 case-control studies nested within prospective cohorts. Information on basic demographics, lifestyle, and environmental risk factors was collected by self-report using in-person interviews and/or self-administered structured questionnaires. We used risk factor information at the reference time, which was defined as study entry or blood collection for nested case-control studies and 1–2 years before diagnosis for case-control studies to ensure exposures were assessed before cancer diagnoses.
Individual level data from all studies were centrally harmonized. We carried out a multi-step data harmonization procedure, reconciling each study’s unique protocols and data-collection instruments (25). First, common data elements (CDEs) were defined for key demographic and environmental risk factors. Next, questionnaires and data dictionaries from each study were examined to identify study-specific data elements that could be mapped to the CDEs. Through an iterative process, we communicated with each data contributor to obtain relevant data and coding information. The data elements were written to a common data platform, transformed, and combined into a single dataset with common definitions, standardized permissible values, and standardized coding (25). The mapping and resulting data were reviewed for quality assurance, and range and logic checks were performed to assess data and data distributions within and between studies. Outlying measures were truncated to the minimum or maximum value of the established range for each variable.
Study Subjects
Case participants were diagnosed with invasive CRC. Appendix or non-invasive (stage 0 or in situ) CRC cases were excluded. Control participants were required to be free of invasive CRC and non-invasive CRC at the time of selection into the study. Case and control participants were excluded if they had prior history of CRC at baseline. CRC cases were confirmed by medical records, pathologic reports, or death certificates. Participants provided consent for participation in each of the included studies. The overall project was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Alcohol consumption and key covariates
The primary exposure was alcohol consumption during the referent time, measured in grams of alcohol per day (g/day). As part of the data harmonization process, we converted consumption of alcoholic beverages into grams of alcohol per day by summing the alcohol content of each type of beverage consumed per day. When studies reported alcohol consumption in terms of servings, we converted this assuming an alcohol content of 14 g/serving (6,16,26).
We considered the following potential confounders, measured at reference time: age, race, sex, educational attainment (less than high school graduate, high school graduate or GED, some college or technical school, college graduate or graduate school), body mass index (BMI: kg/m2), physical activity (≥1 hour/week of vigorous/moderate physical activity vs <1 hour/week), smoking (never vs ever smoker and pack-years), regular use of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs), regular use of post-menopausal hormone therapy (PMH) (collected only for post-menopausal women), red/processed meat intake, fruit and vegetable intake, folate intake (including dietary and supplements), calcium intake (including dietary and supplements), dietary fiber intake, screening history (history of sigmoidoscopy or colonoscopy), family history of CRC in first-degree relatives, and history of diabetes. BMI was derived using pre-diagnosis (cases) or referent (controls) weight and height and analyzed as both a continuous and categorical variable [underweight or normal (<24.9), overweight (25–30), obese (>=30)]. Smoking pack-years were calculated for former or current smokers by multiplying the average number of cigarettes per day by the number of years smoked and dividing by 20 (number of cigarettes in a pack). Pack-years and all dietary variables were collapsed into study- and sex-specific quartiles for analysis. These covariates were identified as potential confounders because prior research has shown that they are potentially associated with both alcohol consumption and CRC. To evaluate confounding within our study we conducted chi-square, ANOVA, and t-tests to test for associations between each potential confounder and alcohol consumption among study controls and for associations between each potential confounder and CRC risk among those who reported no alcohol consumption. Covariates that were significantly associated (p<0.05) with both exposure and outcome were adjusted for in the final models as confounders.
Missing data
Subjects with missing data on alcohol consumption (n=2,375) were excluded from the analysis. A sensitivity analysis was conducted to evaluate the impact of high levels of missing data in alcohol consumption on effect estimates. A meta-analysis of only those studies that were missing alcohol information on less than 10% of participants (11 studies) produced similar effect estimates and inference as the full meta-analysis with all 16 studies. Cases missing information on cancer anatomic site or cancer stage were excluded from the respective stratified analyses.
The influence of missing data for key covariates was evaluated separately for each study. Covariates that were missing for more than 25% of subjects were excluded from the model for that study.
Single regression imputation was used to impute values for potential effect modifiers to ensure consistency across imputations for calculating stratified effect estimates. Study-specific single imputation models included alcohol consumption, case/control status, and any identified confounders (age, sex, education, BMI, smoking, aspirin, NSAIDs, red/processed meat intake, fruit and vegetable intake, folate intake, calcium intake, screening history, and history of diabetes) that were available for all study subjects. We then used multiple imputation by chained equations to impute values for all other covariates that were missing for less than 25% of subjects (27). Alcohol consumption, case/control status, and all identified confounders were included in the multiple imputation models. Imputation and data analysis were performed using STATA 14.2.
Analysis
Modeling the shape of the alcohol-CRC dose-response association
To evaluate the association between alcohol and CRC we used minimally adjusted (sex and age) logistic regression to model alcohol as a continuous variable and explored the use of splines and higher-level terms. We created linear splines at intervals of 14 g/day (1–14 g/day, 15–28 g/day, 29–42 g/day, etc.), based on the definition of a standard drink as equaling 14 grams of alcohol (6,16,26). We also created splines at the 25th, 50th, and 75th percentiles of alcohol consumption among subjects with any alcohol consumption. We sequentially added linear splines to the model and also modeled restricted cubic splines with varying numbers of nodes. We used Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to compare each model and selected the models that minimized AIC and BIC. We then created categories of alcohol consumption based on the splines from the models with the lowest AICs and BICs and selected the categorical model that minimized AIC and BIC.
Each study was analyzed separately using multivariable logistic regression models. A Cochran’s Q test was used to evaluate heterogeneity between studies. Study-specific results were combined using fixed-effects meta-analysis methods to obtain summary odds ratios (ORs) and 95% confidence intervals (CIs) across studies. We also conducted separate meta-analyses for case-control and cohort studies.
Evaluation of effect modification
We assessed the following CRC risk factors as potential modifiers of the alcohol-CRC association, identified from the literature: age, sex, BMI, smoking, and family history of CRC. Multiplicative interaction terms were included in the study-specific logistic regression models and a joint test of coefficients was performed to test if the interaction coefficients were equal to 0. Study-specific p-values from the joint test of coefficients test were pooled using Fisher’s combination method to evaluate overall interaction (28). Models were adjusted for any potential confounders that were not observed to modify the association between alcohol and CRC.
Differential effects by CRC anatomic site and cancer stage
Polytomous logistic regression adjusted for confounders was used to evaluate the alcohol-CRC association by cancer anatomic site and by cancer stage. Study-specific results were combined using fixed-effects meta-analysis. Models were adjusted for identified confounders, and stratified ORs and 95% CIs were reported for each anatomic site and cancer stage. Interaction between alcohol and anatomic site was evaluated by conducting a case-only analysis comparing proximal colon cancer (ICD-9: 153.0, 153.1,153.4, 153.6) to distal colon (ICD-9: 153.2, 153.3, 153.7) or rectal cancers (ICD-9: 154.0, 154.1). A joint test of coefficients was performed to test if the coefficients for each level of alcohol consumption were equal to 0, and p-values were pooled across studies using Fisher’s combination method.
Results
Basic characteristics of the participants are shown in Table 1. We were able to harmonize measures of alcohol consumption for 14,276 cases and 15,802 controls. Overall, 47% of participants reported light or moderate alcohol consumption (1.1–28 g/day or up to 2 drinks/day), 6% of participants reported heavy alcohol consumption (28.1–42 g/day or 2–3 drinks/day), 6% reported very heavy alcohol consumption (>42 g/day or more than 3 drinks/day), and 41% reported no alcohol consumption (≤1 g/day). The average alcohol consumption for subjects reporting any alcohol consumption was 10.5 g/day.
Table 1:
Characteristics of Study Population
| Alcohol consumption | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Female | Non/ occasional drinkers (≤1 g/day) |
Light/ moderate drinkers (1.1– 28 g/day) |
Heavy drinkers (28.1–42 g/day) |
Very heavy drinkers (>42 g/day) |
Alcohol consumption (g/day) among drinkers |
||||||||||||
| Study | Study Design |
Country | Cases | Controls | Total | Mean | SD | % | N | % | N | % | N | % | N | % | M | SD |
| ATBC | Cohort | Finland | 221 | 141 | 362 | 57.6 | 4.7 | 0 | 52 | 14.4 | 228 | 63.0 | 40 | 11.1 | 42 | 11.6 | 18.3 | 20.2 |
| COLO2&3 | Case-control | US | 94 | 129 | 223 | 65.0 | 11.3 | 43.5 | 96 | 43.1 | 98 | 44.0 | 16 | 7.2 | 13 | 5.8 | 12.4 | 21.6 |
| CPS-II | Cohort | US | 2,252 | 2,356 | 4,608 | 67.9 | 5.9 | 48.1 | 2,016 | 43.8 | 2,179 | 47.3 | 230 | 5.0 | 183 | 3.97 | 8.3 | 14.9 |
| DACHS | Case-control | Germany | 2,879 | 2,325 | 5,204 | 68.7 | 10.4 | 39.6 | 1,500 | 28.8 | 2,773 | 53.3 | 383 | 7.4 | 548 | 10.5 | 15.3 | 20.6 |
| DALS | Case-control | US | 1,453 | 1,475 | 2,928 | 63.7 | 9.9 | 43.9 | 1,524 | 52.1 | 1031 | 35.2 | 164 | 5.6 | 209 | 7.1 | 11.0 | 22.7 |
| HPFS | Cohort | US | 828 | 1,149 | 1,977 | 64.3 | 8.8 | 0 | 498 | 25.2 | 1187 | 60.0 | 163 | 8.2 | 129 | 6.5 | 12.5 | 15.8 |
| Kentucky | Case-control | US | 905 | 1,052 | 1,957 | 62.8 | 9.4 | 51.6 | 1,364 | 69.7 | 508 | 26.0 | 30 | 1.5 | 55 | 2.8 | 4.6 | 13.5 |
| MCCS | Cohort | Australia | 780 | 699 | 1,479 | 59.5 | 7.6 | 47.9 | 537 | 36.3 | 638 | 43.1 | 156 | 10.6 | 148 | 10.0 | 15.0 | 21.1 |
| MEC | Cohort | US | 345 | 355 | 700 | 63.0 | 8.0 | 48.0 | 268 | 38.3 | 284 | 40.6 | 62 | 8.9 | 86 | 12.3 | 17.6 | 30.9 |
| NFCCS | Case-control | Canada | 319 | 686 | 1,005 | 59.3 | 9.5 | 28.5 | 452 | 45.0 | 431 | 42.9 | 55 | 5.5 | 67 | 6.7 | 11.6 | 22.6 |
| NHS | Cohort | US | 1,107 | 1,685 | 2,792 | 58.7 | 6.7 | 100 | 1,341 | 48.0 | 1,313 | 47.0 | 106 | 3.8 | 32 | 1.2 | 5.4 | 9.7 |
| PLCO | Cohort | US | 434 | 684 | 1,118 | 64.5 | 5.1 | 39.2 | 468 | 41.9 | 491 | 43.9 | 43 | 3.9 | 116 | 10.4 | 13.0 | 24.7 |
| SELECT | Cohort | US | 308 | 308 | 616 | 65.3 | 6.7 | 0 | 221 | 35.9 | 304 | 49.4 | 38 | 6.2 | 53 | 8.6 | 13.1 | 22.6 |
| SMC & COSM | Cohort | Sweden | 566 | 868 | 1,434 | 63.2 | 8.2 | 39.1 | 142 | 9.9 | 1189 | 82.9 | 65 | 4.5 | 38 | 2.7 | 11.1 | 13.0 |
| VITAL | Cohort | US | 358 | 363 | 721 | 66.3 | 6.3 | 45.1 | 316 | 43.8 | 321 | 44.5 | 46 | 6.4 | 38 | 5.3 | 9.8 | 17.5 |
| WHI | Cohort | US | 1,427 | 1,527 | 2,954 | 66.4 | 6.6 | 100 | 1,636 | 55.4 | 1178 | 39.9 | 97 | 3.3 | 43 | 1.5 | 5.3 | 10.3 |
| Total | 14,276 | 15,802 | 30,078 | 64.7 | 8.9 | 50.1 | 12,431 | 41.3 | 14,153 | 47.1 | 1,694 | 5.6 | 1,800 | 6.0 | 10.5 | 18.4 | ||
We observed a J-shaped association between alcohol consumption and CRC risk across multiple spline and restricted cubic-spline models (Figure 1). In each model, the slope of the first spline was negative, indicating a negative association between alcohol consumption and log-odds of CRC at lower levels of alcohol consumption. Subsequent splines had positive slopes, representing an increase in the log-odds of CRC as alcohol consumption increases past light/moderate consumption levels. The categorical model that minimized AIC and BIC had four categories of alcohol consumption: non-/occasional drinkers (≤1 g/day), light/moderate drinkers (1.1–28 g/day or up to 2 drinks/day), heavy drinkers (28.1–42 g/day or 2–3 drinks/day), and very heavy drinkers (>42 g/day or more than 3 drinks/day). This model also had smaller AIC (41,510) and BIC (41,576) compared to the corresponding linear spline model (AIC: 41,528; BIC: 41,577) and the model that included the continuous variable as a single linear term (AIC: 41,549; BIC: 41,583).
Figure 1:
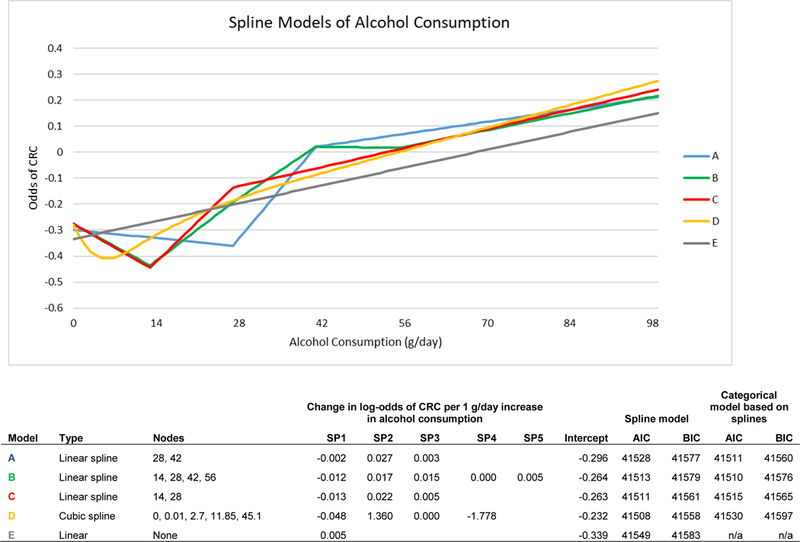
Spline Models of Alcohol Consumption
Using fixed-effects meta-analysis, compared to non-/occasional drinkers, light/moderate alcohol consumption was associated with a decreased risk of CRC (OR: 0.92, 95% CI: 0.88–0.98, p=0.005) (Figure 2A) while very heavy alcohol consumption was associated with an increased risk of CRC (OR: 1.25, 95% CI: 1.11–1.40, P<0.001) (Figure 2C). We observed much weaker evidence of increased risk associated with heavy alcohol consumption (OR: 1.11, 95% CI: 0.99–1.24, p=0.077), compared to non-/occasional drinkers (Figure 2B). A sub-analysis within the light/moderate alcohol consumption group found similar effect estimates across each of four subgroups. Similar effect estimates were also observed in separate sub-analyses of case-control and nested case-control studies (Table 2). There was no evidence of heterogeneity across studies at any of the levels of alcohol consumption.
Figure 2A. Light/moderate (1.1–28 g/day) vs Non/occasional Alcohol Consumption.
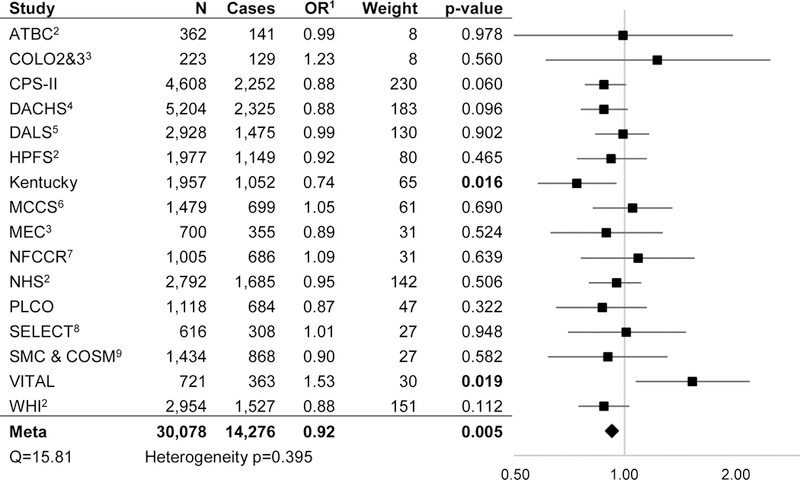
1 All analyses adjusted for sex; age; education; smoking; aspirin; NSAIDS; history of endoscopy; diabetes; BMI; and folate,calcium, fruit, vegetable, processed meat, and red meat intake unless otherwise noted. 2 Adjusted for age; education; smoking; aspirin; NSAIDS; history of endoscopy; diabetes; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake. 3 Adjusted for sex; age; education; smoking; aspirin; NSAIDS; diabetes; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake. 4 Adjusted for sex; age; education; smoking; aspirin; NSAIDS; history of endoscopy; diabetes; BMI; and calcium, fruit, vegetable, processed meat, and red meat intake. 5 Adjusted for sex; age; education; smoking; aspirin; NSAIDS; history of endoscopy; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake. 6 Adjusted for sex; age; education; smoking; history of endoscopy; diabetes; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake. 7 Adjusted for sex; age; education; smoking; aspirin; NSAIDS; history of endoscopy; diabetes; BMI; and folate, calcium, fruit, vegetable, and red meat intake. 8 Adjusted for age; education; smoking; aspirin; NSAIDS; diabetes; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake. 9 Adjusted for sex; age; education; smoking; diabetes; BMI; and folate, calcium, fruit, vegetable, processed meat, and red meat intake
Figure 2C.
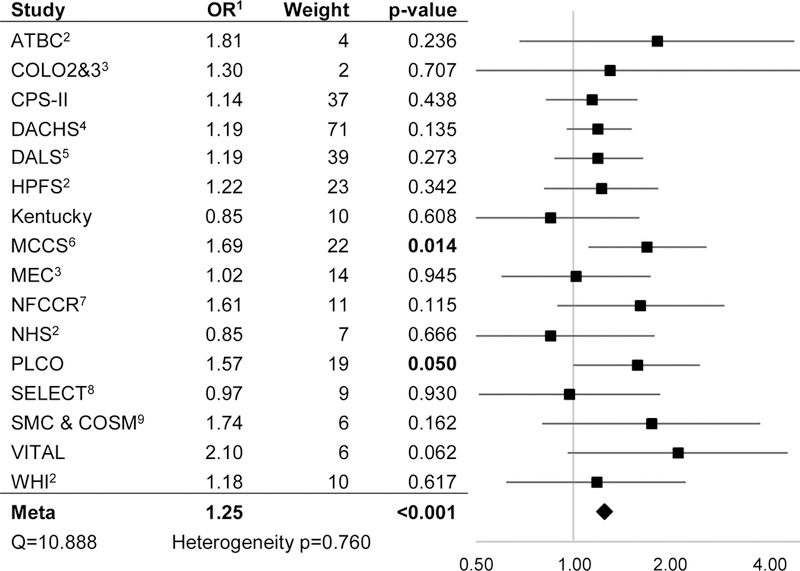
Very heavy (>42 g/day) vs Non/occasional Alcohol Consumption
Figure 2B.
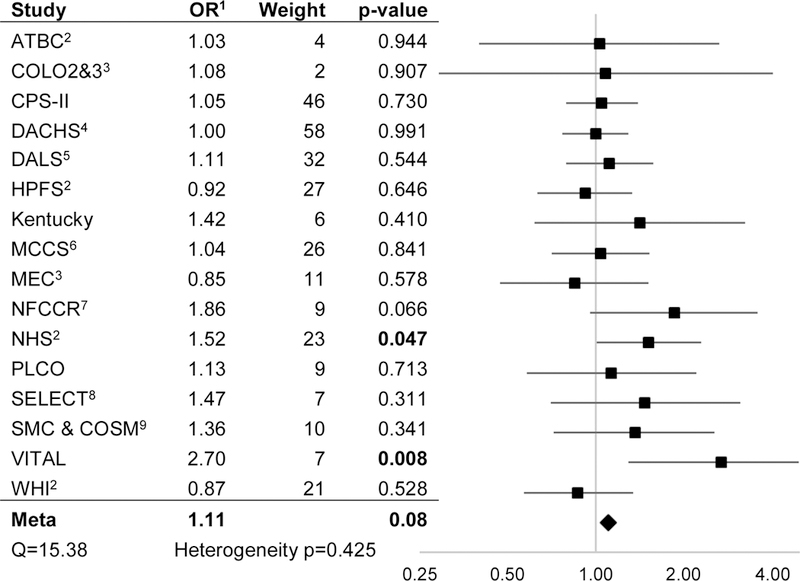
Heavy (28.1–42 g/day) vs Non/occasional Alcohol Consumption
Table 2:
Stratified Odds Ratios for Alcohol Consumption and CRC by Key Covariates1
| Alcohol consumption | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light/moderate (1.1–28 g/day) vs non/occasional |
Heavy (28.1–42 g/day) vs non/occasional |
Very heavy (>42 g/day) vs non/occasional |
Interaction p-value |
|||||||||||
| Meta Analyses | N | OR2 | 95% CI | p-value | OR2 | 95% CI | p-value | OR2 | 95% CI | p-value | ||||
| Overall | 30,078 | 0.92 | 0.88 | 0.98 | 0.005 | 1.11 | 0.99 | 1.24 | 0.077 | 1.25 | 1.11 | 1.40 | <0.001 | |
| Study Design | ||||||||||||||
| Case Control | 11,317 | 0.91 | 0.83 | 1.00 | 0.056 | 1.11 | 0.92 | 1.34 | 0.29 | 1.20 | 1.01 | 1.42 | 0.04 | |
| Cohort | 18,761 | 0.93 | 0.87 | 1.00 | 0.04 | 1.11 | 0.96 | 1.28 | 0.157 | 1.29 | 1.11 | 1.51 | 0.001 | |
| Sex | 0.204 | |||||||||||||
| Female | 15,067 | 0.88 | 0.82 | 0.95 | 0.001 | 1.12 | 0.91 | 1.37 | 0.289 | 1.01 | 0.77 | 1.32 | 0.967 | |
| Male | 15,011 | 0.96 | 0.88 | 1.05 | 0.351 | 1.09 | 0.94 | 1.26 | 0.243 | 1.32 | 1.15 | 1.51 | <0.001 | |
| BMI | 0.736 | |||||||||||||
| Normal or Underweight | 11,254 | 0.88 | 0.80 | 0.96 | 0.005 | 1.11 | 0.91 | 1.34 | 0.301 | 1.13 | 0.91 | 1.40 | 0.275 | |
| Overweight | 12,876 | 0.89 | 0.81 | 0.97 | 0.007 | 1.06 | 0.89 | 1.26 | 0.18 | 1.25 | 1.05 | 1.49 | 0.011 | |
| Obese | 5,928 | 1.07 | 0.93 | 1.22 | 0.366 | 1.04 | 0.76 | 1.43 | 0.812 | 1.38 | 1.04 | 1.83 | 0.025 | |
| Cancer Site | 0.093 | |||||||||||||
| Proximal | 5,562 | 0.94 | 0.87 | 1.01 | 0.094 | 1.05 | 0.90 | 1.23 | 0.511 | 1.13 | 0.96 | 1.32 | 0.145 | |
| Distal | 4,000 | 0.91 | 0.83 | 0.99 | 0.026 | 1.19 | 1.01 | 1.41 | 0.004 | 1.39 | 1.18 | 1.64 | <0.001 | |
| Rectal | 3,109 | 0.91 | 0.82 | 1.01 | 0.063 | 1.17 | 0.97 | 1.41 | 0.099 | 1.46 | 1.22 | 1.76 | <0.001 | |
See footnotes in Figure 2 for adjustment factors
All estimates calculated by fixed effect meta-analysis
Analyses were also conducted separately for men and women. Light/moderate alcohol consumption was associated with decreased risk of CRC compared to non-/occasional consumption among both sexes, although the effect estimate was greater for women (OR: 0.88, 95% CI: 0.82–0.95, p=0.001) than men (OR: 0.96, 95% CI: 0.88, 1.05, p=0.351) (Table 2). Very heavy alcohol consumption was only associated with increased risk of CRC among men (OR 1.32, 95% CI: 1.15–1.51, p<0.001), however there was no evidence of effect modification by sex (interaction p=0.204).
Associations with low levels of alcohol consumption differed slightly when stratified by BMI. Compared with non-/occasional consumption, light/moderate alcohol consumption was not associated with reduced risk among obese individuals (OR: 1.07, 95% CI: 0.93–1.22, p=0.366). However, there was no evidence overall of effect modification by BMI (interaction p=0.736). Additionally, age, smoking (evaluated as ever vs never and pack-years), and family history of CRC were not found to modify the alcohol-CRC association.
Similar protective associations for light/moderate alcohol consumption were observed across proximal colon, distal colon, and rectal cancer sites (Table 2). Heavy alcohol consumption, however, was associated with increased risk of distal colon (OR: 1.19, 95% CI: 1.01–1.45, p=0.004), with a consistent result observed for rectal cancer (OR: 1.17, 95% CI: 0.97–1.41, p=0.099), but not proximal colon cancer (OR: 1.05, 95% CI: 0.90–1.23, p=0.511). Very heavy alcohol consumption was associated with increased risk across all cancer sites. A J-shaped dose-response association was also observed when analyses were stratified by cancer stage. Stronger associations were observed between very heavy alcohol consumption and stage 1 and stage 4 cancers, but overall associations did not differ substantially by cancer stage.
Discussion
In this large meta-analysis, we observed a J-shaped dose-response pattern of association between alcohol consumption and CRC risk. Compared to non-/occasional drinking, light to moderate alcohol consumption (up to 2 drinks/day) was inversely associated with CRC risk, while very heavy consumption (more than 3 drinks/day) was associated with greater risk. While effect estimates varied slightly according to participant sex, BMI, and tumor site, there was no evidence of interaction or of heterogeneity by tumor site. The J-shaped association was consistently observed among both case-control and nested case-control studies.
Alcohol is an established risk factor for CRC and heavy alcohol consumption has been shown to be associated with increased risk of CRC (3,6,8,10,12,16,17,19). Findings are inconsistent for lower levels of alcohol consumption, with some studies reporting minimal or nonsignificant associations (3,6,10,19) and others reporting a reduced risk of CRC for low or moderate drinkers (16,18). We observed a protective association with light/moderate alcohol consumption (up to 2 drinks/day) compared to non- or occasional consumption. A sub-analysis of four subgroups within the light/moderate alcohol consumption group gave similar effect estimates across each of the subgroups, indicating that the observed protective effect is consistent within the low/moderate level of alcohol consumption. Both linear and restricted spline models with nodes at varying cut-points were consistent with a J-shaped association, further supporting this finding. A 2014 meta-analysis of nine studies also reported a J-shaped dose-response relationship between alcohol and CRC mortality, indicating that trends in CRC risk may also be reflected in CRC mortality (17).
The mechanisms by which alcohol consumption influences CRC risk are still being explored (13,14,29). The breakdown of alcohol to acetaldehyde, a carcinogen that has been shown to disrupt DNA replication and repair, is widely believed to play a role in CRC risk (14,30,31). Chronic alcohol consumption has also been shown to disrupt folate metabolism, and folate deficiency is an established risk factor for CRC (8,13,15,30,32). Alcohol consumption can also interfere with the breakdown and absorption of other nutrients, and alcohol metabolism generates reactive oxygen species which can damage DNA, proteins, and lipids (30). Moderate alcohol empirically lowers inflammatory markers and C-peptide (33–35), and a short-term randomized clinical trial observed a benefit of alcohol on insulin parameters (36). A 2017 study of the effects of moderate alcohol consumption in rats observed that moderate levels of alcohol intake did not elevate biological risk factors for CRC development and may provide beneficial effects through reduced inflammation and lower DNA damage (29). As we cannot rule out that the inverse association may be explained by residual confounding or chance a better understanding of the underlying mechanism is necessary to interpret observed protective effects of light/moderate alcohol consumption and inform CRC prevention recommendations.
It has been suggested that the association between alcohol and colorectal cancer may differ by sex, in part due to differences in alcohol metabolism between men and women (37). Although we did not observe sex to significantly modify the association between alcohol and CRC, we did observe some differences in estimated effect sizes. In particular, the protective effect associated with light/moderate alcohol intake was observed among both sexes, this association was most pronounced among women, and the positive association with very heavy alcohol consumption was restricted to men. The potential benefit of moderate alcohol consumption on insulin parameters has been observed to be stronger in women than men (38), which could contribute to the stronger protective effect for CRC observed among women in our analysis. The observed increased risk of CRC associated with very heavy alcohol consumption among men is consistent with other studies that have reported increased risk with heavy drinking among men but not among women (7,18). Studies of the effect of higher levels of alcohol consumption among women are often limited due to the low prevalence of heavy alcohol consumption among women. For example, less than 2% of women (n=275) in our analysis reported very heavy alcohol consumption, compared to over 10% (n=1,525) of men, which limited our power to detect effect modification or identify associations with high levels of alcohol consumption among women.
Few studies have evaluated the alcohol-CRC association by cancer anatomic site. A large-scale cohort study in a Korean population observed that frequent alcohol consumption was associated with increased risk of distal colon cancer but not with proximal colon or rectal cancer (7). A pooled analysis of eight cohort studies from North America and Europe observed elevated risk associated with increased alcohol intake across proximal colon, distal colon, and rectal cancers (10). The highest level of alcohol consumption (>=45 g/day) was associated with a higher risk of distal colon cancer (RR: 1.66, 95% CI: 1.17–2.36) and rectal cancer (RR: 1.49, 95% CI: 1.04–2.12) compared to proximal colon cancer (RR: 1.35, 95% CI: 0.97–1.89) (10). We observed a similar trend, where heavy and very heavy alcohol consumption were associated with a higher risk of distal colon cancer and rectal cancer, compared to proximal colon cancer, however there was no evidence of significant heterogeneity by cancer site. There is some evidence of biological and clinical differences between proximal and distal colon cancer. For example the BRAF mutation and microsatellite instability-high (MSI-high) phenotype have been observed more frequently in proximal colon tumors compared to distal colon tumors (39,40). A case-unaffected sibling study observed that alcohol consumption was associated with increased risk of CRC tumors characterized by MSI-low phenotype but not MSI-high phenotype (41). These findings suggest that etiological pathways may vary by cancer anatomic site and that the role of alcohol may differ by pathway. Further evaluation of alcohol risk by site and tumor characteristics may provide additional insight regarding the biological mechanisms through which alcohol affects CRC risk, and provide more precise estimates of risk for specific anatomic sites.
We are mindful of limitations in our study. Each contributing study used a different questionnaire to obtain information on alcohol consumption and other key covariates. While our centralized data harmonization process improved consistency in measuring these variables across studies, our data are still subject to recall bias, measurement error, and misclassification. Additionally, because we analyzed current alcohol consumption rather than lifetime consumption, former drinkers were included with never drinkers in the referent category of non-/occasional drinkers. There is some evidence that longer duration of alcohol consumption is associated with elevated CRC risk and that past drinkers have a higher risk of CRC compared to non-drinkers (7,42). Thus, inclusion of former drinkers in the referent category could attenuate observed associations and contribute to the observed protective effect associated with light/moderate alcohol consumption. Reassuringly, a pooled analysis of four cohort studies observed a similar J-shaped alcohol-CRC association when former drinkers were included and excluded from the non-drinker reference group (10).
Overall the majority of key covariates were missing for less than 5% of subjects. However there were a few variables that were not collected by certain studies or were only collected for subsets of the study sample. We evaluated the alcohol-CRC association separately within each study and adjusted for all available known and suspected environmental and lifestyle risk factors as potential confounders, thus estimates from some studies may be subject to additional bias due to other confounders, such as screening. However, some large-scale studies have examined the association between alcohol consumption and CRC screening showing opposite results (43,44), suggesting that it is less likely that screening is a confounder. It should also be noted that adjusting for screening is not without problems that cannot be easily addressed. For example, adjusting for screening could remove at least part of the effect of alcohol by removing the adenoma precursor and truncating the disease process, and also lowers the absolute risk of CRC by removing precursors. Finally, the study sample was racially homogenous with over 98% of participants reporting white race; thus, study findings may not be generalizable to more diverse populations.
Our study also had several strengths, including a large sample size and availability of key environmental exposure and other risk factor variables across its contributing studies. This enabled us to adjust for multiple key confounders across studies. Additionally, because we had access to individual- level data from all studies and could select which variables to include in the study-specific multivariate analyses, this allowed for the use of much more consistent models than are typically available for a meta-analysis.
Additionally, a standardized harmonization of data across studies was used to reconcile each study’s unique protocols and data collection instruments. This rigorous, multi-step process contributed to improved data quality and more consistent measures across studies. The use of study- and sex-specific quartiles for smoking (pack-years) and dietary variables also supported improved comparability across studies. There was no evidence of heterogeneity of the alcohol-CRC association across studies, which indicates that our results were not dominated by one or a few studies.
Finally, there are no standard definitions of light, moderate, or heavy alcohol intake that are used consistently in CRC literature. A strength of this study is that the cut-points we selected are based on individual level data that minimized AIC and BIC, reflect similar ranges to those reported in the literature, and are practically applicable as they can be easily interpreted as a number of drinks per day (light/moderate: up to 2 drinks/day, heavy: 2–3 drinks/day, very heavy: >3 drinks/day).
Improving our understanding of the association between alcohol and colorectal cancer is particularly important because alcohol consumption is a modifiable behavioral risk factor that has the potential to be influenced by medical recommendations or behavioral interventions. Our findings indicate that light/moderate drinkers may have a reduced risk of CRC compared to non-/occasional drinkers, but that heavier drinkers are at increased risk. These findings can inform future studies of the mechanisms through which alcohol affects CRC risk.
Novelty and Impact:
While heavy alcohol consumption is an established risk factor for colorectal cancer (CRC) (3–12), associations at moderate levels of consumption are unclear. Studies report a linear relationship, reduced risk for low/moderate drinkers, and a J-shaped association between alcohol and CRC. In this analysis utilizing data from 16 studies, we evaluated linear and cubic spline models of the alcohol-CRC association. We observed protective effects for light/moderate alcohol consumption (≤2 drinks/day), supporting a J-shaped association.
Acknowledgements
The studies included in this analysis are part of the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) and Colorectal Transdisciplinary (CORECT) Study. GECCO is supported by the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (U01 CA137088; R01 CA059045; R01 CA120582). The authors would like to thank all those at the GECCO Coordinating Center for helping bring together the data and people that made this project possible. The authors also acknowledge Deanna Stelling, Mark Thornquist, Greg Warnick, Carolyn Hutter, and team members at COMPASS (Comprehensive Center for the Advancement of Scientific Strategies) at the Fred Hutchinson Cancer Research Center for their work harmonizing the GECCO epidemiological data set. The Colorectal Transdisciplinary (CORECT) Study is supported by the National Cancer Institute, National Institutes of Health under RFA # CA-09–002 (U19 CA148107). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in CORECT, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or CORECT.
ATBC: The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, NIH, and by U.S. Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
COLO2&3: This work is supported by the National Institutes of Health (R01 CA60987).
CPS-II: The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II (CPS-II) cohort. This study was conducted with Institutional Review Board approval. The authors thank the CPS-II participants and Study Management Group for their invaluable contributions to this research. The authors would also like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention National Program of Cancer Registries, and cancer registries supported by the National Cancer Institute Surveillance Epidemiology and End Results program.
DACHS: This work is supported by the German Research Council (Deutsche Forschungsgemeinschaft, BR 1704/6–1, BR 1704/6–3, BR 1704/6–4, BR 1704/6–6 and CH 117/1–1), and the German Federal Ministry of Education and Research (01KH0404, 01ER0814 and 01GL1712). We thank all participants and cooperating clinicians, and Ute Handte-Daub, Utz Benscheid, Muhabbet Celik and Ursula Eilber for excellent technical assistance.
DALS: This work is supported by the National Institutes of Health (R01 CA48998 to M. L. Slattery).
HPFS and NHS: HPFS is supported by the National Institutes of Health (P01 CA055075, UM1 CA167552, U01 CA167552, R01 CA137178, R01 CA151993, R35 CA197735, K07 CA190673, and P50 CA127003). NHS is supported by the National Institutes of Health (R01 CA137178, P01 CA087969, UM1 CA186107, R01 CA137178, R01 CA151993, R35 CA197735, K07 CA190673, and P50 CA127003). We would like to acknowledge Qin (Carolyn) Guo and Lixue Zhu who assisted in programming for NHS and HPFS. We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Kentucky: This work was supported by the following grant support: 1) Clinical Investigator Award from Damon Runyon Cancer Research Foundation (CI-8) and 2) NCI R01CA136726; and, we would like to acknowledge the staff at the Kentucky Cancer Registry
MCCS: The Melbourne Collaborative Cohort Study (MCCS) cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further supported by Australian National Health and Medical Research Council grants 209057, 396414 and 1074383 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database. The MCCS was made possible by the contribution of many people, including the original investigators, the teams that recruited the participants and continue working on follow-up, and the many thousands of Melbourne residents who continue to participate in the study.
MEC: This work is supported by the National Institutes of Health (U01 CA164973).
NFCCR: This work was supported by an Interdisciplinary Health Research Team award from the Canadian Institutes of Health Research (CRT 43821); the National Institutes of Health, U.S. Department of Health and Human Services (U01 CA74783); and National Cancer Institute of Canada grants (18223 and 18226). Funding was provided to Michael O. Woods by the Canadian Cancer Society Research Institute.
PLCO: PLCO is supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff or the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O’Brien and staff, Westat, Inc., and Drs. Bill Kopp and staff, SAIC-Frederick. Most importantly, we acknowledge the study participants for their contributions to making this study possible. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
SELECT: SELECT is funded by Public Health Service grants U10CA37429 and 5UM1CA182883 from the National Cancer Institute. We thank the 35,533 men and many principal investigators and clinical research associates at our 427 clinical sites, whose participation in SELECT has written an important chapter in the history of cancer prevention. We also thank the many personnel of the Southwest Oncology Group (the coordinating group of this Intergroup trial), whose tireless efforts allowed SELECT to successfully complete the test of its primary hypotheses.
SMC & COSM: Swedish Mammography Cohort (SCM) and Cohort of Swedish Men (COSM). This work is supported by the Swedish Research Council /Infrastructure grant, the Swedish Cancer Foundation, and the Karolinska Institutés Distinguished Professor Award to Alicja Wolk.
VITAL: This work is supported by the National Institutes of Health (K05 CA154337).
WHI: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Abbreviations:
- AIC
Akaike Information Criterion
- BIC
Bayesian Information Criterion
- BMI
body mass index
- CDE
common data element
- CI
confidence interval
- CORECT
Colorectal Transdisciplinary
- CRC
colorectal cancer
- GECCO
Genetics and Epidemiology of Colorectal Cancer Consortium
- MSI
microsatellite instability
- NSAIDS
nonsteroidal anti-inflammatory drugs
- OR
odds ratio
- PMH
post-menopausal hormone
- RR
relative risk
References
- 1.Centers for Disease Control and Prevention. CDC - Colorectal Cancer Statistics [Internet]. 2016. [cited 2017 Feb 9]. Available from: https://www.cdc.gov/cancer/colorectal/statistics/
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015. March 1;136(5):E359–386. [DOI] [PubMed] [Google Scholar]
- 3.Cho E, Lee JE, Rimm EB, Fuchs CS, Giovannucci EL. Alcohol consumption and the risk of colon cancer by family history of colorectal cancer1234. Am J Clin Nutr. 2012. February;95(2):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crous-Bou M, Rennert G, Cuadras D, Salazar R, Cordero D, Saltz Rennert H, et al. Polymorphisms in Alcohol Metabolism Genes ADH1B and ALDH2, Alcohol Consumption and Colorectal Cancer. PLoS ONE [Internet]. 2013. November 25 [cited 2017 Feb 7];8(11). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3839967/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasekara H, MacInnis RJ, Room R, English DR. Long-Term Alcohol Consumption and Breast, Upper Aero-Digestive Tract and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis. Alcohol Alcohol Oxf Oxfs. 2016. May;51(3):315–30. [DOI] [PubMed] [Google Scholar]
- 6.Klarich DS, Brasser SM, Hong MY. Moderate Alcohol Consumption and Colorectal Cancer Risk. Alcohol Clin Exp Res. 2015. August;39(8):1280–91. [DOI] [PubMed] [Google Scholar]
- 7.Cho S, Shin A, Park SK, Shin H-R, Chang S-H, Yoo K-Y. Alcohol Drinking, Cigarette Smoking and Risk of Colorectal Cancer in the Korean Multi-center Cancer Cohort. J Cancer Prev. 2015. June;20(2):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Cho YA, Kim D-H, Lee B-H, Hwang D-Y, Jeong J, et al. Dietary intake of folate and alcohol, MTHFR C677T polymorphism, and colorectal cancer risk in Korea. Am J Clin Nutr. 2012. February 1;95(2):405–12. [DOI] [PubMed] [Google Scholar]
- 9.Gao C-M, Ding J-H, Li S-P, Liu Y-T, Cao H-X, Wu J-Z, et al. Polymorphisms in XRCC1 gene, alcohol drinking, and risk of colorectal cancer: a case-control study in Jiangsu Province of China. Asian Pac J Cancer Prev APJCP. 2014. January;14(11):6613–8. [DOI] [PubMed] [Google Scholar]
- 10.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004. April 20;140(8):603–13. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Duan H, Yang H, Lin J. A pooled analysis of alcohol intake and colorectal cancer. Int J Clin Exp Med. 2015. May 15;8(5):6878–89. [PMC free article] [PubMed] [Google Scholar]
- 12.World Cancer Research Fund/American Institute for Cancer Research. Alcoholic drinks and the risk of cancer. In: Continuous Update Project Expert Report 2018. [Internet]. p. 85 Available from: https://www.wcrf.org/sites/default/files/alcoholic-drinks.pdf [Google Scholar]
- 13.Oyesanmi O, Snyder D, Sullivan N, Reston J, Treadwell J, Schoelles KM. Alcohol consumption and cancer risk: understanding possible causal mechanisms for breast and colorectal cancers. Evid ReportTechnology Assess. 2010. November;(197):1–151. [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Alcohol Alert: Alcohol Metabolism: An Update [Internet]. 2007. [cited 2017 May 31]. Available from: https://pubs.niaaa.nih.gov/publications/aa72/aa72.htm
- 15.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006. February;7(2):149–56. [DOI] [PubMed] [Google Scholar]
- 16.Gong J, Hutter CM, Newcomb PA, Ulrich CM, Bien SA, Campbell PT, et al. Genome-Wide Interaction Analyses between Genetic Variants and Alcohol Consumption and Smoking for Risk of Colorectal Cancer. PLoS Genet [Internet]. 2016. October 10 [cited 2017 Feb 7];12(10). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5065124/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai S, Li Y, Ding Y, Chen K, Jin M. Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2014. November;23(6):532–9. [DOI] [PubMed] [Google Scholar]
- 18.Kontou N, Psaltopoulou T, Soupos N, Polychronopoulos E, Xinopoulos D, Linos A, et al. Alcohol consumption and colorectal cancer in a Mediterranean population: a case-control study. Dis Colon Rectum. 2012. June;55(6):703–10. [DOI] [PubMed] [Google Scholar]
- 19.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol Off J Eur Soc Med Oncol. 2011. September;22(9):1958–72. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. CDC - What Are the Risk Factors for Colorectal Cancer? [Internet]. 2016. [cited 2017 Feb 10]. Available from: https://www.cdc.gov/cancer/colorectal/basic_info/risk_factors.htm
- 21.American Cancer Society. Colorectal Cancer Risk Factors [Internet]. [cited 2017 Feb 10]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/causes-risks-prevention/risk-factors.html [Google Scholar]
- 22.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009. July 1;125(1):171–80. [DOI] [PubMed] [Google Scholar]
- 23.Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer Oxf Engl 1990. 2006. January;42(2):216–27. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Zhu Y, Wang PP, West R, Buehler S, Sun Z, et al. Interaction between alcohol drinking and obesity in relation to colorectal cancer risk: a case-control study in Newfoundland and Labrador, Canada. BMC Public Health. 2012;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, Duggan D, et al. Characterization of Gene–Environment Interactions for Colorectal Cancer Susceptibility Loci. Cancer Res. 2012. April 15;72(8):2036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC - Frequently Asked Questions - Alcohol [Internet]. 2018. [cited 2019 Feb 11]. Available from: https://www.cdc.gov/alcohol/faqs.htm
- 27.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple Imputation by Chained Equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011. March 1;20(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher’s method. In: Wikipedia [Internet]. 2017. Available from: https://en.wikipedia.org/w/index.php?title=Fisher%27s_method&oldid=769494613 [Google Scholar]
- 29.Klarich DS, Penprase J, Cintora P, Medrano O, Erwin D, Brasser SM, et al. Effects of moderate alcohol consumption on gene expression related to colonic inflammation and antioxidant enzymes in rats. Alcohol. 2017. June 1;61:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute. Alcohol and Cancer Risk [Internet]. National Cancer Institute; [cited 2017 May 31]. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet [Google Scholar]
- 31.Purohit V, Khalsa J, Serrano J. Mechanisms of alcohol-associated cancers: introduction and summary of the symposium. Alcohol Fayettev N. 2005. April;35(3):155–60. [DOI] [PubMed] [Google Scholar]
- 32.Johns Hopkins Medicine. Folic Acid & Colon Cancer [Internet]. Johns Hopkins Medicine Colorectal Cancer. [cited 2017 May 31]. Available from: http://www.hopkinscoloncancercenter.org/CMS/CMS_Page.aspx?CurrentUDV=59&CMS_Page_ID=1293D614-71B1-4A5A-8CFD-7BF8760295FA [Google Scholar]
- 33.Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, et al. Development and validation of empirical indices to assess the insulinemic potential of diet and lifestyle. Br J Nutr. 2016. November 8;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and Validation of an Empirical Dietary Inflammatory Index123. J Nutr. 2016. August;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006. May 1;186(1):113–20. [DOI] [PubMed] [Google Scholar]
- 36.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002. May 15;287(19):2559–62. [DOI] [PubMed] [Google Scholar]
- 37.Thomasson HR. Gender Differences in Alcohol Metabolism. SpringerLink. 2002;163–79. [DOI] [PubMed] [Google Scholar]
- 38.Schrieks IC, Heil ALJ, Hendriks HFJ, Mukamal KJ, Beulens JWJ. The Effect of Alcohol Consumption on Insulin Sensitivity and Glycemic Status: A Systematic Review and Meta-analysis of Intervention Studies. Diabetes Care. 2015. April 1;38(4):723–32. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012. June;61(6):847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides, or a continuum? Gut. 2012. June;61(6):794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009. October;18(10):2745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995. February 15;87(4):265–73. [DOI] [PubMed] [Google Scholar]
- 43.Mu L, Mukamal KJ. Alcohol consumption and rates of cancer screening: Is cancer risk overestimated? Cancer Causes Control CCC. 2016. February;27(2):281–9. [DOI] [PubMed] [Google Scholar]
- 44.Cohen SS, Murff HJ, Signorello LB, Blot WJ. Obesity and colorectal cancer screening among black and white adults. Cancer Causes Control CCC. 2012. May;23(5):709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


