Summary
Although varicoceles are a widely accepted identifiable male factor in infertile couples, the benefit of varicocele repair in improving pregnancy and live birth rates remains uncertain. The Study for Future Families obtained semen and reproductive hormone samples from US men whose partners were currently pregnant. In our analysis cohort of 709 men, a varicocele was detected by clinical exam in 56 (8%) of men. Men with varicocele had smaller left testis, and lower total and total motile sperm counts than men without varicocele. Gonadotropin levels were higher as well in men with varicocele. Interestingly, testosterone levels were also slightly higher in men with varicocele. Despite these differences, there was no difference between the groups in the time to achieve the study pregnancy or percentage of men with a previous pregnancy. We conclude that even in fertile men varicoceles are associated with some degree of testicular hypofunction. This would support current recommendations to consider varicocele repair in male partners in infertile couples who demonstrate both a varicocele and abnormal semen parameters and after evaluation for treatable female factors.
Keywords: Varicocele, semen, fertile, men, male hormones
Introduction
Although varicoceles are the most widely accepted identifiable male factor in infertile couples, the benefit of varicocele repair in improving pregnancy and live birth rates remains uncertain (Chiba, Ramasamy, Lamb & Lipshultz, 2016; Kroese, de Lange, Collins & Evers, 2012; Will et al., 2011). While it is acknowledged that varicoceles can be present in fertile men, the majority of studies describing semen parameters and reproductive hormone profiles in men with varicocele have been in men referred for fertility evaluation (WHO 1992). Studies in men of known fertility are more limited and most were done prior to the introduction of current semen analysis techniques and reference standards (DeCastro & Mastrorocco, 1984; Redmon, Carey & Pryor, 2002).
The Study for Future Families (SFF) recruited men living in five geographic areas in the US whose female partners were currently pregnant in order to examine geographic difference in semen parameters (Swan et al. 2003). Men participating in SFF underwent a physical examination which included evaluation for presence or absence of varicocele and estimation of testicular size. Men also provided semen and blood samples for determination of semen parameters and reproductive hormone levels. Extensive data were also obtained from both partners on demographic factors, health history and habits, prior reproductive history and details on fertility attempts culminating in the study pregnancy.
In this report we describe semen parameters, reproductive hormone levels and other characteristics of SFF men with and without varicocele.
Materials and Methods
Study population and design
The design and research protocol for the SFF has been previously described (Brazil et al., 2004a; Redmon et al., 2013; Swan et al., 2003). Briefly, women pregnant with unassisted pregnancies (median pregnancy stage 27 weeks) attending prenatal clinics affiliated with SFF clinical sites in Los Angeles (Harbor-UCLA and Cedar Sinai Medical Centers); Minneapolis (University of Minnesota); Columbia, Missouri (University Physicians) and New York (Mount Sinai School of Medicine) were prospectively recruited between September, 1999 and June, 2002. In 2002, the University of Iowa (Iowa City, Iowa) was added as a fifth SFF clinical site and enrolled couples between September, 2002 and February, 2005. Due to unforeseen circumstances the center at Mount Sinai was closed early in the study resulting in limited recruitment at the New York study site. Human subject approvals were obtained from Institutional Review Boards at all participating institutions.
Participating couples completed questionnaires providing demographic data; lifestyle and habits; reproductive and other medical history; and occupational history. Detailed information was obtained characterizing the couples fertility attempts for each month beginning with the last menstrual period (LMP) month of the study pregnancy and extending back for the previous twelve months prior to the LMP month.
Male partners underwent a physical examination by a study physician or other experienced study personnel including measurement of height, weight and testicular volume by orchidometer. By site, examiners were urologists (NY site), an adult endocrinologist/andrologist and a urologist (MN site), an experienced urology nurse (IA site), a reproductive endocrinologist and a trained research nurse (MO site) and an experienced andrology research assistant (CA site). Men were examined standing for presence of absence of clinical varicocele. Varicoceles were graded as 1 (only detected during Valsalva), 2 (palpable but not visible) or 3 (visible) (Dubin & Amelar, 1970).
Semen Analysis
The detailed methods for collection and analyses of semen samples were previously described (Brazil et al., 2004a). Men were asked to observe a 2 – 5 day abstinence period and then provide semen samples by masturbation at study clinics. Samples were not rejected if a man deviated from the requested abstinence period, but for this analysis we arbitrarily excluded 34 men with very short (< 2 hours) or very long (> 240 hours) reported abstinence times. Almost all samples (95%) were analyzed within 45 minutes of collection. Semen volume was measured by weight and sperm concentration was determined by counting using a hemacytometer. Morphology was assessed both by WHO, 1999 criteria (strict criteria) and by older WHO, 1987 criteria in the central laboratory by two observers, one for each morphology classification as previously described (Swan et al., 2003).
To assure comparability in analysis of semen samples among sites, the study’s central andrology laboratory at University California Davis provided central training of all lab technicians, and proficiency testing and quarterly quality control testing was performed throughout the study period (Brazil et al., 2004b).
Hormone measurements
Venous blood samples were obtained for hormone measurements and stored at - 80° C as previously described (Mendiola et al., 2011). While blood sampling was not specifically scheduled for morning hours, approximately 85% of samples were obtained between 7 am and noon. FSH, LH, SHBG, total testosterone and total estradiol levels were measured by immunoassay at the Ringshospitalet (Copenhagen, Denmark). Free testosterone (FT) was calculated using the equation of Vermeulen (Vermeulen, Verdonck & Kaufman, 1999).
Statistical Analysis
Groups were compared with respect to continuous outcomes initially using one-factor ANOVA and for categorical outcomes by chi-square or Fisher’s exact test. We tested for associations between varicocele status and semen and hormone parameters using multiple linear regression adjusted for potential confounders. For semen parameters the model adjusted for center, age of the man, BMI, current smoker (yes/no) and abstinence time for the semen sample. The model for analysis of reproductive hormone parameters adjusted for center, age of the man, BMI, smoking status and time of day of the blood sample for hormone measurements. Since sperm concentration, total sperm count and total motile sperm count were highly skewed, these data were log transformed before analysis, and the results back-transformed for reporting. In order to integrate the various semen parameters into a single measure, we employed methodology recently described by Damsgaard et al. to characterize each man’s semen analysis as “low” (sperm concentration < 15 million/ml and/or motility < 32% and/or morphology (strict) < 4%), “high” (sperm concentration > 40 million/ml and motility > 50% and morphology (strict) > 9%), or “intermediate” (all others) (Damsgaard et al., 2016). P values ≤ 0.05 were considered significant.
Results
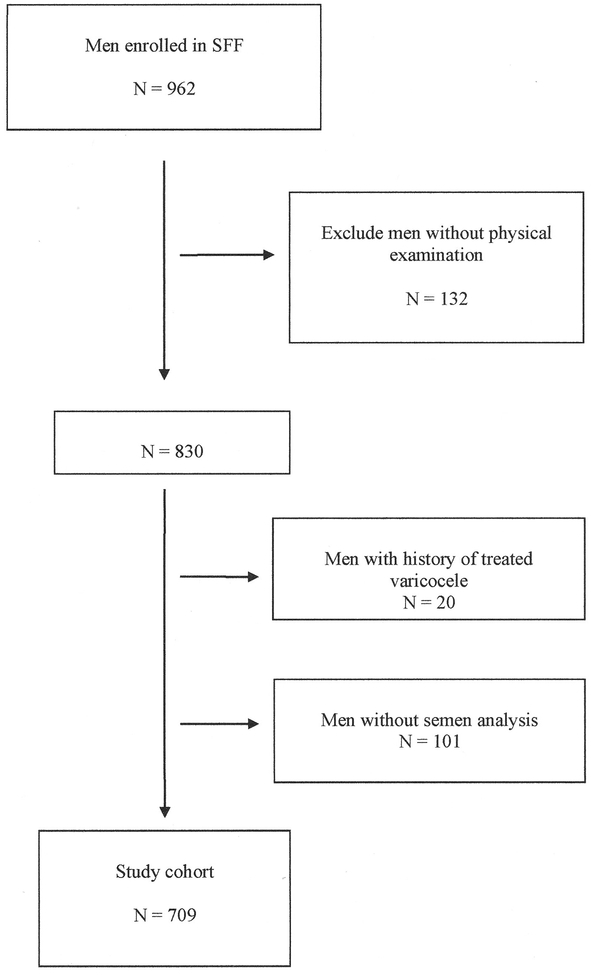
Nine hundred and sixty two men enrolled in SFF. For this analysis we excluded 132 men (14%) who declined a physical exam, 20 men (2%) who reported a prior history of treated varicocele and 101 men (10%) who declined to give a semen sample or who provided a sample with very short (< 2 hours) or very long (> 240 hours) abstinence time, giving a total of 709 men in the final analysis cohort (Figure 1).
Figure 1.
Flow diagram showing the disposition of men in the study cohort.
Table I shows characteristics of men in the cohort. Fifty-six (8%) of men were noted to have a varicocele on exam. Three-quarters of these were unilateral left varicoceles and three-quarters were detected by palpation (grade 2). Unilateral right or bilateral varicoceles were found in 12% of men. There was a significant difference in the prevalence of varicocele among study centers ranging from <1% (1/179 men) at the CA site to 23% (8/35 men) at the NY site.
Table I. Characteristics of study cohort.
Categorical data shown as number of men (%). Percents of the row total are given for the varicocele groups; percents of the total N (709) are given in the total column. Continuous data shown as mean ± SD.
| Varicocele | No varicocele | Total | P Value | |
|---|---|---|---|---|
| Number of men | 56 (8) | 653 (92) | 709 | |
| Varicocele | ||||
| Left only | 42 (75) | |||
| Right only | 7 (12) | |||
| Bilateral | 7 (12) | |||
| Varicocele grade | ||||
| Palpable with Valsalva | 9 (14) | |||
| Palpable | 47 (75) | |||
| Visible | 7 (11) | |||
| Age (yr) | 33 ± 5 | 31 ± 6 | 32 ± 6 | 0.03 |
| BMI (kg/m2) | 28.8 ± 5.9 | 28.5 ± 5.3 | 28.6 ± 5.4 | 0.75 |
| Current smoker | 12 (8) | 133 (92) | 145 (21) | 0.88 |
| Center | ||||
| CA | 1 (1) | 178 (99) | 179 (25) | |
| MN | 23 (12) | 169 (88) | 192 (27) | |
| MO | 17 (9) | 180 (91) | 197 (28) | |
| NY | 8 (23) | 27 (77) | 35 (5) | |
| IA | 7 (7) | 99 (93) | 106 (15) | < 0.0001 |
| Race | ||||
| Hispanic/Latino | 4 (4) | 109 (96) | 113 (16) | |
| White | 46 (9) | 461 (91) | 507 (72) | |
| Black | 6 (11) | 49 (89) | 55 (8) | |
| Other | 0 (0) | 32 (100) | 32 (5) | 0.06 |
| Previous pregnancy | 33 (7) | 414 (93) | 447 (63) | 0.47 |
| Time to pregnancy (mos) | 5.3 ± 4.8 | 5.4 ± 4.8 | 5.4 ± 4.8 | 0.92 |
| Left testes volume (ml) | 19 ± 6 | 22 ± 7 | 22 ± 7 | 0.003 |
| Right testes volume (ml) | 22 ± 5 | 23 ± 6 | 23 ± 6 | 0.15 |
Men with varicocele were slightly older than the men without as were their pregnant partners. There was no difference in BMI in men with or without varicocele. No varicoceles were noted in men who reported their race as Asian/Other, although the number of men in this group was small. There was no difference between men with and without varicocele in time to conceive the SFF pregnancy or percentage with a previous pregnancy with their partner. Left testicular volume was significantly smaller for men with varicocele (19 ± 6 vs 22 ± 7 ml, p = 0.003; mean ± SD).
Table II shows semen parameters for men by presence or absence of varicocele. Average abstinence time for the study semen analysis was 3 days and was slightly shorter for men with varicocele although the difference was minimal. Total sperm count (165 vs 214 million, adjusted p = 0.04) and total motile sperm count (78 vs 107 million, adjusted p = 0.03) were significantly lower in men with varicocele compared to men without. Average semen parameters for men with varicocele still fell within the range typically seen in fertile men. However when we categorized semen quality as low, intermediate or high, men with varicocele were more likely to have overall lower quality semen than men without varicocele using this integrated measure.
Table II. Semen parameters.
Categorical data shown as number of subjects (%). Continuous data shown as mean ± SD, (5th-50th-95th percentiles). Means for sperm concentration, total sperm count and total motile sperm count are geometric means. Adjusted P values from the multivariate model reflect adjustment for center, age of the man, BMI, current smoker (yes/no) and abstinence time for the semen sample.
| No | Unadjusted | Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Varicocele | Varicocele | Total | P Value | P Value | ||||||
| Number of men | 56 (8) | 653 (92) | 709 | |||||||
| Abstinence time (days) | 2.9 | 3.3 | 3.2 ± 1.3 | 0.02 | -- | |||||
| Semen volume (ml) | 3.5 ± 1.6 | 3.9 ± 1.6 | 3.9 ± 1.6 | 0.11 | 0.29 | |||||
| Sperm concentration | 51 | 60 | 60 | 0.15 | 0.10 | |||||
| (x106/ml) | (12-67-192) | |||||||||
| Total sperm count | 165 | 214 | 210 | 0.04 | 0.04 | |||||
| (x106) | (32-240-765) | |||||||||
| Motile sperm (%) | 49 ± 11 | 51 ± 11 | 51 ±11 | 0.12 | 0.09 | |||||
| (29-52-67) | ||||||||||
| Total motile sperm count | 78 | 107 | 104 | 0.03 | 0.03 | |||||
| (x106) | (14-129-395) | |||||||||
| Normal sperm (%) | 10 ± 5 | 11 ± 5 | 11 ± 5 | 0.20 | 0.71 | |||||
| Semen quality (%) | ||||||||||
| Low | 12 (11) | 100 (89) | 112 (16) | |||||||
| Intermediate | 33 (9) | 328 (91) | 361 (51) | |||||||
| High | 11 (5) | 225 (95) | 236 (33) | 0.03 | ||||||
Blood samples for hormone measurements were provided by 682 of the 709 men (96%). Table III shows hormonal parameters by varicocele status. Gonadotropin levels were slightly higher in men with varicocele, although the differences were small and mean values were well within the normal range for both groups. In contrast both total and free testosterone were significantly higher in men with varicocele compared to men without.
Table III. Hormonal parameters.
Categorical data shown as number of subjects (%). Continuous data shown as mean ± SD. Adjusted P values from the multivariate model reflect adjustment for center, age of the man, BMI, current smoker (yes/no) and time of day of blood sampling.
| No | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| Varicocele | Varicocele | Total | P Value | P Value | |
| Number of men | 54 (8) | 628 (92) | 682 | ||
| Age (yr) | 33 ± 5 | 31 ± 6 | 32 ± 6 | 0.02 | -- |
| BMI (kg/m2) | 28.7 ± 5.9 | 28.4 ± 5.2 | 28.4 ± 5.2 | 0.73 | -- |
| Current smoker | 12 (9) | 127 (91) | 139 (20) | 0.75 | -- |
| LH (IU/L) | 4.0 ± 1.5 | 3.6 ± 1.4 | 3.6 ± 1.4 | 0.05 | 0.03 |
| FSH (IU/L) | 3.9 ± 2.5 | 3.3 ± 1.9 | 3.4 ± 2.0 | 0.05 | 0.06 |
| Inhibin b (pg/ml) | 197 ± 75 | 215 ± 83 | 214 ± 82 | 0.12 | 0.4 |
| SHBG (nM) | 32 ± 14 | 30 ± 14 | 30 ± 14 | 0.2 | 0.5 |
| Testosterone (ng/dL) | 617 ± 298 | 540 ± 200 | 546 ± 211 | 0.01 | 0.005 |
| Free Testosterone (ng/dL) | 14 ± 7 | 12 ± 4 | 12 ± 5 | 0.03 | 0.004 |
Discussion
We compared semen and hormone parameters in relation to the presence or absence of varicocele in 709 fertile men participating in the SFF. A palpable varicocele was found in 8% of men. The majority (75%) were unilateral, left sided varicoceles. Sperm counts, both total and total motile, were significantly lower in men with varicocele and men with varicocele were more likely to have overall poorer semen quality using an integrated measure, although the clinical importance of this is unclear. Most men with varicoceles had semen parameters within the range seen in fertile men and there was no difference in time to achieve the SFF pregnancy or in history of previous pregnancy in men with or without a varicocele.
The majority of varicoceles (88%) involved the left testis and left testis volume was significantly lower in men with varicocele. Gonadotropin levels were higher, although only the difference in LH levels reached significance after adjustment. Paradoxically, total and free testosterone levels were significantly higher in men with varicocele.
Most studies of reproductive parameters in men with varicocele have been in men presenting for fertility evaluation (WHO 1992). DeCastro found a palpable varicocele in 97 of 598 men with self-reported fertility presenting for vasectomy (16%) (DeCastro & Mastrorocco, 1984). Only 20 of these 97 men had partners currently pregnant at the time of the study. Overall they found no difference in semen parameters between men with and without a varicocele with the exception of a small difference in percentage of oval-headed sperm. Men without varicocele were more likely to have a sperm concentration ≥ 40 million/ml.
In the largest study to date we are aware of in a population of unknown fertility status, Damsgaard et al. recently reported on the prevalence of varicocele and associated reproductive parameters in over 7,000 young men from six European countries undergoing an examination for military service (Damsgaard et al., 2016). The prevalence of varicocele was 16%. Men with varicocele had lower sperm concentration, total sperm count and percent progressively motile sperm and normal forms. No data were provided as to fertility history of the men.
Studies of reproductive hormone levels in men with varicocele have shown inconsistent findings. Some studies find no difference in testosterone levels (Swerdloff & Walsh, 1975; WHO 1992) or differences in selected subgroups but not all men with varicocele (Comhaire & Vermeulen, 1974) while other studies report lower levels of testosterone in men with varicocele (Tanrikut et al., 2011). Similarly there are inconsistent findings with respect to differences in gonadotropin levels (Hudson, 1988; Swerdloff & Walsh, 1975). Damsgaard et al found that presence of a varicocele was also associated with smaller testes, higher gonadotropins and lower inhibin b (Damsgaard et al., 2016). In that study, testosterone levels did not differ by varicocele status. Our findings are generally consistent with those of Damsgaard et al suggesting some degree of primary testicular hypofunction, at least with respect to spermatogenesis, in men with varicocele. SFF men with varicocele had higher LH and (non-significantly) higher FSH levels and lower inhibin b levels, although their mean testosterone levels were higher than men without varicocele. This latter result may represent a chance finding as mean gonadotropin and testosterone levels were well in the normal range for both groups.
We did not find evidence that presence of a varicocele was associated with any difficulty conceiving in our cohort. The percentage of couples with a previous pregnancy and the time to conceive the SFF pregnancy were not different between men with and without a varicocele. Other studies in men outside of the infertility clinic setting have also failed to find evidence of impaired fertility in men with varicocele (Redmon, Carey & Pryor, 2002).
Strengths of our study include a large number of fertile men (as evidenced by currently pregnant partners) recruited from a wide geographic area in the US and a variety of racial and ethnic groups. Demographic and other data related to the couple and study pregnancy were collected uniformly across sites using standardized questionnaires. Semen samples were collected on site using strict protocols with regular quality control monitoring.
One limitation of our study is the relatively small number of men with a varicocele. The prevalence of varicocele in our cohort was 8% (10% if 20 men with a history of treated varicocele were included). This is in the lower part of the range of other estimates of varicocele prevalence from the literature observed outside the infertility clinic setting (4% - 30%) (Redmon, Carey & Pryor, 2002).
The diagnosis of varicocele in our study was based on clinical exam. We did not perform ultrasound or other imaging studies to detect subtle, non-palpable varicoceles. This is consistent with current recommendations of major professional societies such as the American Society for Reproductive Medicine, the American Urological Association and the European Association of Urology as only clinically palpable varicoceles are considered clearly associated with infertility (Shridharani, Owen, Elkelany & Kim, 2016). Previous studies of the prevalence of varicocele in both unselected men and men undergoing fertility evaluation also used the same diagnostic criteria of palpable varicocele on clinical exam (Damsgaard et al. 2016; DeCastro & Mastrorocco 1984; Redmon, Carey & Pryor, 2002; WHO 1992). Nonetheless, absence of ultrasound confirmation of varicocele status is a limitation of our study and may have contributed to our relatively low prevalence estimate and the observed variability in prevalence between study centers.
There was a significant variation in varicocele prevalence across the SFF study sites – ranging from <1% at the CA site to 23% at the NY site. The CA site did have three men with a history of treated varicocele who were excluded from the analysis. This site also had a higher proportion of Hispanic/Latino men than the other sites, although we are not aware of any data on differences in varicocele prevalence by race/ethnicity. We suspect the differences in prevalence reflect interobserver variability in the diagnosis of varicocele by clinical exam. Even between experienced clinicians, varicocele prevalence estimates on examination of the same men may vary by almost two-fold (DeCastro & Mastrorocco, 1984). In the report by Damsgaard et al the prevalence rates of varicocele varied by 4-fold among the study centers (9% - 38%) (Damsgaard et al., 2016). Misclassification of men in our cohort as to varicocele status would, if anything, reduce our ability to detect differences between the two groups.
Conclusion
In a cohort of fertile men with currently pregnant partners, men with a clinical varicocele had lower total and total motile sperm counts. They also had physical examination findings and reproductive hormone parameters consistent with some degree of mild primary spermatogenic hypofunction compared to men without varicocele. Despite these differences, there was no difference between the groups in the time to achieve the study pregnancy or percentage of men with a previous pregnancy. We conclude that while varicoceles may be associated with some degree of testicular hypofunction, they may not result in semen and reproductive hormone parameters that fall outside the ranges seen in fertile men and may not impact on fertility. This would support current recommendations to consider varicocele repair in men experiencing infertility who demonstrate both a varicocele and abnormal semen parameters.
Acknowledgements
In addition to the authors the Study for Future Families Research Group includes the following: B.S. Carter, D.J. Kelly, S.L. Stewart and T.M. Simmons (University of Missouri); J. W. Overstreet, C. Brazil, C Treece, C Tollner (University of California, Davis); R.S. Swerdloff, L. Lumbreras, S. Villanueva, M. Diaz-Romero, A. Victoroff, R. Sandoval, S. Bravarian, A. Leung and A.L. Nelson (Harbor-UCLA Medical Center); C. Hobel and B. Brock (Cedar-Sinai Medical Center); M. Hatch, M. Pfeiffer, L. Quinones, K. Polgar and A. Brembridge (Mt Sinai School of Medicine); C. Kwong, A. Muehlen, T. Perrier, T. Srb, J. Pryor and C. DeJonge (University of Minnesota). M. Swanson, T. Grider, L. Fisher, M. Maifeld, J. Whitham, A. Wolf, J. Sandlow (University of Iowa); F. Liu (Icahn School of Medicine at Mount Sinai). The authors also wish to acknowledge Dr. Niels Jørgensen and his laboratory at University of Copenhagen, Rigshospitalet for performance of the hormonal assays.
This work was supported by the following grants from the National Institutes of Health: R01-ES09916 to the University of Missouri; M01-RR00400 to the University of Minnesota; M01-RR0425 and UCLA CTSI Grant UL1TR000124 to the Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center and the Cedars-Sinai Research Institute. Support was also provided by the University of Iowa Center for Health Effects of Environmental Contamination cooperative project grant.
References
- 1.Brazil C, Swan SH, Drobnis E, Liu F, Wang C, Redmon JB, Overstreet JW, 2004a. Standardized methods for semen evaluation in a multicenter research study. J Androl. 25, 635–44. [DOI] [PubMed] [Google Scholar]
- 2.Brazil C, Swan SH, Tollner CR, Treece C, Drobnis E, Wang C, Redmon JB, Overstreet JW, 2004b. Quality control of laboratory methods for semen evaluation in a multicenter research study. J Androl.. 25, 645–56. [DOI] [PubMed] [Google Scholar]
- 3.Chiba K, Ramasamy R, Lamb D, Lipshultz L, 2016. The varicocele: diagnostic dilemmas, therapeutic challenges and future perspectives. Asian J Androl. 18, 276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comhaire F, Vermeulen A, 1974. Plasma testosterone in patients with varicocele and sexual inadequacy. J Clin Endocrinol Metab. 40, 824–9. [DOI] [PubMed] [Google Scholar]
- 5.Damsgaard J, Joensen U, Carlsen E, Erenpreiss J, Jensen M, Matulevicius V, Zilaitiene B, Olesen IA, Perheentupa A, Punab M, Salzbrunn A, Toppari J, Virtanen HE, Juul A, Skakkebaek NE, Jorgensen N, 2016. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur J Urol. 70, 1019–29. [DOI] [PubMed] [Google Scholar]
- 6.DeCastro M, Mastrorocco D, 1984. Reproductive history and semen analysis in prevasectomy fertile men with and without varicocele. J Androl. 5, 17–20. [DOI] [PubMed] [Google Scholar]
- 7.Dubin L, Amelar R, 1970. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 21, 606–09. [DOI] [PubMed] [Google Scholar]
- 8.Hudson R, 1988. The endocrinology of varicoceles. Fertil Steril. 49, 199–208. [DOI] [PubMed] [Google Scholar]
- 9.Kroese A, de Lange N, Collins J, Evers J, 2012. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 10 CD000479. [DOI] [PubMed] [Google Scholar]
- 10.Mendiola J, Jorgensen N, Andersson A-M, Calafat A, Silva M, Redmon JB, Sparks A, Drobnis EZ, Wang C, Liu F, Swan SH, 2011. Association between urinary metabolites of di(2-ethylhexyl)phthalate and reproductive hormones in fertile men. Intl J Androl. 34, 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmon J, Carey P, Pryor J, 2002. Varicocele – the most common cause of male factor infertility? Hum Reprod Update. 8, 53–8. [DOI] [PubMed] [Google Scholar]
- 12.Redmon J, Thomas W, Ma W, Drobnis E, Sparks A, Wang C, Brazil C, Overstreet JW, Liu F, Swan SH, 2013. Semen parameters in fertile US men: the Study for Future Families. Andrology. 1, 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shridharani A, Owen R, Elkelany O, Kim E, 2016. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl. 18, 269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swan S, Brazil C, Drobnis E, Liu F, Kruse R, Hatch M, Redmon JB, Wang C, Overstreet JW, Study for Future Families Research Group., 2003. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 111, 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swerdloff R, Walsh P, 1975. Pituitary and gonadal hormones in patients with varicocele. Fertil Steril. 26, 1006–12. [DOI] [PubMed] [Google Scholar]
- 16.Tanrikut C, Goldstein M, Rosoff J, Lee R, Nelson C, Mulhall J, 2011. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 108, 1480–4. [DOI] [PubMed] [Google Scholar]
- 17.Trussell J, Ohl D, Krawetz S, Snyder P, Polotsky A, Patrizio P, et al. , 2014. We need a prospective varicocelectomy trial. Fertil Steril. 101, 1563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen A, Verdonck L, Kaufman J, 1999. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 84, 3666–72. [DOI] [PubMed] [Google Scholar]
- 19.WHO., 1992. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 57, 1289–93. [PubMed] [Google Scholar]
- 20.Will M, Swain J, Fode M, Sonksen J, Christman G, Ohl D, 2011. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 95, 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]