Abstract
Latently infected cells that persist in HIV-infected individuals on antiretroviral therapy (ART) are a major barrier to cure. One strategy to eliminate latency is by activating viral transcription, commonly called latency reversal. Several small non-randomised clinical trials of latency reversing agents (LRAs) in HIV-infected individuals on ART increased viral production, but disappointingly did not reduce the number of latently infected cells or delay time to viral rebound following cessation of ART. More recent approaches aimed at reversing latency include compounds that both activate virus and also modulate immunity to enhance clearance of infected cells. These immunomodulatory LRAs include toll-like receptor agonists, immune checkpoint inhibitors and some cytokines. Here we provide a brief review of the rationale for transcription-activating and immunomodulatory LRAs, discuss recent clinical trials and some suggestions for combination approaches and research priorities for the future.
Graphical abstract
Introduction: HIV persistence and latency
Antiretroviral therapy (ART) can suppress HIV replication but treatment is required lifelong due to the persistence of long-lived and replicating CD4+ T cells that contain integrated HIV DNA, termed a provirus. Although the majority of integrated HIV DNA that persists on ART is defective, 2–5% of proviruses are intact and potentially replication-competent [1–3]. HIV DNA and replication competent virus has been detected in essentially every CD4+ T cell subset analyzed in HIV-infected individuals on ART, including naïve T cells [4–7]. Cells other than CD4+ T cells, including macrophages, astrocytes, and hepatocytes may also contribute to HIV persistence [8–12]. Finally, the frequency of HIV-infected cells that persist on ART is much higher in lymphoid tissue, including lymphoid follicles in lymph node and in the gastrointestinal tissue [13–20]. The heterogeneity of where and how HIV persists on ART has significant implications for developing a robust strategy to eliminate HIV.
Latency reversal and intracellular blocks
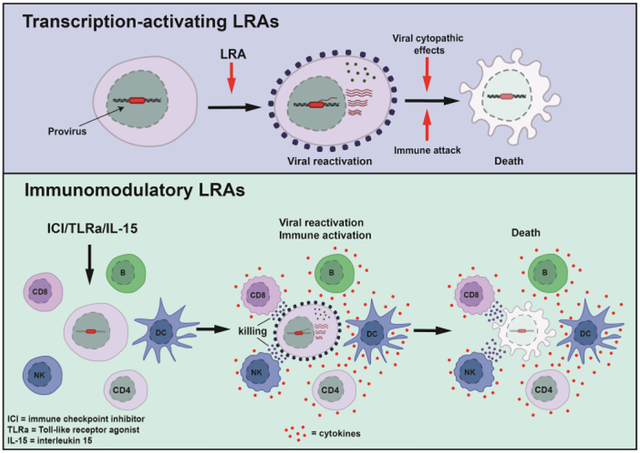
Reversing HIV latency to eliminate latently infected cells has been an actively pursued strategy in HIV cure research for the last 10 years. This approach, known as ‘shock and kill’, involves activating latent HIV through administration of a latency reversing agent (LRA) with the aim of facilitating cell death by viral cytopathic effects or immune-mediated killing [21,22]. This is done in the presence of ART so there are no further rounds of HIV replication.
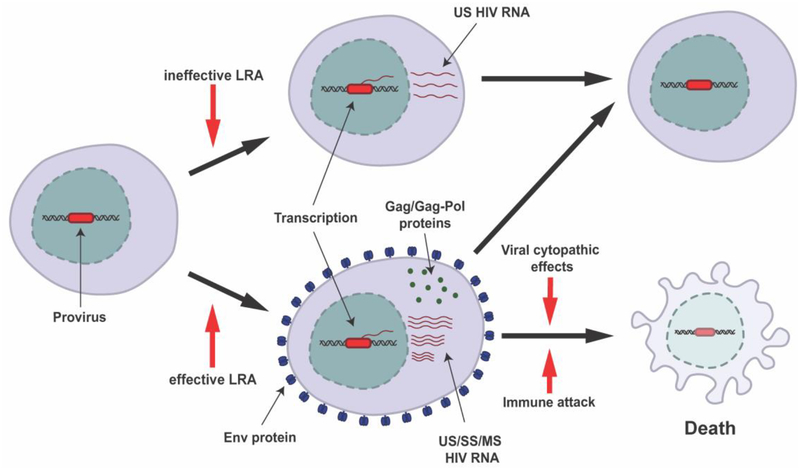
HIV latency is defined as cells that contain integrated HIV DNA but are transcriptionally silent and therefore there is no protein expression or virus production. It is now apparent that there is a spectrum of transcriptional activity in latently infected cells in HIV-infected individuals on fully suppressive ART with some cells truly transcriptionally silent while others produce low levels of cell-associated HIV RNA or HIV proteins [23–25]. In order for an HIV-infected cell to produce progeny virions, more than 40 different mRNA species are produced as a result of RNA splicing [26]. Unspliced (US) HIV RNAs encode the structural proteins, gag and pol, and serve as the genome for new virions. Singly spliced (SS) or incompletely spliced HIV RNAs encode the accessory proteins, Vif, Vpr, Vpu, as well as Env. Multiply spliced (MS) HIV RNAs encode the regulatory proteins Tat, Rev, and Nef [26]. For virion production to occur efficiently, all HIV RNA species need to be produced (Figure 1).
Figure 1: Shock and kill strategy to eliminate latently infected cells.
Following stimulation with a LRA, some latently infected cells will produce only cell-associated HIV RNA (top) while others will go on to become productively infected (bottom). Cells that only produce cell-associated HIV RNA are likely to live, while productively infected cells can either die via cytopathic effects or immune effector mechanisms (common) or survive (uncommon). Env = envelope protein, US = unspliced, SS = singly spliced, MS = multiply spliced.
HIV latency is maintained largely by transcriptional repression through a variety of mechanisms [27,28]. The HIV LTR promotor can be suppressed through increased acetylation or reduced methylation leading to epigenetic silencing [29,30] or due to the limited availability of key transcription factors in resting CD4+ T cells [31]. HIV RNA trafficking from the nucleus, a key step for the production of multiply spliced HIV RNA, can also be impaired through cellular inhibition of Rev [32,33]. Unspliced HIV RNAs are readily detectable in total CD4+ T cells in most individuals on ART, but the majority of these are incomplete transcripts, highlighting that HIV transcription in latently infected cells is inefficient [24,34,35]. In blood CD4+ T cells there is a higher frequency of short transcripts followed by a successively lower frequency of complete transcripts, polyadenylated transcripts and multiply spliced transcripts [24]. These transcriptional blocks differ in CD4+ T cells isolated from blood and rectal tissue [36]. Collectively, these data demonstrate that latency is not all or nothing as originally thought, but rather there is a spectrum with some cells actively transcribing low levels of the viral genome and others maintaining a deeper state of latency. The implications of this are that there are multiple blocks to viral RNA and protein expression that must be overcome to efficiently reverse latency and these differ in different tissue sites.
Transcription-activating LRAs
Multiple classes of drugs have been shown to activate HIV transcription in vitro, including epigenetic modifiers (such as histone deacetylase inhibitors (HDACi), methyl transferase inhibitors, methylation inhibitors and bromodomain inhibitors), protein kinase C agonists, activators of the PI3K pathway (including disulfiram and mTOR inhibitors), NFkB agonists (including SMAC mimetics and maraviroc) [reviewed in [37]]. Multiple early non-randomised observational clinical trials of HDACi and disulfiram in HIV-infected individuals on ART all demonstrated a modest increase in cell-associated HIV RNA and in some cases plasma HIV RNA, however, there was no decline in infected cells or time to virus rebound during ART interruption (reviewed in [38]). It thus appears unlikely that these agents will significantly impact the latent reservoir on their own, but they may still have a role in combination with other interventions. Ongoing studies are testing this concept (Table X).
Table X.
Ongoing combination clinical trials including a latency reversing agents in HIV
| LRA | Additional intervention | n | Institution | Status | Identifier |
|---|---|---|---|---|---|
| HDACi | |||||
| Nicotinamide | Dendritic cell vaccine + auranofin + ART intensification (DTG + MVC) | 30 | Federal University of Sao Paolo | Recruiting | |
| Romidepsin | 3BNC117 | 30 | Rockefeller University | Recruiting | |
| Romidepsin | MVA.HIVconsv vaccine | 15 | IrsiCaixa | Completed | |
| Panobinostat | Peg-IFNa-2a | 34 | Massachusetts General Hospital | Recruiting | |
| Vorinostat | ChAdV63.HIVconsv (ChAd) prime and MVA.HIVconsv boost vaccines | 60 | Imperial College London | Active, not recruiting | |
| Vorinostat | Hydroxychloroquine + maraviroc | 15 | Thai Red Cross AIDS Research Centre | Ongoing, not recruiting | |
| Romidepsin | MVA vector HIV vaccine + HIVACAR01 (personalised HIV RNA vaccine) + 10-1074 | 56 | David Garcia Cinca, Hospital Clinic of Barcelona | Not yet recruiting | |
| Vorinostat | Disulfiram | 15 | Peter Doherty Institute for Infection and Immunity | Suspended during review of AE | |
| Valproic acid | Pyrimethamine | 28 | Erasmus Medical Center | Recruiting | |
| Vorinostat | HXTC (HIV antigen expanded specific T cell therapy) | 12 | University of North Carolina, Chapel Hill | Recruiting | |
| Vorinostat | Tamoxifen | 30 | NIAID | Active, not recruiting | |
| Romidepsin | 3BNC117 | 60 | Aarhus University Hospital | Recruiting | |
| Interleukin | |||||
| IL-2 | Ex vivo activated NK cells | 5 | University of Minnesota | Recruiting | |
| TLR9 agonist | |||||
| Lefitolimod (MGN1703) | 10-1074 + 3BNC117 | 48 | Aarhus University Hospital | Not yet recruiting | XXXX |
HDACi: histone deacetylase inhibitor; TLR: toll-like receptor; IL: interleukin; NK: natural killer; DTG: dolutegravir; MVC: maraviroc
PKC agonists non-specifically alter gene expression levels and can also induce non- specific T cell activation. They include prostratin [39], bryostatin-1 (BRY–1) [40], Euphorbia kansui [41], ingenol-3-angelate (PEP005) [42], Ingenol–B [43], and ingenol 3,20-dibenzoate [44] and have been evaluated in both in vitro and animal models. Only BRY–1 has been evaluated in a clinical trial [45]. While BRY–1 was well-tolerated, it was only administered at a low concentration and there was no activation of PKC or reversal of viral latency [45]. More recently, the HIV entry inhibitor maraviroc (MVC) was shown to increase cell-associated unspliced HIV RNA, potentially mediated through activation of NF–κB via the CCR5 receptor [46]. An intensification study of MVC demonstrated an increase in NF–kB activation, and a modest increase in cell-associated unspliced HIV RNA [47], but the effect on plasma viremia or frequency of latent infection has not been assessed.
Recently it has been shown that certain biological processes including circadian rhythm and sex hormones can affect HIV transcription. These insights may be exploited further. We demonstrated significant variation in cell associated unspliced HIV RNA in HIV-infected individuals on ART which varied with the time of blood collection [48] and changes in the expression of the circadian-locomotor-output-cycles-kaput (CLOCK)-associated gene and brain-and-muscle-ARNT-like-1 (BMAL1), a circadian regulator of gene expression [49]. Using an in vitro system we then demonstrated that CLOCK and BMAL1 can activate HIV transcription through direct activation of the HIV LTR [49]. Further studies will reveal whether these processes can be targeted to optimize latency reversal.
Sex hormones may also significantly affect HIV transcriptional activity, as illustrated by the higher levels of cell-associated HIV RNA, residual plasma viremia, immune activation, and immune exhaustion in men compared to women on ART [50]. Moreover, estrogen and other agonists of estrogen receptor-1 (ESR–1), including β-estradiol, inhibit HIV latency reversal in contrast to antagonists of ESR–1 such as tamoxifen, which activate HIV transcription [51]. These findings suggest that control of latent infection differ in men and women and therefore LRAs may act differently. Further work is underway to determine if targeting ESR–1 could lead to a novel LRA.
LRAs studied to date in clinical trials (specifically HDACi, disulfiram and maraviroc) may not have effectively cleared latently infected cells for several reasons. These include limited potency when used alone, some dose-limiting toxicities, potential insufficient delivery to lymphoid tissue, post-transcriptional blocks limiting viral protein expression [52], competing mechanisms of HIV persistence such as clonal expansion [53,54] and immune exhaustion limiting immune-mediated clearance of virus-expressing cells [55–57]. Based on these findings, there is an ongoing search for new drug targets and also active efforts to test LRAs in combination with other LRAs or immune enhancement strategies (Table X).
Immunomodulatory latency reversing agents
Newer approaches to target latent HIV that appear to be more promising, at least in animal models, include compounds that activate immune function in combination with induction of viral expression. These include toll-like receptor (TLR) agonists, immune checkpoint (IC) inhibitors and cytokine-based therapy such as interleukin (IL)–15.
Toll-like receptor agonists
Therapeutic stimulation of TLRs may lead to dendritic cell (DC) maturation, natural killer (NK) cell activation, enhanced antigen presentation, and enhance adaptive immune responses [58,59]. In HIV, TLR agonists may play a role both as LRAs and as immunotherapy.
The TLR7 agonists GS–986 and GS–9620 activated multiple immune cell populations in simian immunodeficiency virus (SIV)-infected rhesus macaques on ART initiated during chronic infection and also induced cyclical increases in CD4+ T cell activation and plasma viremia [60]. Other studies in non-human primate (NHP) models have investigated the combination of GS–9620 with a therapeutic vaccine. When a TLR7 agonist was administered with an Ad26/MVA vector to SIV-infected NHPs who initiated ART during acute infection, there was a marked increase in both the magnitude and breadth of SIV-specific immune responses and also delayed viral rebound following ART discontinuation [61]. Even more exciting results were reported when GS–9620 was administered in combination with the broadly neutralising antibody (bNAb) PGT121 to SHIV-infected NHPs on ART initiated within 7 days of infection [62]. Whereas almost all NHP receiving placebo, GS–9620-only or PGT121-only had viral rebound after ART was stopped, 5/11 NHP receiving both GS–9620 and PGT121 did not rebound during ART interruption, even after CD8-depletion. Whether the TLR7 agonist was acting as an LRA in both combination studies is unclear as blips in plasma virus were not observed. It is possible that the favourable effects of the TLR7 agonist were a result of immune enhancement, potentially through activation of NK cells. GS–9620 is currently being tested in phase 1 studies in HIV-infected individuals on ART ( and ).
The TLR9 agonist lefitolimod (MGN1703) has also been explored in a non-randomised single arm study of HIV-infected individuals on ART. There was a significant induction of antiviral innate immune responses – including activation of plasmacytoid DCs, NK cells and T cells and an increase in plasma levels of interferon–α [63]. Interestingly, in 6 of the 15 study participants, there was a quantifiable increase in plasma HIV RNA during lefitolimod administration ranging from 21–1571 copies/mL, but no change in cell-associated HIV RNA and actually a slight decrease in cell-associated HIV–RNA post-treatment compared to baseline.
In summary, TLR agonists can activate multiple immune cell populations and may also reverse HIV latency, although it is unclear why this effect was primarily seen as increases in plasma HIV RNA and not cell-associated HIV RNA. This is in contrast to clinical trials of HDACi where an increase in cell-associated HIV RNA was observed more frequently than plasma HIV RNA. These findings may relate to timing for collection of specimens or the rapid elimination of cells that express viral RNA or protein. New tools that can detect the number of cells that express HIV RNA could potentially assist in future studies [64]. Whether the combination of TLR7 agonists with vaccines or antibodies has a similar effect on the reservoir in NHPs treated during chronic infection or in human studies remains eagerly awaited. There are multiple ongoing studies that include TLR7 or TLR9 agonists alone or in combination with other interventions (Table X).
Immune checkpoint blockade as a means to reverse HIV latency
Signalling through ICs provides inhibitory signals to T cells and blockade of these pathways has shown great promise in the treatment of some malignancies [65]. In untreated HIV infection, increased expression of ICs is associated with immune exhaustion and disease progression [55,56,66–70]. This may be partially reversed through blocking ICs, which reinvigorates virus-specific T cell function ex vivo and in SIV-infected rhesus NHPs [71–75]. In addition, CD4+ T cells expressing IC, especially PD–1, lymphocyte activation gene 3 (LAG–3) and T cell immunoreceptor with Ig and ITIM domains (TIGIT), are enriched for HIV and therefore these proteins may play a role in the establishment and maintenance of HIV latency [4,56,76]. Using an in vitro model, we recently demonstrated that blocking PD–1 and TIM–3 prior to infection, reduced the establishment of HIV latency [77]. We and others have recently shown that antibodies to PD–1 or PD–L1 can enhance virus production from CD4+ T cells collected from HIV-infected individuals on ART, but only in the presence of an additional stimuli such as bryostatin or Staphylococcal enterotoxin A/B (SEA/SEB) [78]. In contrast, the administration of anti-PD1 alone seems to have limited effects on virus production ex vivo [79].
A limited number of case reports or small case studies have examined the effects of IC blockers on HIV persistence in HIV-infected individuals on ART receiving IC blockade for cancer. These studies have showed an increase in cell-associated unspliced HIV RNA following anti–CTLA4 (ipilimumab) and anti-PD-1 (nivolumab) [77,80] and a decline in the frequency of infected cells after repeated administration of anti-PD-1 [78,81]. In contrast, another study found no consistent change in cell associated or plasma HIV RNA following anti-PD-1 [82].
Collectively, these studies suggest that IC inhibitors may enhance immune effector functions and perturb HIV latency, but larger clinical trials in HIV-infected individuals are still needed to fully determine whether IC blockers can be used to eliminate latently infected cells. A very significant limitation with these antibodies is the risk of immune related adverse events which occur with the currently available antibodies.
Cytokine therapy
Several clinical studies have investigated the effect of IL–2 or IL–7 on HIV latency and have overall not been successful. Three clinical trials of recombinant human IL–7, one in the setting of intensified ART, showed that the homeostatic effects of this cytokine induced proliferation of CD4+ T cells and actually expanded the pool of latently infected cells [83–85]. More recently, IL–15 and IL–15 superagonists have been explored in cure-related research. In rhesus NHPs infected with SHIV or SIV, both native heterodimeric IL–15 and the IL–15 superagonist ALT–803 increased levels of virus-specific CD8+ T cells in lymph node tissue, including in B cell follicles [86,87], an important anatomical site for SIV persistence [88]. These studies were done in infected NHP not on ART and thus were not designed to investigate the latency-reversing effects of IL–15. However, therapeutically relevant concentrations of ALT–803 were previously shown to reverse latency and even sensitise latently infected CD4+ T cells for CD8+ T cell recognition ex vivo [89]. ALT–803 is currently being investigated in a clinical trial for its effect on HIV persistence in HIV infected individuals on ART ().
Combination strategies to optimise shock and kill
Combination LRAs Several in vitro and ex vivo studies have shown that combinations of LRAs can act synergistically to enhance latency reversal [40,90–96], particularly when combining a PKC agonist with either a bromodomain inhibitor or an HDACi [91,93,96]. It is therefore possible that combining LRAs with different mechanisms of action will significantly enhance latency reversal, although safety remains a limiting factor for advancing this approach. Other combination approaches include combining LRAs with therapeutic vaccines, IC inhibitors, TLR agonists, interferon, bNAbs or pro-apoptotic compounds.
Combining LRA with therapeutic vaccination or interferon
The therapeutic peptide-based HIV vaccine, Vacc-4x, given with rhGM-CSF as local adjuvant, was tested in a study where a prime-boost regimen of 6 vaccine administrations were followed by three infusions of the HDACi romidepsin [97]. This combined intervention was associated with a moderate decrease in the frequency of latently infected CD4+ T cells but did not delay time to virus rebound during ATI [97]. Therapeutic HIV vaccination combined with vorinostat or romidepsin is also being investigated in several ongoing studies in HIV-infected individuals who started ART <6 months or <4 weeks after primary HIV infection. Finally, based on post hoc observations of the effects of panobinostat [98], panobinostat is tested in combination with pegylated interferon-α2a in an ongoing study (). Additional studies are investigating other combinations that include an LRA and are summarised in Table X.
Using pro-apoptotic drugs to enhance killing of virus-expressing cells
As latency reversal may effectively shift virus-expressing cells from a pro-survival to a pro-death state, it is conceivable that combining an LRA with a pro-apoptotic drug may promote selective killing of virus-expressing cells, as recently reviewed by Kim et al [37]. Several pro-apoptotic drugs have been developed for use in cancer treatment, including Bcl–2 antagonists, Pi3K/Akt inhibitors, Smac mimetics, and RIG–I inducer [37,99]. Preliminary analyses using the pro-apoptotic BCL–2 antagonists either alone or in combination with an LRA, have demonstrated a decrease in HIV-infected cells, both in vitro and ex vivo [100–105]. However, further evaluation is needed to better understand the efficacy and safety profile of this approach.
Areas for future research
Work to date has showed evidence of latency reversal in vivo in both blood and tissue-derived CD4+ T cells, however it is unclear if LRAs have different effects in clonally expanded infected cells; different T cell subsets; in transcriptionally silent or actively transcribing cells; or on intact or defective proviruses [3,106]. These are all important sources of latent virus to understand. Given that a fraction of latently infected cells contain full-length intact proviruses that are not easily induced even by maximal T cell stimulation [1], it is unclear if these cells can be ignored or need to be specifically targets. It is also unknown whether latently infected CD4+ T cells in anatomic compartments such as lymphoid follicles, respond to LRAs as few studies have examined tissue sites prior to and following LRA treatment. Advances in imaging the reservoir with radiolabelled antibodies that bind to HIV envelope [107] could potentially answer this question should these tools work in human clinical trials. Finally, virus rebound after cessation of ART, can occur even after dramatic reductions in reservoir size, for example following very early ART or stem cell transplantation [108,109]. These sobering case reports of prolonged absence of viremia and sudden rebound many months after cessation of ART suggest that unless every virus is eliminated, potent immune surveillance will be required to keep whatever virus remains, in check.
Other important considerations for future studies include timing of LRA administration and selection of participants who have a high likelihood of response to an LRA. HIV-infected individuals who initiated ART in acute infection have a lower frequency of latently infected cells, better preserved T cell function and little or no accumulation of immune escape mutations [110]. They are therefore more likely to eliminate virus-expressing cells and have a higher likelihood of spontaneous post-treatment control after cessation of ART [111]. These observations underscore the need for a placebo control group when studying this population. Interestingly, modelling and NHP studies have suggested that latently infected cells turn over at a higher rate during productive infection ie before ART is initiated, which has led to the speculation that administration of an LRA intervention during this labile phase might have greater potency [112,113]. An ongoing study of romidepsin and 3BNC117 is investigating this hypothesis (Table X).
Conclusion
Overall, we believe that LRAs play a key role in HIV cure strategies as a component of a combination approach and to provide a mechanism to “expose” virus. The potency of LRAs can be potentially enhanced through development of compounds with increased specificity for infected cells, ideally through an HIV-specific mode of action; by improving delivery to key tissue sites, potentially through nanoparticle technology; or by using LRAs in conjunction with other interventions to enhance killing of virus-expressing cells. Immunomodulatory LRAs have several advantages and results of human clinical trials with these agents are eagerly awaited.
Highlights.
HIV latency persists in long lived and proliferating CD4+ T cells
Latency is maintained through mechanisms that suppress viral transcription and translation
Transcription-activating LRAs activate HIV transcription in vivo but there is no elimination of latently infected cells
Immunomodulatory LRAs have dual effects on latency reversal and immune activation
Clinical trials of immunomodulatory LRAs look promising in animal models
Acknowledegements
None
Financial support and sponsorship
This work was partly supported by funding from from the the National Health and Medical Research Council (NHMRC) of Australia and the National Institutes of Health Delaney AIDS Research Enterprise (DARE) to find a cure collaboratory. S.R.L. is an NHMRC Practitioner Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
SRL’s institution has received funding from the National Health and Medical Research Council (NHMRC) of Australia, National Institutes for Health, American Foundation for AIDS Research; Merck, Viiv and Gilead for investigator-initiated research; Merck, Viiv and Gilead for educational activities. She is on the advisory board of Abivax and Innivirax.
References
- 1.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF: Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, et al. : Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016, 22:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, et al. : A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. : HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009, 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano-Sarabia N, Bateson RE, Dahl NP, Crooks AM, Kuruc JD, Margolis DM, Archin NM: Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol 2014, 88:14070–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, et al. : HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014, 20:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW, Sluis-Cremer N: Naive CD4+ T Cells Harbor a Large Inducible Reservoir of Latent, Replication-Competent HIV-1. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Abbas W, Herbein G: HIV-1 latency in monocytes/macrophages. Viruses 2014, 6:1837–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR: Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 2009, 66:253–258. [DOI] [PubMed] [Google Scholar]
- 10.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC: Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 2014, 7:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandathil AJ, Sugawara S, Balagopal A: Are T cells the only HIV-1 reservoir? Retrovirology 2016, 13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan M, Poluektova LY, Kharbanda KK, Osna NA: Liver as a target of human immunodeficiency virus infection. World J Gastroenterol 2018, 24:4728–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, Li P, Wong LK, Crouch P, Deeks SG, et al. : The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis 2013, 208:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury G, Fromentin R, Solomon A, Hartogensis W, Killian M, Hoh R, Somsouk M, Hunt PW, Girling V, Sinclair E, et al. : Human Immunodeficiency Virus Persistence and T-Cell Activation in Blood, Rectal, and Lymph Node Tissue in Human Immunodeficiency Virus-Infected Individuals Receiving Suppressive Antiretroviral Therapy. J Infect Dis 2017, 215:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. : Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997, 387:183–188. [DOI] [PubMed] [Google Scholar]
- 16.Nolan DJ, Rose R, Rodriguez PH, Salemi M, Singer EJ, Lamers SL, McGrath MS: The Spleen Is an HIV-1 Sanctuary During Combined Antiretroviral Therapy. AIDS Res Hum Retroviruses 2018, 34:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poles MA, Boscardin WJ, Elliott J, Taing P, Fuerst MM, McGowan I, Brown S, Anton PA: Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr 2006, 43:65–68. [DOI] [PubMed] [Google Scholar]
- 18.Gosselin A, Wiche Salinas TR, Planas D, Wacleche VS, Zhang Y, Fromentin R, Chomont N, Cohen EA, Shacklett B, Mehraj V, et al. : HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 2017, 31:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, Tudor D, Charmeteau B, Couedel-Courteille A, Marion S, et al. : HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 2019. [DOI] [PubMed] [Google Scholar]
- 20.Westermann J, Pabst R: Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig 1992, 70:539–544. [DOI] [PubMed] [Google Scholar]
- 21.Deeks SG: HIV: Shock and kill. Nature 2012, 487:439–440. [DOI] [PubMed] [Google Scholar]
- 22.Hamer DH: Can HIV be Cured? Mechanisms of HIV persistence and strategies to combat it. Curr HIV Res 2004, 2:99–111. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand A, Spindler J, Hong FF, Shao W, Cyktor JC, Cillo AR, Halvas EK, Coffin JM, Mellors JW, Kearney MF: Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc Natl Acad Sci U S A 2017, 114:E3659–E3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yukl SA, Kaiser P, Kim P, Telwatte S, Joshi SK, Vu M, Lampiris H, Wong JK: HIV latency in isolated patient CD4(+) T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinzone MR, VanBelzen DJ, Weissman S, Bertuccio MP, Cannon L, Venanzi-Rullo E, Migueles S, Jones RB, Mota T, Joseph SB, et al. : Longitudinal HIV sequencing reveals reservoir expression leading to decay which is obscured by clonal expansion. Nat Commun 2019, 10:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karn J, Stoltzfus CM: Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med 2012, 2:a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbonye U, Karn J: The molecular basis for human immunodeficiency virus latency. Annu. Rev. Virol 2017, 4:261–285. [DOI] [PubMed] [Google Scholar]
- 28.Khoury G, Darcis G, Lee MY, Bouchat S, Van Driessche B, Purcell DFJ, Van Lint C: The Molecular Biology of HIV Latency. Adv Exp Med Biol 2018, 1075:187–212. [DOI] [PubMed] [Google Scholar]
- 29.Pearson R, Kim YK, Hokello J, Lassen K, Friedman J, Tyagi M, Karn J: Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J Virol 2008, 82:12291–12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Lint C, Bouchat S, Marcello A: HIV-1 transcription and latency: an update. Retrovirology 2013, 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mbonye U, Karn J: Control of HIV latency by epigenetic and non-epigenetic mechanisms. Curr HIV Res 2011, 9:554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF: Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog 2006, 2:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarracino A, Gharu L, Kula A, Pasternak AO, Avettand-Fenoel V, Rouzioux C, Bardina M, De Wit S, Benkirane M, Berkhout B, et al. : Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassen KG, Bailey JR, Siliciano RF: Analysis of human immunodeficiency virus type 1 transcriptional elongation in resting CD4+ T cells in vivo. J Virol 2004, 78:9105–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizaka A, Sato H, Nakamura H, Koga M, Kikuchi T, Hosoya N, Koibuchi T, Nomoto A, Kawana-Tachikawa A, Mizutani T: Short Intracellular HIV-1 Transcripts as Biomarkers of Residual Immune Activation in Patients on Antiretroviral Therapy. J Virol 2016, 90:5665–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telwatte S, Lee S, Somsouk M, Hatano H, Baker C, Kaiser P, Kim P, Chen TH, Milush J, Hunt PW, et al. : Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PLoS Pathog 2018, 14:e1007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y, Anderson JL, Lewin SR: Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen TA, Lewin SR: Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS 2016, 11:394–401. [DOI] [PubMed] [Google Scholar]
- 39.Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC: Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem 2004, 279:42008–42017. [DOI] [PubMed] [Google Scholar]
- 40.Perez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, Munoz-Fernandez MA, Moreno S, Castor TP, Calzado MA, Munoz E: Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res 2010, 8:418–429. [DOI] [PubMed] [Google Scholar]
- 41.Cary DC, Fujinaga K, Peterlin BM: Euphorbia Kansui Reactivates Latent HIV. PLoS One 2016, 11:e0168027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warrilow D, Gardner J, Darnell GA, Suhrbier A, Harrich D: HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses 2006, 22:854–864. [DOI] [PubMed] [Google Scholar]
- 43.Pandelo Jose D, Bartholomeeusen K, da Cunha RD, Abreu CM, Glinski J, da Costa TB, Bacchi Rabay AF, Pianowski Filho LF, Dudycz LW, Ranga U, et al. : Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology 2014, 462–463:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spivak AM, Bosque A, Balch AH, Smyth D, Martins L, Planelles V: Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients. Antimicrob Agents Chemother 2015, 59:5984–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez C, Serrano-Villar S, Madrid-Elena N, Perez-Elias MJ, Martin ME, Barbas C, Ruiperez J, Munoz E, Munoz-Fernandez MA, Castor T, et al. : Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 2016, 30:1385–1392. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Huertas MR, Jimenez-Tormo L, Madrid-Elena N, Gutierrez C, Rodriguez-Mora S, Coiras M, Alcami J, Moreno S: The CCR5-antagonist Maraviroc reverses HIV-1 latency in vitro alone or in combination with the PKC-agonist Bryostatin-1. Sci Rep 2017, 7:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madrid-Elena N, Garcia-Bermejo ML, Serrano-Villar S, Diaz-de Santiago A, Sastre B, Gutierrez C, Dronda F, Coronel Diaz M, Dominguez E, Lopez-Huertas MR, et al. : Maraviroc is associated with latent HIV-1 reactivation through NF-kappaB activation in resting CD4(+) T cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy. J Virol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, et al. : Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015, 2:e520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CC, Naranbhai V, Stern J, Roche M, Dantanarayana A, Ke R, Tennakoon S, Solomon A, Hoh R, Hartogensis W, et al. : Variation in cell-associated unspliced HIV RNA on antiretroviral therapy is associated with the circadian regulator brain-and-muscle-ARNT-like-1. AIDS 2018, 32:2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, Pagliuzza A, Milush JM, Baker CA, Girling V, et al. : Sex-Based Differences in HIV-1 Reservoir Activity and Residual Immune Activation. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das B, Dobrowolski C, Luttge B, Valadkhan S, Chomont N, Johnston R, Bacchetti P, Hoh R, Gandhi M, Deeks SG, et al. : Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018, 115:E7795–E7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moron-Lopez S, Kim P, SoGaard OS, Tolstrup M, Wong JK, Yukl SA: Characterization of the HIV-1 transcription profile after romidepsin administration in ART-suppressed individuals. AIDS 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, et al. : Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 2016, 113:1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeves DB, Duke ER, Wagner TA, Palmer SE, Spivak AM, Schiffer JT: A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nat Commun 2018, 9:4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, et al. : TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog 2016, 12:e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, et al. : CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog 2016, 12:e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J, Willberg CB, Robinson N, Brown H, Fisher M, et al. : Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog 2016, 12:e1005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwasaki A, Medzhitov R: Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004, 5:987–995. [DOI] [PubMed] [Google Scholar]
- 59.Kawasaki T, Kawai T: Toll-like receptor signaling pathways. Front Immunol 2014, 5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, et al. : TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, et al. : Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016, 540:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, et al. : Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018, 563:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, Olesen R, Dige A, Agnholt J, Grau J, et al. : Short-course TLR9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals with HIV infection. Clin Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baxter AE, Niessl J, Fromentin R, Richard J, Porichis F, Charlebois R, Massanella M, Brassard N, Alsahafi N, Delgado GG, et al. : Single-Cell Characterization of Viral Translation-Competent Reservoirs in HIV-Infected Individuals. Cell Host Microbe 2016, 20:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wykes MN, Lewin SR: Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018, 18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. : PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443:350–354. [DOI] [PubMed] [Google Scholar]
- 67.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. : Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006, 12:1198–1202. [DOI] [PubMed] [Google Scholar]
- 68.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. : Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007, 8:1246–1254. [DOI] [PubMed] [Google Scholar]
- 69.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. : PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 2007, 109:4671–4678. [DOI] [PubMed] [Google Scholar]
- 70.Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, Liu A, Zhu L, Yuan S, Hu H, et al. : The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J Immunol 2015, 194:3873–3882. [DOI] [PubMed] [Google Scholar]
- 71.Mylvaganam GH, Chea LS, Tharp GK, Hicks S, Velu V, Iyer SS, Deleage C, Estes JD, Bosinger SE, Freeman GJ, et al. : Combination anti-PD-1 and antiretroviral therapy provides therapeutic benefit against SIV. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. : Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009, 458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR: PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest 2012, 122:1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gill AL, Green SA, Abdullah S, Le Saout C, Pittaluga S, Chen H, Turnier R, Lifson J, Godin S, Qin J, et al. : Programed death-1/programed death-ligand 1 expression in lymph nodes of HIV infected patients: results of a pilot safety study in rhesus macaques using anti-programed death-ligand 1 (Avelumab). AIDS 2016, 30:2487–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, et al. : CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006, 108:3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, Perreau M: PD-1 and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016. [DOI] [PubMed] [Google Scholar]
- 77.Evans VA, van der Sluis RM, Solomon A, Dantanarayana A, McNeil C, Garsia R, Palmer S, Fromentin R, Chomont N, Sekaly RP, et al. : Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS 2018, 32:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fromentin R, DaFonseca S, Costiniuk CT, El-Far M, Procopio FA, Hecht FM, Hoh R, Deeks SG, Hazuda DJ, Lewin SR, et al. : PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat Commun 2019, 10:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bui JK, Cyktor JC, Fyne E, Campellone S, Mason SW, Mellors JW: Blockade of the PD-1 axis alone is not sufficient to activate HIV-1 virion production from CD4+ T cells of individuals on suppressive ART. PLoS One 2019, 14:e0211112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, McNeil C, Garsia R, Lewin SR: Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015, 29:504–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guihot A, Marcelin AG, Massiani MA, Samri A, Soulie C, Autran B, Spano JP: Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol 2018, 29:517–518. [DOI] [PubMed] [Google Scholar]
- 82.Scully EP, Rutishauser RL, Simoneau CR, Delagreverie H, Euler Z, Thanh C, Li JZ, Hartig H, Bakkour S, Busch M, et al. : Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Ann Oncol 2018, 29:2141–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, et al. : Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012, 55:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katlama C, Lambert-Niclot S, Assoumou L, Papagno L, Lecardonnel F, Zoorob R, Tambussi G, Clotet B, Youle M, Achenbach CJ, et al. : Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS 2016, 30:221–230. [DOI] [PubMed] [Google Scholar]
- 85.Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy JP, Sekaly RP, Chomont N: Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013, 121:4321–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, Stanton JJ, Legasse AW, Hobbs T, Martin LD, et al. : The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv 2018, 2:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watson DC, Moysi E, Valentin A, Bergamaschi C, Devasundaram S, Fortis SP, Bear J, Chertova E, Bess J Jr., Sowder R, et al. : Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog 2018, 14:e1006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. : B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015, 21:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones RB, Mueller S, O’Connor R, Rimpel K, Sloan DD, Karel D, Wong HC, Jeng EK, Thomas AS, Whitney JB, et al. : A Subset of Latency-Reversing Agents Expose HIV-Infected Resting CD4+ T-Cells to Recognition by Cytotoxic T-Lymphocytes. PLoS Pathog 2016, 12:e1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, Delacourt N, Melard A, Kabeya K, Vanhulle C, et al. : An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog 2015, 11:e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF: Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J. Clin. Invest 2015, 125:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quivy V, Adam E, Collette Y, Demonte D, Chariot A, Vanhulle C, Berkhout B, Castellano R, de Launoit Y, Burny A, et al. : Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol 2002, 76:11091–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, et al. : Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One 2009, 4:e6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burnett JC, Lim KI, Calafi A, Rossi JJ, Schaffer DV, Arkin AP: Combinatorial latency reactivation for HIV-1 subtypes and variants. J Virol 2010, 84:5958–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez-Bonet M, Clemente MI, Serramia MJ, Munoz E, Moreno S, Munoz-Fernandez MA: Synergistic Activation of Latent HIV-1 Expression by Novel Histone Deacetylase Inhibitors and Bryostatin-1. Sci Rep 2015, 5:16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, Fenton A, Melcher GP, Hildreth JE, Thompson GR, et al. : Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS Pathog 2015, 11:e1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leth S, Schleimann MH, Nissen SK, Hojen JF, Olesen R, Graversen ME, Jorgensen S, Kjaer AS, Denton PW, Mork A, et al. : Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 2016, 3:e463–472. [DOI] [PubMed] [Google Scholar]
- 98.Olesen R, Vigano S, Rasmussen T, Sogaard OS, Ouyang Z, Buzon M, Bashirova A, Carrington M, Palmer S, Brinkmann CR, et al. : Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with panobinostat. J Virol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N: Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014, 2014:150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Cummins NW, Sainski AM, Dai H, Natesampillai S, Pang YP, Bren GD, de Araujo Correia MCM, Sampath R, Rizza SA, O’Brien D, et al. : Prime, Shock, and Kill: Priming CD4 T Cells from HIV Patients with a BCL-2 Antagonist before HIV Reactivation Reduces HIV Reservoir Size. J Virol 2016, 90:4032–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell GR, Bruckman RS, Chu YL, Trout RN, Spector SA: SMAC Mimetics Induce Autophagy-Dependent Apoptosis of HIV-1-Infected Resting Memory CD4+ T Cells. Cell Host Microbe 2018, 24:689–702 e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hattori SI, Matsuda K, Tsuchiya K, Gatanaga H, Oka S, Yoshimura K, Mitsuya H, Maeda K: Combination of a Latency-Reversing Agent With a Smac Mimetic Minimizes Secondary HIV-1 Infection in vitro. Front Microbiol 2018, 9:2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lucas A, Kim Y, Rivera-Pabon O, Chae S, Kim DH, Kim B: Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PLoS One 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim Y, Hollenbaugh JA, Kim DH, Kim B: Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat. PLoS One 2011, 6:e21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li P, Kaiser P, Lampiris HW, Kim P, Yukl SA, Havlir DV, Greene WC, Wong JK: Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat Med 2016, 22:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiener B, Eden JS, Horsburgh BA, Palmer S: Amplification of Near Full-length HIV-1 Proviruses for Next-Generation Sequencing. J Vis Exp 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, Connor-Stroud F, Schuster DM, Amancha PK, Hong JJ, et al. : Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods 2015, 12:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, et al. : Antiretroviral-Free HIV-1 Remission and Viral Rebound After Allogeneic Stem Cell Transplantation: Report of 2 Cases. Ann Intern Med 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, Persaud D: Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015, 372:786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. : Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015, 517:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R, Hartogensis W, Jacobson JM, Connick E, Volberding P, et al. : The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J Infect Dis 2018, 218:1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reece JC, Martyushev A, Petravic J, Grimm A, Gooneratne S, Amaresena T, De Rose R, Loh L, Davenport MP, Kent SJ: Measuring turnover of SIV DNA in resting CD4+ T cells using pyrosequencing: implications for the timing of HIV eradication therapies. PLoS One 2014, 9:e93330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kent SJ, Reece JC, Petravic J, Martyushev A, Kramski M, De Rose R, Cooper DA, Kelleher AD, Emery S, Cameron PU, et al. : The search for an HIV cure: tackling latent infection. Lancet Infect Dis 2013, 13:614–621. [DOI] [PubMed] [Google Scholar]