Abstract
One third of smokers diagnosed with cancer continue smoking, perhaps due to low perceived cancer–related benefits of cessation. To examine perceived cancer–related benefits of quitting among newly diagnosed cancer patients who smoke and associations with quit intentions, baseline measures from patients (N = 303) enrolled in a randomized controlled trial were analyzed using hierarchical regression models and bootstrapping. Higher perceived cancer–related benefits of quitting were associated with having a smoking-related cancer and less education. Perceived cancer–related benefits of quitting and quit intentions were positively correlated, particularly among patients with smoking-related cancers. For smokers with smoking-related cancers, perceived cancer–related benefits of quitting are correlated with quit intentions.
Keywords: cancer, health psychology, perception, smoking, smoking cessation
Introduction
Smoking is the leading cause of preventable death in the United States (Centers for Disease Control and Prevention (CDC), 2018). Moreover, it is estimated that 40 percent of all cancers diagnosed in the United States are associated with smoking (CDC, 2016; CDC, 2018). Approximately 1 in 3 patients smoke at or around the time of cancer diagnosis (Sitas et al., 2014). Studies have shown that those who continue to smoke after diagnosis are at higher risk for poorer prognosis, adverse treatmentrelated side effects, and deteriorating quality of life as compared with cancer patients who quit (Florou et al., 2014; Parsons et al., 2010; Trout et al., 2018). In addition, smoking may reduce treatment effectiveness, increase likelihood of recurrence, or promote the development of new primary tumors (CDC, 2016; Sitas et al., 2014; Tseng et al., 2012; United States Surgeon General, 2014). Thus, the delivery of effective smoking cessation services for cancer patients can advance the treatment and outcomes in these patients.
In the general population, studies have shown that current smokers may underestimate the health benefits of quitting smoking. In addition to having an “optimistic bias” about the risks of continued smoking, current smokers perceive fewer health benefits of quitting smoking than do former smokers and nonsmokers (Arnett, 2000; McCoy et al., 1992; Park et al., 2009; Weinstein et al., 2005). To date, less is known about the perceived health benefits of quitting smoking among cancer patients who smoke. Recently, a large survey of cancer patients identified greater perceived risks of continued smoking as a robust predictor of quit intention and cessation (Alton et al., 2018); however, patients’ anticipated benefits of quitting were not assessed. Thus, it remains unclear whether cancer patients’ perceived cancer–related benefits (PCRB) of quitting are associated with their quit intentions.
According to the transtheoretical model (TTM) of behavior change, perceived benefits of change, as well as the salience of these benefits (i.e. proximal or distal), are key factors that can develop and sustain a patient’s readiness to change (Prochaska and Velicer, 1997). In the context of smoking cessation, prior findings suggest that greater perceived benefits of quitting are associated with smoking cessation (Borrelli et al., 2010). Moreover, the TTM suggests that the greater the salience of the consequences of a risky health behavior, the greater the intention to change. For recently diagnosed cancer patients who smoke, quit intentions may, therefore, be higher when (a) PCRB of quitting are higher and (b) they have been diagnosed with a smoking-related cancer. To the best of our knowledge, no study to date has examined the effect of PCRB of quitting on quit intentions.
For cancer patients who smoke, quit intentions may be associated with a variety of factors in addition to PCRB of quitting smoking. Intention to quit may vary by sociodemographic characteristics, including gender, age, marital status, and education. Higher quit intentions are generally reported among women (Guimond et al., 2017; Schnoll et al., 2002), although some findings have been mixed (Ayala-Bernal et al., 2017; Schnoll et al., 2004). Older smokers may be less likely to endorse quit intentions (Yasin et al., 2012), although, again, findings have been mixed (Broms et al., 2004). As compared with married smokers, single smokers may also endorse lower intentions to quit smoking (Broms et al., 2004). In addition, higher quit intentions are associated with higher levels of education and lower levels of financial strain (Martínez et al., 2018; Park et al., 2016; Vinci et al., 2017). Collectively, it would be prudent to control for these potential covariates when examining the unique association between PCRB of quitting and quit intentions.
Moreover, recent evidence suggests that relationships between cognitive-motivational factors and quit intentions are moderated by illness salience. Findings have been mixed about the relationship between cancer stage and intentions to quit smoking (Kumar et al., 2018; Trout et al., 2018). However, emerging evidence suggests that the relationship between perceived risks of smoking and intention to quit is stronger among cancer patients with smoking-related cancers than for nonsmoking-related cancers (Martínez et al., 2018; Schnoll et al., 2003a; Sciamanna et al., 2000). Similarly, the relationship between PCRB of quitting and quit intentions may be also stronger for patients with smoking-related cancers.
To address these gaps, this study sought to (1) describe and identify correlates of PCRB of quitting among newly diagnosed cancer patients who smoke, (2) examine the unique contribution of PCRB of quitting on quit intentions at diagnosis, and (3) explore smoking-relatedness of cancer as potential moderator of the relationship of PCRB of quitting on quit intentions.
Methods
Participants and recruitment
Participants were patients with newly diagnosed or suspected cancers who were identified as current smokers. Eligible participants were adult (age > 18 years) current smokers (having smoked a cigarette, even a puff, within the last 30 days), who were English or Spanish-speaking and receiving their oncology care at any of the three recruiting institutions in the outpatient thoracic, gastrointestinal, genitourinary, breast, head/neck, lymphoma, gynecological, or melanoma clinics. Participants were enrolled in a clinical trial examining the effects of a counseling-enriched smoking cessation treatment (vs standard treatment) on smoking cessation. Recruitment occurred at two major academic medical centers in the Northeast (Massachusetts General Hospital/Dana Farber Cancer Institute in Boston, MA, and the Memorial Sloan Kettering Cancer Center in New York, NY). Detailed protocol methods, eligibility criteria, and consent procedures have been published previously (Park et al., 2016). This research has been approved by the institutional review boards at each participating site.
A total of 4709 patients were identified and prescreened. Overall, 2455 patients were ineligible (chief reason at chart prescreen was receiving health care elsewhere (n = 614), chief reason at confirmation screen was no tobacco use in the past 30 days (n = 317)), 1808 patients refused to be screened, 84 patients refused to enroll (chief reason was being too upset (n = 19)), and 59 patients were never randomized, resulting in a sample of N = 303 enrolled and randomized patients.
Measures and data collection
Participants completed self-report baseline questionnaires upon study entry and medical information was abstracted from participants’ electronic health records. Participants reported their highest level of education attained, insurance status, partnership status (married/living with partner as married), employment status, and smoking rate (cigarettes per day), but age, gender, history of a smoking-related disease, cancer type, and stage were collected from the electronic health record. As has been done previously (Kalkhoran et al., 2018), our investigative team created a composite “smoking-related cancer” variable by categorizing cancer types into smoking-related (lung, esophageal, head and neck, bladder, kidney, liver, pancreatic, colorectal, anal, small intestinal, gastric, or cervical) and nonsmoking-related (prostate, testicular, penile, breast, lymphoma, melanoma, or noncervical gynecologic cancer), guided by conclusions from the US Surgeon General (United States Surgeon General, 2014).
PCRB of quitting were assessed using an investigator-developed, 4-item numerical rating scale ranging from 0 (“Not at all”) to 10 (“Very much”) (Appendix 1). Items assessed the extent to which the participants believed that quitting smoking would lead to the following cancer-related health benefits: (1) improve his or her chances of getting the full benefit from his or her cancer treatment; (2) reduce his or her chances of having complications from treatment; (3) reduce his or her chances of developing the same cancer again; and (4) reduce his or her chances of developing a new type of cancer. Items were summed to create a total composite score, with a potential range from 0 to 40 and higher scores indicating greater PCRB of quitting smoking. In cases where a single item response was missing, the value was mean-imputed with the average of the other three PCRB of quitting items. In the present sample, internal consistency for the 4-item total PCRB of quitting score was acceptable (Cronbach’s alpha = .73).
Quit intentions were assessed using the well-validated Contemplation Ladder (Biener and Abrams, 1991). Participants rated their intention to quit smoking on a rating scale ranging from 0 (“No thought of quitting”) to 10 (“Taking action to quit”).
Data analyses
Analyses using baseline PCRB of quitting and quit intentions data were conducted using SPSS v24. All variables were examined for normality. Descriptive statistics were computed to summarize demographic, medical, and smoking-related characteristics. Zero-order correlations were computed to examine associations among baseline PCRB of quitting smoking, readiness to quit, and sociodemographic and medical factors. An ordinary least squares (OLS) regression model with pairwise deletion was conducted to identify the unique contribution of PCRB of quitting smoking on quit intentions in a stepwise fashion. Step 1 included sociodemographic variables, Step 2 added medical factors, Step 3 added mean-centered PCRB of quitting and cancer type (smoking-related vs other), and Step 4 added an interaction term of Step 3 variables. Changes in R2 of the full model were computed between each step. Finally, using the PROCESS macro (Hayes, 2012), the conditional effects of PCRB of quitting on quit intentions were probed as a function of cancer type (smoking-related vs nonsmoking-related). Post hoc regression models computed simple slopes and used the Johnson-Neyman Procedure to probe the significant interaction in the OLS model and identify values at which the divergence in regression models crossed the threshold of statistical significance (Hayes and Matthes, 2009).
Results
Table 1 presents the participant sociodemographic and baseline PCRB of quitting and quit intentions. About half of the participants were female (56.11%) and most were White (87.46%), had some college education (68.47%), and were middle aged (M = 58.34, standard deviation (SD) = 9.71). Approximately, half of the participants were recently diagnosed with a smoking-related cancer (59.74%). Participants predominantly reported high PCRB of quitting overall (M = 34.67, SD = 7.20) and for each PCRB item: receiving full benefit of treatments (M = 9.20, SD = 1.95), reducing risk for developing a new cancer (M = 8.78, SD = 2.10), reducing risk of complications from cancer treatment (M = 8.55, SD = 2.62), and reducing risk of cancer recurrence (M = 8.14, SD = 2.87). In the overall sample, quit intentions were moderate (M = 5.90, SD = 1.78). Missingness on measures of PCRB of quitting and quit intentions was minimal (<10%), and there were no discernable differences between participants with complete data and those without (ps > .05).
Table 1.
Patient characteristics (N = 303).
| Variable | M (SD)/n (%) | Range |
|---|---|---|
| Age (years) | 58.34 (9.71) | 21–86 |
| Female | 170 (56.11) | |
| Race | ||
| American Indian/Alaska | 3 (1.00) | |
| Native | ||
| Asian | 2 (.66) | |
| Black/African American | 31 (10.23) | |
| White | 265 (87.46) | |
| Other | 2 (.66) | |
| Ethnicity (Hispanic/Latino) | 11 (3.70) | |
| Partnered | 164 (55.59) | |
| Full-time employed | 102 (34.34) | |
| Education (some college or more) | 202 (68.47) | |
| Cigarettes per day | 14.08 (9.89) | 1–70 |
| Smoking-related disease | 148 (48.84) | |
| Smoking-related cancer | 181 (59.74) | |
| Cancer type | ||
| Anal | 4 (1.32) | |
| Bladder | 18 (5.94) | |
| Breast | 77 (25.41) | |
| Cervical | 2 (.66) | |
| Colorectal | 8 (2.64) | |
| Esophageal | 7 (2.31) | |
| Gastric | 1 (.33) | |
| Noncervical gynecologic cancer | 5 (1.65) | |
| Head and neck | 31 (10.23) | |
| Kidney | 8 (2.64) | |
| Liver | 7 (2.31) | |
| Lung | 88 (29.04) | |
| Lymphoma | 9 (2.97) | |
| Melanoma | 6 (1.98) | |
| Pancreatic | 6 (1.98) | |
| Penile | 1 (.33) | |
| Prostate | 23 (7.59) | |
| Small intestinal | 1 (.33) | |
| Testicular | 1 (.33) | |
| Cancer stage | ||
| 0 | 17(6.16) | |
| I | 86 (31.16) | |
| II | 67 (24.28) | |
| III | 53 (19.20) | |
| IV | 53 (19.20) | |
| Indolenta | 3 (.99) | |
| Aggressivea | 5 (1.65) | |
| NA/unknown | 19 (6.27) | |
| Perceived cancer–related benefits of quitting | ||
| (1) Complications of treatments | 8.55 (2.62) | 0–10 |
| (2) Full benefit of treatments | 9.20 (1.95) | 0–10 |
| (3) Recurrence | 8.14 (2.87) | 0–10 |
| (4) New cancer | 8.78 (2.10) | 0–10 |
| Total score | 34.67 (7.20) | 8–40 |
| Quit intentions | 5.90 (1.78) | 1–9 |
SD: standard deviation.
Indolent and aggressive are bifurcations used for nonsolid tumors only.
Table 2 presents the results of the zero-order correlations. PCRB of quitting were negatively associated with education (Spearman’s rho = −.16, p = .009) but were not associated with age (r = −.01, p = .91), gender (Spearman’s rho = .01, p = .91), partnership status (Spearman’s rho = .08, p = .16), or employment status (Spearman’s rho = −.10, p = .10). In terms of medical characteristics, PCRB of quitting were positively associated with the diagnosis of a smoking-related cancer (Spearman’s rho = .18, p = .003), yet were not associated with the number of cigarettes participants smoked per day (r = .01, p = .88), the diagnosis of a smoking-related disease (Spearman’s rho = .09, p = .11), or cancer stage (Spearman’s rho = .02, p = .76). PCRB of quitting was positively associated with greater quit intentions (r = .17, p = .007).
Table 2.
Correlates of perceived cancer–related benefits of quitting.
| Perceived cancer–related benefits of quitting Pearson r/Spearman’s rho (p) | |
|---|---|
| Age | −.01 (.91) |
| Female | .01 (.91) |
| Partnered | .08 (.16) |
| Full-time employed | −.10 (.10) |
| Some college or more | −.16 (.009) |
| Cigarettes per day | .01 (.88) |
| Smoking-related disease | .09 (.11) |
| Smoking-related cancer | .18 (.003) |
| Cancer stage | .02 (.76) |
| Quit intentions | .17 (.007) |
Spearman’s rho was computed for the following dichotomous variables: female, partnered, full-time employed, some college or more, smoking-related disease, smoking-related cancer, and cancer stage.
Table 3 reports the findings from a four-step hierarchical linear regression, which identified predictors of baseline quit intentions. In the overall sample, the final regression model was statistically significant, (F(10, 256) = 1.99, p = .03, R2 = .07). In Step 1, age, gender, marital status, employment, and education were included in the model, but no participant demographics were significantly associated with quit intentions. In Step 2, cigarettes smoked per day and diagnosis of a smoking-related disease were included in the model, and again these variables were not significantly associated with quit intentions. In Step 3, diagnosis of a smoking-related cancer and PCRB of quitting (mean-centered) were included in the model. Greater PCRB of quitting significantly predicted greater quit intentions (B = .04, t = 2.54, p = .01) but having a smoking-related cancer did not (B = .30, t = 1.21, p = .23). In Step 4, an interaction was found such that greater PCRB of quitting and diagnosis of a smoking-related cancer (0 = no, 1 = yes) explained a statistically significant proportion of variance in quit intentions (B = .08, t = 2.59, p = .01).
Table 3.
Predictors of quit intentions at diagnosis.
| B (SE) | t (p) | 95% CI | |
|---|---|---|---|
| Step 1 | |||
| Age | <.01 (.01) | −.04 (.97) | −.02, .02 |
| Female | −.05 (.22) | −.23 (.82) | −.49, .39 |
| Partnered | .31 (.22) | 1.39 (.17) | −.13, .75 |
| Employed full-time | .24 (.25) | .99 (.32) | −.24, .23 |
| Education | .02 (.10) | .21 (.84) | −.18, .22 |
| Step 2 | |||
| Cigarettes per day | −.01 (.01) | −.58 (.57) | −.03, .02 |
| Smoking-related disease | −.02 (.24) | −.07 (.95) | −.48, .45 |
| Step 3 | |||
| (A) Smoking-related cancer | .30 (.24) | 1.21 (.23) | −.18, .77 |
| (B) PCRB of quitting | .04 (.02) | 2.54 (.01) | .01, .07 |
| Step 4 | |||
| A × B interaction | .08 (.03) | 2.59 (.01) | .02, .15 |
| Full model F, p | Constant | R (R2) | A × B ΔR2, p |
| F (10, 256) = 1.99, p = .03 | 5.47 (.84) | .27 (.07) | .02, .01 |
B: unstandardized coefficient; SE: standard error; CI: confidence interval; PCRB: perceived cancer–related benefits (mean-centered).
Values in each step control for the cumulative effects of variables in concurrent and previous steps.
To explore this interaction further, a post hoc analysis was conducted to probe the conditional effect of PCRB of quitting on quit intentions by type of cancer. For nonsmoking-related cancers, the effect of PCRB of quitting on quit intentions was nonsignificant (B < .01, t = .01, p = .99, 95% confidence interval (CI), −.04, .04). However, for participants with smoking-related cancers, there was a significant conditional effect (B = .08, t = 3.52, p = .001, 95% CI, .04, .12). To probe for points of statistical significance at these levels, a Johnson-Neyman Procedure was conducted. As depicted in Figure 1, patients with a smoking-related cancer reported significantly greater quit intentions than patients with a nonsmoking-related cancer when they reported a mean above 37.38 (49.62% of the sample; B = .52, t = 1.97, p = .05, 95% CI, .00, 1.03). Interestingly, patients with a smoking-related cancer had significantly lower intentions to quit than patients with a nonsmoking-related cancer when they reported PCRB of quitting below a mean of 13.70 (1.92% of the sample; B = −1.35, t = 1.97, p = .05, 95% CI, −2.71, .00).
Figure 1.
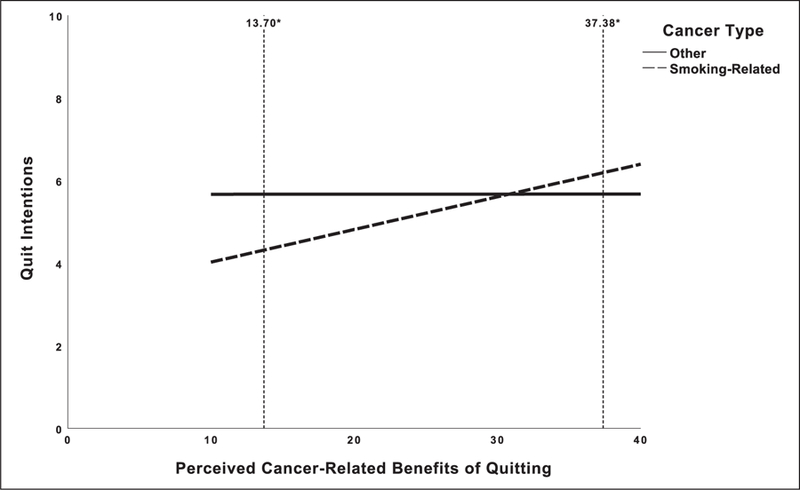
Adjusted quit intentions by perceived cancer–related benefits of quitting and cancer type. Values reflect uncentered estimated marginal means after controlling for age, gender, partnership status, employment, education, smoking (cigarettes per day), and presence of a smoking-related disease.
*Points of statistically significant difference (p = .05) for the conditional effect of perceived cancer–related benefits of quitting by cancer type on quit intentions.
Discussion
The purpose of this study was to describe and investigate associations between PCRB of quitting smoking, smoking-related cancer diagnosis, and intentions to quit smoking for newly diagnosed patients with cancer. Overall, we observed high levels of perceived benefits of quitting smoking among newly diagnosed cancer patients who reported current smoking. Our exploratory moderation analyses revealed that greater perceived benefits of quitting were associated with greater intention to quit smoking and that this relationship was principally accounted for by patients diagnosed with smoking-related cancers. Collectively, these findings have key theoretical implications with regard to models of health behavior change, as well as practical clinical implications for health care providers working with cancer patients who smoke.
Patients tended to endorse high levels of belief that quitting smoking would (1) improve their cancer treatment outcomes and would mitigate the risk for (2) developing a new cancer, (3) incurring treatment-related complications, and (4) cancer recurrence. Consistent with the 2014 Surgeon General’s Report (United States Surgeon General, 2014), these findings support that cancer patients are well aware of the benefits of quitting smoking following cancer diagnosis. We found that patient characteristics significantly associated with greater perceived benefits included lower education and being diagnosed with a smoking-related cancer. Patients with greater educational attainment may be more likely to doubt the accuracy of cancer-related testing (Hall et al., 2018), and this skepticism may generalize to having more modest perceived benefits of quitting smoking. Alternatively, smokers with less education may be more susceptible to exhibiting unrealistic optimism with regard to cancer-related benefits of quitting smoking. Indeed, smokers with less education may perceive fewer risks associated with continued smoking as well (Dillard et al., 2006). We also observed that patients with cancers that were smoking-related (vs not smoking-related) endorsed higher PCRB of quitting, discussed further below. Broadly, these findings build on recent evidence that cancer patients’ risk perceptions about smoking vary as a function of the smoking-relatedness of one’s cancer (Martínez et al., 2018). To the best of our knowledge, this study is the first to report the positive association between having a smoking-related cancer (vs nonsmoking-related) and higher PCRB of quitting smoking.
Our final regression model accounted for modest, yet significant degree of variance in cancer patients’ ratings of their intentions to quit. Post hoc analyses revealed that the strongest association between PCRB of quitting and quit intentions were among patients diagnosed with smoking-related cancers, after controlling for age, gender, partnership status, employment, education, smoking heaviness (i.e. cigarettes per day), and the presence of a comorbid smoking-related disease. Across participants diagnosed with a smoking-related cancer, low levels of perceived benefits (−1 SD) corresponded with a score of approximately 5 on the Contemplation Ladder, “I often think about changing the way that I smoke, but I have not planned to change it yet.” In contrast, high levels of perceived benefits (+1 SD) corresponded with a Contemplation Ladder score of approximately 7, “I definitely plan to change my smoking, and I am ready to make some plans about how to change.” For cancer patients who smoke, this difference in quitting readiness is substantial and may be critical to initiating and sustaining a quit attempt (Martínez et al., 2018). Collectively, these results implicate PCRB of quitting as a potentially modifiable target for future smoking cessation counseling interventions with cancer patients.
These findings provide useful theoretical implications for understanding how patients with differing cancer types process PCRB of quitting smoking. As stated previously, a central component of behavior-change models, such as TTM, focuses on the perceived benefits of a behavior to facilitate transition from pre-contemplation to contemplation to action (Kaufman et al., 2018). Within the context of smoking cessation, TTM-based interventions have provided mixed findings (Aveyard et al., 2009). One reason posited for these mixed findings is that the proposed smoking-related benefits within these interventions are not adequately matched to the stage of behavior change expressed by the patient. As such, patients who do not perceive the benefits as proximally beneficial are less likely to want to contemplate behavior change. Construal level theory (CLT) proposes that there is an interrelation between temporal distance and psychological distance (i.e. how proximal or distal a health outcome is perceived to be relates to how concrete or abstract decision-making is about the behaviors that can prevent or promote that health outcome) (Trope and Liberman, 2010). Previous studies utilizing CLT have primarily focused on the relationship between risk and proximity. For example, Chandran and Menon (2004) found support for greater perceptions of temporal proximity of risk increasing compliance with more difficult preventive behaviors by making a health threat seem more threatening. In addition to perceived risks of continued smoking, patients may also contemplate potential benefits associated with quitting, and the temporal proximity of these perceived benefits may influence their salience. Thus, we hypothesized that participants with a smoking-related cancer would have greater intention to quit smoking than nonsmoking-related cancers due to a greater proximal understanding of the PCRB of quitting. Indeed, results supported this hypothesis, demonstrating that this effect was strongest among participants who reported high PCRB of quitting, which suggests that even within smoking-related cancers there was a high degree of heterogeneity. One possible explanation for this finding is that may have been different perceptions of proximity based on the type of perceived benefit. For example, from a descriptive standpoint the mean score on the item assessing “receiving the full benefit of one’s cancer treatment” was among the most highly endorsed items and, potentially, was perceived to be a more proximal benefit of quitting smoking than the recurrence of a participant’s cancer. As participants in this study had only recently been diagnosed, the PCRB of reducing risk of recurrence may have seemed a more distal, and less important, benefit to promote greater contemplation about quitting.
This study’s focus on the PCRB of quitting also provides further practical implications for clinicians counseling cancer patients who smoke. Previous studies have shown an inconsistent association between risk perceptions among cancer patients and successful cessation. Alton et al. (2018) found an association between current smokers’ perceptions that smoking negatively affects quality of life, survival and fatigue and was strongly associated with smoking cessation. However, other studies that have utilized patient education materials to promote cessation, in which increasing risk perception did not successfully result in cessation (Strecher et al., 2008). As such, fear appeal and risk management theories suggest that communicating risk to cancer patients, who may already possess strong perceptions of threat as a result of their diagnosis, may be a complex process (Dillard and Nabi, 2006). While there remains limited empirical research on preferences of communication type among patients with a smoking-related cancer, focusing on the benefits of quitting aligns with the need to build efficacy and self-confidence in quitting. Without doing so, it could lead to potentially treatment-interfering coping strategies, such as denial and defensive avoidance, that could indirectly lead to worse health outcomes. Therefore, communication between cancer patients and clinicians and researchers should highlight that a diagnosis is a teachable moment (Park et al., 2014). As such, evidence-based shared decision-making at the point of diagnosis may be a useful strategy for distilling the cancer-related benefits of cessation to patients and may lead to meaningfully improved quit intentions.
Benefit finding of quitting is a cognitive activity that requires patients to make predictions about the future, specifically their future health and survival. For patients who are also managing mental health challenges, these predictions will likely be vulnerable to cognitive influences of hopelessness (depression) or fear (anxiety). This is an important consideration as anxiety and depressive psychopathology is much more common among smokers than among the general nonpsychiatric population (Goodwin et al., 2017; Piper et al., 2010; Zvolensky et al., 2018). Similarly, mood and anxiety disorders may interfere with patients’ estimates of their own self-efficacy and intentions to quit. Although mood and anxiety disorders were not assessed in the present report, there is mounting evidence that smoking cessation interventions that reduce anxiety and depression prior to a quit attempt enhance cessation success (Leventhal and Zvolensky, 2015; Lubetkin et al., 2018; Smits et al., 2016) and among patients managing chronic illness (OʼCleirigh et al., 2018).
Limitations and strengths
This study has several limitations. First, the results reported here reflect cross-sectional associations, so no causality can be inferred between correlates of quit intentions and eventual cessation. However, multiple studies, supported by behavior change theories, have shown that intention to perform a behavior is the strongest predictor of successful completion of that behavior (Webb and Sheeran, 2006). Second, there is limited generalizability to racial and ethnic minority groups as the study sample was predominantly nonHispanic/Latino White. A strength of this study, though, was diverse participation of patients across socioeconomic status. Third, generalizability is also limited as enrollment was part of a cessation trial. However, patients who did not wish to quit at the point of enrollment were eligible to participate in the trial, providing a more robust representation of the relationship between cancer patients who continue to smoke and quit intentions in a nontrial setting. Fourth, a variety of factors not examined in this report (e.g. cancer-related distress, pain, psychological treatment, perceived and actual availability of smoking cessation resources, and social support) may also influence quit intentions among cancer patients who smoke and should be assessed in future research examining PCRB of smoking cessation in this population. Finally, a strength of this study was the diversity of cancer types in this sample. Most literature has investigated head/neck and/or lung cancer patients with regard to smoking. Findings from this study therefore address calls for research on differences among patients with diverse cancer types to elucidate the observed differences in smoking rates (Schnoll et al., 2003b; Wakefield et al., 2004).
Conclusion
This study is the first to explore the relationship between PCRB of quitting and intention to quit smoking among recently diagnosed cancer patients. The main study finding was that PCRB of quitting was associated with intention to quit, particularly among patients with smoking-related cancer, highlights the importance of informing patients about the benefits of smoking cessation that are specific to their cancer treatment and prognosis. Moreover, PCRB of quitting can vary greatly based on cancer type and have a dependent relationship with quit intentions based on the smoking-relatedness of one’s cancer. Therefore, future research should explore the efficacy of tailoring specific PCRB of cessation (i.e. proximal vs distal benefits) to match a patient’s preference and cancer diagnosis.
Acknowledgements
The authors wish to thank the patients who participated in this study. Requests for access to study materials can be directed to the corresponding author.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute at the National Institutes of Health (R01CA166147; K24CA197382, and P30CA008748). Dr Hall was supported by the National Center for Complementary and Integrative Health at the National Institutes of Health (T32AT000051).
Appendix 1
Measure of perceived cancer-related benefits of quitting smoking
On a scale of 0–10, with 0 being “not at all” and 10 being “very,” how much would quitting smoking reduce your chances of having complications from your treatment?
On a scale of 0–10, with 0 being “not at all” and 10 being “very,” how much would quitting smoking improve your chances of getting the full benefit from your cancer treatment?
On a scale of 0–10, with 0 being “not at all” and 10 being “very,” how much would quitting smoking reduce your chances of developing your cancer again?
On a scale of 0–10, with 0 being “not at all” and 10 being “very,” how much would quitting smoking reduce your chances of developing a new cancer?
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alton D, Eng L, Lu L, et al. (2018) Perceptions of continued smoking and smoking cessation among patients with cancer. Journal of Oncology Practice 14(5): e269–e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ (2000) Optimistic bias in adolescent and adult smokers and nonsmokers. Addictive Behaviors 25(4): 625–632. [DOI] [PubMed] [Google Scholar]
- Aveyard P, Massey L, Parsons A, et al. (2009) The effect of transtheoretical model based interventions on smoking cessation. Social Science & Medicine 68(3): 397–403. [DOI] [PubMed] [Google Scholar]
- Ayala-Bernal D, Probst-Hensch N, Rochat T, et al. (2017) Factors associated with cessation of smoking among Swiss adults between 1991 and 2011: Results from the SAPALDIA cohort. Swiss Medical Weekly 147: w14502. [DOI] [PubMed] [Google Scholar]
- Biener L and Abrams D (1991) The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology 10: 360–365. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Hayes RB, Dunsiger S, et al. (2010) Risk perception and smoking behavior in medically ill smokers: A prospective study. Addiction 105(6): 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Lahelma E, et al. (2004) Smoking cessation by socioeconomic status and marital status: The contribution of smoking behavior and family background. Nicotine & Tobacco Research 6(3): 447–455. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2016) CDC press releases Available at: https://www.cdc.gov/media/releases/2016/p1110-vital-signs-cancer-tobacco.html (accessed 8 September 2018).
- Centers for Disease Control and Prevention (CDC) (2018) Smoking and Tobacco Use; Fact Sheet; Fast Facts Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/ (accessed 1 October 2018).
- Chandran S and Menon G (2004) When a day means more than a year: Effects of temporal framing on judgments of health risk. Journal of Consumer Research 31(2): 375–389. [Google Scholar]
- Dillard AJ, McCaul KD and Klein WM (2006) Unrealistic optimism in smokers: Implications for smoking myth endorsement and self-protective motivation. Journal of Health Communication, 11(suppl. 1): 93–102. [DOI] [PubMed] [Google Scholar]
- Dillard JP and Nabi RL (2006) The persuasive influence of emotion in cancer prevention and detection messages. Journal of Communication 56(suppl. 1): S123–S139. [Google Scholar]
- Florou AN, Gkiozos ICH, Tsagouli SK, et al. (2014) Clinical significance of smoking cessation in subjects with cancer: A 30-year review. Respiratory Care 59(12): 1924–1936. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Wall MM, Garey L, et al. (2017) Depression among current, former, and never smokers from 2005 to 2013: The hidden role of disparities in depression in the ongoing tobacco epidemic. Drug and Alcohol Dependence 173: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond A-J, Croteau VA, Savard M-H, et al. (2017) Predictors of smoking cessation and relapse in cancer patients and effect on psychological variables: An 18-month observational study. Annals of Behavioral Medicine 51(1): 117–127. [DOI] [PubMed] [Google Scholar]
- Hall DL, Lennes IT, Carr A, et al. (2018) Lung cancer screening uncertainty among patients undergoing LDCT. American Journal of Health Behavior 42: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2012) PROCESS: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling Lawrence, KS: University of Kansas. [Google Scholar]
- Hayes AF and Matthes J (2009) Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods 41(3): 924–936. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, Kruse GR, Rigotti NA, et al. (2018) Electronic cigarette use patterns and reasons for use among smokers recently diagnosed with cancer. Cancer Medicine 7: 3484–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AR, Persoskie A, Twesten J, et al. (2018) A review of risk perception measurement in tobacco control research. Tobacco Control Epub ahead of print 6 February. DOI: 10.1136/tobaccocontrol-2017-054005. [DOI] [PubMed] [Google Scholar]
- Kumar A, Tiwari A, Gadiyar A, et al. (2018) Assessment of readiness to quit tobacco among patients with oral potentially malignant disorders using transtheoretical model. Journal of Education and Health Promotion 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM and Zvolensky MJ (2015) Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin 141(1): 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetkin EI, Guidry JA, Webb A, et al. (2018) Examining transdiagnostic vulnerabilities among HIV positive smokers seen at three inner city community based organizations. AIDS Care 30(2): 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy SB, Gibbons FX, Reis TJ, et al. (1992) Perceptions of smoking risk as a function of smoking status. Journal of Behavioral Medicine 15(5): 469–488. [DOI] [PubMed] [Google Scholar]
- Martínez Brandon ÚTH, Sutton SK, et al. (2018) Associations between the smoking-relatedness of a cancer type, cessation attitudes and beliefs, and future abstinence among recent quitters. Psycho-oncology 27(9): 2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OʼCleirigh C, Zvolensky MJ, Smits JAJ, et al. (2018) Integrated treatment for smoking cessation, anxiety, and depressed mood in people living with HIV: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 79(2): 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ER, Ostroff JS, Perez GK, et al. (2016) Integrating tobacco treatment into cancer care: Study protocol for a randomized controlled comparative effectiveness trial. Contemporary Clinical Trials 50: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ER, Ostroff JS, Rakowski W, et al. (2009) Risk perceptions among participants undergoing lung cancer screening: Baseline results from the national lung screening trial. Annals of Behavioral Medicine 37(3): 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ER, Streck JM, Gareen IF, et al. (2014) A qualitative study of lung cancer risk perceptions and smoking beliefs Among national lung screening trial participants. Nicotine & Tobacco Research 16(2): 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Daley A, Begh R, et al. (2010) Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 340: b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, et al. (2010) Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology 78(1): 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO and Velicer WF (1997) The transtheoretical model of health behavior change. American Journal of Health Promotion 12(1): 38–48. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Calvin J, Malstrom M, et al. (2003a) Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Annals of Behavioral Medicine 25(3): 214–221. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Miller SM, Unger M, et al. (2002) Characteristics of female smokers attending a lung cancer screening program: A pilot study with implications for program development. Lung Cancer 37(3): 257–265. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Rothman RL, Lerman C, et al. (2004) Comparing cancer patients who enroll in a smoking cessation program at a comprehensive cancer center with those who decline enrollment. Head & Neck 26(3): 278–286. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Zhang B, Rue M, et al. (2003b) Brief physician-initiated quit-smoking strategies for clinical oncology settings: A trial coordinated by the eastern cooperative oncology group. Journal of Clinical Oncology 21(2): 355–365. [DOI] [PubMed] [Google Scholar]
- Sciamanna CN, Hoch JS, Duke GC, et al. (2000) Comparison of five measures of motivation to quit smoking among a sample of hospitalized smokers. Journal of General Internal Medicine 15(1): 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitas F, Weber M, Egger S, et al. (2014) Smoking cessation after cancer. Journal of Clinical Oncology 32: 3593–3595. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Zvolensky MJ, Davis ML, et al. (2016) The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosomatic Medicine 78(3): 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher VJ, McClure JB, Alexander GL, et al. (2008) Web-based smoking-cessation programs: Results of a randomized trial. American Journal of Preventive Medicine 34(5): 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y and Liberman N (2010) Construal-level theory of psychological distance. Psychological Review 117(2): 440–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout S, Goldstein AO, Marks L, et al. (2018) Treating tobacco use in patients with incurable malignancies: Should we even start the conversation? Journal of Palliative Medicine 21(6): 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T-S, Lin H-Y, Moody-Thomas S, et al. (2012) Who tended to continue smoking after cancer diagnosis: The national health and nutrition examination survey 1999–2008. BMC Public Health 12: 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Surgeon General (2014) The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Washington, DC: Department of Health and Human Services. Available at: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf [Google Scholar]
- Vinci C, Guo L, Spears CA, et al. (2017) Socioeconomic indicators as predictors of smoking cessation among Spanish-Speaking Mexican Americans. Ethnicity & Health 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M, Olver I, Whitford H, et al. (2004) Motivational interviewing as a smoking cessation intervention for patients with cancer: Randomized controlled trial. Nursing Research 53(6): 396–405. [DOI] [PubMed] [Google Scholar]
- Webb TL and Sheeran P (2006) Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin 132(2): 249–268. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, Marcus SE and Moser RP (2005) Smokers’ unrealistic optimism about their risk. Tobacco Control 14(1): 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin SM, Masilamani R, Ming MF, et al. (2012) Can initial perceptions about quitting predict smoking cessation among Malaysian smokers? The Southeast Asian Journal of Tropical Medicine and Public Health 43: 501–509. [PubMed] [Google Scholar]
- Zvolensky MJ, Jardin C, Wall MM, et al. (2018) Psychological distress among smokers in the United States: 2008–2014. Nicotine & Tobacco Research 20(6): 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]


