Abstract
Purpose
Atherosclerosis in the carotid arteries is one common cause of ischemic stroke. We examined atherogenesis in the left carotid artery with and without interrupted blood flow of C57BL/6 (B6) and C3H Apoe-deficient (Apoe−/−) mouse strains.
Methods
Blood flow was interrupted by ligating the common carotid artery near its bifurcation in one group of mice and other group was not interrupted.
Results
Without interference with blood flow, C3H Apoe−/− mice developed no atherosclerosis in the carotid artery while B6 Apoe−/− mice formed advanced atherosclerotic lesions (98,019 ± 10,594 μm2/section) after 12 weeks of Western diet. When blood flow was interrupted by ligating the common carotid artery near its bifurcation, C3H Apoe−/− mice showed fatty streak lesions 2 weeks after ligation and by 4 weeks fibrous lesions formed though smaller than B6 Apoe−/− mice. Neutrophil adhesion to endothelium and infiltration in lesions was observed in ligated arteries of both strains. Treatment of B6 Apoe−/− mice with antibody against neutrophils had little effect on lesion size.
Conclusions
These findings demonstrate the dramatic influences of genetic backgrounds and blood flow on atherogenesis in the carotid artery of hyperlipidemic mice.
Keywords: Atherosclerosis, carotid artery, hyperlipidemia, genetic background, blood flow
Introduction
Stroke is the leading cause of disability in adults and the fourth most common cause of death in the United States [1]. Ischemic stroke, resulting from obstruction of blood flow to the brain, accounts for 83% of all stroke cases. Atherosclerosis in the carotid arteries leads to changes ranging from minor wall thickening to hemodynamically significant luminal stenosis and constitutes one of the major causes of ischemic stroke, including transient ischemic attacks and cerebral infarction [2],[3],[4]. Carotid atherosclerosis may occur concomitantly with atherosclerosis in other arterial beds, such as coronary and femoral arteries, or occur in isolation [5],[6],[7]. Because atherosclerosis in the aorta is easy to measure in mice, previous studies of this disease with the rodent have almost entirely focused on this vessel.
We and others have established a mouse model of carotid atherosclerosis in which blood flow in the common carotid artery of C57BL/6 (B6) Apoe null (Apoe−/−) mice is interrupted by partially or completely ligating the vessel or its branches [8][9][10][11]. This leads to the development of atherosclerosis at a faster rate than common atherosclerosis in the vessel without interruption of blood flow. Lesions in the ligated artery rapidly progress from fatty streak consisting of macrophage-derived foam cells to fibrous plaque involving smooth muscle cells to advanced lesion containing neovessels, all of which occur within 4 weeks. Lesion formation in this model is markedly accelerated by feeding a Western diet, as seen in the mouse models of common atherosclerosis [12],[13],[14].
Previous studies from our group and others have shown that there exists a wide range of variation in atherogenesis among mouse strains [15],[14],[16]. B6 and C3H are the most divergent mouse strains in terms of variation in aortic lesion formation. When fed an atherogenic diet containing high-fat/cholesterol and cholate or deficient in Apoe, strain B6 develops much larger atherosclerotic lesions in the aorta than C3H mice [15],[12]. Because atherosclerosis develops in a site-specific manner especially when blood flow is altered [17], the goal of this study was to determine the influence of genetic background on atherosclerosis development in the carotid artery using the Apoe−/− mouse model.
MATERIALS AND METHODS
Mice
B6-Apoe−/− mice purchased from the Jackson Laboratory were bred to generate mice used in this study. C3H-Apoe−/− mice were created in our lab using the classical congenic breeding protocol [14]. Mep1α−/− mice, provided by Dr. Christoph Becker-Pauly from University of Kie, Germany, were bred onto the B6-Apoe−/− background. Mice were weaned at 3 weeks of age onto a chow diet. One group of mice were switched onto a Western diet containing 21% fat, 48.5% carbohydrate, 17% protein, and 0.2% cholesterol (by weight) (Envigo, TD 88137) 1 week before surgery and remained on the diet thereafter. Another group was started with the Western diet at 6 weeks of age and remained on the diet for 12 weeks to evaluate the common type of atherosclerosis in the carotid artery. All animal-related procedures were performed in compliance with the NIH guide for the care and use of laboratory animals and approved by the University of Virginia Institutional Animal Care and Use Committee (animal protocol #: 3109).
Surgical procedure
The procedure for ligating the left common carotid artery was performed as described [8]. Briefly, 4~8 week-old mice of both sexes were anesthetized by intramuscular injection with ketamine (80 mg/kg body weight; Ketaset, Aveco Inc.) and xylazine (8 mg/kg; AnaSed, Lloyd Laboratories). Under sterile conditions, a midline incision was made in the front of the neck, then the left common carotid artery was dissected under a microscope and ligated near the bifurcation to completely block blood flow (Fig. 1). The surround tissues were then returned and the incision of the skin was closed with a surgical glue (VETCLOSETM, Henry Schein Animal Health). After the procedure, mice continued on the Western diet.
Figure 1.
A schematic diagram showing the location of ligation on the left common carotid artery of mice and the levels of 3 cross-sections used for morphometric analysis.
Tissue preparation and lesion quantification
Mice receiving carotid artery ligation were euthanized at 1, 3 days, 1, 2, or 4 weeks after surgery with prolonged exposure to isoflurane inhalation, as reported [8]. The vasculature was flushed first with saline and then with 10% formalin via the left ventricle of the heart. The neck was dissected en bloc and further fixed in 10% formalin for >48 h. After fixation, the front soft tissues of the neck including the left and right common carotid arteries were dissected out, embedded in OCT compound (Tissue-Tek, Miles Inc.), and cross-sectioned in 10-μm thickness. Serial sections were collected, starting from disappearance of the ligation suture, and mounted on poly-d-lysine–coated slides with 8 sections per slide. Approximately 300 sections were collected from each mouse. Three evenly spaced slides were chosen for hematoxylin and eosin (H&E) staining (Fig. 1). One section on each stained slide was chosen for morphometric measurements of the ligated left common carotid artery and the contralateral right common carotid artery using Zeiss AxioVision 4.8 software. Luminal area and areas encircled by the internal and external elastic laminae were measured. Lesion area was calculated by subtracting the luminal area from the area within the internal elastic lamina, and the medial area of the arterial wall was calculated by subtracting the area encircled by the internal elastic laminae from the area within the external elastic laminae. Measurements made from 3 separate slides were averaged for each vessel and this average was used for statistical analysis. For visualization of neutral lipid, selected sections were stained with oil red O and hematoxylin, counterstained with fast green [18]. Mice that received no surgery were euthanized after 12 weeks of Western diet to measure primary atherosclerosis in the left common carotid artery and its main branches as previously reported [19].
Immunohistochemical analysis
Immunohistochemical staining for leukocyte specific antigens and smooth muscle cell α-actin was performed on frozen sections of the carotid arteries using the following primary antibodies: rat anti-mouse Ly6G antibody (RB6–8C5, eBioscience), rat anti-mouse CD4 antibody (MABF415, Millipore), rabbit anti-mouse CD8 IgG (D4W2Z, Cell Signaling), rat anti-mouse macrophage/monocyte IgG, clone MOMA-2 (MCA519GT, Serotec), mouse anti-human α-smooth muscle actin IgG (M085129, Dako), goat polyclonal antibody for FPR1 from Santa Cruz (sc-13198), and rat anti-mouse Mac-3 IgG1 (Clone M3/84) from BD Biosciences. Subsequent incubations with biotinylated secondary antibody and visualization with VECTASTAIN Elite ABC HRP Kit (Vector Laboratories) were performed as previously described [20]. Sections stained with goat anti-rat IgG (H+L) alexa fluor 488 (4416S, Cell Signaling), donkey anti-goat IgG (H+L) antibody-NL557 (NL001, R&D Systems), or DAPI fluoromount-G (0100–20, Southern Biotech) were directly visualized under fluorescence microscopy.
Plasma lipid measurements
Fasting blood was collected via retro-orbital sinus puncture under isoflurane anesthesia immediately before mice were euthanized. Enzymatic assays for total and HDL cholesterol and triglycerides were performed using the Thermo DMA (Louisville, CO) cholesterol and triglyceride kits [13]. Non-HDL was calculated as the difference between total and HDL cholesterol levels.
Flow cytometric analysis of blood neutrophils and T lymphocytes
Blood was collected from non-fasted male B6-Apoe−/− and C3H-Apoe−/− mice under isoflurane anesthesia through retro-orbital plexus puncture. Red blood cells were lysed with ACK buffer (ThermoFisher, A1049201). The remaining cells were subjected to flow cytometric analysis of neutrophils and T cells as reported [21][22]. Neutrophils were evaluated with fluoroscence conjugated monoclonal antibodies, including BV421-conjugated rat anti-mouse Ly6G (BD Biosciences, Cat. # 562737), APC-conjugated rat anti-mouse CD11b (eBiosciences, Cat. # 17–0012-81), and Percp-conjugated anti-mouse CD45 (eBioscience, Cat. # 45–0451-80). T cells were determined with FITC-conjugated anti-CD4 (eBioscience, Cat. # 11–0043-81), efluor 450-conjugated CD8α (eBioscience, Cat. # 48–0081-80), and Percp-conjugated anti-mouse CD45. Neutrophils were defined as cells double positive for CD11b and Ly6G within a CD45-positive population. T cells were identified as cells positive for CD4 or CD8 for a CD45-positive population.
Antibody treatment
For neutrophil depletion experiments, Mep1α−/− Apoe−/− mice on a B6 genetic background, which show increased neutrophil infiltration in atherosclerotic lesions (unpublished data), were intraperitoneally (I.P.) injected with 200 μg of monoclonal rat anti-mouse Ly6G antibody (Clone: 1A8, Biolegend) per mouse every other day for 4 weeks, starting the day before surgery. For mice that were not treated with the antibody, an equivalent amount of saline was administered as reported [23].
Statistical analysis
Data were expressed as mean ± SE, with “n” indicating the number of animals. Analysis of variance (ANOVA) or Student’s t test was used to determine statistical significance for differences between strains or treatments in various measurements. Differences were considered statistically significant at P ≤ 0.05.
Results
Fasting plasma lipid levels of two Apoe−/− mouse strains
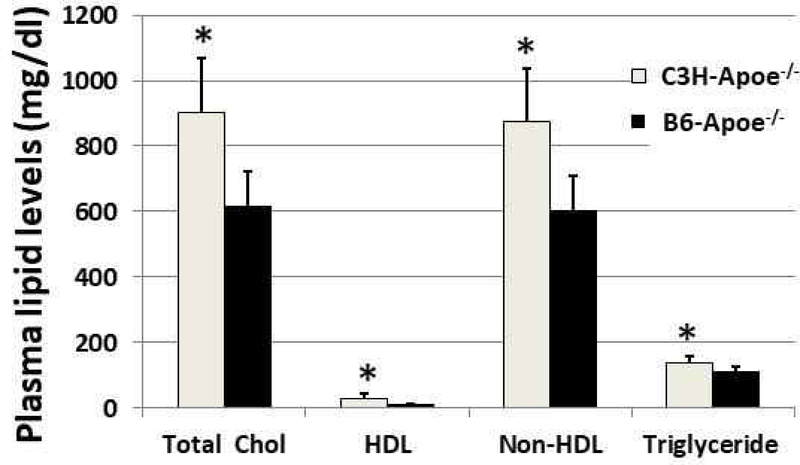
Fasting plasma levels of total and HDL cholesterol and triglyceride were measured for female B6-Apoe−/− and C3H-Apoe−/− mice after being fed 12 weeks of Western diet. Non-HDL cholesterol was calculated as the difference between total and HDL cholesterol levels. Both strains developed severe dyslipidemia with elevated non-HDL cholesterol levels and reduced HDL cholesterol levels (Fig. 2). C3H-Apoe−/− mice (n=8) had significantly higher plasma levels of total (902.7 ± 59.2 vs. 681.4 ± 36.5 mg/dl; P= 0.0016), non-HDL (875.1 ± 56.6 vs. 606.3 ± 36.7 mg/dl; P=0.002), HDL cholesterol (27.5 ± 5.5 vs. 12.1 ± 0.6 mg/dl; P= 0.025) and triglyceride (136.6 ± 8.2 vs. 112.1 ± 5.3 mg/dl; P= 0.028) than B6-Apoe−/− mice (n=8).
Figure 2.
Plasma levels of total cholesterol (total chol), HDL cholesterol, non-HDL cholesterol, and triglyceride in female B6-Apoe−/− (n=8) and C3H-Apoe−/− mice (n=8) fed a Western diet. Values are means ± SE. * P<0.05.
Atherosclerosis in the carotid artery without interrupted blood flow
Atherosclerotic lesions in the carotid arteries of B6-Apoe−/− and C3H-Apoe−/− mice were examined after being fed 12 weeks of Western diet. Atherosclerosis around the bifurcation of the right and left common carotid arteries was grossly observable in B6-Apoe−/− mice (Fig. 3A). Atherosclerotic lesions in the left common carotid artery were measured on oil red O stained sections (Fig. 3B, 3C). B6-Apoe−/− mice developed advanced atherosclerotic lesions containing lipid, fibrous component, calcification and necrotic areas. The average lesion size of these mice was 98,019 ± 10,594 μm2/section (n=14). In striking contrast, C3H-Apoe−/− mice developed no lesion (Fig. 3D; P=4.4E-7) (n=8).
Figure 3.
Common atherosclerosis developed in the carotid arteries without interruption of blood flow in B6-Apoe−/− and C3H-Apoe−/− mice fed 12 weeks of Western diet. A, Gross appearance of atherosclerosis in the carotid arteries of a B6-Apoe−/− mouse. Black arrows point toward atherosclerotic plaque in the bifurcation (left) and branch (right). B and C, oil red O stained sections of the left common carotid artery of a B6-Apoe−/− and a C3H-Apoe−/− mouse. B6-Apoe−/− mouse developed advanced lesion containing fatty, fibrous, necrotic, and calcium components, while C3H-Apoe−/− mouse had no lesion. D, Morphometric measurement of atherosclerotic lesions in the left carotid artery of B6-Apoe−/− and C3H-Apoe−/− mice. Results are means ± SE of 14 female B6-Apoe−/− and 8 female C3H-Apoe−/− mice.
Lesion formation in the carotid artery with interrupted blood flow
Morphometric measurements of the ligated left common carotid artery and the contralateral right common carotid artery were compared between B6-Apoe−/− and C3H-Apoe−/− mice at 1, 2 and 4 weeks after surgery. At all 3 time points examined, B6-Apoe−/− mice developed larger intimal lesions than C3H-Apoe−/− mice in the ligated artery (Fig. 4A). The difference between the two strains in lesion size at 4 weeks was highly significant (P=0.0017) (n=5 per strain). The medial area of ligated artery in B6-Apoe−/− mice at this time point was also larger than that of C3H-Apoe−/− mice although the difference did not reach statistical significance (Fig. 4B; P=0.138). Compared to contralateral control artery, the ligated artery showed a significant increase in medial area at 4 weeks after ligation in B6-Apoe−/− mice (Fig. 4C; P=0.0245).
Figure 4.
Quantitative measurements of intimal (A) and medial areas (B) in ligated left common carotid artery and medial areas in the contralateral right common carotid artery (C) of B6-Apoe−/− and C3H-Apoe−/− mice fed a Western diet. Cross-sectional areas encircled by the external elastic lamina (D) and internal elastic lamina (E) in the ligated common artery were also measured. Measurements were made on the carotid arteries 1 (3 mice per strain), 2 (5 C3H-Apoe−/− and 4 B6-Apoe−/− mice) and 4 weeks (5 mice per strain) after ligation. Values are means ± SE for each group. * P < 0.05 versus C3H-Apoe−/− mice. + P < 0.05 versus the contralateral common carotid artery.
The areas encircled by the external or internal elastic lamina of the ligated artery were comparable between the two strains at 1 and 2 weeks of ligation (Fig. 4D, 4E). At 4 weeks, the areas were significantly larger in B6 Apoe−/− mice than in C3H Apoe−/− mice (P=0.0036 and 0.0045, respectively), indicating that the ligated artery of B6 Apoe−/−mice underwent a positive remodeling.
There was no intimal lesion in the contralateral right carotid artery of both strains. The medial area of the artery was comparable between the two strains at all 3 time points examined (Fig. 4C).
Histological analysis showed that lesions occurred in ligated arteries of B6-Apoe−/− mice as early as 1 week after ligation and 2 weeks in C3H-Apoe−/− mice (Fig. 5). These lesions were small and consisted of 1 or 2 layers of cells overlaying the inner surface of the arteries. The lesions of B6-Apoe−/− mice looked foamy, while the lesions of C3H-Apoe−/− mice consisted of mononuclear cells adhering to the surface of the endothelial lining (Fig. 5A, 5E). With increasing duration, the lesions progressed to intermediate or fibrous lesions containing multiple layers of foam cells, smooth muscle cells and fibrous elements. The fibrous lesions were observable at 2 weeks after ligation in B6-Apoe−/−mice and 4 weeks in C3H-Apoe−/− mice (Fig. 5B, 5F). By 4 weeks, the lesions of B6-Apoe−/− mice progressed to more advanced lesions, containing neo-vessels and substantially narrowing the vessel lumen (Fig. 5C).
Figure 5.
Representative pictures of cross-sections of ligated common carotid artery from B6-Apoe−/− and C3H-Apoe−/− mice 1, 2, and 4 weeks after ligation. The top row shows cross-sections of the vessels from B6-Apoe−/− mice (A, B, C), and the bottom row shows cross-sections of C3H-Apoe−/− mice (D, E, F). Black arrows point toward foam cells, red arrows toward fibrous cap, yellow arrows at neovessels. Foam cells can be observed in the medial area of the ligated vessel (B, green arrow). Sections were stained with the standard hematoxylin-eosin (H&E) method. Original magnification: ×40.
The deposition of lipids in the arterial walls of the ligated carotid artery and the contralateral carotid artery was evaluated using oil red O stain. Lipid deposition was observed in the medial wall of ligated arteries, starting at day 1 and was increasing over time in B6-Apoe−/− mice (Fig. 6). The contralateral artery also showed staining in the medial wall at day 3 and later time points after surgery, though less prominent compared to the ligated artery. Lipid deposition was also seen in the medial wall of the ligated artery at all time points tested and was increasingly prominent at later time points in C3H-Apoe−/− mice. The contralateral carotid artery showed less staining in the medial wall with oil red O.
Figure 6.
Oil red O stained sections of ligated common carotid artery and contralateral right carotid artery of B6-Apoe−/− and C3H-Apoe−/− mice fed a Western diet. Intimal lesions stain red. Note the time-dependent increase in red intensity for the medial layer of both ligated and control arteries of B6-Apoe−/− mice and the differences in red stain between the medial arterial wall of left and right carotid arteries of C3H-Apoe−/− mice over time. Original magnification: ×10.
Cytological differences between the intimal lesions of B6-Apoe−/− mice and C3H-Apoe−/− mice
To evaluate possible mechanisms that may explain strain-dependent differences in lesion formation, we characterized major cellular components that potentially play roles in atherosclerosis. Specific cellular components were determined by immunohistochemical analyses. At the earlier time examined (day 1 through 1 week), sporadic neutrophil adhesion to the endothelium was observed in both strains (Fig. 7A). At later time, neutrophils were observed within the lesions of both strains though C3H-Apoe−/− mice had much more.
Figure 7.
Immunohistochemical detection of neutrophils (A), macrophages (B, C), CD4+ (D), CD8+ T cells (E), and smooth muscle cells (F) in ligated carotid arteries of B6-Apoe−/− and C3H-Apoe−/− mice fed a Western diet at different time points after ligation. A, Section stained with the standard Avidin-Biotin Complex (ABC) method using an anti-Ly6G antibody. Arrows point to stained neutrophils. B, Sections stained with rat monoclonal macrophage antibody MOMA-2. Arrows point to stained macrophages. C, Fluorescence double immunostaining showing the presence of macrophages in ligated arterial wall of B6-Apoe−/− mice 1 week after ligation. Macrophages were stained with antibodies against Mac3 and FPR1 antigens. Nuclei were stained with DAPI. D, Note the presence of CD4+ cells in intimal lesions of C3H-Apoe−/− mice but not B6-Apoe−/− mice. E, Immunostaining for CD8+ T cells: No CD8+ cells were detectable in the lesions. F, Immunostaining for α-smooth muscle actin. Black arrows point at smooth muscle in the caps and white arrows point at disorganized smooth muscle at the bottom of the lesion. Note the low immunoreactivity to α-smooth muscle actin of the medial arterial wall in B6-Apoe−/− mouse 4 weeks after ligation.
Although foam cells were observed histologically as early as 1 week after ligation, immunostaining for macrophages with the MOMA-2 antibody were only apparent at 2 and 4 weeks after ligation when significant intimal lesions formed (Fig. 7B). Fluorescent-labeled antibodies targeting Mac3 and FPR1 antigens confirmed the presence of macrophages in ligated arterial walls of B6-Apoe−/− mice 1 week after ligation (Fig. 7C).
T lymphocytes were detected with antibodies against CD4 and CD8 antigens. Immunoreactivity to the CD4 antigen was observed in the lesions of C3H Apoe−/− mice but not B6 Apoe−/− mice (Fig. 7D). CD8+ T cells were not detectable in the lesions of either strain (Fig. 7E).
Immunostaining for smooth muscle was initially observed in the caps of the lesion at 2 weeks after ligation for B6-Apoe−/− mice (Fig. 7F). As lesions progressed, a sheet of disorganized smooth muscle was observed at the bottom of the lesion and more smooth muscle was seen in the cap of the lesion. The two smooth muscle layers were separated by a layer of foam cells containing fewer smooth muscle cells. For C3H-Apoe−/− mice, smooth muscle cells were intermingled with foam cells or other components of the lesions in ligated artery 4 weeks after ligation. A decline in the immunoreactivity to α-smooth muscle actin was observed in the medial layer of ligated arteries for both strains (Fig. 7F). At 4 weeks, the medial layer underlying the lesions showed little staining although smooth muscle within the lesions showed strong staining in B6-Apoe−/− mice.
Flow cytometric analysis of blood neutrophils and T lymphocytes
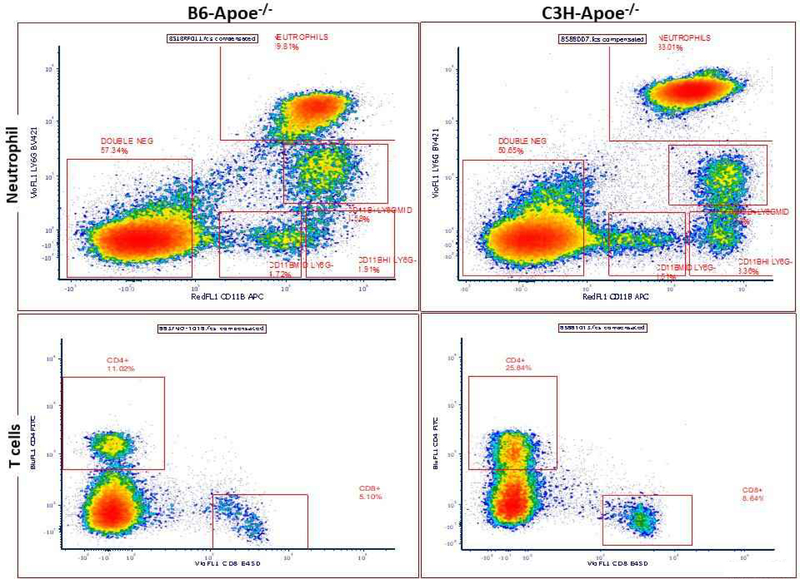
Peripheral blood neutrophils and T cells from 3 male B6-Apoe−/− and C3H-Apoe−/− mice were analyzed by flow cytometry. Neutrophils were identified as CD11b and Ly6G double positive cells and T cells were identified as CD4+ or CD8+ cells following positive selection for the common leukocyte antigen CD45 (Fig. 8A). Neutrophils accounted for 34.4 ± 0.8% of total leukocytes in C3H-Apoe−/− mice, significantly higher than 11.0 ± 4.5% in B6-Apoe−/− mice (P=0.036; Fig. 8B). C3H-Apoe−/− mice also had a higher percentage of CD4+ cells (27.1 ± 0.9 vs. 12.1 ± 1.0; P=0.0004) and CD8+ cells (8.8 ± 0.4 vs. 4.5 ± 0.4; P=0.0014) compared to B6-Apoe−/− mice. The number of leukocytes was counted after removal of red blood cells: C3H-Apoe−/− mice had significantly more leukocytes than B6-Apoe−/− mice (5.27 ± 0.59 vs. 1.53 ± 0.12 × 106/ml; P=0.021) (Supplementary data).
Figure 8.
Flow cytometric analysis of neutrophils, CD4+ and CD8+ T cells in peripheral blood of male B6-Apoe−/− and C3H-Apoe−/− mice fed a chow diet. A, representative results of flow cytometry analysis of neutrophils, CD4+ and CD8+ T cells on a CD45 positive gate. Neutrophils were gated for expression of both CD11b and Ly6G antigens, T cells were gated for expression of CD4+ or CD8+ antigen. B, mean percentage of neutrophils, CD4+ and CD8+ cell population as a percentage of CD45 positive leukocytes. Results are means ± SE of 3 mice per strain. * P < 0.05.
Treatment with antibody targeting neutrophils
To evaluate the role of neutrophils in lesion formation, 4-week-old Mep1α−/− Apoe−/− mice with the B6 genetic background began receiving a 4-week treatment with the anti-Ly6G antibody every other day. Antibody treatment led to elimination of neutrophils in atherosclerotic lesions in ligated carotid artery and aorta (Fig. 9). Mice treated with the antibody did not show a significant difference in lesion size from control mice (72,714 ± 24,265 vs. 51,414 ± 21,621 μm2/section; P=0.537; n=4 per group).
Fig. 9.
Effect of anti-Ly6G antibody on lesion formation in the ligated carotid artery of B6-Apoe−/− mice. Mice were injected with 200 μg antibody every other day for 4 weeks. Immunostaining was performed to determine the presence of neutrophils in atherosclerotic lesions in ligated carotid artery and aortic root. Arrows point to stained neutrophils. Bar graph on the right shows the lesion size in the ligated artery of antibody treated and control mice 4 weeks after ligation. Results are means ± SE of 4 mice (both sexes) per group.
Discussion
To date, strain-dependent differences in atherosclerotic formation among mouse strains have primarily been observed from the aortic root or other parts of the aorta [15],[12],[16],[14],[24]. These differences have provided the bases of linkage mapping for finding genetic factors in atherogenesis [25],[26],[27]. Few studies have been performed to look at strain-dependent differences in atherosclerosis at sites beyond the aorta in mice [28]. One major reason for the rarity of studies is that mouse strains do not develop atherosclerosis at sites beyond the aorta unless they are genetically modified to become deficient in Apoe or Ldlr or to overexpress Apoe3 or Pcsk9. Our effort to find genetic factors in atherosclerosis has led us to the creation of multiple Apoe−/− mouse strains [14]. Although a number of loci for carotid atherosclerosis have been mapped in intercrosses derived from the Apoe−/− mouse strains [19],[27],[29], the characterization of carotid atherosclerosis with these strains has not been reported. In this study we used two different models to characterize atherosclerotic formation in the carotid artery of B6-Apoe−/− mice and C3H-Apoe−/− mice and demonstrated the dramatic effect of genetic background and blood flow on atherogenesis in the vessel. The lesions formed in the carotid artery of B6-Apoe−/− mice with normal blood flow are similar to those in humans: they occurred at the bifurcation of the vessel, contained lipid, fibrous components, calcification and necrosis, and were dramatically affected by genetic background (Fig. 3). The lesions developed in ligated carotid artery are also similar to what are seen in humans, progressing from fatty streak consisting of foam cells to fibrous lesion comprised of foam cells and fibrous element to advanced lesion containing neovessels.
An intriguing finding of this study is that C3H-Apoe−/− mice were totally resistant to atherosclerosis, developing no atherosclerosis in the carotid artery even after being fed 12 weeks of Western diet. In contrast, B6-Apoe−/− mice developed advanced atherosclerotic lesions in the vessel. On the Western diet, B6-Apoe−/− and C3H-Apoe−/− mice developed severe hypercholesterolemia largely due to elevations in non-HDL cholesterol. As C3H-Apoe−/− mice had higher non-HDL cholesterol and triglyceride levels and a comparable HDL cholesterol level relative to B6-Apoe−/− mice, their resistance to atherosclerosis should not be attributable to plasma lipids. In a recent study, we found that C3H-Apoe−/− mice fed 12 weeks of Western diet developed considerable atherosclerotic lesions in the aortic root [14]. Local factors, particularly hemodynamic forces [30], are probably responsible for the difference between the two sites in plaque formation. Indeed, blood flow in the aortic sinus is highly unsteady and turbulent [31] compared to the flow in the carotid bifurcation.
This study shows that C3H-Apoe−/− mice developed both early and intermediate atherosclerotic lesions in the carotid artery once blood flow was interrupted. Foam cells, the hall mark of early atherosclerosis were observed histologically on H&E sections. Immunocytochemical analysis demonstrates the presence of lipid-laden macrophages in the lesions. In mice, foam cells are primarily macrophage-derived and only a tiny fraction of them is derived from smooth muscle cells [32]. Smooth muscle cells are slow in outgrowth [33], and these cells were only observed in the lesions 2 weeks after ligation. The MOMA-2 antibody is effective in detecting monocytes and macrophages in various mouse tissues, but it is less sensitive in detecting marginal zone macrophages. As FPR1 and Mac3 are also specifically expressed by macrophages [21][34], we used these antigens to demonstrate the infiltration of macrophages in arterial walls at the early stage of plaque formation. As the lesions progressed, smooth muscle cells appeared and made up the fibrous caps of the plaques. Though rare, neovessels and necrotic areas were also observed in the lesions.
Compared to B6-Apoe−/− mice, C3H-Apoe−/− mice developed smaller lesions in the ligated artery at the time points examined and showed delays in plaque initiation and progression. Foam cells were observed as early as 1 week after ligation in B6-Apoe−/− mice but 2 weeks in C3H-Apoe−/−mice. Fibrous caps formed 2 weeks after ligation in B6-Apoe−/− but 4 weeks in C3H Apoe−/− mice. By 4 weeks, the last time point of observation, B6-Apoe−/− mice developed advanced lesions containing numerous neovessels and occluding the lumen of the affected vessel.
A previous study has shown that intimal growth in ligated carotid artery is minimal in wild-type B6 and C3H mice 4 weeks after ligation [35]. In contrast, C3H-Apoe−/− and B6-Apoe−/− mice developed medium and large sized intimal lesions in the ligated artery, respectively. The absence of Apoe and the resultant hyperlipidemia should be responsible for increased lesion formation in the Apoe−/− strains. Indeed, Apoe inhibits and hyperlipidemia promotes smooth muscle cell proliferation and vascular remodeling [36],[18][13].
Lipid retention in the medial wall of the ligated artery showed a progressive increase in both Apoe−/− strains as denoted on oil red O staining. The contralateral control artery also showed mild staining in B6-Apoe−/− mice but the staining was less obvious in C3H-Apoe−/− mice. This finding is consistent with our previous observation that B6-Apoe−/− mice have more ApoB-containing lipoproteins in the aortic wall than C3H-Apoe−/− mice [37]. Deposition of lipoproteins, specifically LDL, in the arterial walls is the initial event in the development of atherosclerosis. The deposited LDL undergoes oxidative modification by vascular cells to become oxidized LDL, which stimulates vascular wall cells to express proinflammatory molecules and promotes foam cell formation.
Ligation in the distal end near the bifurcation causes blood flow cessation in the common carotid artery, although the vessel may still experience arterial blood pressure and pulsation. This would increase the interactions between white blood cells and the endothelium in the ligated artery. Neutrophils were observed to adhere to the lining endothelium and to be present in the lesions. Activated neutrophils release superoxide and pro-inflammatory molecules at the sites of adhesion that promote the recruitment of monocytes and alter endothelial cell properties [38]. The role of neutrophils in lesion formation was determined through depleting mice of the cells with anti-Ly6G antibody. The effectiveness of neutrophil depletion with specific anti-Ly6G antibody in mice has been confirmed in multiple studies [39],[40],[41]. In this study we confirmed the elimination of neutrophils in atherosclerotic lesions of antibody treated mice. The present finding that mice treated with the anti-Ly6G antibody showed a little increase in plaque size suggests that neutrophils play a minor protective role in atherosclerosis. The finding that C3H-Apoe−/− mice had more neutrophils in the lesions supports this speculation. We previously observed that F1 mice reconstituted with bone marrow from C3H-Apoe−/− mice developed a carotid lesion comparable to those reconstituted with B6-Apoe−/− bone marrow following endothelium denudation injury [42], suggesting that bone marrow-derived cells contribute little to the difference between the two strains in carotid atherosclerosis. Previous studies also reported the presence of neutrophils in atherosclerotic lesions of Apoe−/− mice [43][44], while depletion of neutrophils with Ly6G antibody only reduced early but had no effect on advanced aortic lesions in Apoe−/− mice [44].
T helper (CD4) cells can promote or protect against atherosclerosis depending on experimental models used and the stage of plaque formation [45]. We observed the presence of CD4 cells in intimal lesions of C3H-Apoe−/− but not B6-Apoe−/− mice, although their significance to the difference between the two strains in lesion formation remains to be defined. The current cytometric analysis of peripheral blood leukocytes showed that C3H-Apoe−/− mice have more neutrophils and CD4+ T cells than B6-Apoe−/− mice, which is in agreement with the results observed by Petkova et al [46] in wild-type B6 and C3H mice. The higher percentage of neutrophils and CD4+ T cells in peripheral blood could be partially responsible for the enhanced infiltration of Cd4+ T cells and neutrophils in the intimal lesions of C3H-Apoe−/− mice. The absence of CD8+ T cells in atherosclerotic lesions precludes their substantial role in plaque formation.
The unresponsiveness of arterial wall cells to oxidized LDL may be responsible for the resistance of C3H-Apoe−/− mice to lesion formation in the ligated artery. We previously observed that endothelial cells and vascular smooth muscle cells of B6 mice exhibit a dramatic induction of MCP-1, CSF-1, VCAM-1, and heme oxygenase-1 in response to oxidized LDL while cells from C3H mice showed little induction [12],[47],[33]. MCP-1, CSF-1, and VCAM-1are primarily associated with the recruitment and differentiation of monocytes. Thus, if arterial wall cells of C3H mice were unable to recruit monocytes to the artery wall and promote their differentiation into macrophages, it might be expected that these mice would develop less atherosclerosis. Through aorta and bone marrow transplantations, we demonstrated that the arterial wall is a major source of variation in atherosclerosis susceptibility of the two strains [12][48].
Wild-type mouse strains, including B6 and C3H, show significant negative remodeling or shrinkage of the ligated carotid artery [35]. In contrast, this phenomenon was not found in the two Apoe−/− strains; rather the ligated artery of B6-Apoe−/− mice showed an increase in the areas encircled by the internal or external elastic lamina 4 weeks after ligation. This finding is consistent with compensatory enlargement of atherosclerotic coronary arteries observed in humans [49]. Smooth muscle cells are the main cellular component of intimal lesions in ligated carotid artery of wild-type mice [50], while the intimal lesions contain macrophages and other inflammatory cells in Apoe−/− mice. Smooth muscle cells and the extracellular matrix produced by the cells restrict vessel distension [51], while macrophages produce MMP-9, MMP-12, and other enzymes that degrade the extracellular matrix [52]. The degradation of the extracellular matrix underlying atherosclerotic plaques is expected to weaken the vessel wall and enhance blood pressure-induced outward remodeling of vessels [52],[53]. Indeed, a higher macrophage count and lipid content is significantly associated with positive remodeling in human coronary artery with plaques [54],[55].
In this study, the mice that received the left common carotid artery ligation developed no symptoms of stroke. This finding is consistent with the observation of Kumar and Lindner made from wild-type mice [50]. The brain receives blood from two sets of arteries: the vertebral arteries, which arise from the subclavian arteries, and the internal carotid arteries, which arise from the common carotid arteries. The right and left vertebral arteries merge on the front surface of the brainstem at the midline to form the basilar artery, which joins the blood supply from the internal carotid arteries through the circle of Willis. The conjoining of the two major sources of cerebral blood supply via the circle of Willis improves the chances of any region of the brain to continue receiving blood when one of the major arteries becomes occluded. In mice both the left and right common carotid arteries have to be occluded to induce stroke and brain damage [56].
In summary, the present study has demonstrated the significance of genetic background, blood flow, and inflammation in the development of atherosclerosis in the carotid artery of hyperlipidemic mouse strains. Despite severe hyperlipidemia developed on the Western diet, C3H-Apoe−/− mice formed no atherosclerosis in the carotid artery with normal blood flow. Once blood flow was interrupted, C3H-Apoe−/− mice developed moderate atherosclerotic lesions in the ligated artery though smaller than B6-Apoe−/− mice. Thus, blood flow is a major force that determines atherosclerosis susceptibility in the carotid arteries. Interruption of blood flow increases the chances of attachment and recruitment of white blood cells to the arterial wall in which lipoproteins accumulate and are subsequently oxidized. Oxidized lipids induce atherosclerosis by promoting inflammation and foam cell formation. The ligation model relates to clinical situations of obstructive arterial disease and restenosis in humans. It is useful for unraveling the cellular and molecular processes of atherosclerosis, a complex human disease.
Supplementary Material
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by National Institutes of Health grants DK116768 and Commonwealth Health Research Board [CHRB] Award.
Andrew Grainger is a recipient of the Robert R. Wagner Fellowship from the University of Virginia School of Medicine.
Footnotes
Disclosure of conflicting interests
None.
Data availability: All data presented in this article are provided in Supplementary materials.
References
- [1].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association, Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- [2].Paraskevas KI, Mikhailidis DP, Liapis CD: Internal carotid artery occlusion: association with atherosclerotic disease in other arterial beds and vascular risk factors, Angiology. 2007;58:329–335. [DOI] [PubMed] [Google Scholar]
- [3].Paciaroni M, Caso V, Venti M, Milia P, Kappelle LJ, Silvestrelli G, Palmerini F, Acciarresi M, Sebastianelli M, Agnelli G: Outcome in patients with stroke associated with internal carotid artery occlusion, Cerebrovasc Dis. 2005;20:108–113. [DOI] [PubMed] [Google Scholar]
- [4].Rothwell PM, Warlow CP: Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group, Stroke. 2000;31:622–630. [DOI] [PubMed] [Google Scholar]
- [5].Manzano JJ, De Silva DA, Pascual JL, Chang HM, Wong MC, Chen CP: Associations of ankle-brachial index (ABI) with cerebral arterial disease and vascular events following ischemic stroke, Atherosclerosis. 2012;223:219–222. [DOI] [PubMed] [Google Scholar]
- [6].Razzouk L, Rockman CB, Patel MR, Guo Y, Adelman MA, Riles TS, Berger JS: Co-existence of vascular disease in different arterial beds: Peripheral artery disease and carotid artery stenosis--Data from Life Line Screening((R)), Atherosclerosis. 2015;241:687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Paraskevas KI, Mikhailidis DP, Liapis CD: Internal carotid artery occlusion: association with atherosclerotic disease in other arterial beds and vascular risk factors, Angiology. 2007;58:329–335. [DOI] [PubMed] [Google Scholar]
- [8].Chang Z, Huangfu C, Grainger AT, Zhang J, Guo Q, Shi W: Accelerated atherogenesis in completely ligated common carotid artery of apolipoprotein E-deficient mice, Oncotarget. 2017;8:110289–110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eschert H, Sindermann JR, Scheld HH, Breithardt G, Weissen-Plenz G: Vascular remodeling in ApoE-deficient mice: diet dependent modulation after carotid ligation, Atherosclerosis. 2009;204:96–104. [DOI] [PubMed] [Google Scholar]
- [10].Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H: Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis, Am J Physiol Heart Circ Physiol. 2009;297:H1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shin IJ, Shon SM, Schellingerhout D, Park JY, Kim JY, Lee SK, Lee DK, Lee HW, Ahn BC, Kim K, Kwon IC, Kim DE: Characterization of partial ligation-induced carotid atherosclerosis model using dual-modality molecular imaging in ApoE knock-out mice, PLoS One. 2013;8:e73451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shi W, Wang NJ, Shih DM, Sun VZ, Wang X, Lusis AJ: Determinants of atherosclerosis susceptibility in the C3H and C57BL/6 mouse model: evidence for involvement of endothelial cells but not blood cells or cholesterol metabolism, Circ Res. 2000;86:1078–1084. [DOI] [PubMed] [Google Scholar]
- [13].Tian J, Pei H, James JC, Li Y, Matsumoto AH, Helm GA, Shi W: Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility, Biochem Biophys Res Commun. 2005;329:1102–1107. [DOI] [PubMed] [Google Scholar]
- [14].Liu S, Li J, Chen MH, Liu Z, Shi W: Variation in Type 2 Diabetes-Related Phenotypes among Apolipoprotein E-Deficient Mouse Strains, PLoS One. 2015;10:e0120935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P: Variation in susceptibility to atherosclerosis among inbred strains of mice, Atherosclerosis. 1985;57:65–73. [DOI] [PubMed] [Google Scholar]
- [16].Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, Pan C, Packard RR, Eskin E, Yan M, Kirchgessner T, Wang Z, Li X, Gregory JC, Hazen SL, Gargalovic PS, Lusis AJ: Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains, PLoS Genet. 2015;11:e1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nigro P, Abe J, Berk BC: Flow shear stress and atherosclerosis: a matter of site specificity, Antioxid Redox Signal. 2011;15:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tian J, Pei H, Sanders JM, Angle JF, Sarembock IJ, Matsumoto AH, Helm GA, Shi W: Hyperlipidemia is a major determinant of neointimal formation in LDL receptor-deficient mice, Biochem Biophys Res Commun. 2006;345:1004–1009. [DOI] [PubMed] [Google Scholar]
- [19].Li Q, Li Y, Zhang Z, Gilbert TR, Matsumoto AH, Dobrin SE, Shi W: Quantitative trait locus analysis of carotid atherosclerosis in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice, Stroke. 2008;39:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan Z, Pei H, Roberts DJ, Zhang Z, Rowlan JS, Matsumoto AH, Shi W: Quantitative trait locus analysis of neointimal formation in an intercross between C57BL/6 and C3H/HeJ apolipoprotein E-deficient mice, Circ Cardiovasc Genet. 2009;2:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Kundu B, Zhong M, Huang T, Li J, Chordia MD, Chen MH, Pan D, He J, Shi W: PET imaging detection of macrophages with a formyl peptide receptor antagonist, Nucl Med Biol. 2015;42:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, Wang Q, Chai W, Chen MH, Liu Z, Shi W: Hyperglycemia in apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility, Cardiovasc Diabetol. 2011;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bruhn KW, Dekitani K, Nielsen TB, Pantapalangkoor P, Spellberg B: Ly6G-mediated depletion of neutrophils is dependent on macrophages, Results Immunol. 2015;6:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tomita H, Zhilicheva S, Kim S, Maeda N: Aortic arch curvature and atherosclerosis have overlapping quantitative trait loci in a cross between 129S6/SvEvTac and C57BL/6J apolipoprotein E-null mice, Circ Res. 2010;106:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Su Z, Li Y, James JC, McDuffie M, Matsumoto AH, Helm GA, Weber JL, Lusis AJ, Shi W: Quantitative trait locus analysis of atherosclerosis in an intercross between C57BL/6 and C3H mice carrying the mutant apolipoprotein E gene, Genetics. 2006;172:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Z, Rowlan JS, Wang Q, Shi W: Genetic analysis of atherosclerosis and glucose homeostasis in an intercross between C57BL/6 and BALB/cJ apolipoprotein E-deficient mice, Circ Cardiovasc Genet. 2012;5:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rowlan JS, Zhang Z, Wang Q, Fang Y, Shi W: New quantitative trait loci for carotid atherosclerosis identified in an intercross derived from apolipoprotein E-deficient mouse strains, Physiol Genomics. 2013;45:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shim J, Handberg A, Ostergren C, Falk E, Bentzon JF: Genetic susceptibility of the arterial wall is an important determinant of atherosclerosis in C57BL/6 and FVB/N mouse strains, Arterioscler Thromb Vasc Biol. 2011;31:1814–1820. [DOI] [PubMed] [Google Scholar]
- [29].Grainger AT, Jones MB, Chen MH, Shi W: Polygenic Control of Carotid Atherosclerosis in a BALB/cJ x SM/J Intercross and a Combined Cross Involving Multiple Mouse Strains, G3 (Bethesda). 2017;7:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].VanderLaan PA, Reardon CA, Getz GS: Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators, Arterioscler Thromb Vasc Biol. 2004;24:12–22. [DOI] [PubMed] [Google Scholar]
- [31].Back M, Gasser TC, Michel JB, Caligiuri G: Biomechanical factors in the biology of aortic wall and aortic valve diseases, Cardiovasc Res. 2013;99:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Allahverdian S, Pannu PS, Francis GA: Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation, Cardiovasc Res. 2012;95:165–172. [DOI] [PubMed] [Google Scholar]
- [33].Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ: Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice, Circulation. 2000;102:75–81. [DOI] [PubMed] [Google Scholar]
- [34].Khazen W, M’bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C: Expression of macrophage-selective markers in human and rodent adipocytes, FEBS Lett. 2005;579:5631–5634. [DOI] [PubMed] [Google Scholar]
- [35].Harmon KJ, Couper LL, Lindner V: Strain-dependent vascular remodeling phenotypes in inbred mice, Am J Pathol. 2000;156:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu B, Kuhel DG, Witte DP, Hui DY: Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice, Am J Pathol. 2000;157:1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brown MD, Jin L, Jien ML, Matsumoto AH, Helm GA, Lusis AJ, Frank JS, Shi W: Lipid retention in the arterial wall of two mouse strains with different atherosclerosis susceptibility, J Lipid Res. 2004;45:1155–1161. [DOI] [PubMed] [Google Scholar]
- [38].Soehnlein O, Weber C, Lindbom L: Neutrophil granule proteins tune monocytic cell function, Trends Immunol. 2009;30:538–546. [DOI] [PubMed] [Google Scholar]
- [39].Abbitt KB, Cotter MJ, Ridger VC, Crossman DC, Hellewell PG, Norman KE: Antibody ligation of murine Ly-6G induces neutropenia, blood flow cessation, and death via complement-dependent and independent mechanisms, J Leukoc Biol. 2009;85:55–63. [DOI] [PubMed] [Google Scholar]
- [40].Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC: The role of neutrophils during mild and severe influenza virus infections of mice, PLoS One. 2011;6:e17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE: Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice, J Leukoc Biol. 2008;83:64–70. [DOI] [PubMed] [Google Scholar]
- [42].Shi W, Pei H, Fischer JJ, James JC, Angle JF, Matsumoto AH, Helm GA, Sarembock IJ: Neointimal formation in two apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility, J Lipid Res. 2004;45:2008–2014. [DOI] [PubMed] [Google Scholar]
- [43].Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Bjorkstrom NK, Malmberg KJ, Lindbom L, Eriksson EE: Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice, Am J Pathol. 2010;177:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O: Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis, Circulation. 2010;122:1837–1845. [DOI] [PubMed] [Google Scholar]
- [45].Gronberg C, Nilsson J, Wigren M: Recent advances on CD4(+) T cells in atherosclerosis and its implications for therapy, Eur J Pharmacol. 2017;816:58–66. [DOI] [PubMed] [Google Scholar]
- [46].Petkova SB, Yuan R, Tsaih SW, Schott W, Roopenian DC, Paigen B: Genetic influence on immune phenotype revealed strain-specific variations in peripheral blood lineages, Physiol Genomics. 2008;34:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miyoshi T, Tian J, Matsumoto AH, Shi W: Differential response of vascular smooth muscle cells to oxidized LDL in mouse strains with different atherosclerosis susceptibility, Atherosclerosis. 2006;189:99–105. [DOI] [PubMed] [Google Scholar]
- [48].Pei H, Wang Y, Miyoshi T, Zhang Z, Matsumoto AH, Helm GA, Tellides G, Shi W: Direct evidence for a crucial role of the arterial wall in control of atherosclerosis susceptibility, Circulation. 2006;114:2382–2389. [DOI] [PubMed] [Google Scholar]
- [49].Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ: Compensatory enlargement of human atherosclerotic coronary arteries, N Engl J Med. 1987;316:1371–1375. [DOI] [PubMed] [Google Scholar]
- [50].Kumar A, Lindner V: Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow, Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. [DOI] [PubMed] [Google Scholar]
- [51].Wagenseil JE, Mecham RP: Vascular extracellular matrix and arterial mechanics, Physiol Rev. 2009;89:957–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shi W, Brown MD, Wang X, Wong J, Kallmes DF, Matsumoto AH, Helm GA, Drake TA, Lusis AJ: Genetic backgrounds but not sizes of atherosclerotic lesions determine medial destruction in the aortic root of apolipoprotein E-deficient mice, Arterioscler Thromb Vasc Biol. 2003;23:1901–1906. [DOI] [PubMed] [Google Scholar]
- [53].Hayashi K, Makino A, Kakoi D: Remodeling of arterial wall: Response to changes in both blood flow and blood pressure, J Mech Behav Biomed Mater. 2018;77:475–484. [DOI] [PubMed] [Google Scholar]
- [54].Ota H, Magalhaes MA, Torguson R, Negi S, Kollmer MR, Spad MA, Gai J, Satler LF, Suddath WO, Pichard AD, Waksman R: The influence of lipid-containing plaque composition assessed by near-infrared spectroscopy on coronary lesion remodelling, Eur Heart J Cardiovasc Imaging. 2016;17:821–831. [DOI] [PubMed] [Google Scholar]
- [55].Varnava AM, Mills PG, Davies MJ: Relationship between coronary artery remodeling and plaque vulnerability, Circulation. 2002;105:939–943. [DOI] [PubMed] [Google Scholar]
- [56].Shibata M, Ohtani R, Ihara M, Tomimoto H: White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion, Stroke. 2004;35:2598–2603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.