Abstract
Hepatic encephalopathy (HE) can cause major morbidity despite standard of care (SOC; rifaximin/lactulose). Fecal microbial transplant (FMT) enemas post-antibiotics are safe but the effect of FMT without antibiotics using the capsular route requires investigation.
Aim:
To determine the safety, tolerability and impact on mucosal/stool microbiota and brain function in HE after capsular FMT in a randomized, single-blind, placebo-controlled clinical trial in Virginia.
Methods:
Cirrhotic patients with recurrent HE with MELD<17 on SOC were randomized 1:1 into receiving 15 FMT capsules vs placebo from a single donor enriched in Lachnospiraceae and Ruminococcaceae. Endoscopies with duodenal and sigmoid biopsies, stool analysis, cognition, serum lipopolysaccharide-binding protein (LBP) and duodenal anti-microbial peptide(AMP) expression at baseline. Clinical follow-up with SOC maintenance was performed till 5 months. FMT-assigned patients underwent repeat endoscopies 4-weeks post-enrollment.
Results:
20 subjects on lactulose/rifaximin were randomized 1:1. MELD score was similar at baseline (9.6 vs 10.2) & study end (10.2 vs 10.5). 6 patients in the placebo group required hospitalizations compared to one in FMT, which was deemed unrelated to FMT. Infection/HE episodes were similar between groups. Baseline microbial diversity was similar in all tissues between groups. Post-FMT, duodenal mucosal diversity(p=0.01) increased with higher Ruminococcaceae, Bifidobacteriaceae and lower Streptococcaceae and Veillonellaceae. Reduction in Veillonellaceae were seen post-FMT in sigmoid(p=0.04) and stool(p=0.05). Duodenal E-cadherin (p=0.03) and Defensin A5 (p=0.03) increased while IL-6(p=0.02) and serum LBP(p=0.009) reduced post-FMT. EncephalApp performance improved post-FMT only(p=0.02).
Conclusion:
In this phase 1 study, oral FMT capsules are safe and well-tolerated in patient with cirrhosis and recurrent HE. FMT was associated with improved duodenal mucosal diversity, dysbiosis and AMP expression, reduced LBP and improved EncephalApp performance. Further studies are needed to prove efficacy.
Trial registration:
www.clinicaltrials.gov number
Keywords: Microbiome, Cirrhosis, Anti-microbial peptides, Cognitive testing, Tolerability
Hepatic encephalopathy (HE) is a major source of morbidity and healthcare expenditure in cirrhosis(1). HE is associated with alterations in gut-liver-brain axis with intestinal barrier dysfunction and gut microbial dysbiosis, and current therapies are mostly directed towards alleviating these impairments(2, 3). However, despite current standard of care (SOC) therapy i.e. lactulose and rifaximin in the USA, there remains a subset of patients who continue to suffer recurrence, which leads to further cognitive impairment, sarcopenia and readmissions(4-6). In addition, these patients are often not given priority for liver transplant, leading to a vicious cycle of readmissions and deterioration(7, 8). A prior study of antibiotics followed by fecal microbial transplant (FMT) enema was associated with improved short-term and long-term outcomes and enhanced brain function in patients with recurrent HE randomized to that arm compared to those on SOC(9). However, this approach was complicated because of pre-FMT antibiotics and lack of a placebo control. Also, patients randomized to FMT enema would have preferred a capsule for future studies. Capsular FMT, which is a viable treatment for C.difficile, needs to be evaluated in patients with recurrent HE(10).
Our aim was to determine the safety and tolerability of oral capsular FMT in patients with recurrent HE compared to placebo in a multi-center, placebo-controlled, randomized trial. Secondary endpoints were related to microbiota and intestinal barrier changes and cognitive function changes
Methods:
Participants:
Cirrhotic outpatients with recurrent HE defined by at least 2 episodes, within the last year, on lactulose and rifaximin, were recruited after obtaining informed consent from the Virginia Commonwealth University Medical Center and the McGuire VA Medical Center Hepatology clinics. We excluded those who were unable to consent, MELD>17, with current absorbable antibiotics, those with autoimmune diseases, and those with history of dysphagia or contra-indications to endoscopic procedures. Complete eligibility criteria are in supplementary table 2. Randomization was performed according to a random number generator at the level of the Investigational Pharmacy and concealed by keeping this with the pharmacy. Subjects and outcome assessors (persons performing the cognitive tests, those analyzing the microbiota, expression of intestinal expression and serum tests) were blinded to the allocation.
Study interventions:
FMT was prepared from one stool sample from one healthy donor stool sourced from a universal stool bank (OpenBiome, Cambridge, MA, USA). At OpenBiome, all potential stool donors underwent a rigorous 240-item donor health questionnaire and a laboratory assessment including comprehensive stool-based and serological assays for pathogenic organisms (Supplement). Placebos appear identical to the FMT capsules. The donor for this FMT was the same donor with high Lachnospiraceae and Ruminococcaceae relative abundance who was selected for our prior enema study and all FMT recipients received capsules derived from a single stool sample from this donor (Supplement)(9).
Study Procedures:
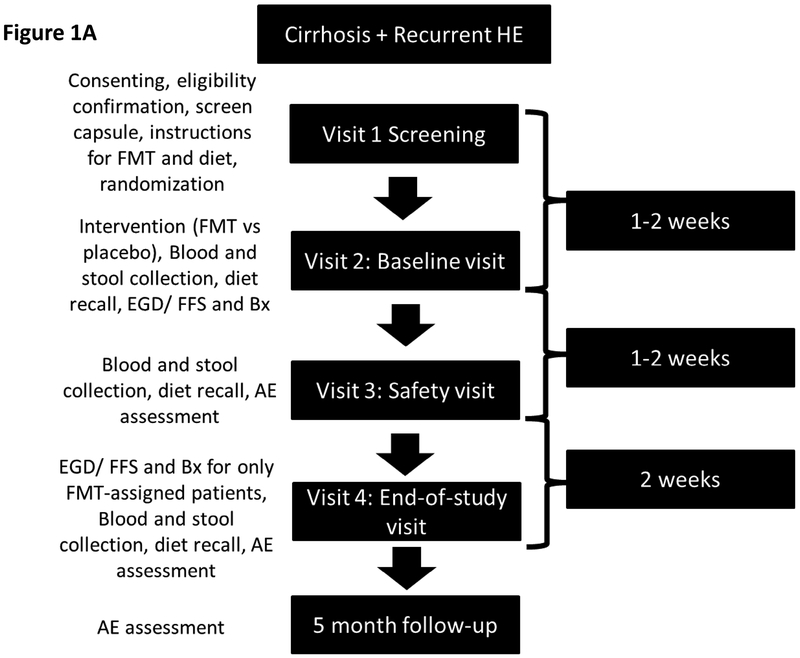
After consent, all subjects were followed for 5 months (Figure 1A, protocol in supplement). In summary, after enrollment and documenting eligibility, a placebo safety capsule was given to every potential subject to ensure that they could swallow this as part of the continued eligibility. Patients were then randomized 1:1 to FMT capsules or placebo and were administered two cognitive tests, psychometric hepatic encephalopathy score (PHES) and EncephalApp Stroop (Supplement)(1, 11, 12). Dietary recalls were performed at each visit. At visit 2 subjects brought in stool samples and underwent upper endoscopy and flexible sigmoidoscopy with biopsies of the duodenum and sigmoid colon for analysis of microbiota. Duodenal inflammatory expression and expression of antimicrobial peptides (AMP) were also analyzed (Supplement). Serum lipopolysaccharide binding protein (LBP) were analyzed at baseline and visit 4 post-FMT or placebo. Subjects were then administered 15 capsules of placebo or FMT as per randomization. Symptom diaries were given to all patients to record adverse events (Supplementary table 3). During the subsequent visit, stools were collected and subjects were inquired about potential adverse events (AE) and serious adverse events (SAEs). At visit 4 subjects underwent cognitive testing, review of symptoms and diet and evaluation of AE/SAEs. After these were performed, only those who had been assigned to FMT underwent repeat upper and lower endoscopy and biopsies. Importantly, all patients came prepared for endoscopies since they were blinded and were only told regarding the need for endoscopy after they had finished the cognitive tests and symptom/AE assessment.
Figure 1A:
Schematic of Study Design
Outcomes:
The primary outcomes were safety and tolerability of FMT compared to placebo related to serious adverse events (SAEs consisting of hospitalizations and ER visits), especially those deemed to be related to the FMT by the Data Safety Monitoring Board (DSMB). Secondary outcomes were AEs not resulting in hospitalization/ER visits, especially those related to FMT, changes in mucosal and stool microbiota, changes in cognitive testing on PHES and EncephalApp, serum LBP, and changes in duodenal mucosal expression of inflammatory cytokines, barrier proteins and AMPs.
Duodenal mucosal analysis was compared between baseline FMT and placebo and pre and post-FMT. We performed RT-quantitative PCR for the quantification of mRNA expression of constitutive and inducible Paneth cell anti-microbial peptides (AMPs), including DefA5, DefA6, for IL-6 and for junctional protein E-cadherin encoded by CDH1 gene(13) and ELISA for LBP(Supplement). Cognitive testing analysis was performed with OffTime+OnTime for EncephalApp and PHES total score at baseline and repeated as outcomes.
This protocol was approved by the IRBs at both centers. The trial was registered at www.clinicaltrials.gov number and performed under FDA Investigational New Drug guidance as a Phase 1 trial. Sample size was based on our prior study where 80% of SOC group and 2 of the FMT group developed SAEs(9). Using a power of 0.80 and alpha of 0.05, we would require 10 patients in each group for a similar study
Microbiota analysis (Supplement):
Microbial DNA was isolated from stool and biopsy samples as previously described and 16s rRNA analysis with V1-V2 region primers were performed(14). Alpha diversity (Shannon diversity index), individual taxa analysis (Linear discriminant analysis effect size LEFSe)(15), principal component analysis (PCA) and Kruskal-Wallis tests for specific taxa (Lachnospiraceae, Ruminococcaceae, Veillonellaceae and Streptococcaceae) that were either high in the donor, or are associated with negative outcomes or worse severity in cirrhosis were performed(9, 14, 16).
Statistical analysis:
Baseline analyses were compared using Mann Whitney and unpaired t-tests as appropriate while pre/post intervention analyses were performed using Wilcoxon signed rank matched pairs tests respectively. Non-parametric analyses (Mann-Whitney and Fisher exact) tests were used to evaluate outcomes between groups. In case a data point was not available, we used the last point carry forward.
Results:
We included 20 patients with recurrent HE who were statistically similar with respect to baseline characteristics (table 1). Subjects were enrolled from VCU and McGuire VA Medical Center from June 2017 to May 2018. Laboratory values, including MELD score and liver-related enzymes remained stable between groups at the safety-visit and the 30-day visit (Table 2).
Table 1:
Baseline Comparison between groups
| FMT | Placebo | P value | |
|---|---|---|---|
| Age | 63.3±4.2 | 64.2±6.2 | 0.71 |
| Gender (M/F) | 8/2 | 8/2 | 1.0 |
| Race (Caucasian/African-American/Hispanic) | 7/3/0 | 7/3/0 | 1.0 |
| Etiology of cirrhosis (HCV/Alcohol/HCV+Alcohol/NASH/Others) | 2/1/3/2/2 | 3/1/2/2/1 | 0.78 |
| PPI use | 10 | 10 | 1.0 |
| Lactulose | 10 | 10 | 1.0 |
| Rifaximin | 10 | 10 | 1.0 |
| Number of prior HE episodes (median, range) | 2 (2-6) | 2 (2-5) | 0.9 |
| Time of last HE episode before enrollment (months) | 3.3±5.7 | 4.1±4.0 | 0.72 |
| MELD score | 9.5±2.6 | 10.9±4.2 | 0.39 |
| AST | 48.4±13.8 | 40.9±20.8 | 0.36 |
| ALT | 39.0±16.4 | 21.9±15.5 | 0.33 |
| Alkaline Phosphatase | 144.8±66.7 | 126.9±40.7 | 0.48 |
| INR | 1.27±0.17 | 1.30±0.16 | 0.69 |
| Bilirubin | 1.26±0.80 | 1.46±0.80 | 0.53 |
| Serum albumin | 3.3±0.5 | 3.3±0.6 | 0.67 |
| WBC (103/ml) | 4.65±1.43 | 5.4±2.0 | 0.37 |
| Hemoglobin (g/dl) | 13.5±1.9 | 12.9±2.6 | 0.21 |
| Platelet count(103/ml) | 113.0±48.5 | 140.4±70.5 | 0.33 |
Table 2:
Changes in laboratory parameters over time between groups
| Safety visit | P value | 30-day visit | P value | |||
|---|---|---|---|---|---|---|
| Placebo | FMT | Placebo | FMT | |||
| MELD score | 11.7±3.9 | 10.2±4.5 | 0.44 | 11.3±3.9 | 8.7±2.9 | 0.11 |
| AST | 41.0±21.4 | 49.4±11.1 | 0.29 | 43.4±26.0 | 50.2±16.3 | 0.50 |
| ALT | 30.9±12.4 | 39.0±13.2 | 0.18 | 35.1±14.6 | 40.4±13.6 | 0.42 |
| Alkaline Phosphatase | 133.7±57.3 | 145.8±67.0 | 0.67 | 132.2±57.6 | 132.9±59.7 | 0.98 |
| INR | 1.32±0.22 | 1.29±0.16 | 0.73 | 1.31±0.23 | 1.23±0.14 | 0.40 |
| Bilirubin | 1.57±0.72 | 1.49±0.74 | 0.81 | 1.62±0.92 | 1.29±0.59 | 0.336 |
| Serum albumin | 3.17±0.57 | 3.33±0.48 | 0.51 | 3.32±0.67 | 3.52±0.49 | 0.46 |
| WBC (103/ml) | 5.1±2.2 | 4.9±1.5 | 0.83 | 5.1±2.0 | 5.0±1.3 | 0.93 |
| Hemoglobin (g/dl) | 11.7±2.8 | 13.4±1.8 | 0.13 | 12.5±2.9 | 13.9±1.5 | 0.20 |
| Platelet count(103/ml) | 146.4±85.6 | 108.4±31.8 | 0.22 | 142.9±76.5 | 116.0±53.3 | 0.38 |
Safety visit is 1-2 weeks after the initial EGD and Colonoscopy; comparisons performed using unpaired t-tests
Clinical course:
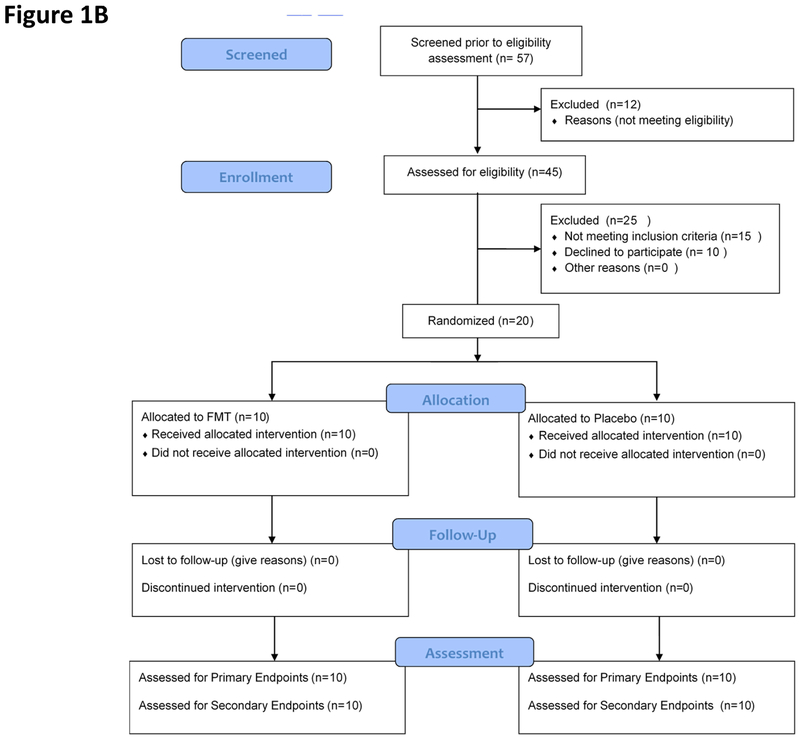
All subjects tolerated the procedures, including EGD and sigmoidoscopy as well as safety capsule and capsule ingestion without any issues (Figure 1B). Total SAEs and number of patients with SAEs tended to be higher in the placebo compared to FMT group (Figure 2C, Table 3). Most SAEs were hospitalizations and ER visits related to liver disease progression. Six placebo patients experienced SAEs; one patient had 6 events (5 HE episodes described below, 1 renal insufficiency without HE), the remaining 5 of these had one SAE (two with infection, two with HE, one with electrolyte abnormalities). Four placebo-assigned patients did not have SAEs. One FMT-assigned patient had one episode of HE as the SAE; the remaining nine did not have SAEs during the follow-up period. One placebo-assigned patient required ER visits/hospitalization for altered electrolytes, which resolved within 24 hours. One placebo-assigned patient (with the 6 admissions) was transferred to hospice and died four months post-enrollment. None of the FMT-assigned patients died during the follow-up period.
Figure 1B:
CONSORT Flow chart
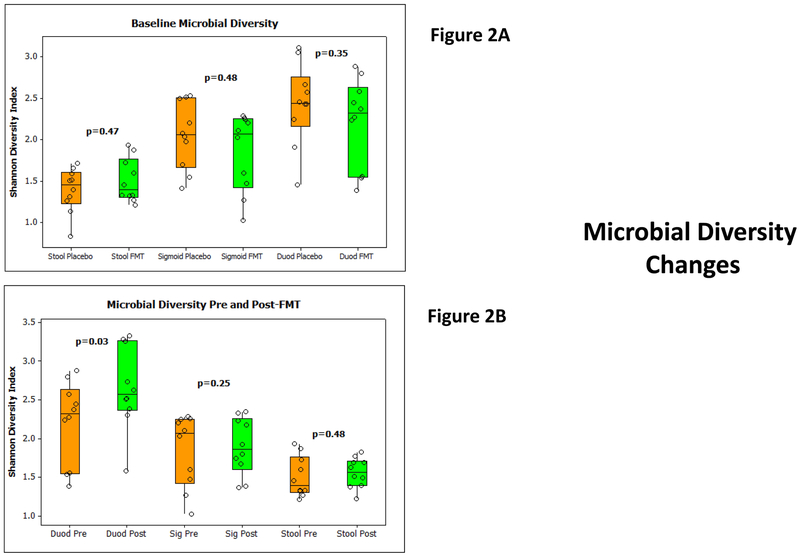
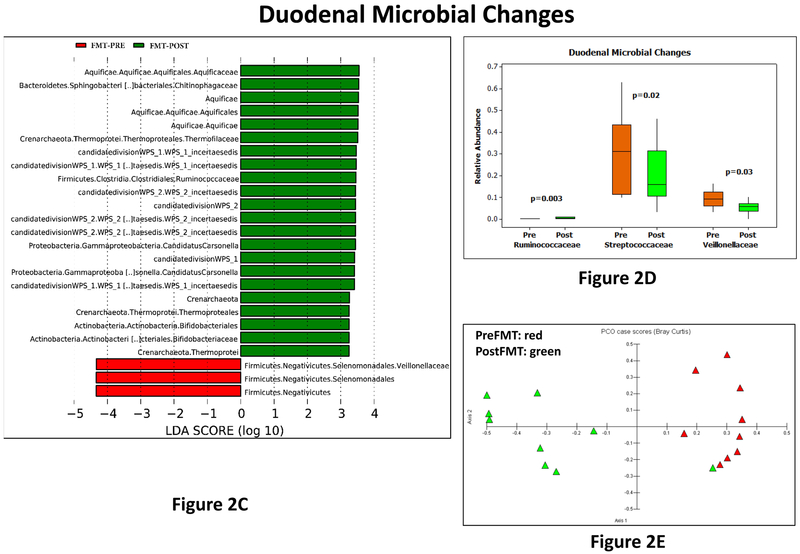
Figure 2:
2A: Median and 95% CI of Shannon microbial diversity in stool, sigmoid and duodenal microbiota at baseline between groups did not show any baseline significant differences. FMT group is in orange while placebo-assigned group is in the green. P values compared using Mann Whitney tests are displayed.
2B: Median and 95% CI of Shannon microbial diversity within the FMT group before and 4 weeks after FMT in the stool, sigmoid and duodenal microbiota. There was a significant increase only in the duodenal but not sigmoid or stool diversity on Wilcoxon signed rank pairs test. Orange bars are pre and green are post-FMT values.
2C: LEFSe results pre (red bars) and post (green bars) FMT in duodenal microbiota
2D: Median and 95% CI of selected taxa pre (orange) and post (green) FMT in duodenal microbiota. P values based on Wilcoxon signed rank pairs test, which showed a higher Ruminococcaceae and lower Veillonellaceae and Streptococcaceae in the post-FMT mucosa compared to pre-FMT.
2E: PCA shows pre-FMT duodenal microbiota (red) clustered apart from the post-FMT microbiota (green)
Table 3:
Details of Adverse events and Serious adverse events
| Placebo Group (n=10) |
FMT group (n=10) |
|
|---|---|---|
| Number of SAEs (median, range) | 11 (1.0, 0-6) | 1 (0, 0-1)** |
| Number of Patients with SAEs | 6 | 1* |
| Hepatic Encephalopathy | ||
| HE episodes (number of total episodes) | 7 | 1 |
| HE episodes requiring hospitalization/ER visits | 7 | 1 |
| HE episodes managed as an outpatient | 0 | 0 |
| Number of Patients with HE | 3 | 1 |
| Infections | ||
| Infections (number of total infections) | 3 (Pneumonia, n=1, Cellulitis n=1, Fever +diarrhea n=1; all hospitalized) | 2 (Pneumonia, n=1, UTI, n=1; not hospitalized) |
| Number of patients with infections | 3 | 2 |
| Deaths | 1 | 0 |
| Patients with Self-limited AEs | ||
| Constipation (number) | 1 | 2 |
| Diarrhea (number) | 1 | 1 |
| Bloating (number) | 0 | 1 |
| Nausea/vomiting (number) | 1 | 0 |
p<0.05 between groups on Fisher test,
p<0.05 on Mann-Whitney test. SAE: serious adverse events (hospitalization/ER visits), AE: adverse events, none of SAEs or AEs in the FMT group were considered related to the FMT by the Data Safety Monitoring Board.
HE episodes were similar between groups on follow-up. Three placebo patients developed HE; one patient had a single HE episode within 30 days due to an unknown precipitant, who recovered within two days. Another patient also had a single episode that required dose adjustment. Another placebo-randomized patient experienced five HE episodes precipitated by acute kidney injury in two episodes, pneumonia in one episode, anasarca in another and lactulose non-adherence in the remaining episode. This patient was also admitted another time without HE with renal insufficiency. One FMT patient was admitted within 30 days for HE, which was considered related to transjugular intra-hepatic porto-systemic shunt(TIPS) placed 1.5 months prior. This resolved after TIPS was reduced in size and was considered unrelated to FMT by the DSMB. This patient did not undergo repeat EGD and flexible sigmoidoscopy because he was admitted during that time for TIPS closure; therefore, values from baseline were extended forward.
Infections were seen in three placebo and two FMT-assigned patients. The three placebo patients who got infections were as follows, one developed pneumonia as a precipitating factor for HE (mentioned above), another patient developed calf cellulitis that required hospitalization and one patient who had diarrhea and fever that lasted for 2 days. One patient had an incidentally diagnosed urinary tract infection with K.pneumoniae two months post-FMT that responded to outpatient antibiotics, the second patient, undergoing routine alpha-1 anti-trypsin infusions was found to have a pneumonia which was also treated as an outpatient. Both these episodes were considered unrelated to FMT and due to their outpatient management, not considered SAEs by the DSMB.
There were also self-limited gastrointestinal adverse events that did not require treatments and were similar across groups (Table 3). None of the patients developed symptoms related to dysphagia, belching, palatability concerns, bloating, reflux or aspiration.
Microbiota changes:
No changes in baseline Shannon diversity indices were seen between groups (Figure 2A). Stool collected at day 30 did not show any differences in diversity again between groups. Since endoscopies were only repeated in the FMT group, we studied changes in diversity pre/post FMT and found an increase in duodenal mucosal diversity but no changes in stool or sigmoid mucosal diversity at day 30 post-FMT compared to pre-FMT (Figure 2B).
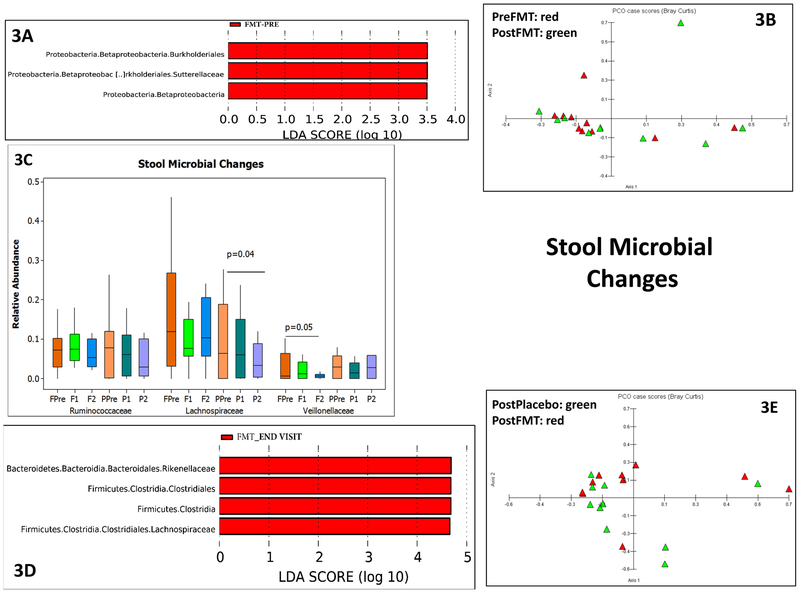
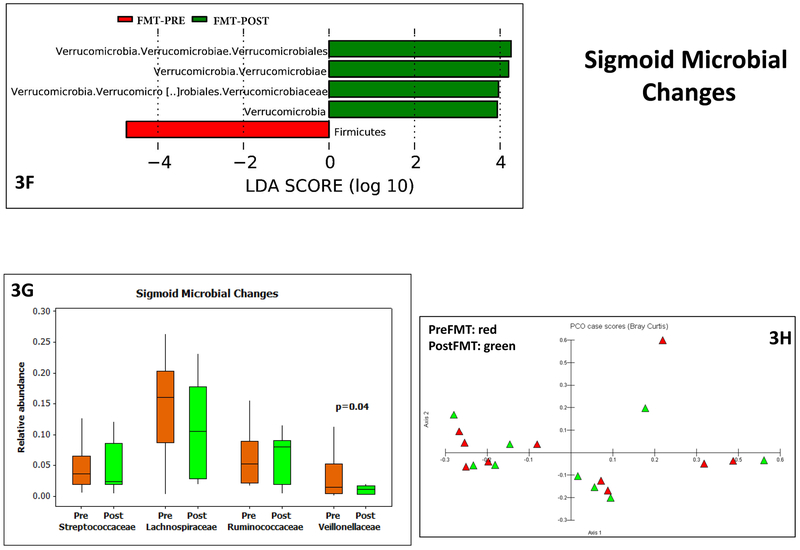
There were changes between pre vs post FMT duodenal, sigmoid and stool microbial taxa. In the duodenum, there was separate clustering, higher Ruminococcaceae and Bifidobacteriaceae and lower Veillonellaceae post-FMT compared to pre-FMT (Figure 2D-E). This was also seen in the Krukal-Wallis comparison, where there was also a lower Streptococcaceae post-FMT. Since stool was collected both pre and post-intervention in both placebo and FMT groups (Figures 3A-E), we found higher Lachospiraceae and Rikenellaceae in FMT group at day 30 compared to placebo, which was accompanied by relatively separate clustering on PCA. This was likely related to a reduction in Lachnospiraceae in placebo over time as seen in Kruskal-Wallis comparisons, which was accompanied by a reduction in Veillonellaceae post-FMT. On LEFSE there was higher Sutterellaceae baseline compared to post-FMT values. In the sigmoid on LEFSe, higher Verrucomicrobiaceae and lower Firmicutes were seen (Figure 3F-H). Veillonellaceae were again reduced but no changes in relative abundance of Streptococcaceae, Lachnospiraceae and Ruminococcaceae nor separate clustering on PCA were seen.
Figure 3:
3A: LEFSe in pre vs post FMT (red bars) in stool microbiota within the FMT group
3B: PCA shows relative clustering of pre-FMT (red) compared to the post-FMT stool microbiota (green)
3C: Median and 95% CI of selected taxa pre (orange) and post (green) FMT in stool microbiota. FPre: FMT group pre-FMT, F1: first post-FMT visit in FMT group, F2: end of study visit FMT group, PPre: Placebo group baseline, P1: first post-placebo visit, P2: end of study visit placebo group. P values based on Kruskal-Wallis tests showed reduction in Lachnospiraceae over time in placebo group and reduction of Veillonellaceae over time in the FMT group.
3D: LEFSe of end study visit in placebo vs FMT group at end of study visit showed a relatively higher abundance of Lachnospiraceae in the FMT compared to placebo group on the stool.
3E: PCA shows relative clustering of post-FMT away from post-placebo in stool microbiota
3F: LEFSe results pre (red bars) and post (green bars) FMT in sigmoid mucosal microbiota
3G: Median and 95% CI of selected taxa pre (orange) and post (green) FMT in sigmoid mucosal microbiota showed a lower Veillonellaceae post-FMT compared to baseline. P values based on Wilcoxon signed rank pairs test.
3H: PCA shows little separation between pre (red) and post-FMT (green) sigmoid microbiota
Brain function:
Baseline performances on EncephalApp and PHES were similar between groups (Table 4). After FMT, there was a significant improvement in OffTime+OnTime due to improvement in OnTime on EncephalApp in the FMT group compared to baseline. Despite a trend towards improvement on NCT-A and Digit symbol compared to baseline in the FMT group, there was no significant improvement on PHES in the FMT group. No significant changes were seen in placebo group regarding EncephalApp or PHES.
Table 4:
Cognitive testing changes
| Mean±SD | Pre-FMT | Post-FMT | Pre-Placebo | Post-Placebo |
|---|---|---|---|---|
| OffTime (seconds) | 120.0±47.3 | 103.6±20.5 | 120.5±45.6 | 136.3±72.9 |
| OnTime (seconds) | 157.9±78.7 | 124.1±36.2* | 198.5±143.0 | 183.6±98.3 |
| OffTime+OnTime (seconds) | 277.8±123.5 | 226.7±56.1* | 318.9±181.0 | 308.9±169.5 |
| PHES score total | −6.8±6.3 | −5.7±5.4 | −8.4±5.2 | −8.9±4.7 |
| NCT-A (seconds) | 89.5±83.5 | 60.70±30.85 | 69.4±22.1 | 72.4±43.9 |
| NCT-B (seconds) | 192.3±144.9 | 173.7±124.6 | 179.8±98.1 | 193.5±123.5 |
| Digit Symbol (raw score) | 39.6±20.4 | 43.4±15.4 | 35.3±16.5 | 33.8±15.1 |
| Serial dotting (seconds) | 88.8±48.4 | 82.3±30.6 | 118.2±51.1 | 105.7±54.2 |
| Line tracing time (seconds) | 126.5±85.4 | 116.4±65.5 | 112.8±27.9 | 124.7±46.1 |
| Line tracing errors (raw score) | 54.6±22.6 | 43.3±25.3 | 57.0±34.1 | 66.6±48.3 |
pre vs post p<0.05 on Wilcoxon signed rank test. There were no significant differences in cognitive tests between groups at the pre-FMT testing time period. Higher score on digit symbol test indicates better performance while a high score or time taken in the remaining tests indicates poor performance.
Duodenal AMP, barrier proteins and inflammatory cytokines (Figure 4):
Figure 4:
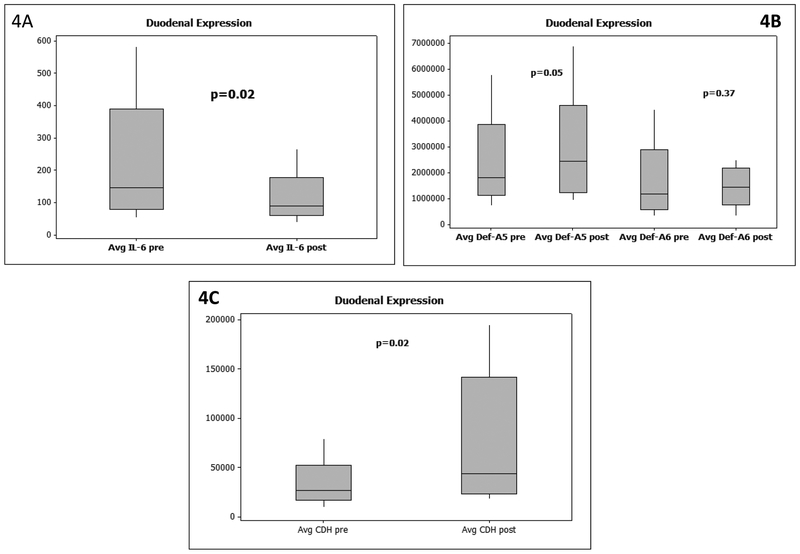
Data presented as median and 95% CI. P values based on Wilcoxon signed rank pairs test. 4A: mRNA expression of duodenal IL-6 pre and post-FMT showed a significant reduction.
4B: mRNA expression of duodenal defensin A-5 and A-6 pre and post-FMT showed a significant increase in A-5 but not A-6 expression
4C: mRNA expression of duodenal e-cadherin protein (CDH) pre and post-FMT showed a significant increase in CDH.
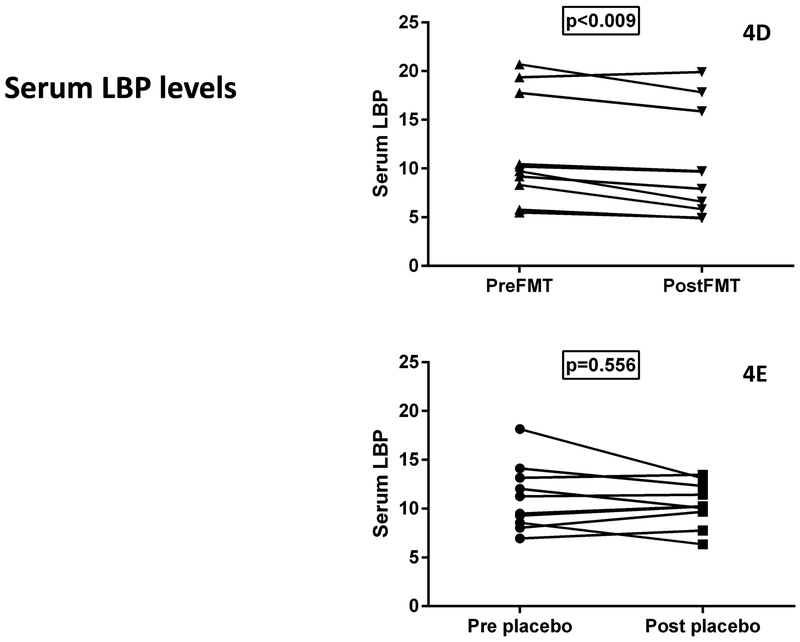
4D: Serum LBP showed significant reduction post-FMT compared to baseline
4E: Serum LBP showed no significant change post-placebo compared to baseline
There were no significant differences at baseline between groups with respect to AMP expression such as DefA5 (3618975 ± 2326814 placebo vs 2477000 ± 1863218 FMT), DefA6 (1981985 ± 1527905 placebo vs 1806600 ± 1570451 FMT), Reg3a (563121 ± 338844 placebo vs 517450 ± 440001 FMT) or Reg3g (12773 ± 9726 placebo vs 13201 ± 15720 FMT). Similarly, median IQR expression of inflammatory cytokines IL-6 (92.2 ± 73.6 placebo vs 100.3 ± 391.7 FMT) and junctional protein E-cadherin CDH expression (37150 ± 28625 placebo vs 35600 ± 96175 FMT) were not significantly different. Since only the FMT group underwent repeat endoscopies, comparisons showed a significant increase in DefA5 and CDH expression and reduced IL-6 expression post-FMT compared to baseline. A non-significant trend towards increased expression of DefA6 was observed.
LBP levels:
there were no significant differences at baseline between groups (p=0.98) but LBP levels significantly reduced post-FMT compared to baseline but not post-placebo (Figure 4D/E)
Discussion:
The current placebo-controlled, randomized Phase 1 trial demonstrates that the oral route of FMT was safe, well tolerated and was associated with a favorable change in serum LBP, duodenal mucosal microbial and barrier expression. There was an improvement in EncephalApp performance in the FMT-assigned patients.
Cirrhosis and recurrent HE pose a considerable medical, psychosocial and societal burden that is currently underserved with available therapies(1). There is a risk of persistent cognitive injury that may be irreversible even after liver transplant as well as readmission, falls and burden on caregivers if this vicious cycle is not interrupted(17, 18). There is an alteration of the gut-liver-brain axis in cirrhosis and recurrent HE(19, 20). In animal models and humans with cirrhosis, there is evidence of small bowel inflammatory expression and impaired barrier function, which is complicated by dysbiosis of the intestinal mucosa and stool(20-24). These are complicit in worsening the systemic inflammatory milieu that drives cirrhosis progression, neuro-inflammation and worsening of HE symptoms(25). While current approved therapies such as lactulose and rifaximin favorably affect the gut milieu, further beneficial alterations may be needed to improve outcomes(26). Therefore, we continued rifaximin in these patients despite the potential for it affecting the FMT. A prior open-label randomized clinical FMT enema trial which included pre-FMT antibiotics, demonstrated a reduction in hospitalization and improvement in brain function(9) in the short and long-term(27). However, since antibiotics were administered before FMT and it was an open-label study, further exploration was needed. Also from a patient experience perspective, given a choice between capsules and repeat enemas to patients who were randomized to FMT, all 10 patients chose a potential capsule in future studies. The current trial was placebo-controlled, randomized and single-blinded and did not involve pre-intervention antibiotic therapy.
This was a Phase 1 study focused on safety and tolerability of FMT compared to placebo and no FMT-related SAE was observed. SAEs observed were related to the advancing of the disease process in both groups. While not powered for efficacy, there was a trend towards lower hospitalizations but not HE in patients randomized to FMT. This pattern is interesting and likely points towards the role of small bowel intestinal barrier dysfunction that can result in translocation and worsening of the overall liver disease(28). However, larger studies powered to detect a beneficial impact on the likelihood of hospitalization over time with alleviation of dysbiosis and enhancing barrier function are needed.
Stool microbiota, which were studied in both groups at all time points, demonstrated a relatively lower Lachnospiraceae in the placebo group compared to FMT at study end, which likely reflects the donor characteristics. This was accompanied by lower LBP levels, Within the FMT group there was a decrease in Veillonellaceae and the Proteobacteria constituent, Sutterellaceae relative abundance in the stool after FMT. When mucosal composition, which was only analyzed pre/post FMT was studied, the duodenal microbiota showed an increase in Ruminococcaceae and Bifidobacteriaceae, reduction in Streptococaceae and Veillonellaceae and an increase in Shannon diversity compared to their baseline. This was associated with favorable changes in anti-microbial protein expression and intestinal inflammation. These changes are relevant since the FMT capsules are engineered to open in the proximal small bowel and this is the likely site of maximal engraftment. Given that the donor was enriched in Lachnospiraceae and Ruminococcaceae, this relative increase in Ruminococcaceae suggests engraftment of the donor microbiota. In addition to the duodenum, Veillonellaceae was also reduced in the sigmoid after FMT, although the diversity remained similar. Streptococaceae and Veillonellaceae, are usually oral in origin and are found in higher relative abundance in intestinal mucosa and stool of cirrhotic patients, where they are associated with an overall poor prognosis(22, 29-31). Their relative abundance is exacerbated by PPI therapy, which is epidemic in cirrhosis and indeed was seen in all our participants(29, 30). Veillonella spp have been shown to enhance pathogenicity of gram-negative bacilli and are usually reduced with rifaximin therapy and by PPI withdrawal(26, 32, 33). Streptococcaceae express urease that can potentially generate ammonia and have been associated with HE(32, 34). On the other hand, Lachnospiraceae, Ruminococcaceae and Bifidobacteriaceae are over-expressed in healthy controls and taxa belonging to Lachnospiraceae and Ruminococcaceae are associated with short-chain fatty acid and bile acid metabolism(35, 36). These findings in the duodenum, sigmoid and stool all point towards a relative reduction in oral-origin taxa associated with cirrhosis progression and increase in taxa associated with benefit. These changes are even more striking given that the patients in the FMT and placebo group both continued their lactulose, rifaximin and PPI therapy. There was also a simultaneous reduction in the duodenal IL-6 expression and increase in expression of key AMP and intestinal barrier proteins, which was likely mediated by the favorable change in microbial composition(37). These data indicate that the intestinal barrier is favorably modulated from a microbial, inflammatory and antimicrobial peptide aspect after capsular FMT.
There was also an improvement in the cognitive aspect based on EncephalApp but not on overall PHES with a trend towards improvement in number connection-A and digit symbol test. This is intriguing because these testing strategies interrogate psychomotor speed, indicating a potential impact of the FMT on the gut-brain axis(1, 11, 12). Both these tests had improved in the enema FMT trial in a similar cirrhosis population using feces from the same donor as used in this trial(9). Therefore, it is unclear why the other components did not improve but it is likely that studies with a larger sample size comparing the upper versus the lower GI route of delivery are needed. We can speculate that the greater quantity of bacteria in the colon, which could be affected directly by the lower GI route and antibiotics compared to upper GI route, could be driving this change.
The study is limited by the small sample size and focus on Phase 1 safety rather than being powered to detect efficacy. While we collected stool during every visit, we did not perform repeat upper and lower endoscopies on the placebo-randomized patients due to the unacceptable invasive risk of repeating these in patients not receiving the active FMT. Therefore, it could be possible that beneficial changes in sigmoid and duodenal microbiota could be a variation within subjects. However, changes in stool microbial composition between and within groups that were collected simultaneously reflect beneficial microbial change, which was associated with decrease in LBP in the FMT group only. Also, the potential beneficial impact of oral capsules that was greater in the duodenum compared to sigmoid lend biological plausibility towards this being an effect of the FMT. Patients in the placebo arm remained blinded as to the need for repeat endoscopies until after all the symptom questionnaires and cognitive testing had been performed, to minimize subjective changes in these results. The endpoints were objective, and assessors were blinded and therefore unlikely to be affected by the knowledge of subject assignment. All patients were on PPI therapy, as is typical for this stage of cirrhosis. We also only included patients on rifaximin already because these patients had continued HE despite being on maximal FDA-approved therapy. We chose to give all capsules at the same time rather than one daily capsule for 15 days given the similar dosing pattern for C.difficile in such cases. Further studies are needed to analyze the effect of FMT in patients not on rifaximin or PPI and multiple FMT administrations.
We conclude in this placebo-controlled randomized clinical trial that oral capsular FMT from a rationally derived donor was safe in patients with cirrhosis and recurrent HE. This was associated with favorable changes in mucosal and stool microbial composition and enhancement of the intestinal barrier. Further studies with larger sample sizes over a longer period are needed to establish efficacy.
Supplementary Material
Acknowledgements:
The team would like to acknowledge Dr Douglas M Heuman, Dr Michael Climo and Ms HoChong Gilles for their support as members of the Data Safety Monitoring Board along with Dr Brent Gregory and Dr Robin Sculthorpe for their role in the Investigational Pharmacy.
Financial Support: This work was partly supported by NCATS R21TR002024 to JSB and NHS, and VA Merit Review CX10076 to JSB. Capsules, including placebos, were provided by OpenBiome. None of the funders had any role in the decision to publish the findings.
Abbreviations:
- FMT
fecal microbiota transplant
- HE
hepatic encephalopathy
- DSMB
data safety monitoring board
- SAE
serious adverse events
- AE
adverse events
- SOC
standard of care
- EGD
esophagogastroduodenoscopy
- PCA
principal component analysis
- AMP
anti-microbial peptides
- LBP
lipopolysaccharide binding protein
- PHES
Psychometric hepatic encephalopathy score
- LEFSe
Linear discriminant analysis effect size
- ER
emergency room
- TIPS
transjugular intra-hepatic porto-systemic shunt
Footnotes
Presentations: This will be presented at the AASLD section of the Liver Plenary of DDW 2019
References:
- 1.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2012;302:G168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya C, Bajaj JS. Current Management of Hepatic Encephalopathy. Am J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 4.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol 2016;14:1181–1188 e1182. [DOI] [PubMed] [Google Scholar]
- 5.Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, Ma M, et al. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients With Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am J Gastroenterol 2016. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138:2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucidi C, Ginanni Corradini S, Abraldes JG, Merli M, Tandon P, Ferri F, Parlati L, et al. Hepatic encephalopathy expands the predictivity of model for end-stage liver disease in liver transplant setting: Evidence by means of 2 independent cohorts. Liver Transpl 2016;22:1333–1342. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, et al. Fecal Microbiota Transplant from a Rational Stool Donor Improves Hepatic Encephalopathy: A Randomized Clinical Trial. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015;149:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol 2016;111:78–86. [DOI] [PubMed] [Google Scholar]
- 12.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768–773. [DOI] [PubMed] [Google Scholar]
- 13.Liu TC, Gurram B, Megan T. Baldridge RH Vy Lam Chengwei Luo Yumei Cao Pippa Simpson Michael Hayward Holtz Mary L. Pavlos Bousounis Joshua Noe Diana Lerner Jose Cabrera Vincent Biank Michael Stephens Curtis Huttenhower McGovern Dermot P.B. Xavier Ramnik J. Stappenbeck Thaddeus S. and Salzman Nita H. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 2016;1:e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj JS, Betrapally NS, Hylemon PB, Thacker LR, Daita K, Kang DJ, White MB, et al. Gut Microbiota Alterations can predict Hospitalizations in Cirrhosis Independent of Diabetes Mellitus. Sci Rep 2015;5:18559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014. [DOI] [PubMed] [Google Scholar]
- 17.Nardelli S, Allampati S, Riggio O, Mullen KD, Prakash R, Gioia S, Unser A, et al. Hepatic Encephalopathy Is Associated with Persistent Learning Impairments Despite Adequate Medical Treatment: A Multicenter, International Study. Dig Dis Sci 2016. [DOI] [PubMed] [Google Scholar]
- 18.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012;107:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 2012;303:G675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz L, Borrero MJ, Ubeda M, Conde E, Del Campo R, Rodriguez-Serrano M, Lario M, et al. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 22.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 23.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int 2013;33:1457–1469. [DOI] [PubMed] [Google Scholar]
- 24.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest 2012;42:439–446. [DOI] [PubMed] [Google Scholar]
- 25.Tranah TH, Vijay GK, Ryan JM, Shawcross DL. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis 2013;28:1–5. [DOI] [PubMed] [Google Scholar]
- 26.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013;8:e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes after Fecal Microbiota Transplant in Cirrhosis. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol 2018;113:1177–1186. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, Hylemon PB, et al. Systems Biology Analysis of Omeprazole Therapy in Cirrhosis Demonstrates Significant Shifts in Gut Microbiota Composition and Function. Am J Physiol Gastrointest Liver Physiol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep 2016;6:34055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pustelny C, Komor U, Pawar V, Lorenz A, Bielecka A, Moter A, Gocht B, et al. Contribution of Veillonella parvula to Pseudomonas aeruginosa-mediated pathogenicity in a murine tumor model system. Infect Immun 2015;83:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol 2017;23:8355–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol 2013;108:1601–1611. [DOI] [PubMed] [Google Scholar]
- 35.Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 2013;4:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017;67:1084–1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.