Abstract
Objective
This study evaluated the impact of daily ECG (electrocardiogram) self‐recordings on time to documented recurrent atrial fibrillation (AF) or atrial flutter (AFL) and time to treatment of recurrent arrhythmia in patients undergoing catheter radiofrequency ablation (RFA) or direct current cardioversion (DCCV) for AF/AFL.
Background
AF recurrence rates after RFA and DCCV are 20% to 45% and 60% to 80%, respectively. Randomized trials comparing mobile ECG devices to standard of care have not been performed in an AF/AFL population after treatment.
Methods
Of 262 patients consented, 238 were randomized to either standard of care (123) or to receive the iHEART intervention (115). Patients in the intervention group were provided with and trained to use an AliveCor KardiaMobile ECG monitor, and were instructed to take and transmit daily ECG recordings. Data were collected from transmitted ECG recordings and patients’ electronic health records.
Results
In a multivariate Cox model, the likelihood of recurrence detection was greater in the intervention group (hazard ratio = 1.56, 95% confidence interval [CI]: 1.06‐2.30, P = .024). Hazard ratios did not differ significantly for RFA and DCCV procedures. Recurrence during the first month after ablation strongly predicted later recurrence (hazard ratio = 4.53, 95% CI: 2.05‐10.00, P = .0006). Time from detection to treatment was shorter for the control group (hazard ratio = 0.33, 95% CI: 0.57‐2.92, P < .0001).
Conclusions
The use of mobile ECG self‐recording devices allows for earlier detection of AF/AFL recurrence and may empower patients to engage in shared health decision‐making.
Keywords: atrial fibrillation, atrial flutter, atrial arrhythmias, cardioversion, Holter/event recorders
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice.1, 2 AF that remains untreated is associated with an increased risk of stroke,3, 4, 5, 6 myocardial infarction,7, 8 heart failure exacerbations,4, 9 and all‐cause mortality.4, 10, 11 The prevalence of AF will surge in the next decade due to an aging population that is living longer with a high burden of comorbid conditions that are associated with AF.12, 13 Catheter radiofrequency ablation (RFA) is one form of therapy widely used in clinical practice to treat symptomatic AF,2 but postprocedure recurrent AF occurs in 20% to 45% of patients.14, 15 Direct current cardioversion (DCCV) interrupts AF and restores sinus rhythm, but has no durable effect. Even when patients receive additional antiarrhythmic drug therapy to maintain sinus rhythm, about 40% to 60% of the patients have AF recurrence within 3 months and 60% to 80% within 1 year after DCCV.16, 17 AF management and symptom identification is challenging for both patients and providers because symptoms that are commonly associated with AF such as fatigue, shortness of breath, and palpitations may also be associated with coexisting cardiovascular conditions such as heart failure. Many AF patients fail to recognize and distinguish symptoms associated with AF, which may hinder timely treatment and put the patient at risk for complications.18 Additionally, AF can be missed through conventional monitoring approaches, such as 12‐lead ECGs (electrocardiograms) and Holter studies, which only capture a finite period of time.19, 20
Currently available mobile health tools that aim to promote earlier detection and treatment of AF and atrial flutter (AFL), adherence to cardiovascular regimens, and improvement in self‐management behaviors have not been systematically evaluated. The aim of this randomized, prospective study, iPhone Helping Evaluate Atrial fibrillation Rhythm through Technology (iHEART), was to evaluate the impact of frequent cardiac rhythm monitoring by self‐recordings on outcomes in post‐RFA and ‐DCCV populations with at least one known risk factor for AF. We hypothesized that providing patients with a rhythm monitoring device immediately after RFA or DCCV would result in a shorter time from RFA or DDCV to discovery and treatment of AF or AFL recurrence.
2. METHODS
2.1. Recruitment and the informed consent process
Approval for this study by the Columbia University Medical Center Institutional Review Board was obtained before subject enrollment. Subjects were recruited for the iHEART study from the cardiac electrophysiology clinics within the Division of Cardiology at Columbia University Medical Center in New York, NY, United States of America. These individuals were identified as potential study subjects by their health‐care providers who obtained verbal approvals before the study team approached them. The research team discussed the study with them, allowed them to read the informed consent, and answered all questions. Patients who agreed to participate were asked to sign the consent form which was available in both English and Spanish. All patients were given a copy of their signed consent form for their personal records.
2.2. Study subjects
The planned sample size for this study was 300 patients. A total of 262 consecutive patients undergoing either RFA or DCCV and meeting the inclusion criteria were randomized 1:1 using a blocked‐randomization scheme to age‐match patients in the control and intervention groups. Inclusion criteria were age 18 and older with a history of documented AF and at least one AF risk factor (sedentary lifestyle, obesity, hypertension, smoking, and diabetes). Patients also needed to express willingness to participate for the full 6‐month duration of the trial and demonstrate an ability to use a smartphone, send and receive text messages, and successfully use the AliveCor KardiaMobile ECG monitor (AliveCor). Patients with a history of cognitive impairment and those unwilling to have their clinical data collected or receive text messages were excluded from the study.
A total of seven patients (six control and one intervention) did not undergo any procedure on the day of enrollment for various clinical reasons. These patients were not included in this study because the follow‐up period started immediately postprocedure. Two patients were not randomized because they did not convert to sinus rhythm by DCCV, and five (one control and four intervention) withdrew from the study. Ten patients randomized to intervention were discharged without being set up to connect to the Kardia portal to enable ECG transmission and were also excluded. One hundred and fifteen intervention patients and 123 controls remained in the study (Figure 1).
Figure 1.
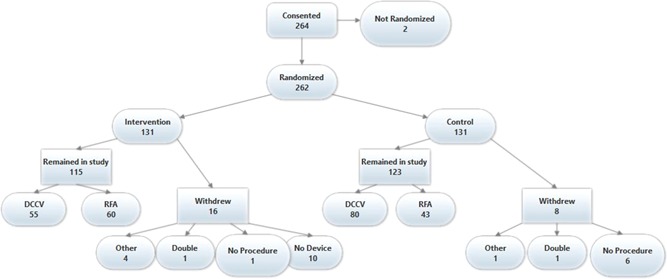
iHEART study consort diagram. DCCV, direct current cardioversion; RFA, radiofrequency ablation
2.3. Procedures at enrollment
All DCCV procedures were carried out after a 12‐hour fasting period, under brief anesthesia with propofol, using a synchronized 20‐J DC biphasic shock. RFA procedures were carried out under general anesthesia using the same uniform protocol by all operators participating in the study. Radiofrequency energy was used for all catheter ablation procedures, and approach for all RFA procedures was wide antral ablation to achieve electrical isolation of all pulmonary veins, verified by entry and exit block.
2.4. Device training
Patients randomized to the iHEART intervention received an iPhone and cellular service plan with unlimited data/text messaging, and the AliveCor KardiaMobile ECG monitor for 6 months.21 If they already owned a smartphone compatible with the KardiaMobile device, they had the option to use the KardiaMobile device with their own phone. Patients also received motivational text messages three times per week relating to management of AF and risk factors (eg, obesity, sedentary lifestyle), for example, “Limit sugary drinks to no more than 36 oz a week.” Patients were trained on how to use the phone; how to use the Kardia application, which connects to the WasKardiaMobile device to record ECGs; and how to record ECGs and symptoms using the KardiaMobile device. Patients were instructed to record a daily ECG and additional ECGs whenever they experienced symptoms perceived to be associated with an atrial arrhythmia. Upon discovery of any arrhythmia, patients contacted their health‐care provider, and all treatment, management, and follow‐up for the arrhythmia were determined by the patient's provider.
2.5. Data collection and outcomes
In addition to the transmitted KardiaMobile recordings, data were collected through a review of patients’ electronic health records (EHR). The study protocol did not include additional monitoring for the control group. When follow‐up data were not available, the study coordinators reached out to patients or their cardiologists for additional information, including AF/AFL recurrence and dates of treatment. All analyses were intention‐to‐treat. Data were analyzed to determine the time from enrollment to first documentation of recurrent atrial arrhythmia, time from enrollment to first treatment for atrial arrhythmia, and time from documentation to treatment. Recurrence was defined as one of the following: a KardiaMobile rhythm strip showing AF/AFL as determined by a physician, an ECG in the EHR displaying an AF/AFL confirmed by a physician, or a note in the EHR from a physician stating that the patient had a recurrent AF/AFL. Patient reports of symptoms were not counted as recurrence unless confirmed by the patient's physician or with an ECG.
Postenrollment treatment was defined as any of the following: repeat RFA after first ablation, RFA scheduled after DCCV, DCCV for AF/AFL not already scheduled at the time of enrollment, or addition of an antiarrhythmic medication not used prior to the time of recurrent arrhythmia. Addition of an AV nodal agent for rate control, changes in dose of existing rhythm control medication, and treatments that were already scheduled before the original procedure, such as future catheter ablations planned for cardioversion patients, were not considered to be new treatments.
Recurrence during the first month after ablation is referred to as “early recurrence”, and any recurrence after the first month was considered to be “late recurrence”. In a secondary analysis, for patients undergoing RFA, data were analyzed both from the time of randomization to 6 months after randomization and also from the end of the first month to 6 months after randomization.
2.6. Statistical analysis
All demographic and clinical data with the exception of age are reported as frequencies and percentages; age is reported as mean and standard deviation. The Fisher exact test was used to assess differences in clinical characteristics, medications, and procedures between those in the intervention group and the control group. Kaplan‐Meier curves were created for recurrence detection in the intervention and control groups over the 6‐month follow‐up period. Differences in time to recurrence between groups were assessed using a Cox proportional hazards model. Baseline variables that differed significantly between groups were included as covariates in the analysis and covariate adjusted Kaplan‐Meier curves were constructed.22 Kaplan‐Meier curves were also created for assessing time to treatment for intervention and control groups. Time to treatment was defined as the time interval from detection of a recurrent arrhythmia to treatment for that arrhythmia. The study was designed to have 80% power to detect a hazard ratio of 2 for recurrence detection (α = .05). Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). A critical P value of .05 was used for significance in all analyses.
3. RESULTS
The clinical characteristics of the AliveCor intervention group and the control group are shown in Table 1. There were no significant differences between the groups with the exception of the procedure at enrollment. The procedure at time of enrollment (RFA or DCCV) was not accounted for in the randomization scheme, and the proportion of patients undergoing DCCV was higher in the control group than in the intervention group. At the time of enrollment, in the control group, the numbers of AF and AFL cardioversions were 63 and 17, respectively, and the numbers of AF, AFL, and AF/AFL ablations were 32, 9, and 2. In the intervention group, the numbers of AF and AFL cardioversions were 40 and 15, respectively, and the numbers of AF, AFL, and AF/AFL ablations were 48, 8, and 4. Follow‐up data were not obtained in five of the control patients. Among the remaining 118 controls, 49 (41.5%) had a documented recurrence within the 6‐month study period. This is a lower bound for actual recurrence. In the intervention group, 58 (50.4%) had a documented recurrence during the study period. The Kaplan‐Meier curves for recurrence stratified by procedure at time of enrollment (covariate differing significantly between groups) are shown in Figure 2. The covariate adjusted Kaplan‐Meier curves for all control vs all intervention patients are shown in Figure 3. The likelihood of recurrence detection was significantly greater in the intervention group (hazard ratio = 1.56, 95% CI: 1.06‐2.30, P = .024). In this multivariable model, the likelihood of recurrence detection was significantly lower for patients undergoing RFA at the time of enrollment (hazard ratio = 0.65, 95% CI: 0.44‐0.97, P = .036). The hazard ratio for the intervention group did not differ significantly for those undergoing RFA and DCCV (P = .92). Regardless of whether patients underwent DCCV or RFA, recurrence was detected earlier in the intervention group. The increase in recurrence detection rate was largest among patients with a history of persistent AF, with recurrence rates in the intervention and control groups at 74.3% and 44.7%, respectively, for persistent AF patients compared to 54.1% and 50.0% for paroxysmal AF patients.
Table 1.
Clinical characteristics
| Variable | Intervention group (N = 115) | Control group (N = 123) | P value |
|---|---|---|---|
| Age (mean + SD), y | 61 + 12 | 61 + 12 | .88 |
| #/N (%) | #/N (%) | ||
| Males | 88/115 (77) | 96/123 (78) | .88 |
| Race | |||
| White | 88/115 (77) | 93/123 (76) | ⋯ |
| Black or African American | 3/115 (3) | 8/123 (7) | |
| Asian | 1/115 (1) | 5/123 (4) | |
| Unknown or not reported | 23/115 (20) | 17/123 (14) | |
| Ethnicity | |||
| Hispanic or Latino | 10/115 (9) | 17/123 (14) | ⋯ |
| Not Hispanic or Latino | 61/115 (53) | 67/123 (54) | |
| Unknown or not reported | 42/115 (37) | 44/123 (36) | |
| Language | |||
| English | 114/115 (53) | 120/123 (98) | .62 |
| Spanish | 1/115 (37) | 3/123 (2) | |
| Procedure at enrollment | |||
| DCCV | 55/115 (48) | 80/123 (65) | .009 |
| RFA | 60/115 (52) | 43/123 (35) | |
| Type of atrial fibrillation | |||
| Paroxysmal | 74/109 (68) | 74/121 (61) | .34 |
| Persistent | 35/109 (32) | 47/121 (39) | |
| Comorbidities | |||
| Hx of stroke/TIA | 11/115 (10) | 10/123 (8) | .82 |
| Hx of congestive heart failure | 22/115 (19) | 26/123 (26) | .75 |
| Cardiovascular risk factors | |||
| Diabetes | 14/115 (12) | 17/123 (14) | .85 |
| Hypertension | 66/115 (57) | 77/123 (63) | .43 |
| Obesity | 38/103 (37) | 47/107 (44) | .33 |
| Hx of smoking (current) | 5/115 (4) | 4/123 (3) | .74 |
| Hx of smoking (past) | 38/115 (33) | 39/123 (32) | .89 |
| Sleep apnea | 27/115 (23) | 27/123 (22) | .88 |
| PR interval > 200 | 17/81 (21) | 25/89 (28) | .29 |
| Medications | |||
| Anticoagulants | 105/115 (87) | 112/123 (91) | 1.0 |
| Beta blockers | 74/115 (64) | 93/123 (76) | .07 |
| Antiarrhythmics | 34/115 (30) | 32/123 (26) | .56 |
| Diuretics | 18/115 (16) | 25/123 (20) | .40 |
| Calcium channel blockers | 22/115 (19) | 21/123 (17) | .74 |
| ACE/ARB | 34/115 (30) | 37/123 (30) | 1.0 |
| Digoxin | 8/115 (7) | 13/123 (11) | .37 |
| LVEF | |||
| Normal | 62/95 (65) | 57/94 (61) | .55 |
| Decreased | 33/95 (35) | 37/94 (39) | |
| LA diameter | |||
| Normal | 26/56 (46) | 27/66 (41) | .59 |
| Enlarged | 30/56 (54) | 39/66 (59) | |
Abbreviations: ACE/ARB, angiotensin converting enzyme inhibitors/angiotensin‐receptor blockers; DCCV, direct current cardioversion; LA, left atrium; LVEF, left ventricular ejection fraction; RFA, radiofrequency ablation; TIA, transient ischemic attack.
Bold P values are statistically significant.
Figure 2.
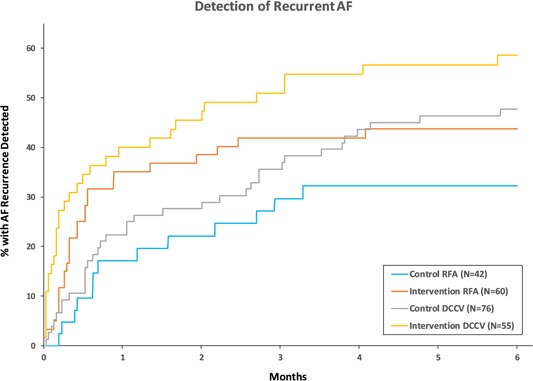
Kaplan‐Meier analysis of time from procedure at enrollment to first detection of recurrent AF/AFL stratified by procedure at enrollment. Recurrent arrhythmias were detected earlier in the intervention group. AF, atrial fibrillation; AFL, atrial flutter; DCCV, direct current cardioversion; RFA, radiofrequency ablation
Figure 3.

Kaplan‐Meier curves adjusted for the procedure at enrollment of time from enrollment to first detection of recurrent AF/AFL for control and intervention groups. Recurrent arrhythmias were detected earlier in the intervention group. The P value shown is for control vs intervention after adjusting for procedure at enrollment (RFA or DCCV). 95% CI, 95% confidence interval; AF, atrial fibrillation; AFL, atrial flutter; DCCV, direct current cardioversion; RFA, radiofrequency ablation
Kaplan‐Meier curves for time from AF/AFL detection to treatment are shown in Figure 4. Patients with recurrent AF/AFL in the intervention group were less likely to be treated than those in the control group (hazard ratio = 0.33, 95% CI: 0.57‐2.92, P < .0001). Among control patients with recurrence detected, 35 (71.4%) were treated during the study period while only 21 (36.2%) were treated in the intervention group.
Figure 4.
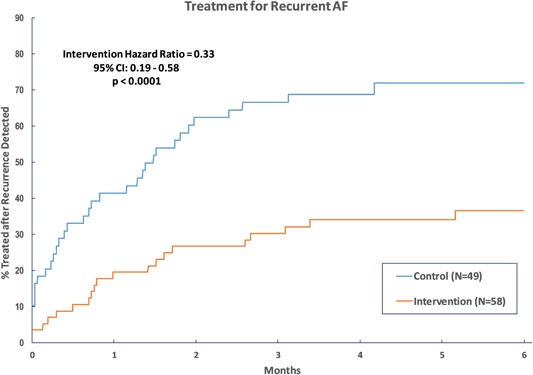
Kaplan‐Meier analysis of time from first detection of recurrent AF/AFL to first treatment of arrhythmia. Recurrent arrhythmias were treated earlier in the control group. AF, atrial fibrillation; AFL, atrial flutter
Patients in the control arm were not monitored for the purposes of the study. Nineteen of these patients had LINQs, pacemakers, or ICDs for clinical reasons unrelated to our protocol, which allowed for remote monitoring. Additionally, at least two control patients purchased the Alivecor device for themselves separate from the study during the follow‐up period. However, the majority of patients did not have any remote monitoring and had postprocedure follow‐up at the discretion of their electrophysiologist, typically 1, 3, and 6 months after procedure. The patients in the control group experienced a higher number of all‐cause hospitalizations (56) and emergency room visits (13) than the intervention group (45 hospitalizations and three emergency room visits). However, this difference is not statistically significant.
While there was greater arrhythmia detection in the intervention group, the difference was not significant when only late recurrences (post 1 month) are considered. (hazard ratio = 1.29, 95% CI: 0.57‐2.92, P = .54). However, in patients undergoing RFA, early recurrence of AF/AFL was a strong predictor of later recurrence within the first 6 months after the procedure. Fifty‐two percent of RFA patients who experienced early arrhythmias went on to experience later arrhythmias compared to 16% among those who did not experience early recurrence (hazard ratio = 4.53, 95% CI: 2.05‐10.00, P = .0006).
Patient usage data are shown in Table 2. Forty‐one (36%) recorded greater than 180 times, on average once per day, and 77 (67%) used the device in the last month of their study period. Ninety‐three (81%) averaged transmission at least once per week and 86 (75%) used the device in the second half of the study. Of the 41 patients who transmitted once per day on average, 19 (46%) had no documented recurrence. There was no significant relationship between any variables collected and number of days used or number of tracings taken. Further, the majority of patients did not use the KardiaMobile device to report their symptoms: only 11 (10%) patients transmitted symptom data along with their ECGs. Ten of these patients reported experiencing AF symptoms when the associated ECG revealed sinus rhythm.
Table 2.
AliveCor usage statistics (n = 115)
| Variable | N (%) |
|---|---|
| Days from enrollment to last tracing | |
| >150 | 77 (67) |
| 121‐150 | 6 (5) |
| 91‐120 | 3 (3) |
| 61‐90 | 4 (3) |
| 31‐60 | 2 (2) |
| 1‐30 | 11 (10) |
| 0 | 7 (6) |
| Unknown | 5 (4) |
| Total tracings sent | |
| >360 | 15 (13) |
| 181‐360 | 26 (23) |
| 91‐180 | 23 (20) |
| 61‐90 | 9 (8) |
| 31‐60 | 20 (17) |
| 1‐30 | 10 (9) |
| 0 | 7 (6) |
| Unknown | 5 (4) |
4. DISCUSSION
As hypothesized, time to documentation of recurrence from enrollment was shorter for the intervention group compared to the control group. This was true mostly for early recurrences, and especially for patients with persistent AF; after the first month there was no significant difference in time to detection of new recurrence. Difference in time to documentation likely represents a difference in time from initiation of the arrhythmia to documentation of the arrhythmia, as there is no reason to expect any difference in actual time to recurrence between the two groups. Nonetheless, early recurrence was a strong predictor of later recurrence.
The second part of our hypothesis, that the intervention group would be treated more quickly than the control group, was not confirmed; the control group had a shorter time from documentation of a recurrence to treatment. There are several potential explanations for this finding. For many control patients, the first documentation of an arrhythmia was when they came in for treatment. A patient coming in for a DCCV after experiencing symptoms would not have documentation of the arrhythmia until they arrived for the procedure. Arrhythmias that would have gone unnoticed in asymptomatic patients were more likely to be documented for intervention patients. Patients with short AF recurrences that spontaneously converted back to sinus rhythm are more likely to be documented in the intervention group, yet physicians are less likely to proceed to immediate treatment for early paroxysmal recurrences during the first month. Patients who spontaneously converted to sinus after a short recurrence, but later had an episode of AF requiring treatment, would be documented earlier in the intervention group than the control group. This may also contribute to a greater detection to treatment time in the intervention group. Another potential factor may be the higher rate of DCCV patients in the control group. Patients are more likely to receive a second treatment sooner after DCCV because DCCV is not a long‐term treatment for AF, and physicians may wait several months after RFA to see if arrhythmias resolve before proposing further treatment.
In a secondary analysis for patients who underwent RFA procedures at enrollment, early and late recurrences were differentiated to account for arrhythmias that take place during the healing process as a result of inflammation and edema, which usually resolve within a few weeks.23 While detection of recurrence in the intervention group was greater after the first month, too few patients had recurrences during the latter 5 months for the differences to be significant. The Heart Rhythm Society, the European Heart Rhythm Association, and the European Cardiac Arrhythmia Society recommend a 3‐month blanking period,24 but there is debate on the usefulness of blanking periods,25 as recurrence within the first month is a strong predictor of later recurrence. Early reablation has been shown to improve long‐term freedom from AF.23, 26, 27
In agreement with other studies,28 we found that early recurrence was a strong predictor for recurrence during the later months.23 Therefore, detection of early recurrence has clinical prognostic value and could aid in tailoring treatment plans and determining when an ablation should be considered successful, or if repeat ablation and continued anticoagulation is required. Although early recurrent arrhythmias do not necessarily signify a failed ablation, when they are identified early, it enables physicians and patients to start planning future treatments and propose alternative strategies such as pill‐in‐pocket antiarrhythmic therapy, all of which will minimize time spent in AF. Patients who spend less time in AF are potentially less likely to suffer strokes and other complications, more likely to maintain cardiac function, and, if symptomatic, more likely to have improved quality of life.
Mobile heart monitor devices may be useful not only for their positive predictive value but also for their ability to eliminate false positives. In this study, we were not able to assess how many patients believed they were in AF, but discovered that they were in sinus rhythm using the device. Similarly, we do not know how many patients scheduled a cardioversion only to spontaneously convert to sinus, discovered it using the device, and avoided an unnecessary trip to the hospital. Nonetheless, the limited‐symptom data we were able to collect indicates that some patients experience symptoms they associate with AF while in sinus rhythm. These symptoms may be attributable to comorbidities (eg, shortness of breath is a symptom of both AF and heart failure). The true benefit to the patient may be greater than our results indicate as a result of the negative predictive value of the device.
Randomized trials comparing mobile ECG devices to standard of care have not been performed in an AF population after treatment. Other studies have found that mobile health technologies have varying degrees of usefulness for diagnosing and monitoring AF.29 The Apple Heart study found that continuous monitoring of the general population using Apple Watch tachygram analysis was 34% effective at diagnosing AF, and 86% accurate at identifying AF in patients wearing a remote monitor, but did not investigate how many patients had AF that was not picked up by the watch. The REHEARSE‐AF study found that in a population without an AF diagnosis, patients using the AliveCor KardiaMobile Monitor had a higher rate of AF diagnosis than control (hazard ratio, 3.9).30 As one would expect, our hazard ratio was lower because a population of patients already being treated for AF will be seen for follow‐up visits, increasing the likelihood of AF discovery in the control group.
Although patients were instructed to record daily ECGs, there was a large range in usage with some patients transmitting no recordings and some transmitting several hundred times during the 6‐month window. Forty‐six (40%) patients sent fewer than 90 ECG measurements, or on average less than once every other day during the 6‐month period, and 23 (20%) patients did not transmit after the first 3 months. Possible reasons for lack of usage include the following: patients who no longer felt symptoms or had a long sequence of sinus rhythm recordings may not have felt the need to continue to record ECGs; patients may have found it burdensome to take ECG measurements every day; and patients may have forgotten to take measurements. On the contrary, 41 (36%) patients used the device more frequently than directed, averaging more than one ECG per day, and 19 (46%) of these patients had no documented recurrence during the 6‐month follow‐up window. Fifteen (13%) patients averaged more than twice per day. Many hospitals offer subscription ECG reading services, in which patients pay a monthly fee for nurse practitioners or physicians to read their mobile ECG recordings. These more frequent users should be taken into consideration when these types of services are offered, as they may require a disproportionate amount of hospital resources and time. Given that the use of home monitoring devices is increasing and can now be billed at many hospitals, further research into the cost effectiveness of home ECG monitoring is warranted.
4.1. Limitations
A major limitation involved the accuracy of the time to documentation for the control patients, where we were limited to using their EHR to determine first recurrence. Patients whose AF was documented by doctors outside our network might have gone undetected in our study, despite suffering a recurrent arrhythmia. Time to recurrence was more accurately documented for the intervention patients than for the control patients for obvious reasons. Also, many control patients did not have a documentation of the arrhythmia until they came in for treatment, resulting in an artificially shorter time between discovery and treatment. The greater proportion of control patients who underwent DCCV also introduces bias. Because we did not reach the intended sample size, the statistical power of the study was reduced. This study was also limited by the short duration of the follow‐up period. Even though this investigation was randomized and prospective, it has the recognized limitations of a single‐center study. There may be selection bias, especially for the subgroup chosen by their physicians to undergo catheter ablation therapy. Therefore, there should be caution in generalizing our findings to all patients with atrial fibrillation, as results may differ in other patient populations.
5. CONCLUSION
In this study, we have provided evidence supporting the hypothesis that when AF patients are compliant with daily use of home ECG monitoring, recurrent arrhythmias are discovered earlier when compared to control patients. Our results suggest that the AliveCor KardiaMobile home monitoring device is mostly beneficial for prompt detection of early (first month) recurrence after RFA or DCCV, and that early recurrence predicts late recurrence. Earlier detection of recurrent arrhythmias can empower patients and providers to make informed health decisions and develop treatment plans sooner. Future studies should look at the effects of home ECG monitoring over a longer time period on outcomes including cost effectiveness, quality of life, and long‐term rates of hospitalization, stroke, and death.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENT
This study was funded by R01 from the National Institute of Nursing Research (R01NR014853).
Goldenthal IL, Sciacca RR, Riga T, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;30:2220‐2228. 10.1111/jce.14160
References
REFERENCES
- 1. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071‐2104. [DOI] [PubMed] [Google Scholar]
- 2. Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(11):e101‐e198. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049‐1056. [DOI] [PubMed] [Google Scholar]
- 4. Stewart S, Hart CL, Hole DJ, McMurray JJV. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley Study. Am J Med. 2002;113(5):359‐364. [DOI] [PubMed] [Google Scholar]
- 5. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach. Chest. 2010;137(2):263‐272. [DOI] [PubMed] [Google Scholar]
- 6. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983‐988. [DOI] [PubMed] [Google Scholar]
- 7. Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyasaka Y, Barnes ME, Gersh BJ, et al. Coronary ischemic events after first atrial fibrillation: risk and survival. Am J Med. 2007;120(4):357‐363. [DOI] [PubMed] [Google Scholar]
- 9. Miyasaka Y, Barnes ME, Gersh BJ, et al. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community‐based study over two decades. Eur Heart J. 2006;27(8):936‐941. [DOI] [PubMed] [Google Scholar]
- 10. Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries: 1993‐2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation. J Am Coll Cardiol. 2007;49(9):986‐992. [DOI] [PubMed] [Google Scholar]
- 12. Wolowacz SE, Samuel M, Brennan VK, Jasso‐Mosqueda JG, van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. 2011;13(10):1375‐1385. [DOI] [PubMed] [Google Scholar]
- 13. Garg PK, O'Neal WT, Ogunsua A, et al. Usefulness of the American Heart Association's Life Simple 7 to predict the risk of atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke [REGARDS] Study). Am J Cardiol. 2018;121:199‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darby AE. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9(1):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sultan A, Lüker J, Andresen D, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep. 2017;7(1):16678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alcaraz R, Hornero F, Rieta JJ. Noninvasive time and frequency predictors of long‐standing atrial fibrillation early recurrence after electrical cardioversion. Pacing Clin Electrophysiol. 2011;34(10):1241‐1250. [DOI] [PubMed] [Google Scholar]
- 17. Elesber AA, Rosales AG, Herges RM, et al. Relapse and mortality following cardioversion of new‐onset vs. recurrent atrial fibrillation and atrial flutter in the elderly. Eur Heart J. 2006;27(7):854‐860. [DOI] [PubMed] [Google Scholar]
- 18. McCabe PJ, Schad S, Hampton A, Holland DE. Knowledge and self‐management behaviors of patients with recently detected atrial fibrillation. Heart Lung. 2008;37(2):79‐90. [DOI] [PubMed] [Google Scholar]
- 19. Hickey KT, Riga TC, Mitha SA, Reading MJ. Detection and management of atrial fibrillation using remote monitoring. Nurse Pract. 2018;43(3):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193‐194. [DOI] [PubMed] [Google Scholar]
- 21. Hickey KT, Hauser NR, Valente LE, et al. A single‐center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. 2016;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35(6):437‐443. [DOI] [PubMed] [Google Scholar]
- 23. Dixit S. Early recurrences during the blanking period after atrial fibrillation ablation. J Atr Fibrillation. 2018;10(5):1726‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. Heart Rhythm. 2007;4(6):816‐861. [DOI] [PubMed] [Google Scholar]
- 25. Themistoclakis S, China P. Early recurrences of atrial tachyarrhythmias after ablation of atrial fibrillation. JACC: Clin Electrophysiol. 2017;3(6):577‐579. [DOI] [PubMed] [Google Scholar]
- 26. Andrade JG, Khairy P, Macle L, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014;7(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 27. Mariani MA, Pozzoli A, Maat G, Alfieri OR, Benussi S. What does the blanking period blank? J Atr Fibrillation. 2015;8(4):1268‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrade JG, Khairy P, Verma A, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35(1):106‐116. [DOI] [PubMed] [Google Scholar]
- 29. Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786‐789. [DOI] [PubMed] [Google Scholar]
- 30. Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor Heart Monitor to screen for atrial fibrillation: the REHEARSE‐AF Study. Circulation. 2017;136(19):1784‐1794. [DOI] [PubMed] [Google Scholar]


