Abstract
There is an urgent need to understand the molecular signaling pathways that drive or mediate the development of hepatocellular carcinoma (HCC). The focal adhesion kinase (FAK) gene, PTK2, is amplified in 16.4% of The Cancer Genome Atlas HCC specimens, and its amplification leads to increased FAK messenger RNA expression. It is not known whether the overexpression of FAK alone is sufficient to induce HCC or whether it must cooperate in some ways with other oncogenes. In this study, we found that 34.8% of human HCC samples with FAK amplification also show β-catenin mutations, suggesting a co-occurrence of FAK overexpression and β-catenin mutations in HCC. We overexpressed FAK alone, constitutively active forms of β-catenin (CAT) alone, or a combination of FAK and CAT in the livers of C57/BL6 mice. We found that overexpression of both FAK and CAT, but neither FAK nor CAT alone, in mouse livers was sufficient to lead to tumorigenesis. We further demonstrated that FAK’s kinase activity is required for FAK/CAT-induced tumorigenesis. Furthermore, we performed RNA sequencing analysis to identify the genes/signaling pathways regulated by FAK, CAT, or FAK/CAT. We found that FAK overexpression dramatically enhances binding of β-catenin to the promoter of androgen receptor (AR), which leads to increased expression of AR in mouse livers. Moreover, ASC-J9, an AR degradation enhancer, suppressed FAK/β-catenin-induced HCC formation. Conclusion: FAK overexpression and β-catenin mutations often co-occur in human HCC tissues. Co-overexpression of FAK and CAT leads to HCC formation in mice through an increased expression of AR. This mouse model will be useful for further studies of the molecular mechanisms in the pathogenesis of HCC and could lead to the identification of new therapeutic targets.
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and the second leading cause of cancer deaths worldwide.(1) The 5-year overall survival of patients with HCC is less than 12%, and most patients with HCC have limited treatment options.(2) A major obstacle to treatment is that diagnosis often occurs at an advanced stage of the disease, and current systemic treatments provide only a modest survival benefit.(3) There is an urgent need to develop new and more effective therapeutic strategies and agents to treat HCC, but these efforts are limited by our incomplete understanding of the molecular signaling pathways that drive or mediate the development of the disease.
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that can be phosphorylated and activated by integrins and growth factors.(4,5) FAK targets multiple downstream signaling pathways to regulate different cellular functions.(6) FAK is often overexpressed in HCC specimens,(7,8) offering a potential target for the treatment of HCC. We have recently reported that deletion of Fak in hepatocytes or the administration of a FAK inhibitor blocks tumor proliferation and development and prolongs the survival of experimental animals in a mesenchymal epithelial transition factor (c-Met)/β-catenin-driven HCC mouse model,(9,10) suggesting that FAK is required for c-Met/β-catenin-driven hepatocarcinogenesis. However, it is not yet known whether the overexpression of FAK by itself is sufficient for hepatocarcinogenesis or whether there is a need for cooperation with other oncogene products to induce HCC.
In this study, we show that concomitant FAK overexpression and β-catenin mutations often occur in human HCC samples. We further demonstrate that neither the overexpression of FAK nor constitutively active β-catenin (CAT), each by themselves, will lead to the development of liver tumors in mice, whereas co-overexpression of these two genes results in hepatocarcinogenesis. Most tumors that developed in FAK/β-catenin-overexpressed mice displayed histologic features reminiscent of and expression markers characteristic of human HCC. Using RNA sequencing (RNA-seq) analysis, we found that many gene sets related to hepatocarcinogenesis are enriched in FAK/β-catenin-induced HCCs. Finally, we found that overexpression of FAK enhances the expression of androgen receptor (AR) by β-catenin in mouse livers and also that inhibition of AR dramatically suppresses FAK/β-catenin-induced HCC initiation. Considered together, the data presented here support an important role for both the FAK and β-catenin pathways in human HCC development. The FAK/β-catenin mouse model might be useful both to further understand the biology of HCC and to test novel potential therapies.
Materials and Methods
Plasmids
The plasmids pT3-IRES-GFP, pT3-FAK-IRES-GFP, pT3-FAK (K454R)-IRES-GFP, pT3-CAT, and pT3-MET were generated by Gateway cloning as described.(9,10) The plasmids were purified using GeneJET Plasmid Maxiprep Kit (Thermo Fisher Scientific) for hydrodynamic tail vein injection.
Mice and Treatments
All animals received humane care according to the Guide for the Care and Use of Laboratory Animals (http://oacu.od.nih.gov/ac_cbt/guide3.htm). The procedures for all animal experiments were approved ahead of performance of the experiments by the Institutional Animal Care and Use Committee of Loyola University Chicago. The mice were housed in micro-isolator cages in a room illuminated from 7:00 am to 7:00 pm (12:12-hour light-dark cycle) and were allowed access to water and chow ad libitum.
Five male and five female C57BL/6J mice were injected hydrodynamically with control (45 µg pT3-EF1α-GFP + 5 µg HSB2 transposase plasmid), FAK (22.5 µg pT3-EF1α-FAK + 22.5 µg pT3-EF1α-GFP + 5 µg HSB2), MET (22.5 µg pT3-EF1α-MET + 22.5 µg pT3-EF1α-GFP + 5 µg HSB2), CAT (22.5 µg pT3-EF1α-CAT + 22.5 µg pT3-EF1α-GFP + 5 µg HSB2), FAK/MET (22.5 µg pT3-EF1α-FAK + 22.5 µg pT3-EF1α-MET + 5 µg HSB2), FAK/CAT (22.5 µg pT3-EF1α-FAK + 22.5 µg pT3-EF1α-CAT + 5 µg HSB2), or FAK (K454R)/CAT (22.5 µg pT3-EF1α-CAT+ 22.5 µg pT3-EF1α-FAK [454R] + 5 µg HSB2) to study the role of FAK in hepatocarcinogenesis. Mice were sacrificed either 24 weeks or 2 weeks after treatment, and livers were collected. The liver weight and body weight for each mouse were measured.
For ASC-J9 treatment, 2 weeks after the injection of FAK/CAT, we treated 5 male and 5 female mice with vehicle (30% Captisol) or 75 mg/kg ASC-J9 by intraperitoneal injection every other day for 8 weeks. Mice were sacrificed 14 weeks after the last treatment with either vehicle or ASC-J9. To test the potential toxicity of ASC-J9, 5 male and 5 female mice were injected with pT3-GFP and 1 week later were treated with vehicle (30% Captisol) or 75 mg/kg ASC-J9 by intraperitoneal injection every other day for 8 weeks. These mice were sacrificed 1 day after the last treatment with vehicle or ASC-J9. All livers were collected for analysis.
Western Blotting
Western blotting was performed as described.(9,10,11,12) Primary antibodies against FAK (#3285) and phospho-β-catenin (S675) (#4176) were purchased from Cell Signaling (Danvers, MA). The β-catenin antibody (#610153) was purchased from BD Transduction Laboratories (San Jose, CA). The AR antibody (#sc-7305) was purchased from Santa Cruz (Santa Cruz, CA), and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (#G8795) was purchased from Sigma-Aldrich (St Louis, MO). The p-β-catenin (Y654) antibody (PA5–38440) was purchased from Life Technology (Grand Island, NY).
Quantitative Real-Time Polymerase Chain Reaction
Cellular mRNA was extracted using Zymo mini-columns (Zymo Research Corp., Irvine, CA). Quantitative real-time polymerase chain reactions (RT-PCRs) were performed as described.(11,13) Primers used for RT-PCR are listed in Supporting Table S1.
Immunohistochemical Staining
Immunohistochemical (IHC) staining was performed as described.(9,10) Cells with positive staining were scored in at least five fields at 400× or 200× magnification and reported as mean ± standard deviation (SD). Three mice were used per group. The Ki-67 antibody (#RM-9106) was purchased from Fisher Scientific (Hampton, NH), and the alpha-fetoprotein (AFP) antibody (#A0008) was purchased from DAKO (Troy, MI).
RNA-seq and Analysis
RNA from livers of mice injected with control, FAK, CAT, or FAK/CAT for 2 or 24 weeks was extracted using Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA). RNA-seq was performed by the bioinformatics core of the University of Chicago and analyzed by the bioinformatics core of Loyola University Chicago. Detailed information is provided in Supporting Procedures. Gene set enrichment analysis (GSEA) was performed using the GSEA software (http://software.broadinstitute.org/gsea/index.jsp).
Chromatin immunoprecipitation assay
Mice hydrodynamically injected with control, FAK, CAT, or FAK/CAT were euthanized at 2 weeks after injection, and chromatin immunoprecipitation (ChIP) assays were performed using a β-catenin antibody (#610153, BD, San Jose, CA). Details are provided in Supporting Procedures. The primers are listed in Supporting Table S1.
Human Sample Analysis
Alterations of FAK (PTK2) and β-catenin DNA were analyzed from publicly available The Cancer Genome Atlas (TCGA) data from cBioPortal (http://www.cbioportal.org/). The overall survival data (Kaplan-Meier estimates) were analyzed by Dr. Cara Joyce, a statistician from Loyola University Chicago.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software. Data are presented as means ± SDs. Statistical significance was calculated using the Student t test. P < 0.05 was considered to be significant. The means ± SDs are shown in the figures where applicable.
Results
FAK amplification commonly occurs in human HCC patients.
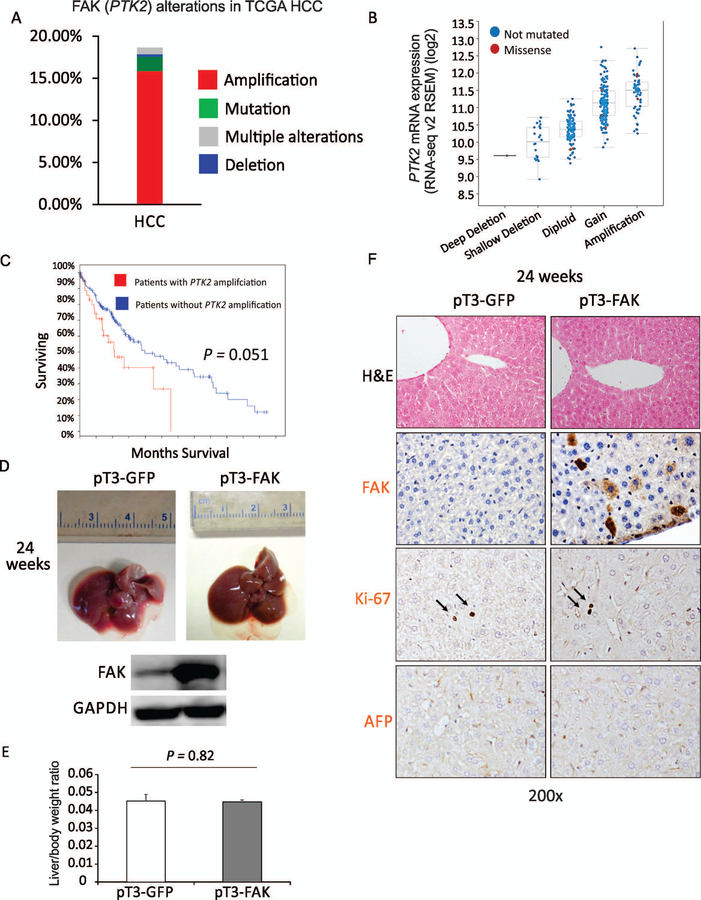
We first analyzed FAK DNA dysregulation (PTK2) in HCC from publicly available TCGA data (provisional) from cBioPortal (http://www.cbioportal.org/). (13,14) We found a high incidence of PTK2 gene alteration observed in human HCCs. In 366 HCC samples with sequencing and copy number alteration data, 16.4% of HCC cases showed amplification of PTK2 DNA copy number, and 2.5% (9) had one missense mutation (Fig. 1A). Interestingly, 6/9 of mutated samples (N629S, M102V, I497T, D604G, R550L, and N135K) showed gain or amplification of DNA copy number. Deletion of PTK2 was found in only 0.3% (1/366) of HCC specimens. Importantly, gain or amplification of FAK DNA copy number was correlated with increased FAK mRNA expression in HCC specimens (Fig. 1B). Moreover, FAK alteration in HCC specimens very closely approached statistical significance (P = 0.051) in being correlated with poor prognosis (Fig. 1C). In general, these data indicate that FAK amplification/overexpression commonly occurs in human HCC and is very closely correlated with poor prognosis.
FIG. 1.
FAK amplification occurs regularly in human HCC patients, but overexpression of FAK is insufficient to induce HCC in mice. (A) PTK2 gene alterations in the TCGA HCCs. (B) Correlation of PTK2 mRNA expression level with gene alterations in the TCGA HCCs. (C) Overall Kaplan-Meier survival estimates in TCGA HCC patients with or without PTK2 amplification. (D) Top, photographs of the livers of C57B6/J mice 24 weeks after injection of pT3-GFP or pT3-FAK. Bottom, expression of FAK and GAPDH proteins in the livers of C57B6/J mice 24 weeks after injection of pT3-GFP or pT3-FAK. (E) Liver weight/body weight ratios were analyzed in mice from (D) (5 males and 5 females per group). (F) H&E and IHC staining for FAK, AFP, and Ki-67 were analyzed in mice from (D) (n = 5).
Overexpression of FAK is not sufficient to induce HCC in mice.
It remains unknown whether overexpression of FAK alone is sufficient to induce HCC. To address this question, we hydrodynamically injected a previously generated FAK transposon vector, pT3-EF1α-FAK-IRES-GFP (pT3-FAK),(9) or a control plasmid (pT3-EF1α-IRES-GFP (pT3-GFP) with the transposase vector (HSB2) into C57BJ/6 mice. We first confirmed overexpression of FAK in approximately 15% of hepatocytes in the livers injected with pT3-FAK compared to those injected with control (Fig. 1D,F). However, no tumors, nodules, or lesions were found in the mice even 24 weeks after injection of pT3-FAK (Fig. 1D,E), suggesting that overexpression of FAK alone is insufficient to induce HCC in mice. Consistent with the physical inspection of livers, IHC staining for Ki-67 (a proliferation marker) or AFP, a common HCC marker, in the livers showed these organs to be unaffected by the overexpression of FAK (Fig. 1F).
Concurrent overexpression of FAK and β-Catenin mutation is evident and correlated with poorer prognosis in human HCC specimens.
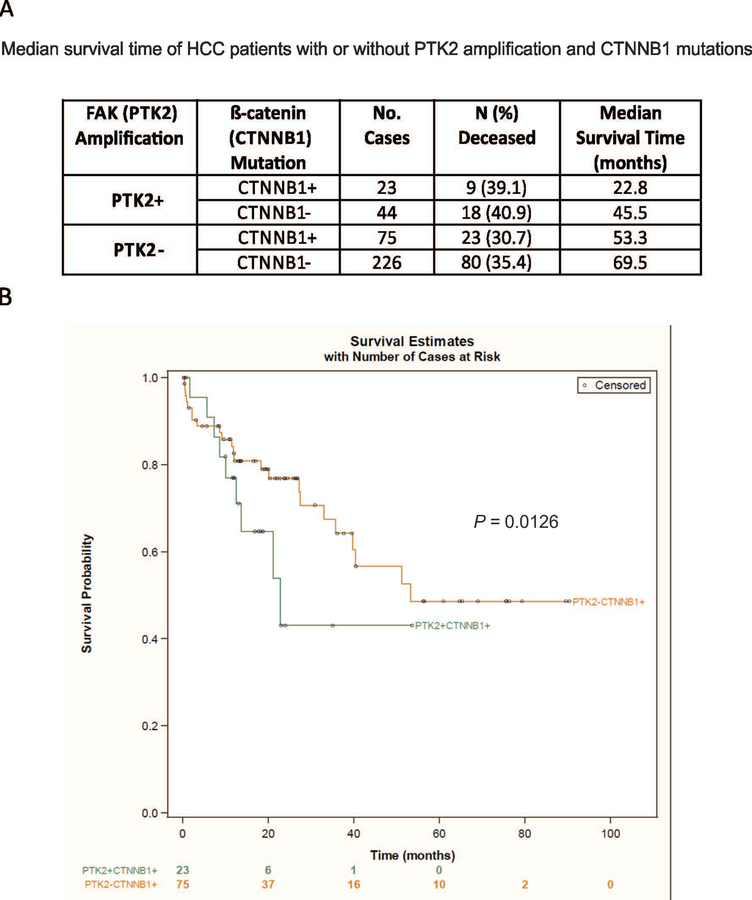
Cooperation among various oncogenes is commonly required for hepatocarcinogenesis.(15) For example, c-MET and CAT induce hepatocarcinogenesis only when administered together.(16) Also, coactivation of protein kinase B (AKT) and CAT, but neither AKT nor CAT alone, rapidly induces HCC in C57BL/6 mice.(17) Thus, it is possible that overexpression of FAK needs to cooperate with other oncogenes to induce HCC. We therefore analyzed the enrichment of mutations among the HCC specimens showing FAK amplification in the TCGA data (provisional). Intriguingly, we found the most enriched mutation in HCC patient samples with FAK amplification is β-catenin (34.8%, 23/66; Supporting Table S2). On the other hand, FAK amplification is also one of the most highly enriched instances of DNA dysregulation (23.7%, 23/97) in HCC patient samples showing β-catenin mutations (Supporting Table S2). Notably, β-catenin is mutated in 26.5% (97/366) of HCC samples, and 82.5% mutations occurred within exon 3 of β-catenin (18.4% mutations at S45, 15.5% at S33, and 15.5% at D32), which contains multiple glycogen synthase kinase 3β (GSK3β) phosphorylation sites. Overall, 6.3% (23/366) of TCGA HCC specimens reveal co-occurrence of FAK amplification and β-catenin mutations (Supporting Table S2).
We further analyzed the TCGA database to ascertain whether co-occurrence of FAK amplification and β-catenin mutations is associated with a poorer prognosis for HCC patients. Indeed, we found that HCC patients with both alterations had reduced survival compared to patients with either one alteration or no alternations (Fig. 2A,B). Altogether, this novel observation suggests a possible cooperation between the FAK and β-catenin signaling pathways in promoting the development of human HCC.
FIG. 2.
Concurrent overexpression of FAK and β-catenin mutation is evident in human HCC specimens. (A) The median survival time in TCGA HCC patients who have PTK2 amplification with or without CTNNB1 mutations or who have CTNNB1 mutations with or without PTK2 amplification. (B) Overall Kaplan-Meier survival estimates for TCGA HCC patients who have CTNNB1 mutations with or without PTK2 amplification.
Co-expression of FAK and CAT induces HCC in mice
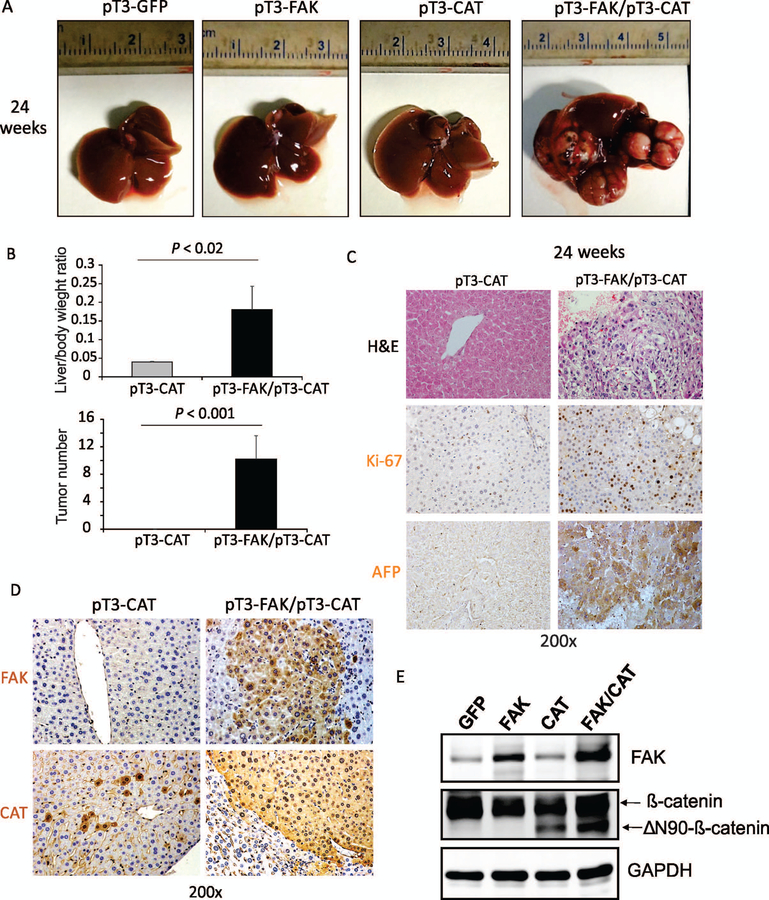
We further determined whether co-expression of FAK and active β-catenin induces hepatocarcinogenesis. We hydrodynamically delivered to the livers of mice a combination of FAK and CAT (ΔN90/β-catenin, CAT, exon 3 of β-catenin is deleted) or ΔN90/β-catenin alone. As reported,(17) the overexpression of ΔN90/β-catenin did not lead to any tumor formation in mice even 24 weeks after injection (Fig. 3A,B). In striking contrast, co-expression of FAK and ΔN90/β-catenin, which will be referred to henceforth as FAK/CAT, led to a lethal burden of liver tumors by 24 weeks after injection in 100% of mice (both males and females were affected; Fig. 3A,B). Macroscopically, multiple HCC nodules formed in the livers of FAK/β-catenin mice; these varied in size from 200 to 500 μm3. The tumors showed a trabecular growth pattern with increased cellular density, cytological atypia, enlarged pleomorphic nuclei, and frequent mitoses (Fig. 3C and Supporting Fig. S1). Consistently, the tumors showed high expression of Ki-67, AFP, arginase1, glypican 3, and HepPar1, which are all typical features of HCC (Fig. 3C and Supporting Fig. S1). We also confirmed the overexpression of FAK and active β-catenin in these FAK/CAT-induced tumors (Fig. 3D,E). Considered altogether, these data indicate that co-overexpression of FAK and CAT induces HCC in mice.
FIG. 3.
Co-expression of FAK and CAT induces HCC in mice. (A) Top, photographs of the livers of C57B6/J mice 24 weeks after injection of pT3-GFP, pT3-GFP/pT3-FAK, pT3-GFP/pT3-CAT, or pT3-FAK/pT3-CAT. (B) Liver weight/body weight ratios and tumor numbers were analyzed in the mice from (A) (5 males and 5 females per group). (C) Staining of H&E, Ki-67, and AFP was analyzed in the mice from (A). (D) IHC staining of FAK and β-catenin was analyzed in the mice from (A). (E) Expression of FAK, β-catenin, and GAPDH proteins in the livers of C57B6/J mice 24 weeks after injection of pT3-GFP, pT3-GFP/pT3-FAK, pT3-GFP/pT3-CAT, or pT3-FAK/pT3-CAT.
Co-overexpression of FAK and c-MET does not induce HCC in mice
β-catenin does not phosphorylate FAK, but FAK was required for β-catenin-induced cyclin D1 expression in a kinase-independent fashion.(10) We therefore examined whether FAK can mimic β-catenin in cooperating with MET to induce HCC. We hydrodynamically injected a combination of pT3-FAK and pT3-MET or pT3-GFP and pT3-MET into C57BJ/6 mice. We did not observe tumor formation in the mice injected with either pT3-FAK/pT3-MET or pT3-GFP/pT3-MET by 24 weeks after injection (Supporting Fig. S2A,B). In a consistent manner, overexpression of FAK did not affect cell proliferation in mouse livers injected with pT3-MET (Supporting Fig. S2C). These data suggest that co-expression of FAK and c-MET in mouse livers is not sufficient to induce HCC. Consistent with this, only 0.82% (3/366) of TCGA HCC patients with FAK amplification contain MET amplification, suggesting that FAK does not cooperate with c-MET to induce HCC in humans.
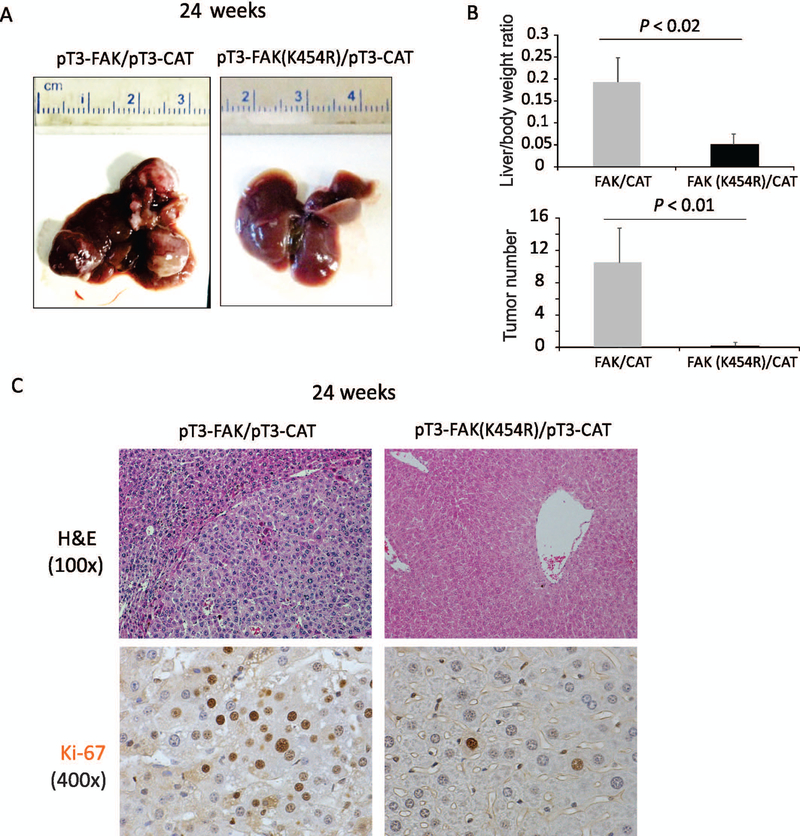
FAK activity is required for FAK/CAT-induced hepatocarcinogenesis
FAK is a non-receptor tyrosine kinase, and its kinase activity plays a critical role in many of its physiological functions.(4,5) However, FAK also has kinase-independent functions important for other cellular processes.(18,19) To investigate whether FAK/CAT-induced tumor formation requires FAK’s kinase activity, we examined whether co-expression of a FAK kinase-null mutant (FAK [K454R]) together with CAT induces HCC in mice. Strikingly, combined injection of FAK (K454R) and ΔN90/β-catenin led to a complete abrogation of tumorigenesis in mice as late as 24 weeks after injection (Fig. 4A–C). This observation highlights the critical co-requirement for β-catenin in the induction of HCC by FAK’s kinase activity.
FIG. 4.
FAK kinase activity is required for FAK/CAT-induced hepatocarcinogenesis. (A) Photographs of the livers of C57B6/J mice 24 weeks after injection of pT3-FAK/pT3-CAT or pT3-FAK (K454R)/pT3-CAT. (B) Liver weight/body weight ratios and tumor numbers were analyzed in the mice from (A) (5 males and 5 females per group). (C) Staining for H&E and Ki-67 was analyzed in the mice from (A).
Genetic pathway analysis of overexpression of FAK alone, CAT alone, and FAK/CAT in the liver
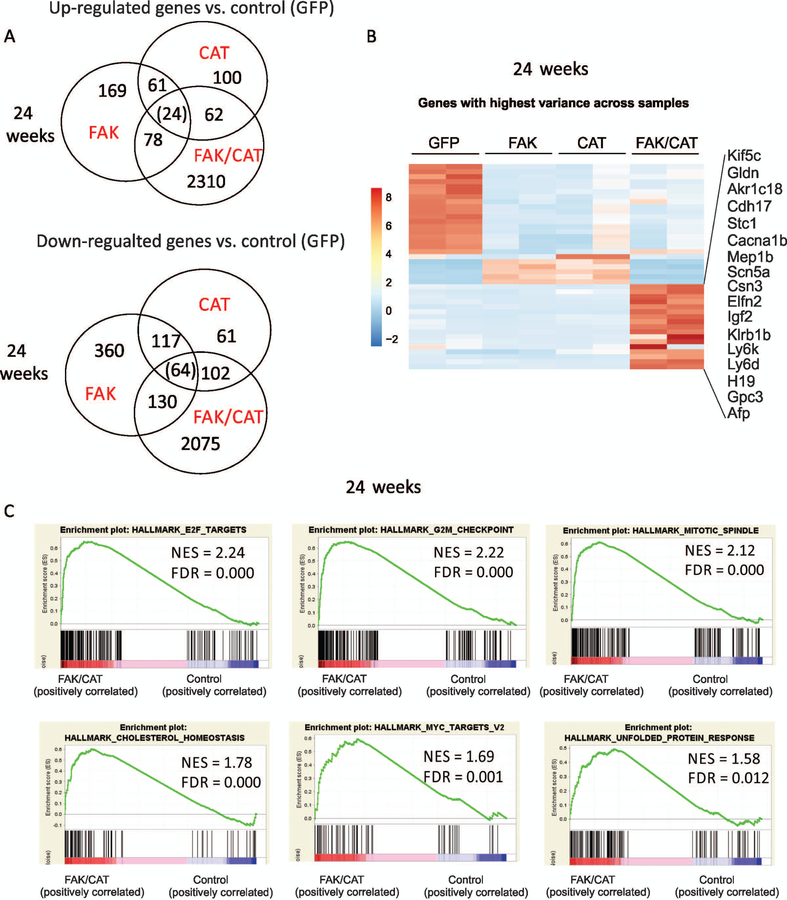
To further understand the molecular mechanisms by which cooperation of FAK overexpression with β-catenin activation induces HCC, we performed RNA-seq analysis to analyze the molecular signaling pathways in our liver samples injected with control, FAK, CAT, or FAK/CAT for 24 weeks. Interestingly, many more genes were up-regulated and down-regulated in mouse livers by the combination of FAK + CAT than by FAK alone or CAT alone (Fig. 5A,B). We then performed a GSEA. We found that many gene sets were significantly affected by the overexpression of FAK in mouse livers. The most significantly up-regulated gene sets included oxidative phosphorylation, fatty acid metabolism, Myc targets, adipogenesis, mechanistic target of rapamycin complex 1 (Mtocr1) signaling, and heme metabolism (Supporting Fig. S3). Activation of β-catenin affected some of the same pathways as FAK, such as oxidative phosphorylation, Myc targets, adipogenesis, and heme metabolism (Supporting Fig. S4). However, β-catenin activation also affected different signaling pathways, including protein secretion and unfolded protein response (Supporting Fig. S4). On the other hand, the combined activation of FAK and β-catenin significantly affected different pathways, including transcriptional factor E2F targets, G2/M checkpoint, mitotic spindle formation, and cholesterol hemostasis (Fig. 5C and Supporting Fig. S5,6), suggesting that there are unique gene signatures for the combined activities of FAK and CAT. In addition, we further analyzed the RNA-seq data to identify genes that are altered by FAK or CAT alone and more significantly altered by FAK/CAT co-transfection. We used the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database to analyze the enriched pathways among these genes. We found a number of enriched signaling pathways, including cell cycle, cellular senescence, chronic myeloid leukemia, Foxo signaling, and phosphoinositide 3-kinase (PI3K)/AKT signaling (Supporting Fig. S7). These signaling pathways may contribute to tumorigenesis in FAK/CAT-induced HCC.
FIG. 5.
Gene pathway analysis in mouse livers 24 weeks after overexpression of FAK alone, CAT alone, and FAK/CAT. (A) Diagram of up-regulated and down-regulated genes affected by the overexpression of FAK, CAT, or FAK/CAT for 24 weeks. (B) A heat map indicates genes with highest variances across samples 24 weeks after injection with GFP, FAK, CAT, or FAK/CAT. (C) GSEA shows gene sets up-regulated by FAK/CAT at 24 weeks.
FAK enhances β-catenin-induced AR expression in the promotion of hepatocarcinogenesis
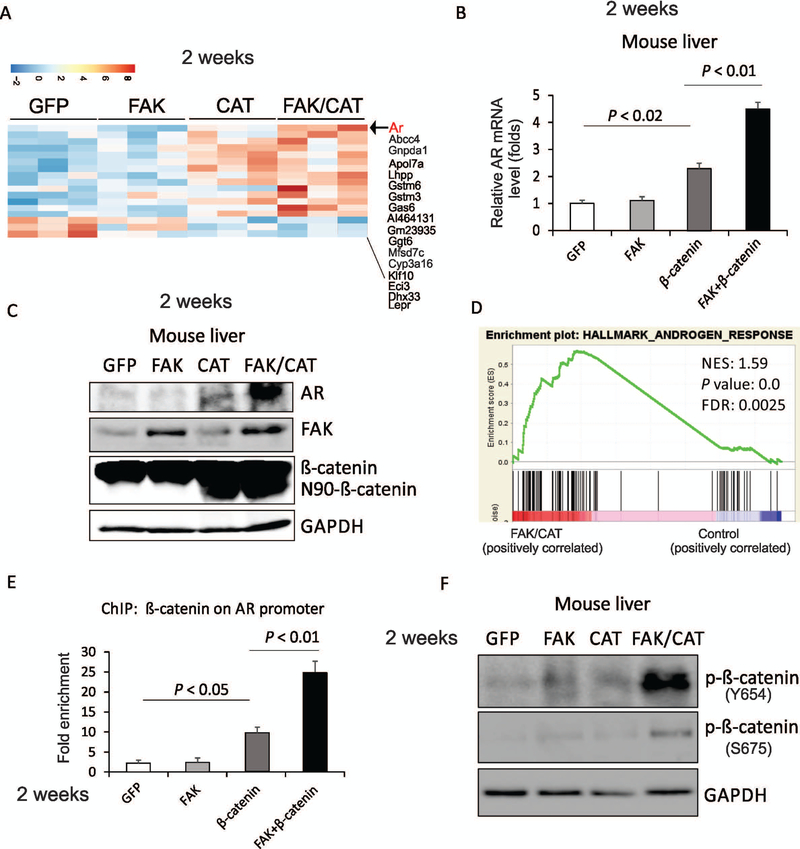
Because tumors had already formed in FAK/CAT-treated samples by 24 weeks after administration, it was possible that many of the changes in gene expression observed in these samples were the passengers rather than the drivers of tumor growth. To look for the early drivers of tumorigenesis by FAK/CAT, we collected liver samples injected with control, FAK, CAT, or FAK/CAT 2 weeks following administration, a point in time when there was as yet no tumor formation in any of the groups. Interestingly, AR is among the genes with the highest variance affected by FAK/CAT, but by neither FAK nor CAT singly (Fig. 6A), which was further confirmed by RT-PCR and western blot data (Fig. 6B,C). This was consistent with GSEA data, which indicated that the AR gene set was enriched in the liver samples of mice treated by the combination of FAK + CAT compared to the samples injected with control (Fig. 6D). We also confirmed that AR expression was higher in FAK/CAT-induced liver tumors compared to FAK- or CAT-overexpressed mouse livers (Supporting Fig. S8). AR signaling is known to be involved in the initiation of carcinogen- or hepatitis B virus (HBV)-related HCC.(20,21) It has been reported that AR regulates a number of downstream targets, such as Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit (EZH2),(22) mature microRNA (miR)-520f,(23) telomerase reverse transcriptase,(24) ETS Proto-Oncogene 1 Transcription Factor (ETS-1),(25) Nanog,(26) reactive oxygen species (ROS),(20) and p53,(20) to promote hepatocarcinogenesis. Therefore, it is possible that the combination of FAK plus β-catenin induces AR to promote the development of HCC. AR has been shown to be a direct transcriptional target of β-catenin/transcription factor 4 (TCF4).(27) We therefore hypothesized that FAK overexpression may enhance the binding of β-catenin to the promoter of AR. Indeed, ChIP assays revealed that overexpression of FAK enhances the binding of β-catenin to the promoter of AR (−377 to approximately −322) in the mouse liver (Supporting Fig. S9 and Fig. 6E). These data indicate that FAK enhances β-catenin-mediated AR transcription in the livers of mice.
FIG. 6.
Two weeks after FAK overexpression enhances the induction of AR by β-catenin in mouse livers. (A) A heat map indicates genes with highest variances across the mouse liver tissues 2 weeks after injection with GFP, FAK, CAT, or FAK/CAT. (B) mRNA level of Ar in the mouse liver tissues that were injected with GFP, FAK, CAT, or FAK/CAT for 2 weeks. (C) Protein levels of AR, FAK, β-catenin, and GAPDH in the mouse liver tissues that were injected with GFP, FAK, CAT, or FAK/CAT for 2 weeks. (D) GSEA shows androgen response pathway is enriched in mouse liver tissues after overexpression of FAK/CAT for 2 weeks. (E) ChIP analysis reveals the binding of β-catenin to the Ar promoter in mouse liver tissue injected with GFP, FAK, CAT, or FAK/CAT for 2 weeks. (F) Phosphorylation of β-catenin on Y654 and S675 was examined by western blot in mouse liver tissues injected with GFP, FAK, CAT, or FAK/CAT for 2 weeks.
Phosphorylation of β-catenin by tyrosine kinases is important in Wnt signaling.(28) Phosphorylation of β-catenin on tyrosine 654 results in this protein’s release from cadherins and provokes a conformational change in the C-terminus of β-catenin, enhancing the phosphorylation of β-catenin on serine 675 by protein kinase A (PKA), and results in an increase in TCF-mediated transcriptional activity.(29) We found that FAK kinase activity is critical for FAK/β-catenin induced hepatocarcinogenesis (Fig. 5). Therefore, we hypothesized that FAK, like other tyrosine kinases, such as Src,(28) might phosphorylate β-catenin on tyrosine 654, thereby enhancing β-catenin’s transcriptional activity. Indeed, phosphorylation of β-catenin on tyrosine 654 was enhanced by the co-overexpression of FAK and β-catenin in mouse livers (Fig. 6F). In agreement with this notion, FAK overexpression also increased the phosphorylation of β-catenin on serine 675 (Fig. 6F). These results suggest that FAK phosphorylates β-catenin on tyrosine 654, thereby enhancing β-catenin’s transcriptional activation of the Ar gene.
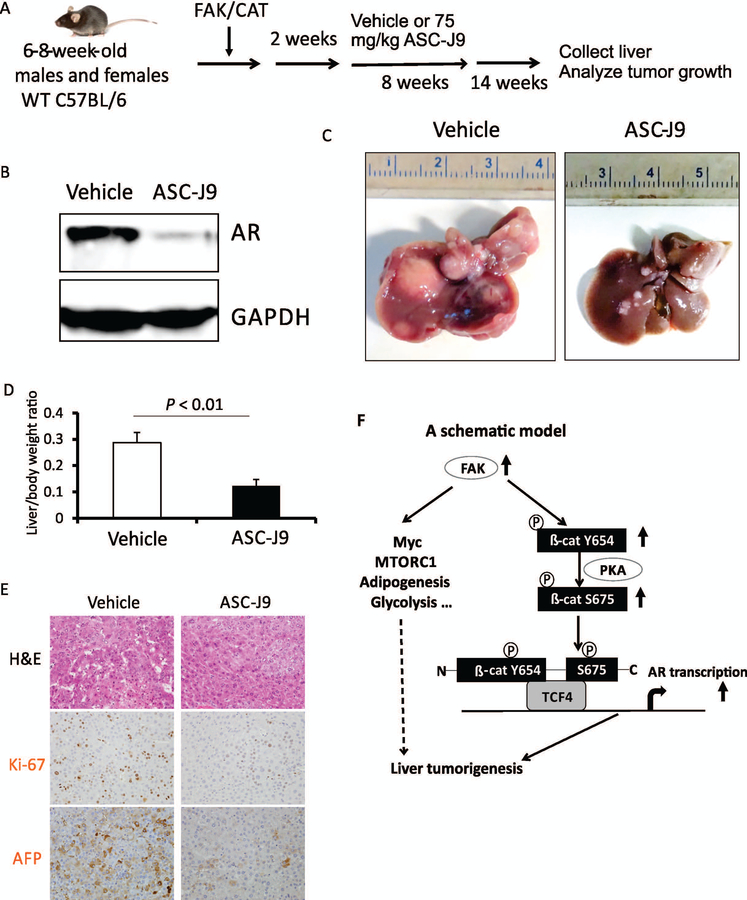
To further determine if enhanced AR expression contributes to hepatocarcinogenesis induced by the co-expression of FAK and CAT, we treated both male and female mice with ASC-J9, an AR degradation enhancer,(30) for 8 weeks; injection of ASC-J9 was initiated 2 weeks after the injection of FAK/CAT into the animals (Fig. 7A). Mouse weight was not significantly affected by ASC-J9 treatment (Supporting Fig. S10), suggesting that AR inhibition is minimally toxic, as has been reported.(30) We confirmed the degradation of AR by ASC-J9 in the livers of mice injected with FAK/CAT (Fig. 7B). Importantly, FAK/CAT-induced tumor development was significantly inhibited by ASC-J9 in both male and female mice (Fig. 7C,D; data not shown), suggesting that AR promotes HCC development in an androgen-independent manner. Proliferation of tumors was also decreased by ASC-J9 (Fig. 7E). Overall, these results indicate that FAK/β-catenin induces hepatocarcinogenesis by enhancing AR expression (Fig. 7F).
FIG. 7.
AR inhibitor ASC-J9 inhibits FAK/CAT-induced hepatocarcinogenesis. (A) Diagram of the experimental protocol. (B) Expression of AR protein in the livers of C57B6/J mice treated with vehicle or ASC-J9 in FAK/CAT-injected mice. (C) Photographs of the livers of C57B6/J mice treated with vehicle or ASC-J9 for 8 weeks in FAK/CAT-injected mice. (D) Liver weight/body weight ratios and tumor numbers were analyzed in the mice from (C) (5 males and 5 females per group). (E) H&E, Ki-67, and AFP staining were analyzed in the mice from (C). (F) A schematic model: FAK overexpression and activation results in phosphorylation of β-catenin on Y654, which enhances the phosphorylation of β-catenin on serine 675 by protein kinase A (PKA). These lead to an enhancement of the transcriptional activity of β-catenin with respect to AR, thereby promoting hepatocarcinogenesis. FAK might also promote liver tumorigenesis by up-regulating Myc, MTORC1, adipogenic, and glycolytic signaling pathways.
Discussion
We reported that FAK is required for c-MET/β-catenin-induced HCC tumor initiation and progression,(9,10) although it was not known whether overexpression of FAK alone was sufficient for HCC development or if additional oncogene products were needed to induce HCC. In this study, we showed that concomitant FAK overexpression and β-catenin mutations occur in human HCC samples, and we demonstrated that overexpression of both FAK and active β-catenin, but neither FAK nor β-catenin alone, results in hepatocarcinogenesis in a mouse model. In addition, most tumors that developed in FAK/β-catenin mice display histologic features reminiscent of human HCC and display the expression of markers that are also consistent with this disease.
The finding that AR expression was synergistically increased by the combination of FAK and β-catenin provides a plausible pathway for FAK/CAT-induced tumorigenesis. Our data indicate that FAK overexpression enhances the binding of β-catenin to the promoter of AR, thereby enhancing the transcription of AR. Interestingly, expression of other β-catenin-targeted genes, such as Axin2, glutamine synthetase, and regucalcin, were not enhanced by FAK overexpression (Supporting Fig. S11), suggesting that FAK does not enhance β-catenin/TCF4’s overall transcriptional activity and that the regulation of β-catenin-induced AR by FAK is gene-specific. It is notable that AR is increased by the combination of FAK + β-catenin in both male and female mice. Accordingly, the AR inhibitor ASC-J9 suppresses FAK/CAT-induced liver tumor development in both genders. These data suggest that AR promotes HCC development in an androgen-independent manner. Despite the well-known mechanism of AR activation by androgen, AR has been shown to be activated by intracellular kinase signals, cytokines, and osteoblast-derived factors.(31–33) For example, interleukin 6 (IL-6) increases transactivation of AR through interactions with signal transducer and activator of transcription 3 (STAT3).(32) Epidermal growth factor (EGF) signaling enhances ligand-independent AR transactivation and promotes the activation of Src and the Ras-Raf-mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway.(34) Indeed, we found that ERK activation is enhanced in the FAK/CAT-overexpressed mouse liver compared to the CAT-only treatment in both male and female mice (Supporting Fig. S12A). Therefore, AR might be activated by cytokines (e.g., IL-6) or growth factors (e.g., EGF) in FAK/β-catenin-overexpressed livers, thereby promoting tumor development.
For the first time, the gene expression patterns affected by FAK overexpression in the mouse liver have been revealed. The gene sets that are most significantly up-regulated by FAK overexpression include oxidative phosphorylation, Myc targets, fatty acid metabolism, adipogenesis, and MTORC1 signaling. Myc-, mTORC1-, and adipogenesis-related signaling have been shown to promote tumor growth and progression.(35–37) It is possible that FAK promotes HCC development through up-regulating these signaling pathways. FAK overexpression also up-regulates the glycolysis gene set (Supporting Fig. S13). Expression of a number of genes that regulate glycolysis, including Tpi1, Pklr, Eno1, Prkaca, Aldob, and Aldoc, was increased by FAK overexpression. FAK has recently been shown to promote tumor glucose metabolism in human pancreatic ductal adenocarcinoma cells (PDACs).(38) In that study, the authors found that the overexpression of FAK increased enolase 1 (ENO1) expression in PDAC lines, which has now been further supported by our data from in vivo models. FAK overexpression/activation promoting tumorigenesis by modulating glycolysis, maintaining the energy and carbon supplies necessary to meet high metabolic requirements during tumor growth and survival, might indeed be a common mechanism across different tumor tissue types.(39) An examination of whether the inhibition of FAK could suppress glycolysis, thereby stifling HCC growth, would clearly seem to be warranted in future studies. The regulation of oxidative phosphorylation by FAK was unexpected. Oxidative phosphorylation, which is important for cancer cells to meet their energy demands by enhancing glycolysis, is often impaired or reduced in cancer cells.(40) Indeed, oxidative phosphorylation is down-regulated in FAK/CAT-induced liver tumors compared to FAK-overexpressed livers (data not shown). As a result, it is possible that the increase in oxidative phosphorylation induced by FAK overexpression alone might halt tumorigenesis. Therefore, FAK overexpression can positively and negatively regulate signaling pathways that are critical for hepatocarcinogenesis, which may result in a balance of cell proliferation and controlled cell growth.
Many genes related to the development of human HCC were dysregulated in FAK/CAT-induced tumors. For example, cadherin 17 (Cdh17), a member of the cadherin family, is among the genes with the greatest increase in expression in FAK/CAT-treated samples compared to control-, FAK- or CAT-treated samples (Fig. 5B). Cdh17 regulates intercellular adhesion, and overexpression of CDH17 has been found in many types of cancer, including HCC.(41) However, the precise mechanistic role of CDH17 in hepatocarcinogenesis remains unknown. Our FAK/CAT-induced HCC model should be suitable for studying the role of CDH17 in HCC initiation as well as testing the effects of CDH17 inhibitors on HCC development. H19, a long noncoding RNA and an imprinted gene, regulates cell proliferation, and its overexpression has been shown to be associated with cancer initiation, progression, and metastasis.(42) It has been reported that H19 suppresses polyploidy, which results in genomic instability and leads to tumorigenesis in polyploid bone marrow mesenchymal stromal cells and mouse liver cells.(43,44) c-Myc directly induces H19 by allele-specific binding.(45) Indeed, we found that c-Myc protein expression was increased (Supporting Fig. S12B) and c-Myc targets were up-regulated (Fig. 5C) in FAK/CAT-induced tumors. Therefore, it is possible that H19 expression was increased in FAK/CAT-induced HCC through enhancing the expression of c-Myc. Of note, the expression of insulin-like growth factor 2 (IGF2), another imprinted gene closely linked to H19,(46) was also significantly increased in FAK/CAT-induced tumors. Up-regulation of IGF2 occurs in both childhood and adult malignancies.(47) The transcription factor E2F3 directly regulates IGF2 expression during development by binding and activating fetal IGF2 promoter sequences.(48) The expression of E2F3 was also increased in FAK/CAT-induced tumors (RNA-seq data), suggesting a possible mechanism for IGF2 induction. In general, many instances of gene dysregulation occurring in mouse FAK/CAT-induced HCC closely resemble the changes observed in human HCCs. Therefore, the FAK/β-catenin HCC mouse model may represent a relevant preclinical model for studying some of the mechanisms of hepatocarcinogenesis and should be useful for testing candidate therapeutic drugs to treat HCC.
In conclusion, FAK overexpression and β-catenin mutations co-occur in human HCC tissues. Our study shows that co-expression of FAK and active β-catenin is sufficient to induce HCC by enhancing AR expression. The FAK/β-catenin-driven HCC model represents a clinically relevant preclinical model that may be useful for studying the mechanisms of hepatocarcinogenesis and testing the efficacy of potentially therapeutic drugs to treat human HCC.
Supplementary Material
Acknowledgment:
We thank Dr. Shang and Mr. Wang for performing experiments, analyzing data, and writing the paper; Mr. Bank and Mr. Perara for breeding mice and performing experiments; Dr. Joyce, Dr. Kuffel, and Dr. Zilliox for analyzing data; Dr. Cotler, Dr. Dhanarajan, and Dr. Breslin for critically revising the paper; and Dr. Qiu for designing experiments, analyzing data, and writing the paper. We also thank Dr. Mitchell F. Denning, Dr. Nancy Zeleznik-Le, Dr.Jiwang Zhang, and Dr. Manuel Diaz of Loyola University Chicago for their helpful discussions and advice.
Financial Support: This study was supported in part by grants to W.Q. (AASLD Liver Scholar Award, ACS RSG-18-107, and NIH R03CA195183, R03CA184652, and R01CA197128).
Abbreviations:
- AFP
alpha-fetoprotein
- AKT
protein kinase B
- AR
androgen receptor
- c-MET
mesenchymal epithelial transition factor
- CAT
constitutively active β-catenin
- ChIP
chromatin immunoprecipitation
- FAK
focal adhesion kinase
- H&E
hematoxylin and eosin
- HCC
hepatocellular carcinoma
- GSEA
gene set enrichment analysis
- IGF2
insulin-like growth factor 2
- IHC
immunohistochemical
- mRNA
messenger RNA
- MTORC1
mammalian target of rapamycin complex 1
- RNA-seq
RNA sequencing
- RT-PCR
real-time polymerase chain reaction
- SD
standard deviation
- TCF4
transcription factor 4
- TGCA
The Cancer Genome Atlas
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1).Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- 2).Johnson PJ. Non-surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 4).Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005;6:56–68. [DOI] [PubMed] [Google Scholar]
- 5).Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011;63:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, et al. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res 2004;10:2812–2817. [DOI] [PubMed] [Google Scholar]
- 8).Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, et al. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol 2004;41:104–111. [DOI] [PubMed] [Google Scholar]
- 9).Shang N, Arteaga M, Zaidi A, Cotler SJ, Breslin P, Ding X, et al. FAK kinase activity is required for the progression of c-MET/beta-catenin-driven hepataocellular carcinoma. Gene Expr 2016;17:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shang N, Arteaga M, Zaidi A, Stauffer J, Cotler SJ, Zeleznik-Le NJ, et al. FAK is required for c-Met/beta-catenin-driven hepatocarcinogenesis. Hepatology 2015;61:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Arteaga M, Shang N, Ding X, Yong S, Cotler SJ, Denning MF, et al. Inhibition of SIRT2 suppresses hepatic fibrosis. Am J Physiol Gastrointest Liver Physiol 2016;310:G1155–G1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Shang N, Arteaga M, Chitsike L, Wang F, Viswakarma N, Breslin P, et al. FAK deletion accelerates liver regeneration after two-thirds partial hepatectomy. Sci Rep 2016;6:34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol 2014;184:912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A 2007;104:14771–14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, et al. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res 2011;71:2718–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol 2010;189:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 2008;29:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008;135:947–955, 955 e941-e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ma WL, Lai HC, Yeh S, Cai X, Chang C. Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocr Relat Cancer 2014;21:R165–R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Song H, Yu Z, Sun X, Feng J, Yu Q, Khan H, et al. Androgen receptor drives hepatocellular carcinogenesis by activating enhancer of zeste homolog 2-mediated Wnt/beta-catenin signaling. EBioMedicine 2018;35:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Xiao Y, Sun Y, Liu G, Zhao J, Gao Y, Yeh S, et al. Androgen receptor (AR)/miR-520f-3p/SOX9 signaling is involved in altering hepatocellular carcinoma (HCC) cell sensitivity to the Sorafenib therapy under hypoxia via increasing cancer stem cells phenotype. Cancer Lett 2019;444:175–187. [DOI] [PubMed] [Google Scholar]
- 24).Li CL, Li CY, Lin YY, Ho MC, Chen DS, Chen PJ, et al. Androgen receptor enhances hepatic telomerase reverse transcriptase gene transcription after hepatitis B virus integration or point mutation in promoter region. Hepatology 2019;69:498–512. [DOI] [PubMed] [Google Scholar]
- 25).Ren H, Ren B, Zhang J, Zhang X, Li L, Meng L, et al. Androgen enhances the activity of ETS-1 and promotes the proliferation of HCC cells. Oncotarget 2017;8:109271–109288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Jiang L, Shan J, Shen J, Wang Y, Yan P, Liu L, et al. Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma. Oncotarget 2016;7:36814–36828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene 2006;25:3436–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J 2012;31:2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, et al. beta-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut 2011;60:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, et al. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia 2012;14:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem 1999;274:7777–7783. [DOI] [PubMed] [Google Scholar]
- 32).Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem 2002;277:7076–7085. [DOI] [PubMed] [Google Scholar]
- 33).Blaszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, Duncan CP, et al. Osteoblast-derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin Cancer Res 2004;10:1860–1869. [DOI] [PubMed] [Google Scholar]
- 34).Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 1994;54:5474–5478. [PubMed] [Google Scholar]
- 35).Lin CP, Liu CR, Lee CN, Chan TS, Liu HE. Targeting c-Myc as a novel approach for hepatocellular carcinoma. World J Hepatol 2010;2:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol 2014;60:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Zhang Z, Scherer PE. Adipose tissue: the dysfunctional adipocyte—a cancer cell’s best friend. Nat Rev Endocrinol 2018;14:132–134. [DOI] [PubMed] [Google Scholar]
- 38).Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene 2016;35:1926–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev 2009;23:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 41).Liu LX, Lee NP, Chan VW, Xue W, Zender L, Zhang C, et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology 2009;50:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One 2007;2:e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Shoshani O, Massalha H, Shani N, Kagan S, Ravid O, Madar S, et al. Polyploidization of murine mesenchymal cells is associated with suppression of the long noncoding RNA H19 and reduced tumorigenicity. Cancer Res 2012;72:6403–6413. [DOI] [PubMed] [Google Scholar]
- 44).Ravid O, Shoshani O, Sela M, Weinstock A, Sadan TW, Gur E, et al. Relative genomic stability of adipose tissue derived mesenchymal stem cells: analysis of ploidy, H19 long non-coding RNA and p53 activity. Stem Cell Res Ther 2014;5:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, et al. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res 2006;66:5330–5337. [DOI] [PubMed] [Google Scholar]
- 46).Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev 2008;19:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Livingstone C IGF2 and cancer. Endocr Relat Cancer 2013;20:R321–R339. [DOI] [PubMed] [Google Scholar]
- 48).Lui JC, Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc Natl Acad Sci U S A 2013;110:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.