Abstract
Neurofibromatosis 1 (NF1) is an autosomal dominant genetic disorder that presents with variable phenotypes as a result of mutations in the neurofibromatosis type 1 (NF1) gene and subsequently, abnormal function of the protein product, neurofibromin. Patients with NF1 are at increased risk for central nervous system (CNS) manifestations including structural, functional and neoplastic disease. The mechanisms underlying the varied manifestations of NF1 are incompletely understood, but the loss of functional neurofibromin, resulting in sustained activation of the oncoprotein RAS, is responsible for tumorigenesis throughout the body, including the CNS. Much of our understanding of NF1-related CNS manifestations is from a combination of data from animal models and natural history studies of people with NF1 and CNS disease. Data from animal models suggest the importance of both Nf1 mutations and somatic genetic alterations, such as Tp53 loss, for development of neoplasms, as well as the role of the timing of the acquisition of such alterations on the variability of CNS manifestations. A variety of non-neoplastic structural (macrocephaly, hydrocephalus, aqueductal stenosis, vasculopathy) and functional (epilepsy, impaired cognition, attention deficits and autism spectrum disorder) abnormalities occur with variable frequency in individuals with NF1. In addition, there is increasing evidence that similar appearing CNS neoplasms in people with and without the NF1 syndrome are due to distinct oncogenic pathways. Gliomas in people with NF1 show alterations in the RAS/MAPK pathway, generally in the absence of BRAF alterations (common to sporadic pilocytic astrocytomas) or IDH or histone H3 mutations (common to diffuse gliomas subsets). A subset of low grade astrocytomas in these patients remain difficult to classify using standard criteria, and occasionally demonstrate morphologic features resembling subependymal giant cell astrocytomas that afflict patients with tuberous sclerosis complex (“SEGA-like astrocytomas”). There is also emerging evidence that NF1-associated high grade astrocytomas have frequent co-existing alterations such as ATRX mutations and an alternative lengthening of telomeres (ALT) phenotype responsible for unique biologic properties. Ongoing efforts are seeking to improve diagnostic accuracy for CNS neoplasms in the setting of NF1 versus sporadic tumors. In addition, MEK inhibitors, which act on the RAS/MAPK pathway, continue to be studied as rational targets for the treatment of NF1-associated tumors, including CNS tumors.
Keywords: Neurofibromatosis, Neurofibromin, Glioma, Brain Tumor, vasculopathy, hydrocephalus, seizure
Introduction
Neurofibromatosis type 1 (NF1) is an autosomal dominant genetic disorder characterized by alterations in the NF1 gene, resulting in phenotypically heterogeneous systemic manifestations. The prevalence of NF1 is estimated at approximately 1 in 3,000 worldwide [115,41], and therefore it represents the most common and well known neurocutaneous disorder. Approximately half of the patients develop sporadically without a known family history. NF1 is diagnosed clinically by two or more features including: the presence of > six café-au-lait macules, skinfold freckling, Lisch nodules, characteristic lesions of the bone, optic pathway gliomas, neurofibromas of the skin or deep nerve, and a first-degree relative with NF1 [73]. Central nervous system (CNS) manifestations of NF1 include neoplasms, learning disabilities, macrocephaly, hydrocephalus and seizures.
NF1 affects a variety of organs and tissues, in the form of neoplasms and non-neoplastic manifestations (Table 1). Neurofibromas are among the most common manifestations in these patients. They are composed predominantly of a neoplastic Schwann cell, but typically have a variety of soft tissue and nerve components, including perineurial cells, axons, mast cells and fibroblasts that likely contribute to tumor growth. They are predominantly of the cutaneous form, and they may be numerous. More worrisome are large plexiform neurofibromas, which have a propensity to transform to malignant peripheral nerve sheath tumors (MPNST), a significant cause of mortality in these patients. Ocular manifestations are also important for the clinical diagnosis of NF1. Lisch nodules (asymptomatic hamartomatous aggregates of melanin-containing cells on surface of iris) occur in almost all patients, and are evaluable through ophthalmologic exam. More recently, choroidal abnormalities representing hamartomatous thickening (“ganglioneuroma”) have been highlighted in the pediatric literature, and modern imaging techniques disclose abnormalities in greater than 80% of patients, which also has diagnostic implications [118].
Table 1.
Clinical manifestations and pathology of NF1
| Non-Neoplastic Manifestations | |
|---|---|
| Ophthalmic |
|
| Central Nervous System |
|
| Skin |
|
| Musculoskeletal |
|
| Cardiovascular |
|
| Central Nervous System Tumors |
|
| Peripheral Nervous System Tumors |
|
| Other Tumors |
|
The current review presents updates about CNS manifestations of NF1. An overview of the genetics, genomics and molecular pathways in NF1 is followed by discussion of current findings in non-neoplastic manifestations (neurocognitive function, vasculopathy, epilepsy) and CNS neoplasms with a focus on diagnostic and molecular pathology.
Genetics and Signaling Pathways
The NF1 gene spans approximately 60 exons and is located on chromosome 17q11.2. It encodes neurofibromin, a GTPase-activating protein that is expressed in many cell types, including neurons, astrocytes, and oligodendrocytes. People with NF1 are born with one inactivated NF1 allele and develop tumors when the second allele is lost [78].
Challenges in NF1 genetic screening in the clinical setting are compounded by the gene size, variety of pathogenic gene variants (including over 2600 reported) and lack of clear genotype phenotype correlations [41,113]. Sabbagh and colleagues analyzed 565 index cases from the NF-France Network and achieved high detection of NF1 pathogenic gene variants [92]. Tsipi and colleagues have more recently reported 70 novel genetic alterations through a peripheral blood-based screening assay utilizing next generation sequencing (NGS) and multiplex ligation-dependent probe amplification (MLPA) [113]. However, only limited genotype-phenotype correlations have been extrapolated from these studies [92,113]. Patients with large NF1 deletions may have more severe phenotypes and structural CNS anomalies [58], although the extent of this association is still being clarified.
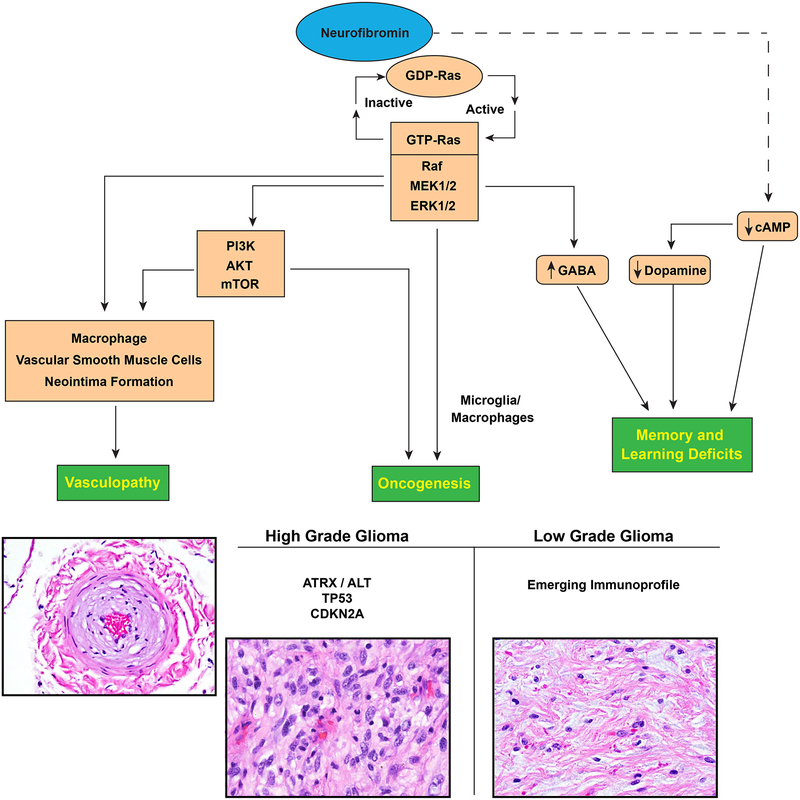
Although the broad functions of neurofibromin remain to be defined, it is known that neurofibromin directly inhibits RAS activation by converting the active form of GTP-bound RAS to its inactive, GDP-bound state [41,23,113]. Inactivation of NF1 leads predominantly to unchecked RAS signaling, its best studied function. GTP-bound RAS leads to activation of mitogen-activated protein kinases (MAPK), extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2). The end result of RAF/MAPK activation is stimulation of transcription and cell growth [40,54,24,29,30] (Figure 1). Unchecked RAS activation can also lead to cross-activation of another important pathway for cell proliferation and survival, the PI3K-mTOR pathway. For example, GTP-bound RAS can bind and activate PI3K leading to survival and proliferation effects through AKT and mTOR activity. ERK can also facilitate mTOR activation through phosphorylation of TSC2, which in turn drives cell growth and survival [70]. Thus, neurofibromin loss can lead to disease through multiple pathways.
Figure 1.
CNS manifestations resulting from neurofibromin loss and aberrant signaling pathways.
Neurofibromin function has also been linked with altered activity in the cAMP signaling pathway. Nf1-deficient drosophila, zebrafish and mice exhibit reduced cAMP levels in the brain [126,49,13]. In the mammalian brain, neurofibromin may regulate cAMP through RAS-mediated activation of protein kinase C zeta (PKCζ) outside of the traditional RAS/MEK signaling pathway. RAS is believed to activate PKCζ, which in turn leads to inhibition of Gαs and subsequently decreased cAMP production in the brain [1]. Warrington and colleagues also reported that reduced cAMP levels may lead to gliomagenesis in a genetically engineered mouse mode of optic pathway gliomas [122]. As such, cAMP modulation in NF1 may have pharmacological utility to both neurocognitive and neoplastic treatment strategies.
Neurodevelopmental and cognitive abnormalities in NF1
A variety of non-neoplastic CNS manifestations are encountered in patients with NF1 through imaging studies, including macrocephaly (~50%), hyperintense T1/T2 lesions, ventricular dilatation[116], and cerebellar hypoplasia [109]. Individuals with NF1 frequently develop MR hyperintensities that are asymptomatic and should not be mistaken for neoplasms (“unidentified bright objects (UBOs)[116] These UBOs histologically demonstrate myelin vacuolation with possible increased water content [110,27]. Neurocognitive deficits are common in people with NF1 and autism spectrum disorder has been also increasingly recognized in children [37,121]. Alterations in white matter tracts in the frontal lobes identified by diffusion tensor imaging may be a contributing factor [55]. At the molecular level, cognitive abnormalities are thought to be related to alterations in neurofibromin production. Decreased cAMP levels in the brain are implicated in learning and memory deficits as well as alterations in neural and glial development [126,46,49,12]. Hyman and colleagues in an observational study of 81 Individuals with NF1 and 49 unaffected sibling controls found that children with NF1 suffered from deficits in reading, spelling, mathematics, attention, executive functioning, receptive and expressive language, and motor skills, compared to controls [51]. Although the causes of neurocognitive deficits are not fully explained, investigations using animal models have revealed possible mechanisms of such deficits.
Epilepsy in NF1
Epilepsy is another recognized neurologic complication in patients with NF1, with a prevalence estimated at 4–13% [59,8]. Observational studies suggest that epileptic seizures in individuals with NF1 are often associated with intracranial tumors or structural abnormalities, including hippocampal sclerosis and polymicrogyria[8]. However, in a subset of cases the etiology of seizures and/or an epileptic focus is not identified [36,50,77]. Although the brain in NF1 has been shown to be hyperexcitable due to alterations in GABA signaling and ion channel dysfunction in mice, this hyperexcitability has not been convincingly implicated as a direct contribution to epileptogenesis in NF1 [72,101].
Cerebrovascular Disorders in NF1
NF1 has been associated with CNS vasculopathy in both adult and pediatric populations. Multiple retrospective imaging studies have found a variety of vascular malformations including vessel ectasia, moyamoya, aneurysm, hypoplasia, and vessel narrowing, including severe stenosis [90,17,74] (Figure 2a–c). However, intracranial aneurysms were not shown to be associated with NF1 in a large Finnish population-based study aimed at assessing Individuals with NF1 with intracranial aneurysms or aneurysmal subarachnoid hemorrhage [60].
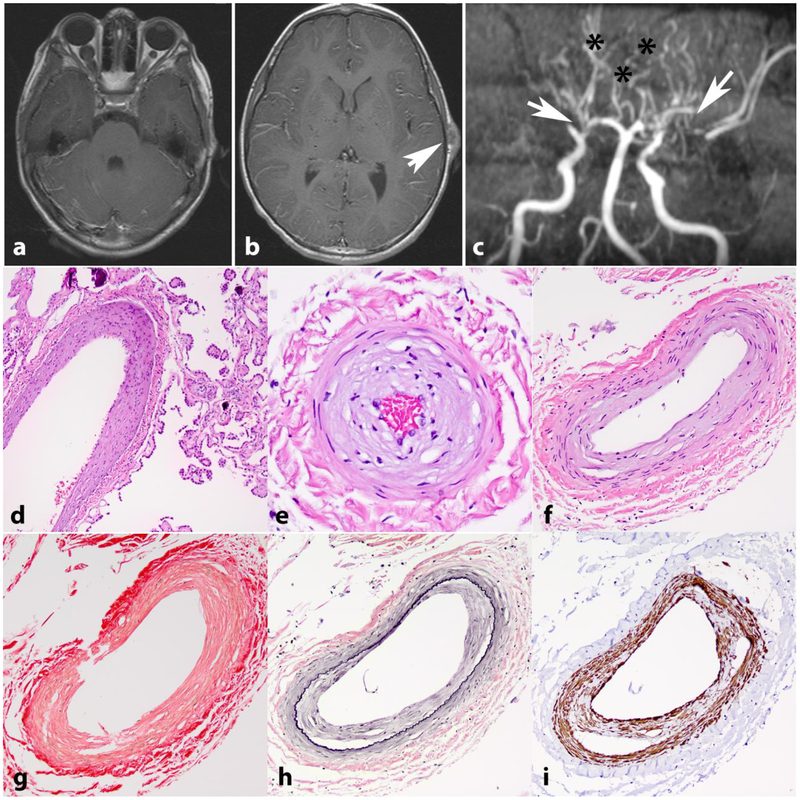
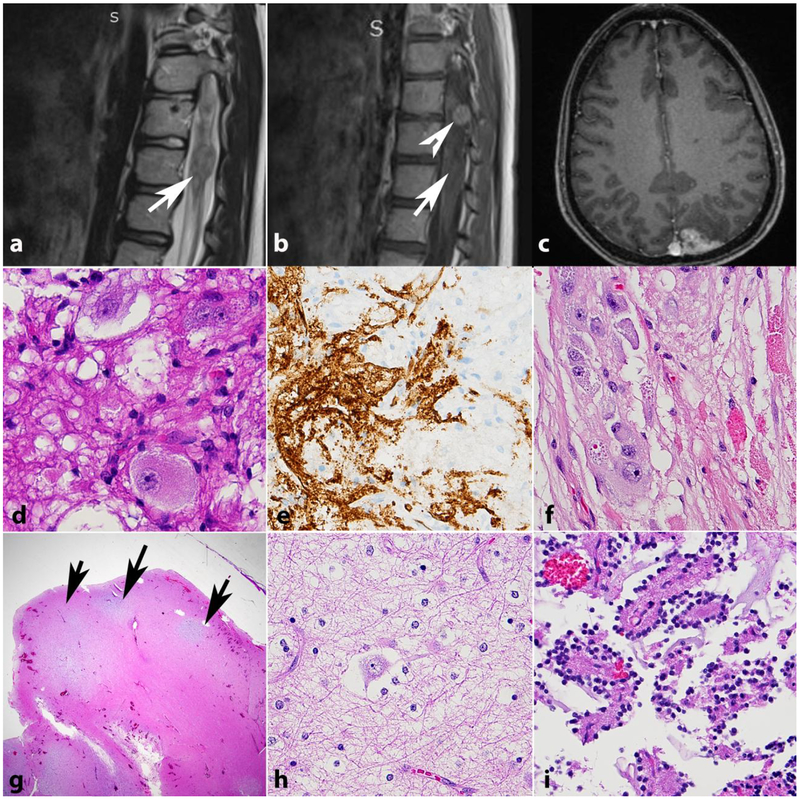
Figure 2. Intracranial vasculopathy in NF1.
Axial T1 weighted image with contrast in a 15-year-old boy with NF1 shows bilateral optic nerve gliomas, larger on the right (a). Axial T1 weighted image with contrast also shows a left frontoparietal scalp soft tissue lesion representing a cutaneous neurofibroma (arrow) (b). MR angiography of the circle of Willis shows stenosis of the distal internal carotid arteries, with no flow in the right middle cerebral artery and severe narrowing of the left middle cerebral artery (arrows). Many collateralized vessels in a moya-moya pattern are seen (asterisks) (c). Histologic sections from a 21 year-old patient with NF1 demonstrating intimal thickening involving vessels in the choroid plexus (d) and leptomeninges (e,f). Picrosirius red stain demonstrates normal mural collagen fiber architecture (g). VVG highlights intimal hyperplasia central to internal elastic lamina (black)(h) and SMA immunostain shows positivity in many of these cells (i).
An important potential complication of vascular malformations associated with NF1 is stroke. This complication is compounded by the fact that individuals with NF1 frequently suffer from hypertension [35], a well-documented risk factor for stroke. Terry and colleagues, using a population-based, case-control study of the US Nationwide Inpatient Sample, demonstrated associations of younger mean age of patients suffering from stroke, increased likelihood of hypertension in pediatric patients, and greater likelihood of stroke diagnosis, particularly hemorrhagic strokes, in individuals with NF1 compared to the general population [107]. Causes of hypertension in these patients include renal artery stenosis, and on occasion an underlying pheochromocytoma.
The mechanism underlying vasculopathy in NF1 is poorly understood. At least some vasculopathy encountered, particularly moyamoya, may be attributable to radiotherapy effects [17,38,114]. However, vascular abnormalities in individuals with NF1 are observed in the absence of radiotherapy, suggesting an underlying intrinsic mechanism of vasculopathy. Bajaj et al. studied the effect of neurofibromin loss on endothelial cells. Neurofibromin loss results in increased proliferation and cell cycle entry. Additionally, NF1-deficient cells demonstrated abnormal morphogenesis, did not branch normally in co-culture assays, resulting in few tubules and branches [4].
An early hypotheses for vasculopathy in NF1 focused on possible Schwann cell proliferation within arteries, which was proposed by Salyer and Salyer based on pure morphologic observations describing a putative resemblance to cellular areas of neurofibroma [93]. However, this hypothesis has lost support, with other cell components such as smooth muscle being more likely involved in the vasculopathy [45]. For instance, findings by Li and colleagues in vascular smooth muscle cell cultures from Nf1 +/− mice and human individuals with NF1 suggest increased proliferation and migration of vascular smooth muscle cells as a result of a Ras-induced increase of platelet-derived growth factor (PDGF) secondary to neurofibromin loss [65]. As such, neurofibromin-induced vascular smooth muscle cell dysfunction may contribute to vasculopathy.
Additionally, vasculopathy in NF1 has been hypothesized to be a result of intimal proliferation secondary to macrophage dysfunction. Nf1 +/− and wildtype mice results from Stansfield and colleagues showed Ras-Erk directed recruitment of macrophages, increased macrophage proliferation, migration, and adhesion, and neointimal formation following injury in neurofibromin-deficient mice compared to wild-type controls [103]. Bessler and colleagues further hypothesized that MCP-1, a chemokine anchored to endothelium as well as secreted, and CCR2 positive macrophages are important mediators of neointima formation in the context of NF1 [10].
Histological and ultrastructural assessment of vessels in NF1 have demonstrated thickened intima, smooth muscle nodules and proliferation, fibromuscular hyperplasia, Schwann cell hyperplasia, and neural proliferation in vessel walls [35,71]. Neointimal thickening is identifiable in small caliber vessels in spinal cord, leptomeningeal and choroid plexus vessels of individuals with NF1 [68](Figure 2d–i), and Nf1 deficiency increases neointimal formation in mice after injury [102].
Central Nervous System Neoplasms in NF1
Individuals with NF1 are prone to developing neoplasms of the CNS and are particularly at risk for optic pathway gliomas [34]. Individuals with NF1 are additionally at an increased risk for other CNS tumors [31], including infiltrating gliomas [39]. Rare CNS tumors such as ganglioglioma, astrocytomas resembling SEGA, and tumors that are difficult to classify and grade using standard criteria developed for sporadic tumors also occur in individuals with NF1 [88]. Predilection for the optic pathways (Figure 3a) and multifocality (Figure 3b) are features that may be encountered in low grade gliomas of individuals with NF1. As mentioned above, asymptomatic MR hyperintensities are frequent in these individuals (Figure 3c).
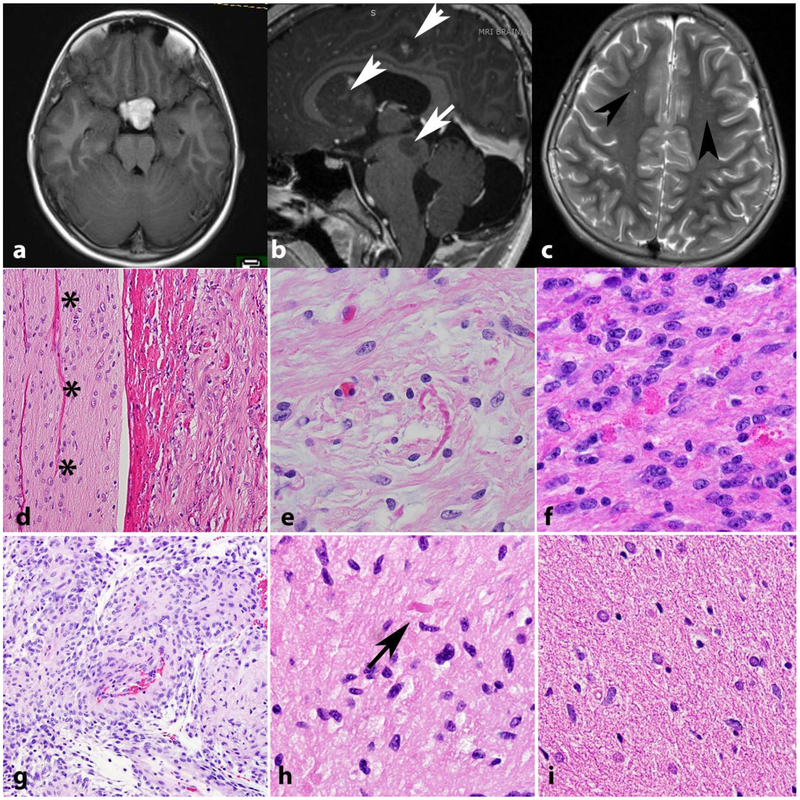
Figure 3. Low grade astrocytomas in NF1.
The optic pathways, including the optic chiasm are favored sites for low grade astrocytomas in NF1. Axial T1 weighted MR image in a 41-year-old woman with NF1 demonstrates a bulky mass involving the optic chiasm (a) that histologically proved to be a pilocytic astrocytoma. Multicentricity (arrows) of pilocytic astrocytomas is a feature of NF1 (arrows) (b). “Unidentified bright objects” represent hyperintensities on MRI that do not grow and frequently regress over time (arrowheads). They are frequent in individuals with NF1 and should not be misinterpreted as tumors (Axial T2-weighted image) (c). Pilocytic astrocytomas involving the optic nerve (asterisks) (optic nerve glioma) frequently extend into the subarachnoid space (d). Most gliomas in individuals with NF1 are pilocytic astrocytomas, characterized by frequent Rosenthal fibers (e) and eosinophilic granular bodies (f). The pilomyxoid variant of pilocytic astrocytoma also may develop in individuals with NF1 (g). Low grade astrocytomas that are difficult to classify, usually having infiltrative features but with occasional piloid features such as rare Rosenthal fibers (arrow) are also relatively frequent (h). Diffuse astrocytomas also occur in individuals with NF1 and resemble sporadic diffuse astrocytomas, particularly single cell infiltration and lack of Rosenthal fibers(i).
Pilocytic Astrocytomas in NF1
Optic pathway gliomas are estimated to affect 15–20% of children with NF1. However, the clinical term, “optic glioma,” is not a pathologic term. Most gliomas of the optic nerve are pilocytic astrocytomas [87] [84]. They are typically followed or treated clinically without pathological confirmation of tumor type or resection, as many tumors behave in an indolent fashion such that many regress without intervention or respond to first line chemotherapy. Anatomic location and the morbidity of the procedure also play a role in the rarity of these biopsies in this special population.
Specific NF1 gene variants may also be important to varying optic glioma behavior and severity. Toonen and colleagues demonstrated distinct neurofibromin levels, proliferation rates, microglial content, and clinical behavior when comparing nonsense (R681X) and missense (G848R) Nf1 mutations in genetically engineered mouse models [111]. Of interest, studies of the tumor microenvironment in optic pathway gliomas have highlighted an important biologic role for microglia. Microglia have been found to constitute a significant constituent of glial tumors, possibly as a result of monocyte chemoattractant protein-1 (MCP-1) release by gliomas [123]. Within the tumor microenvironment, microglia can have immunosuppressive and tumor permissive effects through release of chemokines such as tumor growth factor beta (TGFβ), interleukin 6 (IL-6), prostaglandins, and vascular endothelial growth factor (VEGF), aiding in glioma growth and survival [123]. Interestingly, higher microglia levels and varying axonal injury sensitivity have been observed in female mice compared to male counterparts, a difference thought to be mediated by estrogen effects on estrogen receptor β [112]. Additionally, some studies have shown clinical differences in optic glioma behavior based on sex, with females being more likely than males to have a poorer clinical course [25,112]. Experiments aimed at reducing microglial function and recruitment of microglia to gliomas have demonstrated attenuated optic glioma growth, raising the possibility of immunomodulation of microglia as a possible therapeutic strategy in such neoplasms [81,99,48,56].
A neuropathology-oriented study revealed that PA are the most common CNS tumors in individuals with NF1 [88]. PA are low grade, generally well-circumscribed glial neoplasms often exhibiting a biphasic histologic pattern of compact and loose areas (Figure 3). Immunohistochemically, PA are positive for glial markers such as glial fibrillary acid protein (GFAP) and oligodendrocyte transcription factor 2 (OLIG2) [75].
Within the context of NF1, PA tumorigenesis is thought to arise secondary to altered levels and or activity of neurofibromin, suggesting that NF1-associated PA arise through a distinct tumorigenic pathway versus their sporadic counterparts [54,40,61,34]. Loss of neurofibromin in astrocytes leads to increased RAS activity and subsequently downstream RAS effects hypothesized to result in PA tumorigenesis [43,61,34]. For example, MAPK/ERK activation has been shown across all astrocytoma types, including sporadic and NF1-associated PA; however, MAPK/ERK activation in NF1-associated PA is independent of BRAF alterations [85].
Whereas most PA behave in an indolent manner and are designated as World Health Organization (WHO) Grade I, a subset behave more aggressively and may demonstrate anaplastic features, such as necrosis, increased mitotic activity, and hypercellularity [86]. Recent studies into PA with anaplasia, including those in the context of NF1 syndrome, demonstrated alternative lengthening of telomeres (ALT) or features of alpha thalassemia/mental retardation syndrome X‐ linked (ATRX) loss in a substantial portion of cases. Specifically, ALT was present in half (4 of 8) cases developing individuals with NF1 and ATRX protein loss in 5 (of 8) [86], although NF1 status was not independently associated with a poorer prognosis. Reinhardt et al. also reported on a group of anaplastic astrocytomas with piloid features, and reported ATRX loss or pathogenic gene variants in 45% of their cases. Of interest, MAPK pathway alterations were present in most tumors (75%) and variants in the NF1 gene were the single most frequent alteration [83]. More recently, D’angelo et al. in a comprehensive genomic analysis of gliomas developing in individuals with NF1 reported ATRX pathogenic gene variants in 38% of high grade gliomas compared to 3.1% of low grade gliomas [22].
Overall, NF1-associated PA have a better prognosis than sporadic counterparts in children. [85]. However, it is increasingly recognized that NF1-associated PA in adults may behave more aggressively [16,47,67,91,104].
Diffuse Gliomas
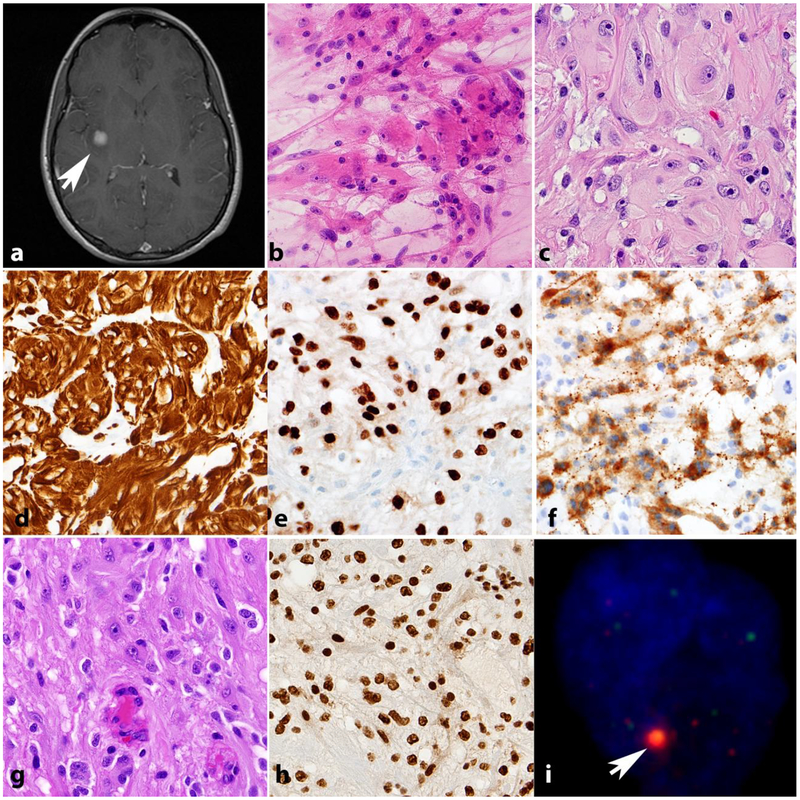
Although not as common as PA, patients with NF1 suffer from an increased risk of diffuse and high grade gliomas, particularly in patients over 10 years of age [44,88,18](Figure 4). A retrospective pathologic study of NF1-associated gliomas found that diffuse astrocytomas accounted for 27% of such cases [88], and they usually develop outside of the optic pathways. Limited data is available on glioblastomas within the context of NF1 syndrome. In a recent report of four NF1-associated glioblastomas, the tumors were well-circumscribed on imaging, had relatively long survival, and did not feature alterations in ATRX, EGFR amplification, IDH1, BRAF V600E, or TERT promoter [95]. All high grade glioma morphologic variants may develop in individuals with NF1, including giant cell glioblastoma, its close mimic anaplastic pleomorphic xanthoastrocytoma and gliosarcoma (Figure 4b–f). The latter is important to have in mind for pathologic diagnosis, given the difficulties in separating from MPNST. Our previous work has also demonstrated a high frequency of ATRX loss and ALT in NF1 associated diffuse/high grade gliomas [89](Figure 4g,h,i), and as mentioned above, ATRX pathogenic variants are typical of high grade gliomas in individuals with NF1.
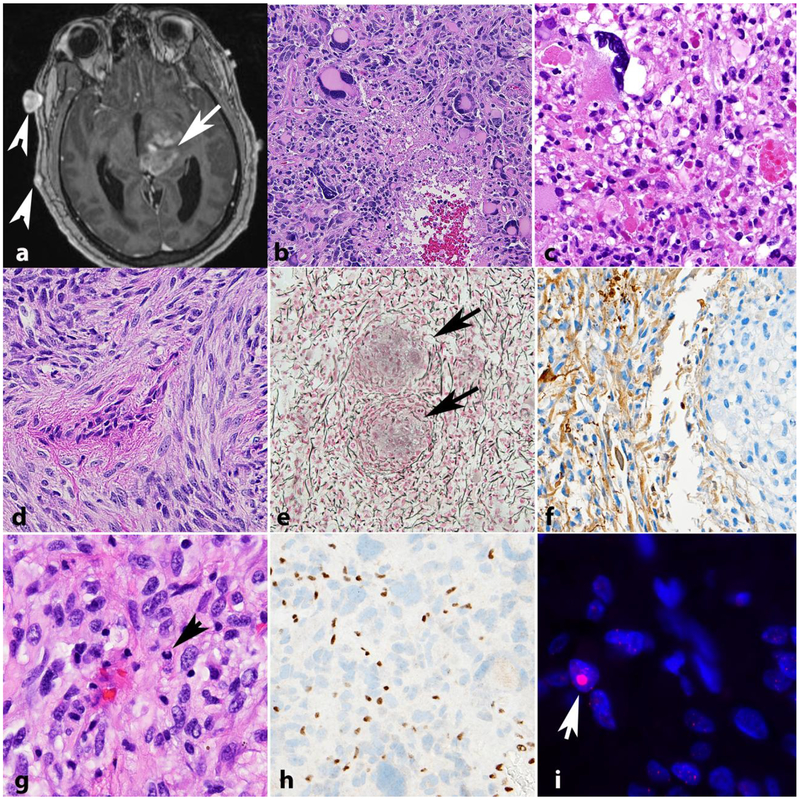
Figure 4. High grade astrocytomas in NF1.
High grade astrocytoma forming a deep contrast enhancing mass (arrow). Other manifestations of NF1 in this patient included neurofibromas of the scalp (arrowheads) (axial T1-weighted post-contrast image)(a). The histology of high grade astrocytomas in individuals with NF1 is also variable, and includes giant cell glioblastoma (b) and anaplastic pleomorphic xanthoastrocytoma (c). NF1 associated gliosarcoma (d) with biphasic components highlighted by reticulin special stain, including islands of reticulin poor glioma cells (arrows) surrounded by reticulin rich sarcomatous areas (e). GFAP immunoreactivity in this tumor is limited to glial areas (left) and is negative in the pleomorphic sarcomatous component (right)(f). Anaplastic astrocytoma in NF1 patient with mitotic activity (arrow)(g), ATRX expression loss by immunohistochemistry (h) and large foci in telomeric FISH (arrow) consistent with alternative lengthening of telomeres (i).
Indeterminate and Rare CNS Neoplasms in NF1
Although the most common CNS neoplasms in NF1 are PA followed by diffuse gliomas, a number of indeterminate and rare neoplasms may occur in individuals with NF1. A diagnostic challenge in the context of NF1 is the evaluation of low grade gliomas lacking definitive characteristics of PA, often with concerning features such as infiltrative growth or increased proliferation (Figure 3h). Nonetheless, such indeterminate low grade gliomas have been shown to follow a similar clinical course to classical PA [88].
Other rare CNS neoplasms observed in Individuals with NF1 include a variety of glial and glioneuronal tumors, such as the pilomyxoid astrocytoma variant of PA, ganglioglioma, desmoplastic infantile ganglioglioma [88], pleomorphic xanthoastrocytoma [108,105], dysembryoplastic neuroepithelial tumor (DNT) [64], and rosette-forming glioneuronal tumor (RGNT) [98,57] (Figures 3–5). Regarding glioneuronal tumors in specific, it is unclear if they are more common in individuals with NF1 since most papers describing them are limited to isolated case reports. In a prior study of 100 patients with NF1, two (2%) glioneuronal tumors were identified: one ganglioglioma and one desmoplastic infantile ganglioglioma [88]. The molecular alterations in these cases have not been adequately studied either.
Figure 5. Glioneuronal tumors in NF1.
Gangliogliomas centered in the conus (a,b,d,e) and occipital cortex (c,f) in individuals with NF1. CD34 expression may be seen (e) as in sporadic gangliogliomas. Dysembryoplastic neuroepithelial tumor forming mucoid cortical nodules (arrows)(g) and containing floating neurons on high power (h). Rosette forming glioneuronal tumors also may occur in individuals with NF1, often outside the posterior fossa (i).
Tumors with morphologic and immunophenotypic features resembling SEGA, a tumor typically developing in patients with tuberous sclerosis complex (TSC) are also known to develop in individuals with NF1 [76](Figure 6). These SEGA-like astrocytomas are predominantly low grade, and characterized histologically by the presence of large, plump cells with ample cytoplasm and macronuclei that stain for GFAP, OLIG2, and S100. They variably express neuronal markers, demonstrate mTOR pathway activation, and most contain NF1 pathogenic gene variants. IDH1, IDH2, and BRAF alterations are not observed [76]. Of interest, NF1 loss results in mTOR pathway activation, and mTOR pathway activation is a molecular basis for the pathogenesis of SEGA. More recently, primary intracranial sarcomas containing DICER1 mutations have been reported predominantly in the sporadic setting but also in at least one individual with NF1 [63].
Figure 6. SEGA-like astrocytoma.
Neoplasms resembling subependymal giant cell astrocytomas (SEGA) typical of tuberous sclerosis occur in a subset of individuals with NF1, although they tend to be hemispheric (a, b,c). As SEGA, they express glial markers (GFAP, d; OLIG2, e) and neuronal markers (synaptophysin, f). SEGA-like astrocytoma in NF1 patient (g) with intact ATRX expression (h) but large telomere foci (red) colocalizing with Promyelocytic bodies (green)(arrow) consistent with alternative lengthening of telomeres (i).
Animal Models of NF1 deficiency
Models of NF1 deficiency have been particularly useful in the study of a variety of non-neoplastic and neoplastic CNS manifestations of the disease. For example, experiments using heterozygous Nf1 +/− mice with and without corrective Ras mutations demonstrated that spatial learning deficits in Nf1 heterozygous mice could be corrected with Ras null mutations. Such learning deficits are thought to result secondary to RAS mediated increases in GABA-mediated inhibition [21]. In addition, Zhu and colleagues in a Cre-LoxP Nf1 model directed against neuronal populations in mice demonstrated abnormal cerebral cortex development and astrogliosis [130]. Other genetically engineered mice modeling heterozygous Nf1 germline loss and somatic inactivation in neuroglial progenitor cells demonstrated abnormalities in early communicative behavior, relevant to autism spectrum disorders [69]. Heterozygous null Nf1 mouse studies implicate neurofibromin-related Ras pathway alterations leading to GABA dysregulation in the brain as a cause of learning deficits and working memory impairment in individuals with NF1 [96,21]. Dopamine dysregulation has also been implicated as a cause of learning and attention deficits in individuals with NF1 [26,14]. Short term trials have found a possible beneficial effect of methylphenidate in individuals with NF1 and ADHD [66] and additional trials of methylphenidate and neurostimulants in these patients are ongoing ( NCT02944032) [82]. Nf1 mutant Drosophila flies have also been used to study memory deficits [15]. A new porcine model developed by White and colleagues recapitulates features of NF1, such as learning and memory deficits, in addition to physical manifestations such as café au lait macules [124]. Such a model may prove useful for future investigation into the mechanistic underpinnings of neurocognitive deficits in NF1.
Modeling NF1 in animal models has demonstrated several technical challenges. For instance, traditional Nf1 knockout mouse model features heterozygous Nf1 genes, as a homozygous knockout genotype is incompatible with life [42,52]. The heterozygous Nf1 +/− mouse model described by Jacks and colleagues demonstrated increased tumorigenesis, including pheochromocytoma and myeloid leukemia, and these neoplasms demonstrated loss of wild type Nf1, a finding consistent with the “two-hit” hypothesis of tumorigenesis in individuals with NF1. However, the heterozygous mouse model described by Jacks et al. did not develop classical phenotypic characteristics of NF1, such as café-au-lait macules, Lisch nodules, and neurofibromas [52] for unknown reasons. Additional studies using the heterozygous Nf1 model have demonstrated increased astrocyte proliferation and growth advantage of Nf1 +/− mice compared to wild type controls through increased activation of the p21-RAS pathway [43,5].
In order to produce peripheral nerve sheath tumorigenesis in mouse models, a chimeric approach has been used in some studies by injecting Nf1−/− cells into a blastocyst. Cichowski and colleagues successfully used this approach to generate plexiform neurofibromas in such a chimeric mouse model [19]. In order to induce malignant peripheral nerve sheath tumors (MPNST), mice heterozygous for Nf1 and Tp53 are used, though when respective mutations are on opposite chromosomes (trans) the genetically engineered mice also developed aggressive sarcomas, in addition to MPNST [19].
The synergistic effects of Nf1 and Tp53 mutations are also utilized in mouse models to generate astrocytomas. Zhu and colleagues using Cre-LoxP recombination with a neural-specific Nf1 mutation and varying Tp53 genotype demonstrated that Tp53 loss before or concurrent with Nf1 loss was crucial for astrocytoma formation, including high grade astrocytoma. The authors concluded that early Tp53 loss provides a selective advantage for astrocytes, which progress to astrocytoma through RAS-pathway activation secondary to Nf1 loss [128]. In this manner, not only is the cooperation of Nf1 and Tp53 alterations important to astrocytoma development in mouse models but, also the timing of such alterations.
Pilocytic astrocytoma of the optic pathway, a common tumor encountered in NF1, is also studied using mouse models. Bajenaru and colleagues demonstrated in a Cre-LoxP Nf1 mouse model that optic pathway glioma formation was dependent on astrocytic Nf1 loss within a heterozygous Nf1 background [6]. Zhu and colleagues demonstrated hyperplastic optic nerves with cellular disorganization using a Cre-LoxP Nf1 mouse model and suggest that such hyperplasia and disorganization is consistent with early optic pathway gliomas [129]. Though optic pathway gliomas produced by the Bajenaru and Zhu groups show some histological features of pilocytic astrocytoma, both lack other common features of pilocytic astrocytoma such as Rosenthal fibers and eosinophilic granular bodies [129,6]. A recent study by Solga and colleagues using a conditional knockout mouse model found that both germline neural progenitor/stem cell Nf1 knockouts and Olig2+ cells with somatic Nf1 loss can give rise to low grade gliomas, though the temporal course of optic glioma tumorigenesis differed [100]. This study suggests the possibility of different progenitor origins contributing to differing tumor behavior among low grade gliomas in individuals with NF1.
Studies of Nf1 effects in zebrafish have used NF1 orthologue knockdown strategies such as morpholino oligonucleotides and zinc finger nucleases as well as targeting induced local lesions in genomes (TILLING) strategies. Shin and colleagues developed lines in zebrafish demonstrating oligodendrocyte progenitor cell (OPC) hyperplasia and proliferation, Schwann cell hyperplasia, myelination abnormalities, upregulation of RAS pathway signaling, and, in combination with Tp53 mutation, high grade glioma and MPNST development [97]. Lee and colleagues have also shown increased OPC proliferation and migration with Nf1 loss using morpholino knockdown in zebrafish [62]. This abnormal accumulation of OPC has additionally been demonstrated in Nf1-mutant mouse models and cell cultures, and again tumorigenic qualities of Nf1 mutants, such as survival and proliferation, were suggested [9]. Such abnormal accumulations of progenitor cells, a consequence of neurofibromin loss, may thus contribute to both neoplastic and neurostructural NF1 manifestations.
Therapeutic Aspects of CNS Manifestations in NF1
Clinical monitoring and treatment of CNS manifestations of NF1 are varied, and many strategies are still in the process of optimization. The most common CNS manifestation, optic pathway gliomas may become symptomatic, but when this happens it usually occurs in young children [11]. Hence, close surveillance of visual acuity as a manifestation of OPG is recommended in children with NF1, and specifically in the context of clinical trials [33]. MRI of the brain is not used as a screening tool for OPG, but patients with suspicious symptoms (deteriorating vision, endocrine issues, headaches, seizures, HC increase) should consider getting a MRI +/− contrast.
A major impetus for treatment in optic pathway gliomas is vision loss, which has spurred interest in the monitoring and assessment of vision deterioration in children affected with NF1 [11]. Fisher and colleagues recommend visual acuity assessment using Teller acuity cards as a primary endpoint with additional assessments of visual acuity depending on the child’s age [33]. A novel approach to visual acuity monitoring is optic coherence tomography, which assesses the thickness of the retinal nerve fiber layer (RNFL), an approach used to assess visual function in the setting of multiple sclerosis [33,11,106]. As RNFL thinning is associated with clinically significant vision loss [106], optic coherence tomography may offer an objective strategy for vision monitoring, particularly in young children for which visual acuity assessment may be difficult [3,11,33].
When optic pathway glioma treatment is needed, chemotherapy is typically used. Symptomatic optic pathway gliomas have traditionally been treated with carboplatin in combination with vincristine [33]. Ongoing chemotherapy trials at the time of this writing include the use of lenalidomide ( NCT01553149), selumetinib ( NCT01089101), vinblastine +/− bevacizumab ( NCT02840409), pomalidomide ( NCT02415153), and pegylated interferon ( NCT02343224) (clinicaltrials.gov).
Among novel NF1 neoplastic therapeutics, selumetinib, an oral MEK1 and MEK2 inhibitor, is of particular interest as there is ongoing investigation of its utility in NF1-related optic pathway gliomas and plexiform neurofibromas. Inhibition of MEK proteins works to mitigate the hyperactivation of RAS signaling secondary to neurofibromin loss in NF1 [53].
An ongoing selumetinib trial in NF1-related plexiform neurofibromas has shown decreased neurofibroma volume with treatment [28]. Selumetinib is also eliciting preliminary treatment responses in sporadic and NF1-associated PA [32,7]. Relatedly, MEK inhibition has shown promise in NF1-deficient glioblastoma cell lines [94], suggesting the applicability of selumetinib therapy to high grade gliomas within the context of NF1 syndrome.
CNS tumor surveillance other than optic pathway glioma surveillance in pediatric NF1-syndrome patients is less defined. For asymptomatic patients, Evans and colleagues recommend that clinicians be aware of the increased risk of low grade and high grade gliomas and that families are educated on clinical warning signs of such CNS disease [31]. For patients who develop high grade gliomas, genetic investigation for constitutional mismatch repair deficiency syndrome (CMMRD), a rare syndrome caused by mutations in mismatch repair genes, is recommended as patients with CMMRD are susceptible to high grade gliomas and show phenotypic overlap with NF1 syndrome. Any of the typical manifestations of NF1 may be present, including café au lait spots, freckling, dermal neurofibromas, plexiform neurofibromas, Lisch nodules and bone abnormalities. However, only a minority of patients satisfy established clinical criteria for NF1 [31,125].
Clinical trials, outcome assessment, and intervention for non-neoplastic CNS manifestations of NF1 is challenging given the multifaceted nature of neurocognitive function. The Neurocognitive Committee of the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) International Collaboration aims to review and provide recommendations for future NF1 trials in the realm of cognitive deficit evaluation and intervention [120].
Sporadic CNS Neoplasms with NF1 Alterations
It is important to note that NF1 alterations in tumorigenesis are not limited to tumors developing in individuals with NF1. Somatic NF1 loss is implicated in a variety of human cancers including glioblastoma, sarcomas, desmoplastic melanoma, ovarian carcinoma, breast cancer, paragangliomas, and acute myeloid leukemia among many others. As such, both animal models and cell lines are employed to investigate sporadic NF1 mutation effects in such neoplasms [80].
Sporadic PA are often characterized molecularly by alterations in the MAPK pathway, particularly by BRAF fusions including KIAA1549:BRAF and point mutations such as BRAF V600E. Other alterations found in PA include FGFR1, FGFR-ITD/fusion, NTRK fusions, and NF1 gene variants [20]. NF1 pathogenic gene variant may occur in PA in the absence of clinical evidence of NF1, where they are usually mutually exclusive with BRAF alterations. More recently, the molecular alterations responsible for anaplasia in PA with anaplasia (anaplastic astrocytoma with piloid features) have been studied. Alterations leading to MAPK activation are frequent, particularly NF1 pathogenic gene variants [83,86](Figure 7)
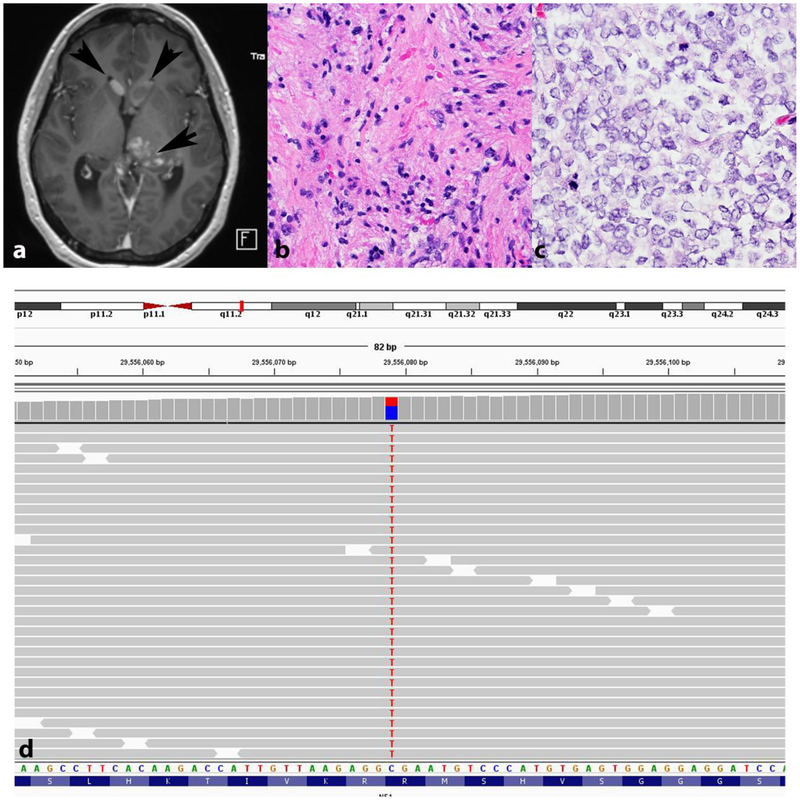
Figure 7. Sporadic pilocytic astrocytoma with anaplasia and NF1 pathogenic gene variant.
Axial T1-weighted MRI demonstrating a thalamic mass with heterogeneous enhancement (arrow), as well as intraventricular nodules consistent with subependymal spread (arrowheads) (a). First biopsy demonstrated a pilocytic astrocytoma (b). Recurrence in the absence of treatment showed a cellular glial neoplasm with brisk mitotic activity consistent with anaplastic progression (c). Next generation sequencing demonstrated a somatic truncating NF1 p.R816* variant with a variant allele frequency (VAF) of 38.5 (c). No other significant gene sequencing variants were identified.
The role of NF1 gene alterations has been studied in sporadic diffuse gliomas. NF1 alterations are associated with the mesenchymal subtype of sporadic glioblastomas, a molecular subtype based on a gene expression signature and associated with short survival [79,117,2,127].
Glioblastoma cell line studies knocking down neurofibromin levels have shown increased secretion of chitinase-3-like protein 1 (CHI3L1), a glycoprotein associated with increased invasion and survival, endoglin (ENG), a TGFβ component implicated in mesenchymal differentiation, and interleukin-8 (IL-8), a cytokine with pro-angiogenic effects [127]. However, a recent review of The Cancer Genome Atlas (TCGA) data did not find a significantly worse prognosis in sporadic glioblastomas with NF1 loss, although sporadic lower grade diffuse gliomas with NF1 loss did demonstrate significantly shorter survival [119]. NF1 pathogenic gene variants occurred in 21 (7%) of the lower grade glioma group. IDH mutations co-occurred with NF1 alterations in this group in only 7 (38%). However, 3 (of 5) patients with follow up data died during the follow-up period, suggesting that the low frequency of IDH mutations alone does not explain the adverse outcome.
Given the increased availability of comprehensive platforms for molecular genetic testing, NF1 sequence variants in brain tumor tissue are increasingly uncovered during routing molecular diagnostics. Criteria for the diagnosis of NF1 remains clinical. However, the identification of NF1 pathogenic variant with associated loss of heterozygosity may provide a rationale to suggest comprehensive clinical evaluation and germline NF1 testing.
Conclusion
Ongoing investigation into NF1-related CNS disease is increasingly geared toward early detection of disease and exploiting therapeutic targets. The complexity of the large NF1 gene and its many known gene variants without clear genotype-phenotype correlation present a challenge for clinicians and researchers alike. However, new drugs such as those targeting the RAS/MAPK pathway show promise in treating NF1-related neoplasia. Efforts at standardizing outcome assessment in trials for non-neoplastic NF1 disease may add increasingly reliable data for investigation into the multifaceted nature of neurocognitive assessment and treatment. Finally, roles of the microenvironment as well as patient characteristics such as sex may further contribute to a precision medicine-based approach to combating CNS disease in NF1 syndrome.
Acknowledgments.
The authors would like to thank contributors to several figures Drs. Doris Lin (figure 2), Bette Kleinschmidt-Demasters (figure 2), Christopher Heaphy (Figure 4 and 6) and Liam Chen (figure 7),
The authors have no conflict of interest to report. All authors participated in the writing, reviewed, and approved the manuscript. The work was funded in part by This work was supported in part by Pilocytic/Pilomyxoid Fund, including Lauren’s First and Goal, and the Stick it to Brain Tumors Annual Women’s Ice Hockey Tournament (F.J.R.) and NIH grant P30 CA006973 to the Sidney Kimmel Comprehensive Cancer Center (PI: W. Nelson).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Anastasaki C, Gutmann DH (2014) Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum Mol Genet 23:6712–6721. doi: 10.1093/hmg/ddu389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appin CL, Brat DJ (2014) Molecular Genetics of Gliomas. Cancer J 20:66–72. doi: 10.1097/ppo.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 3.Avery RA, Hwang EI, Ishikawa H, Acosta MT, Hutcheson KA, Santos D, Zand DJ, Kilburn LB, Rosenbaum KN, Rood BR, Schuman JS, Packer RJ (2014) Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol 132:265–271. doi: 10.1001/jamaophthalmol.2013.7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj A, Li QF, Zheng Q, Pumiglia K (2012) Loss of NF1 expression in human endothelial cells promotes autonomous proliferation and altered vascular morphogenesis. PLoS One 7:e49222. doi: 10.1371/journal.pone.0049222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajenaru ML, Donahoe J, Corral T, Reilly KM, Brophy S, Pellicer A, Gutmann DH (2001) Neurofibromatosis 1 (NF1) heterozygosity results in a cell-autonomous growth advantage for astrocytes. Glia 33:314–323. doi: [DOI] [PubMed] [Google Scholar]
- 6.Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH (2003) Optic Nerve Glioma in Mice Requires Astrocyte Nf1 Gene Inactivation and Nf1 Brain Heterozygosity. Cancer Res 63:8573. [PubMed] [Google Scholar]
- 7.Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 19:1135–1144. doi: 10.1093/neuonc/now282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barba C, Jacques T, Kahane P, Polster T, Isnard J, Leijten FSS, Ozkara C, Tassi L, Giordano F, Castagna M, John A, Öz B, Salon C, Streichenberger N, Cross JH, Guerrini R (2013) Epilepsy surgery in Neurofibromatosis Type 1. Epilepsy Res 105:384–395. doi: 10.1016/j.eplepsyres.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 9.Bennett MR, Rizvi TA, Karyala S, McKinnon RD, Ratner N (2003) Aberrant Growth and Differentiation of Oligodendrocyte Progenitors in Neurofibromatosis Type 1 Mutants. J Neurosci 23:7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessler WK, Kim G, Hudson FZ, Mund JA, Mali R, Menon K, Kapur R, Clapp DW, Ingram DA Jr., Stansfield BK (2016) Nf1+/− monocytes/macrophages induce neointima formation via CCR2 activation. Hum Mol Genet 25:1129–1139. doi: 10.1093/hmg/ddv635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakeley JO, Plotkin SR (2016) Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol 18:624–638. doi: 10.1093/neuonc/nov200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JA, Diggs-Andrews KA, Gianino SM, Gutmann DH (2012) Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner. Mol Cell Neurosci 49:13–22. doi: 10.1016/j.mcn.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JA, Diggs-Andrews KA, Gianino SM, Gutmann DH (2012) Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner. Mol Cell Neurosci 49:13–22. doi: 10.1016/j.mcn.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JA, Xu J, Diggs-Andrews KA, Wozniak DF, Mach RH, Gutmann DH (2011) PET Imaging for Attention Deficit Preclinical Drug Testing in Neurofibromatosis-1 Mice. Exp Neurol 232:333–338. doi: 10.1016/j.expneurol.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchanan ME, Davis RL (2010) A Distinct Set of Drosophila Brain Neurons Required for NF1-Dependent Learning and Memory. J Neurosci 30:10135–10143. doi: 10.1523/JNEUROSCI.0283-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne S, Connor S, Lascelles K, Siddiqui A, Hargrave D, Ferner RE (2017) Clinical presentation and prognostic indicators in 100 adults and children with neurofibromatosis 1 associated non-optic pathway brain gliomas. J Neurooncol 133:609–614. doi: 10.1007/s11060-017-2475-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns AG, North KN (2008) Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatry 79:1165. doi: 10.1136/jnnp.2007.136457 [DOI] [PubMed] [Google Scholar]
- 18.Campian J, Gutmann DH (2017) CNS Tumors in Neurofibromatosis. J Clin Oncol 35:2378–2385. doi: 10.1200/JCO.2016.71.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T (1999) Mouse Models of Tumor Development in Neurofibromatosis Type 1. Science 286:2172. [DOI] [PubMed] [Google Scholar]
- 20.Collins VP, Jones DTW, Giannini C (2015) Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129:775–788. doi: 10.1007/s00401-015-1410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ (2002) Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415:526. doi: 10.1038/nature711 [DOI] [PubMed] [Google Scholar]
- 22.D’Angelo F, Ceccarelli M, Tala, Garofano L, Zhang J, Frattini V, Caruso FP, Lewis G, Alfaro KD, Bauchet L, Berzero G, Cachia D, Cangiano M, Capelle L, de Groot J, DiMeco F, Ducray F, Farah W, Finocchiaro G, Goutagny S, Kamiya-Matsuoka C, Lavarino C, Loiseau H, Lorgis V, Marras CE, McCutcheon I, Nam DH, Ronchi S, Saletti V, Seizeur R, Slopis J, Sunol M, Vandenbos F, Varlet P, Vidaud D, Watts C, Tabar V, Reuss DE, Kim SK, Meyronet D, Mokhtari K, Salvador H, Bhat KP, Eoli M, Sanson M, Lasorella A, Iavarone A (2019) The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 25:176–187. doi: 10.1038/s41591-018-0263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH (2005) Proteomic Analysis Reveals Hyperactivation of the Mammalian Target of Rapamycin Pathway in Neurofibromatosis 1–Associated Human and Mouse Brain Tumors. Cancer Res 65:2755. [DOI] [PubMed] [Google Scholar]
- 24.DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR (1992) Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69:265–273. doi: 10.1016/0092-8674(92)90407-4 [DOI] [PubMed] [Google Scholar]
- 25.Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH (2014) Sex is a major determinant of neuronal dysfunction in Neurofibromatosis Type 1. Ann Neurol 75:309–316. doi: 10.1002/ana.24093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH (2013) Dopamine deficiency underlies learning deficits in Neurofibromatosis-1 mice. Ann Neurol 73:309–315. doi: 10.1002/ana.23793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiPaolo DP, Zimmerman RA, Rorke LB, Zackai EH, Bilaniuk LT, Yachnis AT (1995) Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology 195:721–724. doi: 10.1148/radiology.195.3.7754001 [DOI] [PubMed] [Google Scholar]
- 28.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC (2016) Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N Engl J Med 375:2550–2560. doi: 10.1056/NEJMoa1605943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donovan S, Shannon KM, Bollag G (2002) GTPase activating proteins: critical regulators of intracellular signaling. Biochim Biophys Acta 1602:23–45. doi: 10.1016/S0304-419X(01)00041-5 [DOI] [PubMed] [Google Scholar]
- 30.Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3:11. doi: 10.1038/nrc969 [DOI] [PubMed] [Google Scholar]
- 31.Evans DGR, Salvador H, Chang VY, Erez A, Voss SD, Schneider KW, Scott HS, Plon SE, Tabori U (2017) Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 1. Clin Cancer Res 23:e46. [DOI] [PubMed] [Google Scholar]
- 32.Fangusaro JR, Onar-Thomas A, Young-Poussaint T, Wu S, Ligon AH, Lindeman NI, Banerjee A, Packer R, Kilburn LB, Pollack I, Jakacki R, Qaddoumi IA, Fisher PG, Dhall G, Baxter PA, Kreissman SG, Doyle LA, Smith MA, Dunkel IJ, Fouladi M (2017) A phase II prospective study of selumetinib in children with recurrent or refractory low-grade glioma (LGG): A Pediatric Brain Tumor Consortium (PBTC) study. J Clin Oncol 35:10504–10504. doi: 10.1200/JCO.2017.35.15_suppl.10504 [DOI] [Google Scholar]
- 33.Fisher MJ, Avery RA, Allen JC, Ardern-Holmes SL, Bilaniuk LT, Ferner RE, Gutmann DH, Listernick R, Martin S, Ullrich NJ, Liu GT (2013) Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology 81:S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freret ME, Gutmann DH (2007) Understanding Vision Loss from Optic Pathway Glioma in Neurofibromatosis Type 1. Ann Neurol 61:189–198. doi: 10.1002/ana.21107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE, McManus B, Korf BR (2002) Cardiovascular disease in neurofibromatosis 1: Report of the NF1 Cardiovascular Task Force. Genet Med 4:105. doi: 10.1097/00125817-200205000-00002 [DOI] [PubMed] [Google Scholar]
- 36.Gales J, Prayson RA (2017) Hippocampal sclerosis and associated focal cortical dysplasia-related epilepsy in neurofibromatosis type I. J Clin Neurosci 37:15–19. doi: 10.1016/j.jocn.2016.10.048 [DOI] [PubMed] [Google Scholar]
- 37.Garg S, Green J, Leadbitter K, Emsley R, Lehtonen A, Evans DG, Huson SM (2013) Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics 132:e1642–1648. doi: 10.1542/peds.2013-1868 [DOI] [PubMed] [Google Scholar]
- 38.Grill J, Couanet D, Cappelli C, Habrand JL, Rodriguez D, Sainte-Rose C, Kalifa C (2001) Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 45:393–396. doi: [DOI] [PubMed] [Google Scholar]
- 39.Guillamo JS, for the Réseau NFF, Créange A, for the Réseau NFF, Kalifa C, for the Réseau NFF, Grill J, for the Réseau NFF, Rodriguez D, for the Réseau NFF, Doz F, for the Réseau NFF, Barbarot S, for the Réseau NFF, Zerah M, for the Réseau NFF, Sanson M, for the Réseau NFF, Bastuji‐Garin S, for the Réseau NFF, Wolkenstein P, for the Réseau NFF (2003) Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1)A retrospective study of 104 patients. Brain 126:152–160. doi: 10.1093/brain/awg016 [DOI] [PubMed] [Google Scholar]
- 40.Gutmann DH (2002) Review Article : Neurofibromin in the Brain. J Child Neurol 17:592–601. doi: 10.1177/088307380201700809 [DOI] [PubMed] [Google Scholar]
- 41.Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ (2017) Neurofibromatosis type 1. Nat Rev Dis Primers 3:17004. doi: 10.1038/nrdp.2017.4 [DOI] [PubMed] [Google Scholar]
- 42.Gutmann DH, Giovannini M (2002) Mouse Models of Neurofibromatosis 1 and 2. Neoplasia 4:279–290. doi: 10.1038/sj.neo.7900249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutmann DH, Loehr A, Zhang Y, Kim J, Henkemeyer M, Cashen A (1999) Haploinsufficiency for the neurofibromatosis 1 (NF1) tumor suppressor results in increased astrocyte proliferation. Oncogene 18:4450. doi: 10.1038/sj.onc.1202829 [DOI] [PubMed] [Google Scholar]
- 44.Gutmann DH, Rasmussen SA, Wolkenstein P, MacCollin MM, Guha A, Inskip PD, North KN, Poyhonen M, Birch PH, Friedman JM (2002) Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 59:759. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton SJ, Friedman JM (2001) Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet 58:341–344. doi: 10.1034/j.1399-0004.2000.580501.x [DOI] [PubMed] [Google Scholar]
- 46.Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH (2007) Neurofibromatosis-1 Regulates Neuronal and Glial Cell Differentiation from Neuroglial Progenitors In Vivo by Both cAMP- and Ras-Dependent Mechanisms. Cell Stem Cell 1:443–457. doi: 10.1016/j.stem.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 47.Helfferich J, Nijmeijer R, Brouwer OF, Boon M, Fock A, Hoving EW, Meijer L, den Dunnen WF, de Bont ES (2016) Neurofibromatosis type 1 associated low grade gliomas: A comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol 104:30–41. doi: 10.1016/j.critrevonc.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 48.Helfferich J, Nijmeijer R, Brouwer OF, Boon M, Fock A, Hoving EW, Meijer L, den Dunnen WFA, de Bont ESJM (2016) Neurofibromatosis type 1 associated low grade gliomas: A comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol 104:30–41. doi: 10.1016/j.critrevonc.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 49.Ho IS, Hannan F, Guo H-F, Hakker I, Zhong Y (2007) Distinct Functional Domains of Neurofibromatosis Type 1 Regulate Immediate versus Long-Term Memory Formation. J Neurosci 27:6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh H-Y, Fung H-C, Wang C-J, Chin S-C, Wu T (2011) Epileptic seizures in neurofibromatosis type 1 are related to intracranial tumors but not to neurofibromatosis bright objects. Seizure 20:606–611. doi: 10.1016/j.seizure.2011.04.016 [DOI] [PubMed] [Google Scholar]
- 51.Hyman SL, Shores A, North KN (2005) The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65:1037. [DOI] [PubMed] [Google Scholar]
- 52.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA (1994) Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genet 7:353. doi: 10.1038/ng0794-353 [DOI] [PubMed] [Google Scholar]
- 53.Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim M-O, Dombi E, Sabo J, Hardiman Dudley A, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N (2013) MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest 123:340–347. doi: 10.1172/JCI60578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones DTW, Gronych J, Lichter P, Witt O, Pfister SM (2012) MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci 69:1799–1811. doi: 10.1007/s00018-011-0898-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlsgodt KH, Rosser T, Lutkenhoff ES, Cannon TD, Silva A, Bearden CE (2012) Alterations in white matter microstructure in neurofibromatosis-1. PLoS One 7:e47854. doi: 10.1371/journal.pone.0047854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karmakar S, Reilly KM (2017) The role of the immune system in neurofibromatosis type 1-associated nervous system tumors. CNS Oncol 6:45–60. doi: 10.2217/cns-2016-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kemp S, Achan A, Ng T, Dexter MAJ (2012) Rosette-forming glioneuronal tumour of the lateral ventricle in a patient with neurofibromatosis 1. J Clin Neurosci 19:1180–1181. doi: 10.1016/j.jocn.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 58.Korf BR, Schneider G, Poussaint TY (1999) Structural anomalies revealed by neuroimaging studies in the brains of patients with neurofibromatosis type 1 and large deletions. Genet Med 1:136–140. doi: 10.1097/00125817-199905000-00004 [DOI] [PubMed] [Google Scholar]
- 59.Kulkantrakorn K, Geller TJ (1998) Seizures in neurofibromatosis 1. Pediatr Neurol 19:347–350. doi: 10.1016/S0887-8994(98)00075-7 [DOI] [PubMed] [Google Scholar]
- 60.Kurtelius A, Kallionpää RA, Huttunen J, Huttunen TJ, Helin K, Koivisto T, Frösen J, von Und Zu Fraunberg M, Peltonen S, Peltonen J, Jääskeläinen JE, Lindgren AE (2017) Neurofibromatosis type 1 is not associated with subarachnoid haemorrhage. PloS one 12:e0178711–e0178711. doi: 10.1371/journal.pone.0178711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau N, Feldkamp MM, Roncari L, Loehr AH, Shannon P, Gutmann DH, Guha A (2000) Loss of Neurofibromin Is Associated with Activation of RAS/MAPK and PI3-K/AKT Signaling in a Neurofibromatosis 1 Astrocytoma. J Neuropathol Exp Neurol 59:759–767. doi: 10.1093/jnen/59.9.759 [DOI] [PubMed] [Google Scholar]
- 62.Lee J-S, Padmanabhan A, Shin J, Zhu S, Guo F, Kanki JP, Epstein JA, Look AT (2010) Oligodendrocyte progenitor cell numbers and migration are regulated by the zebrafish orthologs of the NF1 tumor suppressor gene. Hum Mol Genet 19:4643–4653. doi: 10.1093/hmg/ddq395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JC, Villanueva-Meyer JE, Ferris SP, Sloan EA, Hofmann JW, Hattab EM, Williams BJ, Guo H, Torkildson J, Florez A, Van Ziffle J, Onodera C, Grenert JP, Cho SJ, Horvai AE, Jones DTW, Pfister SM, Koelsche C, von Deimling A, Korshunov A, Perry A, Solomon DA (2019) Primary intracranial sarcomas with DICER1 mutation often contain prominent eosinophilic cytoplasmic globules and can occur in the setting of neurofibromatosis type 1. Acta Neuropathol 137:521–525. doi: 10.1007/s00401-019-01960-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lellouch-Tubiana A, Bourgeois M, Vekemans M, Robain O (1995) Dysembryoplastic neuroepithelial tumors in two children with neurofibromatosis type 1. Acta Neuropathol 90:319–322. doi: 10.1007/BF00296517 [DOI] [PubMed] [Google Scholar]
- 65.Li F, Munchhof AM, White HA, Mead LE, Krier TR, Fenoglio A, Chen S, Wu X, Cai S, Yang F-C, Ingram DA (2006) Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet 15:1921–1930. doi: 10.1093/hmg/ddl114 [DOI] [PubMed] [Google Scholar]
- 66.Lion-Francois L, Gueyffier F, Mercier C, Gerard D, Herbillon V, Kemlin I, Rodriguez D, Ginhoux T, Peyric E, Coutinho V, Breant V, des Portes V, Pinson S, Combemale P, Kassai B, Reseau NFRAA-F (2014) The effect of methylphenidate on neurofibromatosis type 1: a randomised, double-blind, placebo-controlled, crossover trial. Orphanet J Rare Dis 9:142. doi: 10.1186/s13023-014-0142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J (2004) Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology 63:1944–1946 [DOI] [PubMed] [Google Scholar]
- 68.Lummus S, Breeze R, Lucia MS, Kleinschmidt-DeMasters BK (2014) Histopathologic features of intracranial vascular involvement in fibromuscular dysplasia, ehlers-danlos type IV, and neurofibromatosis I. J Neuropathol Exp Neurol 73:916–932. doi: 10.1097/NEN.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 69.Maloney SE, Chandler KC, Anastasaki C, Rieger MA, Gutmann DH, Dougherty JD (2018) Characterization of early communicative behavior in mouse models of neurofibromatosis type 1. Autism Res 11:44–58. doi: 10.1002/aur.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36:320–328. doi: 10.1016/j.tibs.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milewicz DM, Kwartler CS, Papke CL, Regalado ES, Cao J, Reid AJ (2010) Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet Med 12:196–203. doi: 10.1097/GIM.0b013e3181cdd687 [DOI] [PubMed] [Google Scholar]
- 72.Moutal A, Dustrude ET, Khanna R (2017) Sensitization of Ion Channels Contributes to Central and Peripheral Dysfunction in Neurofibromatosis Type 1. Mol Neurobiol 54:3342–3349. doi: 10.1007/s12035-016-9907-1 [DOI] [PubMed] [Google Scholar]
- 73.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference (1988). Arch Neurol 45:575–578 [PubMed] [Google Scholar]
- 74.Oderich GS, Sullivan TM, Bower TC, Gloviczki P, Miller DV, Babovic-Vuksanovic D, Macedo TA, Stanson A (2007) Vascular abnormalities in patients with neurofibromatosis syndrome type I: Clinical spectrum, management, and results. J Vasc Surg 46:475–484.e471. doi: 10.1016/j.jvs.2007.03.055 [DOI] [PubMed] [Google Scholar]
- 75.Otero JJ, Rowitch D, Vandenberg S (2011) OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. J Neurooncol 104:423–438. doi: 10.1007/s11060-010-0509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palsgrove DN, Brosnan-Cashman JA, Giannini C, Raghunathan A, Jentoft M, Bettegowda C, Gokden M, Lin D, Yuan M, Lin M-T, Heaphy CM, Rodriguez FJ (2018) Subependymal giant cell astrocytoma-like astrocytoma: a neoplasm with a distinct phenotype and frequent neurofibromatosis type-1-association. Modern Pathol 31:1787–1800. doi: 10.1038/s41379-018-0103-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pecoraro A, Arehart E, Gallentine W, Radtke R, Smith E, Pizoli C, Kansagra S, Abdelnour E, McLendon R, Mikati MA (2017) Epilepsy in neurofibromatosis type 1. Epilepsy Behav 73:137–141. doi: 10.1016/j.yebeh.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 78.Pemov A, Li H, Patidar R, Hansen NF, Sindiri S, Hartley SW, Wei JS, Elkahloun A, Chandrasekharappa SC, Program NCS, Boland JF, Bass S, Laboratory NDCGR, Mullikin JC, Khan J, Widemann BC, Wallace MR, Stewart DR (2017) The primacy of NF1 loss as the driver of tumorigenesis in neurofibromatosis type 1-associated plexiform neurofibromas. Oncogene 36:3168. doi:10.1038/onc.2016.46410.1038/onc.2016.464https://www.nature.com/articles/onc2016464#supplementary-informationhttps://www.nature.com/articles/onc2016464#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173. doi: 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 80.Philpott C, Tovell H, Frayling IM, Cooper DN, Upadhyaya M (2017) The NF1 somatic mutational landscape in sporadic human cancers. Hum Genomics 11:13–13. doi: 10.1186/s40246-017-0109-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH (2013) Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol 73:303–308. doi: 10.1002/ana.23813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pride NA, Barton B, Hutchins P, Coghill DR, Korgaonkar MS, Hearps SJC, Rouel M, Malarbi S, North KN, Payne JM (2018) Effects of methylphenidate on cognition and behaviour in children with neurofibromatosis type 1: a study protocol for a randomised placebo-controlled crossover trial. BMJ Open 8:e021800. doi: 10.1136/bmjopen-2018-021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reinhardt A, Stichel D, Schrimpf D, Sahm F, Korshunov A, Reuss DE, Koelsche C, Huang K, Wefers AK, Hovestadt V, Sill M, Gramatzki D, Felsberg J, Reifenberger G, Koch A, Thomale U-W, Becker A, Hans VH, Prinz M, Staszewski O, Acker T, Dohmen H, Hartmann C, Mueller W, Tuffaha MSA, Paulus W, Heß K, Brokinkel B, Schittenhelm J, Monoranu C-M, Kessler AF, Loehr M, Buslei R, Deckert M, Mawrin C, Kohlhof P, Hewer E, Olar A, Rodriguez FJ, Giannini C, NageswaraRao AA, Tabori U, Nunes NM, Weller M, Pohl U, Jaunmuktane Z, Brandner S, Unterberg A, Hänggi D, Platten M, Pfister SM, Wick W, Herold-Mende C, Jones DTW, von Deimling A, Capper D (2018) Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol 136:273–291. doi: 10.1007/s00401-018-1837-8 [DOI] [PubMed] [Google Scholar]
- 84.Reis GF, Bloomer MM, Perry A, Phillips JJ, Grenert JP, Karnezis AN, Tihan T (2013) Pilocytic astrocytomas of the optic nerve and their relation to pilocytic astrocytomas elsewhere in the central nervous system. Modern Pathol 26:1279. doi: 10.1038/modpathol.2013.79 [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, Gilmer-Flynn H, Sarkaria JN, Jenkins S, Long J, Rodriguez FJ (2011) PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol 121:407–420. doi: 10.1007/s00401-010-0784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodriguez FJ, Brosnan-Cashman JA, Allen SJ, Vizcaino MA, Giannini C, Camelo-Piragua S, Webb M, Matsushita M, Wadhwani N, Tabbarah A, Hamideh D, Jiang L, Chen L, Arvanitis LD, Alnajar HH, Barber JR, Rodríguez-Velasco A, Orr B, Heaphy CM (2019) Alternative lengthening of telomeres, ATRX loss and H3-K27M mutations in histologically defined pilocytic astrocytoma with anaplasia. Brain Pathol 21:126–140. doi: 10.1111/bpa.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez FJ, Ligon AH, Horkayne-Szakaly I, Rushing EJ, Ligon KL, Vena N, Garcia DI, Cameron JD, Eberhart CG (2012) BRAF duplications and MAPK pathway activation are frequent in gliomas of the optic nerve proper. J Neuropathol Exp Neurol 71:789–794. doi: 10.1097/NEN.0b013e3182656ef8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodriguez FJ, Perry A, Gutmann DH, O’Neill BP, Leonard J, Bryant S, Giannini C (2008) Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol 67:240–249. doi: 10.1097/NEN.0b013e318165eb75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez FJ, Vizcaino MA, Blakeley J, Heaphy CM (2016) Frequent alternative lengthening of telomeres and ATRX loss in adult NF1-associated diffuse and high-grade astrocytomas. Acta Neuropathol 132:761–763. doi: 10.1007/s00401-016-1619-0 [DOI] [PubMed] [Google Scholar]
- 90.Rosser TL, Vezina G, Packer RJ (2005) Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology 64:553. doi: 10.1212/01.WNL.0000150544.00016.69 [DOI] [PubMed] [Google Scholar]
- 91.Ryu HH, Jung TY, Lee GJ, Lee KH, Jung SH, Jung S, Baek HJ (2015) Differences in the clinical courses of pediatric and adult pilocytic astrocytomas with progression: a single-institution study. Childs Nerv Syst 31:2063–2069. doi: 10.1007/s00381-015-2887-z [DOI] [PubMed] [Google Scholar]
- 92.Sabbagh A, Pasmant E, Imbard A, Luscan A, Soares M, Blanché H, Laurendeau I, Ferkal S, Vidaud M, Pinson S, Bellanné-Chantelot C, Vidaud D, Parfait B, Wolkenstein P (2013) NF1 Molecular Characterization and Neurofibromatosis Type I Genotype–Phenotype Correlation: The French Experience. Hum Mutat 34:1510–1518. doi: 10.1002/humu.22392 [DOI] [PubMed] [Google Scholar]
- 93.Salyer WR, Salyer DC (1974) The vascular lesions of neurofibromatosis. Angiology 25:510–519. doi: 10.1177/000331977402500803 [DOI] [PubMed] [Google Scholar]
- 94.See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO (2012) Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res 72:3350–3359. doi: 10.1158/0008-5472.CAN-12-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shibahara I, Sonoda Y, Suzuki H, Mayama A, Kanamori M, Saito R, Suzuki Y, Mashiyama S, Uenohara H, Watanabe M, Kumabe T, Tominaga T (2018) Glioblastoma in neurofibromatosis 1 patients without IDH1, BRAF V600E, and TERT promoter mutations. Brain Tumor Pathol 35:10–18. doi: 10.1007/s10014-017-0302-z [DOI] [PubMed] [Google Scholar]
- 96.Shilyansky C, Karlsgodt KH, Cummings DM, Sidiropoulou K, Hardt M, James AS, Ehninger D, Bearden CE, Poirazi P, Jentsch JD, Cannon TD, Levine MS, Silva AJ (2010) Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proceedings of the National Academy of Sciences of the United States of America 107:13141–13146. doi: 10.1073/pnas.1004829107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shin J, Padmanabhan A, de Groh ED, Lee J-S, Haidar S, Dahlberg S, Guo F, He S, Wolman MA, Granato M, Lawson ND, Wolfe SA, Kim S-H, Solnica-Krezel L, Kanki JP, Ligon KL, Epstein JA, Look AT (2012) Zebrafish neurofibromatosis type 1 genes have redundant functions in tumorigenesis and embryonic development. Dis Model Mech 5:881–894. doi: 10.1242/dmm.009779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sieg EP, Payne R, Langan S, Specht CS (2016) Case Report: A Rosette-forming Glioneuronal Tumor in the Tectal Plate in a Patient with Neurofibromatosis Type I. Cureus 8:e857. doi: 10.7759/cureus.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, Gutmann DH (2011) Neurofibromatosis-1 Heterozygosity Increases Microglia in a Spatially- and Temporally-Restricted Pattern Relevant to Mouse Optic Glioma Formation and Growth. J Neuropathol Exp Neurol 70:51–62. doi: 10.1097/NEN.0b013e3182032d37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solga AC, Toonen JA, Pan Y, Cimino PJ, Ma Y, Castillon GA, Gianino SM, Ellisman MH, Lee DY, Gutmann DH (2017) The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget 8:47206–47215. doi: 10.18632/oncotarget.17589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stafstrom CE, Staedtke V, Comi AM (2017) Epilepsy Mechanisms in Neurocutaneous Disorders: Tuberous Sclerosis Complex, Neurofibromatosis Type 1, and Sturge–Weber Syndrome. Front Neurol 8:87. doi: 10.3389/fneur.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing BD, Kapur R, Ingram DA Jr. (2014) Ras-Mek-Erk signaling regulates Nf1 heterozygous neointima formation. Am J Pathol 184:79–85. doi: 10.1016/j.ajpath.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing BD, Kapur R, Ingram DA Jr. (2014) Ras-Mek-Erk signaling regulates Nf1 heterozygous neointima formation. Am J Pathol 184:79–85. doi: 10.1016/j.ajpath.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strowd RE 3rd, Rodriguez FJ, McLendon RE, Vredenburgh JJ, Chance AB, Jallo G, Olivi A, Ahn ES, Blakeley JO (2016) Histologically benign, clinically aggressive: Progressive non-optic pathway pilocytic astrocytomas in adults with NF1. Am J Med Genet A 170:1455–1461. doi: 10.1002/ajmg.a.37622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takei H, Rouah E, Bhattacharjee MB (2015) Cerebellar pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1: a case report and literature review. Int J Clin Exp Pathol 8:7570–7574 [PMC free article] [PubMed] [Google Scholar]
- 106.Talman LS, Bisker ER, Sackel DJ, Long DA Jr., Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying G-S, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ (2010) Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 67:749–760. doi: 10.1002/ana.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terry Anna R, Jordan Justin T, Schwamm L, Plotkin Scott R (2016) Increased Risk of Cerebrovascular Disease Among Patients With Neurofibromatosis Type 1. Stroke 47:60–65. doi: 10.1161/STROKEAHA.115.011406 [DOI] [PubMed] [Google Scholar]
- 108.Thara K, Sharma R, Thiagarajan G, Ramdas A, Varghese RG (2017) Anaplastic Pleomorphic Xanthoastrocytoma in a Case of Neurofibromatosis Type 1: A Case Report. J Clin Diagn Res : JCDR 11:ED23–ED24. doi: 10.7860/JCDR/2017/26685.9713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toelle SP, Poretti A, Weber P, Seute T, Bromberg JE, Scheer I, Boltshauser E (2015) Cerebellar Hypoplasia and Dysmorphia in Neurofibromatosis Type 1. Cerebellum 14:642–649. doi: 10.1007/s12311-015-0658-8 [DOI] [PubMed] [Google Scholar]
- 110.Tognini G, Ferrozzi F, Garlaschi G, Piazza P, Patti A, Virdis R, Bertolino C, Bertolino G, Manfredini D, Zompatori M, Crisi G (2005) Brain apparent diffusion coefficient evaluation in pediatric patients with neurofibromatosis type 1. J Comput Assist Tomogr 29:298–304 [DOI] [PubMed] [Google Scholar]
- 111.Toonen JA, Anastasaki C, Smithson LJ, Gianino SM, Li K, Kesterson RA, Gutmann DH (2016) NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet 25:1703–1713. doi: 10.1093/hmg/ddw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toonen JA, Solga AC, Ma Y, Gutmann DH (2017) Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma–induced retinal pathology. J Exp Med 214:17–25. doi: 10.1084/jem.20160447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsipi M, Poulou M, Fylaktou E, Kosma K, Tsoutsou E, Pons M-R, Kokkinou E, Kitsiou-Tzeli S, Fryssira H, Tzetis M (2018) Phenotypic expression of a spectrum of Neurofibromatosis Type 1 (NF1) mutations identified through NGS and MLPA. J Neurol Sci 395:95–105. doi: 10.1016/j.jns.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 114.Ullrich NJ, Robertson R, Kinnamon DD, Scott RM, Kieran MW, Turner CD, Chi SN, Goumnerova L, Proctor M, Tarbell NJ, Marcus KJ, Pomeroy SL (2007) Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 68:932. doi: 10.1212/01.wnl.0000257095.33125.48 [DOI] [PubMed] [Google Scholar]
- 115.Uusitalo E, Leppävirta J, Koffert A, Suominen S, Vahtera J, Vahlberg T, Pöyhönen M, Peltonen J, Peltonen S (2015) Incidence and Mortality of Neurofibromatosis: A Total Population Study in Finland. J Invest Dermatol 135:904–906. doi: 10.1038/jid.2014.465 [DOI] [PubMed] [Google Scholar]
- 116.Van Es S, North KN, McHugh K, De Silva M (1996) MRI findings in children with neurofibromatosis type 1: a prospective study. Pediatr Radiol 26:478–487 [DOI] [PubMed] [Google Scholar]
- 117.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabrie S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, The Cancer Genome Atlas Research N (2010) An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell 17:98. doi: 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Viola F, Villani E, Natacci F, Selicorni A, Melloni G, Vezzola D, Barteselli G, Mapelli C, Pirondini C, Ratiglia R (2012) Choroidal abnormalities detected by near-infrared reflectance imaging as a new diagnostic criterion for neurofibromatosis 1. Ophthalmology 119:369–375. doi: 10.1016/j.ophtha.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 119.Vizcaíno MA, Shah S, Eberhart CG, Rodriguez FJ (2015) Clinicopathologic implications of NF1 gene alterations in diffuse gliomas(). Hum Pathol 46:1323–1330. doi: 10.1016/j.humpath.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walsh KS, Janusz J, Wolters PL, Martin S, Klein-Tasman BP, Toledo-Tamula MA, Thompson HL, Payne JM, Hardy KK, de Blank P, Semerjian C, Gray LS, Solomon SE, Ullrich N, Collaboration REI (2016) Neurocognitive outcomes in neurofibromatosis clinical trials: Recommendations for the domain of attention. Neurology 87:S21–S30. doi: 10.1212/WNL.0000000000002928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, Castellanos FX, Acosta MT (2013) Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol 55:131–138. doi: 10.1111/dmcn.12038 [DOI] [PubMed] [Google Scholar]
- 122.Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB (2010) Cyclic AMP Suppression Is Sufficient to Induce Gliomagenesis in a Mouse Model of Neurofibromatosis-1. Cancer Res 70:5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watters JJ, Schartner JM, Badie B (2005) Microglia function in brain tumors. J Neurosci Res 81:447–455. doi: 10.1002/jnr.20485 [DOI] [PubMed] [Google Scholar]
- 124.White KA, Swier VJ, Cain JT, Kohlmeyer JL, Meyerholz DK, Tanas MR, Uthoff J, Hammond E, Li H, Rohret FA, Goeken A, Chan C-H, Leidinger MR, Umesalma S, Wallace MR, Dodd RD, Panzer K, Tang AH, Darbro BW, Moutal A, Cai S, Li W, Bellampalli SS, Khanna R, Rogers CS, Sieren JC, Quelle DE, Weimer JM (2018) A porcine model of neurofibromatosis type 1 that mimics the human disease. JCI Insight 3:e120402. doi: 10.1172/jci.insight.120402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wimmer K, Rosenbaum T, Messiaen L (2016) Connections between constitutional mismatch repair deficiency syndrome and neurofibromatosis type 1. Clin Genet 91:507–519. doi: 10.1111/cge.12904 [DOI] [PubMed] [Google Scholar]
- 126.Wolman MA, de Groh ED, McBride SM, Jongens TA, Granato M, Epstein JA (2014) Modulation of cAMP and ras signaling pathways improves distinct behavioral deficits in a zebrafish model of neurofibromatosis type 1. Cell reports 8:1265–1270. doi: 10.1016/j.celrep.2014.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wood MD, Mukherjee J, Pieper RO (2018) Neurofibromin knockdown in glioma cell lines is associated with changes in cytokine and chemokine secretion in vitro. Sci Rep 8:5805. doi: 10.1038/s41598-018-24046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF (2005) Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8:119–130. doi: 10.1016/j.ccr.2005.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF (2005) Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development 132:5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF (2001) Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 15:859–876. doi: 10.1101/gad.862101 [DOI] [PMC free article] [PubMed] [Google Scholar]