Abstract
Purpose
CIPN is a common, debilitating, and dose-limiting side effect of chemotherapy. Here, we describe characteristics of patients with CIPN using both patient-reported outcomes (PRO) and quantitative sensory testing (QST).
Methods
Breast cancer survivors with persistent moderate to severe CIPN defined by a rating of 4 or greater on a 0–10 Numeric Rating Scale (NRS) from two ongoing clinical trials were included. PROs included the Neuropathic Pain Scale (NPS) and Functional Assessment of Cancer Therapy-Gynecologic Oncology Group/Neurotoxicity (FACT/GOG-Ntx). QST included tactile and vibration detection threshold measurements. Data were analyzed using descriptive statistics and Spearman correlation coefficients.
Results
49 female patients with a mean age of 61 years were assessed; 63% were Caucasian. Mean NRS scores were 4.2, 5.7, and 4.3 on 0–10 scale for pain, numbness, and tingling, respectively. Mean NPS score was 41.0 on a 0–100 scale, and the mean FACT/GOG-Ntx score was 25.8 on a 0–44 scale. QST showed mild to moderate impairments in tactile and vibration perception. The FACT/GOG-Ntx subscale for numbness was negatively correlated with tactile and vibration thresholds in both hands and feet (both p < 0.05). NPS was positively correlated with tactile thresholds in the hands and feet (p < 0.05).
Conclusion
Patients with moderate to severe CIPN report moderate pain, numbness, and tingling, and exhibit reduced tactile and vibration perception on QST. Weak to moderate correlations were observed between PRO and QST. These data suggest that QST outcomes are associated with CIPN symptoms and may be useful in helping monitor and manage CIPN treatment.
Keywords: Breast cancer, Chemotherapy, Peripheral neuropathy, Patient-reported outcomes, Quantitative sensory testing
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most common and significant side effects of taxane-based chemotherapy [1]. Taxanes are an established treatment that greatly improves survival rates among breast cancer patients [2, 3]. However, as a neurotoxic agent, taxanes often cause CIPN symptoms that significantly impact patient quality of life and functional ability [4–6]. Though symptoms may subside upon completion of chemotherapy in some, 30–60% of patients have persistent CIPN beyond treatment [1, 7]. Taxane-induced peripheral neuropathy can manifest in any of the three divisions of the peripheral nervous system—motor, sensory, and autonomic—depending on the site of action. Thus, affected patients can demonstrate a large variance in symptom presentation such as numbness, tingling, and pain in a ‘glove and stocking’ distribution, decreased positional, vibratory, and temperature perception, as well as weakness [8–11]. Due to the large variability in symptom presentation, CIPN can be difficult to characterize and monitor in breast cancer patients.
Identifying symptoms and accurately assessing functional disability are crucial for achieving timely intervention and improving the management of CIPN. Currently, CIPN symptoms are graded mostly based on patient-reported outcomes (PRO), which are prone to subjectivity and inconsistency. By comparison, quantitative sensory testing (QST) semi-quantifies gains and losses in somatosensory function by measuring detection and pain thresholds for standardized and systemically delivered sensory input [12]. For example, thinly myelinated A fibers (Aδ) are assessed by cool temperature and pinprick stimuli, and larger fibers (Aα and Aβ) are assessed through mechanical touch (e.g., von Frey filaments) and vibratory stimuli [13]. Several studies have established the validity of using QST in the assessment of chronic pain conditions as well as in the analysis of pain mechanisms [14–16]. A few studies found significant associations between subjective neuropathic pain reports with QST measures in patients with diabetic neuropathy, HIV neuropathy, chronic ischemic neuropathy in Peripheral Artery Disease, and trigeminal neuralgia [17–20]. These findings suggest that using PROs alongside QST may provide a more comprehensive and accurate approach in assessing CIPN symptoms for improved monitoring of treatment response in clinical care and in clinical trials for CIPN interventions. However, further study is needed to understand how QST can be integrated with PROs for the management of CIPN. A previous study found significant association between PROs and QST measures in breast cancer survivors treated with adjuvant paclitaxel chemotherapy [21]. However, the study only compared one component of PROs (FACT/GOG-Ntx) with QST in a population with varying degrees of CIPN. In this study, we undertook a cross-sectional analysis of PROs (NRS, NPS, and FACT/GOG-Ntx) and QST in breast cancer survivors with moderate to severe CIPN symptoms to determine the efficacy of employing both methods to improve CIPN symptom management.
Methods
Study participants
Baseline data of breast cancer survivors with moderate to severe CIPN from two ongoing Institutional Review Board (IRB) approved clinical trials at Memorial Sloan Kettering Cancer Center (MSKCC) were extracted for cross-sectional analysis. Moderate to severe CIPN was defined as patient-reported symptoms of pain, numbness, or tingling that were rated four or greater on a (0–10) numeric rating scale (NRS) at least 3 months after completion of neurotoxic chemotherapy. English-speakers over the age of 18 with no evidence of disease following treatment for stage I–III breast cancer, and not taking any anti-neuropathy medications, were eligible for the study. In total, our analysis included 49 eligible female patients who provided informed consent and completed PRO questionnaires and QST. Both trials were approved by MSKCC IRB and registered at clinicaltrials.gov (, ).
Patient-reported outcomes
CIPN severity was assessed using a three-item questionnaire that uses a 0–10 NRS to quantify the patient’s CIPN symptoms (pain, numbness, tingling) within the last 24 h of completing the survey.
Neuropathic Pain Scale (NPS) is a ten-item questionnaire that uses a 0–10 numeric rating scale to quantify global pain intensity, unpleasantness, and eight other descriptive qualities of neuropathic pain [22, 23]. NPS distinguishes neuropathic pain from non-neuropathic pain [24]. A higher NPS score indicates more severe symptoms with a score range of 0–100. This measure has been validated in patients with neuropathic pain and specifically in patients with taxane-induced CIPN [22, 25]. In this study, patients with ratings between 0 and 30 were considered to have mild pain, ratings between 31 and 70 were considered to have moderate pain, and ratings between 71 and 100 were considered to have severe pain.
Functional Assessment of Cancer Therapy-Gynecologic Oncology Group/Neurotoxicity subscale (FACT/GOG-Ntx) is a questionnaire that assesses sensory, motor, and hearing-related neurotoxic symptoms. There are 11 questions in total, each rated on a 0–4 scale. A lower score indicates more functional disability and more severe neurotoxicity with a 0–44 total score range. This questionnaire has been validated with its reliability assessed in multiple studies of CIPN [26–28]. In this study, symptom severity was categorized based on patient response to each individual question with a score of 0–1 considered mild, 2 considered moderate, and 3–4 considered severe.
QST
A trained research study assistant (RSA) conducted QST in a quiet room with minimal environmental stimuli. Participants were asked not to take any pain, stimulant, or sedative medications at least 12 h prior to the testing session.
Tactile Detection Threshold (TDT) was determined using a set of 20 Von Frey filaments (Touch Test Sensory Evaluators, North Coast Medical, Inc), calibrated to generate a force in grams (g) within a 5% standard deviation. The subjects were asked to close their eyes so that the examination was not influenced by visual input. TDT was assessed at the dorsum of the distal interphalangeal joint of the right and left middle fingers and the right and left big toes. Starting with the smallest size filament, an RSA touched the testing site at 90° angle until the filament bent. The RSA then repeated with ascending filament size until the patient reported tactile sensation at the test site. The force at which touch is perceived was recorded as the tactile threshold [29]. Thresholds for categories of tactile perception were defined by the filament manufacturer as follows: Normal (0.008–0.07 g); Diminished light touch (0.16–0.4 g); Diminished protective sensation (0.6–2 g); Loss of protective sensation (4–180 g); Deep pressure sensation only (300 g) [14]. From this point forward, we will use the following categorical labels: Normal Perception Threshold (0.008–0.07 g), Mild Loss of Perception (0.16–0.4 g), Moderate Loss of Perception (0.6–2 g), and Severe Loss of Perception (4–300 g) for consistency in discussing tactile and vibration thresholds.
Vibration Detection Threshold (VDT) was assessed by a hand-held biothesiometer (Bio-Medical Instrument Company; Newbury, Ohio) at the dorsum of the distal interphalangeal joints of the right and left index fingers and the right and left big toes. The amplitude of device vibration (microns) was gradually increased (1 V/s) until participants first perceived vibratory sensation. Average of three perception thresholds at the dorsal interphalangeal joint of the dominant big toe was recorded as the vibration threshold [30]. The biothesiometer operating manual defines vibration perception for normal subjects to be at 0.42 microns on the index finger and 0.84 microns at the big toe. Based off the distribution of thresholds seen in the study, the following categories for vibration were defined by study authors: Mild Loss of Perception (index finger 0.43–4.0 microns, big toe 0.84–4.0 microns), Moderate Loss of Perception (index finger > 4 microns, big toe 4.0–11.8 microns), and Severe Loss of Perception (only observed at big toe, > 11.8 microns).
Biostatistics analysis
We performed statistical analyses using STATA 15.0 (Stata-Corp, College Station, TX). We summarized and reported demographic and clinical variables as well as study outcomes using standard descriptive statistics. Left- and rightsided QST measurements were averaged since left and right are not distinguished in PRO reports. Spearman’s rank-order correlation was used to determine the association between various PRO scores and QST thresholds for hands and feet, including NPS total score with TT, NPS total score with VT, FACT/GOG-Ntx numbness and tingling subscore items with TT, and FACT/GOG-Ntx numbness and tingling subscore items with VT.
Results
Study participants
Baseline data for 49 patients enrolled in both ongoing clinical trials who completed PRO questionnaires and QST between July 2017 and June 2018 were included. The mean subject age was 61.6 years (range 35.5–86.0) and 63.3% of subjects self-identified as Caucasian. The average BMI of subjects was 27.8 (range 19.1–44.7). 89.8% of subjects received taxane-based therapy and 10.2% received both taxane and platinum-based chemotherapy agents. The median time from chemotherapy completion is 3 (0.26–18) years. Baseline demographic and clinical characteristics of participants are shown in Table 1.
Table 1.
Demographic and clinical characteristics of women after treatment of breast cancer (n = 49)
| Mean age (SD, range) | 61.6 | (10.1, 35.5–86.0) |
| Mean BMI (SD, range) | 27.8 | (5.2, 19.1–44.7) |
| Race [N (%)] | ||
| White | 31 | (63.3%) |
| Black or African American | 9 | (18.4%) |
| Asian | 5 | (10.2%) |
| Other | 1 | (2%) |
| Unknown | 3 | (6.1%) |
| Chemotherapy [N (%)] | ||
| Taxane-based only | 44 | (89.8%) |
| Taxane and platinum combined | 5 | (10.2%) |
| Time (year) from Chemotherapy Completion (Median, Range) | 3 | (0.26–18) |
| Mean subjective measures (SD, Range) | ||
| NRS pain | 4.2 | (2.6, 0–8) |
| NRS numbness | 5.7 | (2.3, 0–10) |
| NRS tingling | 4.4 | (2.7, 0–10) |
| NPS | 41.0 | (22.4, 0–84) |
| FACT/GOG-Ntx | 25.8 | (6.8, 8–40) |
PROs
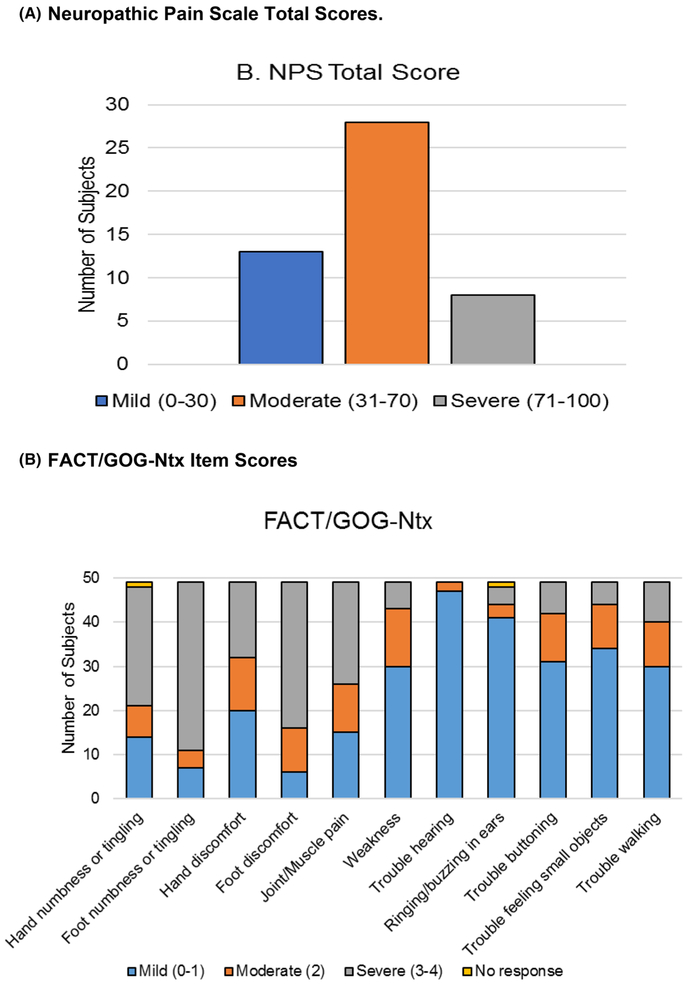
Among 49 participants, the mean NRS scores (0–10 scale) were 4.2 (SD 2.6) for pain, 5.7 (SD 2.3) for numbness, and 4.4 (SD 2.7) for tingling. The mean total NPS score (0–100 scale) for neuropathic pain was 41.0 (SD 22.4) with 57.1% of patients reporting moderate CIPN pain (scores of 31–70) and 16.3% reporting severe CIPN pain (scores of 71–100) (Table 1 and Fig. 1a). The mean FACT/GOG-Ntx score assessing neurotoxicity was 25.8 (SD 6.8) on a 0–44 scale, with lower scores indicate worse CIPN symptoms (Table 1). The FACT/GOG-Ntx subscores indicate that 69.4% and 85.7% of participants experienced moderate to severe numbness or tingling in the hands and feet, respectively. 59.2% and 87.8% of participants reported moderate to severe discomfort in the hands and feet, respectively (Fig. 1b). Up to 40% patients had moderate to severe functional disabilities including trouble buttoning (36.7%), trouble feeling small objects (30.6%), and trouble walking (38.8%).
Fig. 1.
Patient-reported outcomes
QST
Tactile perception
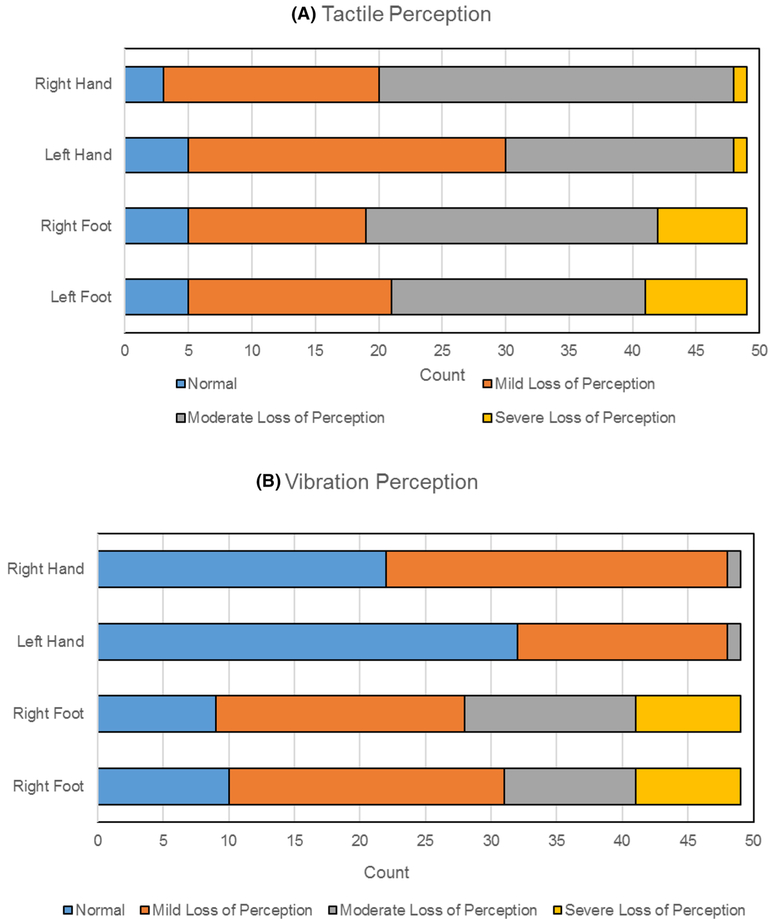
10% of patients had normal tactile perception in upper and lower extremities. Mild loss of tactile perception (or diminished light touch) was seen in 34.7% and 51.2% of right and left hands, respectively, and 28.6% and 32.7% of right and left toes, respectively. Majority of patients had moderate loss (or diminished protective sensation): 57.1% and 36.7% of right and left hands, respectively, and 46.9% and 40.8% of right and left toes, respectively. In our study group, severe loss of tactile perception was observed only in 2% of right and left middle fingers but in 14.3% and 16.3% of patients in the right and left big toe, respectively (Fig. 2a).
Fig. 2.
Quantitative sensory testing outcomes
Vibration perception
Loss of vibration perception was worse in lower extremities compared to upper extremities. Vibration perception was normal in 45% of right and 65% of left finger, whereas vibration perception was preserved in only 18% of right and 20% of left big toe. Mild loss of vibration was observed in 53% and 33% of right and left fingers, respectively, versus 39% and 43% in right and left toes, respectively. Moderate losses of vibration were seen in 2% of both hands, with no severe loss detected in the upper extremities. In contrast, 27% and 20% of right and left toes had moderate loss of vibration, respectively, and 16% in both left and right toes had severe vibration perception loss (Fig. 2b).
Correlation
Statistically significant, weak to moderate correlations were found between PRO and QST measures (Table 2). A negative correlation was found between tactile perception and FACT/GOG-Ntx numbness scores in both upper and lower extremities (rs= − 0.30, rs= − 0.32, respectively, with p ≤ 0.05). A negative correlation was found between vibration perception and FACT/GOG-Ntx numbness in both upper and lower extremities (rs = − 0.41, rs = − 0.41, respectively, with p ≤ 0.05) as well. There was a positive correlation between tactile perception and NPS scores in both upper and lower extremities (rs = 0.30, rs = 0.29, respectively, with p ≤ 0.05). No statistically significant correlation was identified between NPS and vibration perception.
Table 2.
Spearman’s ρ correlation coefficient
| Subjective PROs | Objective QST | |||
|---|---|---|---|---|
| Tactile | Vibration | |||
| Hand | Feet | Hand | Feet | |
| FACT-GOG/NTx numbness sub-score | −0.30 (p = 0.038) | −0.33 (p = 0.023) | −0.41 (p = 0.0039) | −0.41 (p = 0.0040) |
| NPS total score | 0.30 (p = 0.035) | 0.29 (p = 0.043) | 0.28 (p = 0.059) | 0.092 (p = 0.54) |
Discussion
Chemotherapy-induced peripheral neuropathy is a common and disabling adverse effect of lifesaving cancer treatment. Consistent with past clinical reports, our study showed significantly impacted daily functionality in breast cancer patients with moderate to severe CIPN [31]. Specifically, 30.6% to 38.8% of study participants were found to have moderate functional disability as measured by FACT/GOG-Ntx with trouble buttoning, feeling small objects, and walking. These findings emphasize the prevalence and magnitude of impact CIPN symptoms have on patients’ lives and the need for improved diagnostic and management techniques.
While current management of CIPN has focused mainly on subjective reports from patients, this is one of the first studies to establish baseline somatosensory features of CIPN in breast cancer survivors using both PRO and QST measurements. Our study showed that breast cancer patients with moderate to severe CIPN can be characterized by having moderate pain on NRS, moderate numbness and tingling based on FACT/GOG-Ntx subscales, and reduced tactile and vibration perception measured by QST. Furthermore, our study established a correlation between self-reported symptoms of CIPN with quantitative measures of sensory function. Weak to moderate negative correlations between FACT/GOG-Ntx and QST tactile and vibration thresholds were seen in all extremities, indicating that increased functional disability is associated with increased sensory loss. A positive correlation was also observed between NPS report and tactile thresholds, suggesting that increased neuropathic pain is associated with increased loss of tactile perception. The slightly stronger correlation between FACT/GOG-Ntx and QST compared to NPS and QST could be because FACT/GOG-Ntx assesses multiple CIPN symptoms including pain, tingling, numbness, and functional disability while NPS assesses primarily pain.
A previous study of breast cancer patients treated with adjuvant paclitaxel chemotherapy found a similar association (− 0.333, p = 0.026) between QST vibration thresholds and FACT/GOG-Ntx assessed numbness in the hands [21]. Interestingly, our study found a stronger correlation (− 0.41, p = 0.0039) as well as a significant correlation between FACT-Ntx and tactile QST measures (− 0.33, p = 0.023), which was not found in their study. Moreover, our study found vibration perception to be more diminished in feet compared to hands. This finding is consistent with the fact that taxane-based CIPN is known to most commonly cause a length-dependent sensory axonal neuropathy in large nerve fibers, which manifests as a loss in vibration perception in the extremities and sensory ataxia. This is likely due to the “dying back” degenerative nature of the distal terminal of affected sensory neurons [32]. Both length dependency and propensity towards large fiber disruption is evident in our QST findings and is further consistent with studies reporting patients with CIPN to be at significantly higher risk of falling [33].
Although the exact pathogenesis of CIPN is not fully understood, it is well appreciated that the underlying cause of CIPN is multifactorial with various sites of involvement [34]. Physical neuronal damage contributes to functional impairment and hyperexcitability of peripheral nerves through oxidative stress, neuroinflammation, apoptosis, and electrophysiological disturbances [35]. Taxane-based chemotherapy targets cancer cells by inhibiting microtubule depolymerization and causing mitotic arrest [36]. Prospective experimental studies have alluded that the microtubule disruption extends to peripheral sensory neurons [37]. Further histological examination of peripheral nerve sections treated with taxane-based agents showed neuronal apoptosis caused by chemotherapy-induced Ca2+ dysregulation specifically in group A and C fibers [38, 39]. As decreased mechanical and vibration sensation suggests abnormalities in large Aβ fibers [40], our findings of diminished tactile and vibration perception in many of our subjects are consistent with the proposed site of neurotoxicity.
Our study is limited by a small sample size. Furthermore, as only patients with moderate to severe symptoms of CIPN were selected for this cross-sectional analysis, generalizability might be limited. However, patients with moderate to severe CIPN represent approximately 27.7% of breast cancer patients who have received adjuvant chemotherapy [1]. As there is significant variability in CIPN presentations between patients, direct correlations are difficult to generalize within a study group. Ideally, baseline symptoms and functionality for each individual patient before treatment would be compared to reassessed levels post chemotherapy. A notable limitation was that since NRS assesses symptom severity only within the past 24 h, five study patients reported low NRS scores at the time of survey. Though these patients previously experienced CIPN symptom severity categorized by NRS score > 4, their symptoms were not severe within 24 h of the survey. The lower NRS scores reported at the time of survey could possibly skew the strength of our correlation between PRO and QST outcomes. Moving forward, multiple NRS scores can be averaged to create a more comprehensive PRO representation for each patient. In addition, biomarkers such as plasma cytokines could be used to assess how local inflammation at nerve endings relates to abnormal QST results [41]. Furthermore, a larger sample size would also help elucidate any meaningful differences in QST thresholds between left and right testing sites and provide better insights into QST correlations with PROs. Methodologically, QST is dependent on the test subject’s attention and active participation. While this paper only reports a single, cross-sectional QST measurement, further work will be needed to understand test reliability and between-tester variation in QST [42]. Finally, though numerous studies support the validity of FACT-Ntx, the questionnaire does not address autonomic functions such as dizziness after position change and erection disorder. CIPN20 is a more comprehensive questionnaire that assesses motor, sensory, and autonomic functionality and can be included in future studies to potentially demonstrate a stronger correlation with QST.
Despite these limitations, this study showed that both PROs and QST measurements can be used together to improve identification of CIPN symptoms and monitoring of treatment response. Clinical trials evaluating the treatment of CIPN commonly use the Common Terminology Criteria for Adverse Events (CTCAE) to measure the degree of neuropathy. However, the lack of clearly defined criteria leads to inconsistent interpretations of grading and inter-observer variability of 46% [43]. By comparison, PRO assessments provide more information on the effect of chemotherapy on neuropathic symptoms. However, PRO assessments are still limited by subjectivity and inter-subject variability. By demonstrating a significant association between PROs and QST measurements, our study suggests that QST can be used in conjunction with subjective reports as more accurate way to assess CIPN symptom severity. This is demonstrated by the fact that by using both PROs and QST measurements, we were able to more accurately established baseline somatosensory features in patients with moderate to severe CIPN symptoms. This is particularly helpful in designing CIPN targeted clinical trials as both PROs and QST can be used to select appropriate patient populations for specific interventions as well as outcome endpoints. Finally, successful identification of CIPN somatosensory phenotypes has enormous implications for improving diagnosis and management of CIPN and ultimately enhancing patients’ quality of life.
Funding
This work was supported by a Memorial Sloan Kettering Cancer Center P30 Grant [P30-CA008748], the Translational Research and Integrative Medicine Fund at Memorial Sloan Kettering Cancer Center, and the Frueauff Foundation. The funding sources had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of Memorial Sloan Kettering Cancer Institutional Review Board (IRB) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study fully complies with the current law of the country in which it was conducted.
Informed consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E 3rd, Schilsky RL, Wood WC, Muss HB, Norton L (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983 [DOI] [PubMed] [Google Scholar]
- 3.De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L, Pennacchio R, Criscitiello C, Montanino A, Limite G, Bianco AR, De Placido S (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53 [DOI] [PubMed] [Google Scholar]
- 4.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. PAIN® 155:2461–2470 [DOI] [PubMed] [Google Scholar]
- 5.Sun S, Wang LP, Zhang J, Yang XY, Zhang QL, Jia Z, Hu XC, Wang BY (2012) Phase II study of oxaliplatin plus leucovorin and 5-fluorouracil in heavily pretreated metastatic breast cancer patients. Med Oncol 29:418–424 [DOI] [PubMed] [Google Scholar]
- 6.Beijers AJM, Mols F, Vreugdenhil G (2014) A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 22:1999–2007 [DOI] [PubMed] [Google Scholar]
- 7.Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, Wade JL 3rd, Brufsky AM, Julian TB, Margolese RG, McCarron EC, Ganz PA (2018) Long-term Peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst 110(2):djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakitas MA (2007) Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res 56:323–331 [DOI] [PubMed] [Google Scholar]
- 9.Pachman DR, Barton DL, Watson JC, Loprinzi CL (2011) Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 90:377–387 [DOI] [PubMed] [Google Scholar]
- 10.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66:218–228 [DOI] [PubMed] [Google Scholar]
- 12.Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M (2009) Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 25:641–647 [DOI] [PubMed] [Google Scholar]
- 13.Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede R-D, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D (2013) Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. PAIN® 154:1807–1819 [DOI] [PubMed] [Google Scholar]
- 14.Felix ER, Widerstrom-Noga EG (2009) Reliability and validity of quantitative sensory testing in persons with spinal cord injury and neuropathic pain. J Rehabil Res Dev 46:69–83 [PubMed] [Google Scholar]
- 15.Kanzawa-Lee GA, Harte SE, Bridges CM, Brummett C, Clauw DJ, Williams DA, Knoerl R, Lavoie Smith EM (2018) Pressure pain phenotypes in women before breast cancer treatment. Oncol Nurs Forum 45:483–495 [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Almeida Y, Fillingim RB (2014) Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 15:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaaskelainen SK, Teerijoki-Oksa T, Forssell H (2005) Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain 117:349–357 [DOI] [PubMed] [Google Scholar]
- 18.Lang PM, Ruger LJ, Abahji T, Hoffmann U, Crispin A, Irnich D (2009) Correlation between quantitative sensory testing and questionnaires on neuropathic pain for chronic ischemic pain in peripheral arterial disease. Schmerz 23(251–254):256–258 [DOI] [PubMed] [Google Scholar]
- 19.Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, Forer L, Belobradkova J, Olsovsky J, Weber P, Dusek L, Jarkovsky J, Bednarik J (2017) Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain 158:2340–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McArthur JH (1998) The reliability and validity of the subjective peripheral neuropathy screen. J Assoc Nurses AIDS Care 9:84–94 [DOI] [PubMed] [Google Scholar]
- 21.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD (2011) Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 125:767–774 [DOI] [PubMed] [Google Scholar]
- 22.Galer BS, Jensen MP (1997) Development and preliminary validation of a pain measure specific to neuropathic pain: the neuropathic pain scale. Neurology 48:332–338 [DOI] [PubMed] [Google Scholar]
- 23.Jensen MP, Friedman M, Bonzo D, Richards P (2006) The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clin J Pain 22:97–103 [DOI] [PubMed] [Google Scholar]
- 24.Fishbain DA, Lewis JE, Cutler R, Cole B, Rosomoff HL, Rosomoff RS (2008) Can the neuropathic pain scale discriminate between non-neuropathic and neuropathic pain? Pain Med 9:149–160 [DOI] [PubMed] [Google Scholar]
- 25.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL (2011) The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncol Nurs Forum 38:133–142 [DOI] [PubMed] [Google Scholar]
- 26.Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D (2003) Psychometric evaluation of the functional assessment of cancer therapy/gynecologic oncology groupneurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13:741–748 [DOI] [PubMed] [Google Scholar]
- 27.Huang HQ, Brady MF, Cella D, Fleming G (2007) Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxelinduced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer 17:387–393 [DOI] [PubMed] [Google Scholar]
- 28.Castellino SM, Rodday AM, Pei Q, Bush R, Keller FG, Henderson TO, Kelly KM, Cella D, Parsons SK (2019) Performance of FACT-GOG-Ntx to assess chemotherapy-induced peripheral neuropathy (CIPN) in pediatric Hodgkin lymphoma (HL) patients. J Clin Oncol 37:10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drobek W, De Laat A, Schoenaers J (2001) Tactile threshold and pressure pain threshold during treatment of orofacial pain: an explorative study. Clin Oral Invest 5:185–193 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg JM, Lindblom U (1979) Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatry 42:793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, Ohsumi S, Makino H, Katsumata N, Kuranami M, Suemasu K, Watanabe T, Hausheer FH (2012) Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Support Care Cancer 20:3355–3364 [DOI] [PubMed] [Google Scholar]
- 32.Fukuda Y, Li Y, Segal RA (2017) A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci 11:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S (2017) Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol 35:2604–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G (2000) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21:389–393 [PubMed] [Google Scholar]
- 35.Areti A, Yerra VG, Naidu V, Kumar A (2014) Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malacrida A, Meregalli C, RodriguezMenendez V, Nicolini G (2019) Chemotherapy-induced peripheral neuropathy and changes in cytoskeleton. Int J Mol Sci 20:2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shemesh OA, Spira ME (2010) Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta Neuropathol 119:235–248 [DOI] [PubMed] [Google Scholar]
- 38.Jaggi AS, Singh N (2012) Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 291:1–9 [DOI] [PubMed] [Google Scholar]
- 39.Xiao WH, Bennett GJ (2008) Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain 135:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D (2013) Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 154:1807–1819 [DOI] [PubMed] [Google Scholar]
- 41.Schrepf A, Bradley CS, O’Donnell M, Luo Y, Harte SE, Kreder K, Lutgendorf S, Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research (2015) Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun 49:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B (2006) Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain 123:231–243 [DOI] [PubMed] [Google Scholar]
- 43.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK (1998) Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 9:739–744 [DOI] [PubMed] [Google Scholar]