Abstract
Objective:
The ε4 allele of the apolipoprotein E (APOE) gene increases risk for cognitive decline in normal and pathologic aging. However, precisely how APOE ε4 exerts its negative impact on cognition is poorly understood. The present study aimed to determine whether APOE genotype (ε4+ vs. ε4-) modifies the interaction of medial temporal lobe (MTL) resting cerebral blood flow (CBF) and brain structure (cortical thickness [CT], volume [Vo]) on verbal memory performance.
Methods:
Multiple linear regression models were employed to investigate relationships between APOE genotype, arterial spin labeling MRI-measured CBF and FreeSurfer-based CT and Vo in four MTL regions of interest (left and right entorhinal cortex and hippocampus), and verbal memory performance among a sample of 117 cognitively healthy older adults (41 ε4+, 78 ε4-) between the ages of 64 and 89 (mean age=73).
Results:
Results indicated that APOE genotype modified the interaction of CBF and CT on memory in the left entorhinal cortex, such that the relationship between entorhinal CBF and memory was negative (lower CBF was associated with better memory) in non-carriers with higher entorhinal CT, positive (higher CBF was associated with better memory) in non-carriers with lower entorhinal CT, and negative (higher CBF was associated with worse memory) in ε4 carriers with lower entorhinal CT.
Conclusions:
Findings suggest that older adult APOE ε4 carriers may experience vascular dysregulation and concomitant morphological alterations in the medial temporal lobe that interact to negatively affect memory even in the absence overt clinical symptoms, providing potential insight into the mechanistic link between APOE ε4 and detriments in cognition. Moreover, findings suggest a distinct multimodal neural signature in ε4-carriers (higher CBF and lower CT in the entorhinal cortex) that could aid in the identification of candidates for future clinical trials aimed at preventing or slowing cognitive decline. Differential findings with respect to ε4-carriers and non-carriers are discussed in the context of neurovascular compensation.
Keywords: aging, Alzheimer’s disease, APOE ε4, cerebral blood flow, cognitive decline, cortical thickness
1. Introduction
With a rapidly growing older population, identifying risk factors and mechanisms of age-related cognitive decline represents one of the greatest challenges to improving the health and overall independence of older adults. The ε4 allele of the apolipoprotein E gene (APOE) confers risk for pathologic and normal age-related cognitive decline (Bretsky et al., 2003; Richard J. Caselli et al., 2009; Schiepers et al., 2012; Tsuang et al., 2013). Precisely how APOE ε4 exerts its negative impacts on cognition is still poorly understood, but is likely related to its role in a diverse range of biological processes including glucose metabolism, mitochondrial function, synaptic function, neurogenesis, tau phosphorylation, neuronal atrophy, neuroinflammation, and amyloid-β (Aβ) metabolism and aggregation (Kanekiyo, Xu, & Bu, 2014; C.-C. Liu, Kanekiyo, Xu, & Bu, 2013; Mahley & Rall, 2000). Interestingly, APOE ε4’s effects on cognition appear largely dependent on age, with young ε4 carriers equal to or outperforming non-carriers on a wide range of cognitive abilities (Jochemsen, Muller, van der Graaf, & Geerlings, 2012; Marchant, King, Tabet, & Rusted, 2010; Mondadori et al., 2007; Rusted et al., 2013), but older ε4 carriers demonstrating worse cognitive performance (Adamson et al., 2010; De Blasi et al., 2009; Honea, Vidoni, Harsha, & Burns, 2009; Kukolja, Thiel, Eggermann, Zerres, & Fink, 2010; Tuminello & Han, 2011) and accelerated age-related cognitive decline, most notably in episodic memory (Bretsky et al., 2003; R. J. Caselli et al., 2004; Richard J. Caselli et al., 2009; Hayden et al., 2009; C.-C. Liu et al., 2013; Schiepers et al., 2012; Whitehair et al., 2010). Identifying and characterizing APOE-related brain changes could offer insight into the mechanistic link between APOE ε4 genotype and cognitive decline.
APOE ε4 carriers demonstrate alterations in cerebral blood flow (CBF), or the rate of delivery of arterial blood to the capillary bed in a volume of tissue. CBF is an indirect measure of neural function (Buxton, 2009) that has been implicated in both normal aging and AD-related cognitive decline (Bertsch et al., 2009; Hays, Zlatar, & Wierenga, 2016; Heo et al., 2010), demonstrating reliable correlations with cognitive performance across the lifespan (Bangen et al., 2012; Bertsch et al., 2009; Okonkwo et al., 2014; Wierenga et al., 2012). APOE ε4 carriers demonstrate altered resting CBF across widespread medial temporal, frontal, and parietal regions (Tai et al., 2016; Wierenga, Hays, & Zlatar, 2014) and the temporal staging of CBF alteration appears to resemble APOE-related changes in cognition, with ε4 carriers exhibiting higher resting CBF than non-carriers in early adulthood and middle-age, but lower resting CBF in old age (Thambisetty, Beason-Held, An, Kraut, & Resnick, 2010; Wierenga et al., 2013). It has been suggested that higher brain perfusion among ε4 carriers reflects cerebrovascular compensation for the deleterious effects of APOE ε4 (e.g., impaired repair mechanisms, neurovascular disruption) and that lower brain perfusion reflects a breakdown of these compensatory mechanisms (Dai et al., 2009; Hays et al., 2016; Koizumi et al., 2018; Luckhaus et al., 2008; Wierenga et al., 2012). Notably, cross-sectional investigations of cerebral perfusion in cognitively normal older adult ε4 carriers have produced mixed results, with reports of both increased (Bangen et al., 2009; Thambisetty et al., 2010) and decreased CBF (Filippini et al., 2011; Wierenga et al., 2013; Zlatar et al., 2016), relative to non-carriers. Similarly, findings from studies exploring associations between APOE-related CBF alteration and cognition have also been mixed, with reports of both positive and negative relationships between CBF and cognition in older adult ε4 carriers (Bangen et al., 2012; Wierenga et al., 2012; Zlatar et al., 2016). To the extent that some of these discordant findings are due to differences in sample characteristics (e.g., definitions of cognitively normal, middle age versus older adults) or methodology (e.g., imaging modality limitations, statistical or experimental control of confounding variables, regions of interest versus voxel wise analysis, differing methods of partial volume correction), it is important to extend these prior studies using cutting-edge methodologies within large, well-characterized samples to further clarify associations between APOE, CBF, and cognition.
APOE genotype is also associated with alterations in brain morphology. More specifically, ε4 carriers demonstrate reduced cortical thickness in youth relative to non-carriers (Alexander et al., 2012; Ringman, Pope, & Salamon, 2010; Shaw et al., 2007) and accelerated gray matter atrophy in old age, most notably in medial temporal lobe (MTL) regions (Cohen, Small, Lalonde, Friz, & Sunderland, 2001; den Heijer et al., 2002; Jak, Houston, Nagel, Corey-Bloom, & Bondi, 2007; Tohgi et al., 1997). Moreover, ε4 carriers who demonstrate APOE-related alterations in brain structure also exhibit cognitive deficits, compared to non-carriers (Honea et al., 2009; Lind et al., 2006), suggesting that APOE ε4 genotype might confer risk for cognitive decline through changes in brain structure, perhaps through its moderating role in myelination, brain plasticity, and repair functions (Zhong & Weisgraber, 2009). However, it is difficult to reconcile findings of reduced structural integrity in young ε4 carriers with evidence of increased cognitive performance in this same group. Thus, it has been suggested that lower cortical reserve in young carriers of the ε4 allele may represent a neural endophenotype that increases susceptibility to neurodegeneration later in life (Shaw et al., 2007). Therefore, rather than having direct effects on cognition, APOE ε4-related changes in brain structure may interact with concomitant alterations in CBF, exacerbating detrimental effects on cognition. If correct, this could help explain findings of both positive and negative associations between CBF and cognition in ε4-carriers, as this relationship could vary as a function of structural integrity.
Together, this evidence suggests that cognitively normal APOE ε4 carriers experience alterations in CBF, brain structure, and memory, compared to non-carriers. Moreover, APOE-related alterations in MTL CBF and brain structure might interact to negatively impact memory performance in cognitively normal older adult carriers of the ε4 allele. No study, to our knowledge, has explored the interactions among these variables. To bridge this gap in the literature, the current study used arterial spin labeling (ASL) magnetic resonance imaging (MRI) and a high-resolution structural scan among a relatively large and well-characterized sample of cognitively normal older adults to determine whether APOE genotype (ε4+ vs. ε4-) modifies the interaction of medial temporal resting CBF and brain structure (cortical thickness [CT], volume [Vo]) on verbal memory performance. We predicted that the interaction of CBF and CT/Vo would differ by APOE genotype, such that increased CBF (reflecting neurovascular compensation) and reduced CT and/or Vo in MTL regions (entorhinal cortex [EC], hippocampus [Hc]) would interact to predict worse memory performance in APOE e4 carriers, but not in non-carriers. Exploratory analyses also investigated these same relationships in frontal brain regions implicated in memory encoding and retrieval (caudal anterior cingulate cortex [cACC], rostral middle frontal cortex [rMFC]). Such findings may help elucidate the underlying mechanisms of APOE ε4 effects on cognition and enable early intervention strategies aimed at preventing age-related cognitive decline.
2. Material and methods
2.1. Participants
See Table 1 for participant demographic and cognitive characteristics. Participants were community-dwelling older adult volunteers enrolled in a longitudinal study of normal aging at the VA San Diego Healthcare System (VASDHS). A total of 117 cognitively normal participants between the ages of 64 and 89 (mean age = 73.3, SD = 6.2) with available data were included in the current analyses. Forty-one participants were carriers of the APOE ε4 allele (ε3/ε4 = 37, ε4/ε4 = 4) and 76 were non-carriers (ε3/ε3 = 66, ε3/ε2 = 10). All participants were administered a full neuropsychological battery and an MRI scan (mean time interval between neuropsychological testing and MRI scan = 51 days). Normal cognitive function was determined based on a comprehensive neuropsychological test battery. Participants were excluded if performance on more than one measure within a cognitive domain was more than one standard deviation below age-appropriate norms, consistent with the empirically-derived criteria for diagnosis of mild cognitive impairment (MCI) developed by Jak and colleagues (Jak et al., 2009), or if overall performance on the Dementia Rating Scale (DRS) was more than 1 standard deviation below age-appropriate norms (see Sup Table S1 for specific cognitive tests, domains, and normative data; this resulted in the removal of 22 participants). Potential participants were also excluded if they had a history of severe head injury, uncontrolled hypertension, or a DSM-IV diagnosis of learning disability, attention deficit disorder, mood disorder, or substance abuse. In addition, participants were excluded if they had contraindications to MRI scanning such as ferrous implants or a pacemaker. All participants provided written informed consent prior to enrollment and data were collected in accordance with all ethical standards as stipulated by the UCSD and VASDHS institutional review board-approved procedures.
Table 1. Participant demographic, cognitive, and brain characteristics.
Note: APOE= Apolipoprotein E; SD= Standard deviation; t or χ2= either t-statistic or χ2; df= degrees of freedom; DRS= Mattis Dementia Rating Scale; WMS= Wechsler Memory Scale; LM= Logical Memory; SS= Scaled score; CVLT= California Verbal Learning Test; SD= Short delay; LD= Long delay; DKEFS= Delis-Kaplan Executive Function System; df= Degrees of freedom; + tests that were included in the memory composite; *significance at p<0.05; **significance at p< 0.01; *** significance at p< 0.001.
| APOE ε4− (N=76) | APOE ε4+ (N=41) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t or χ2 | df | p-value | |
| Age (years) | 72.98 | 6.03 | 74.02 | 6.62 | 0.83 | 115 | 0.406 |
| Gender (male/female) | 26/50 | -- | 13/28 | -- | 0.08 | 1 | 0.784 |
| Education (years) | 16.52 | 2.27 | 15.90 | 2.2 | 1.44 | 115 | 0.151 |
| NP and MRI time interval (days) | 51.09 | 70.6 | 53.29 | 73.3 | 0.15 | 114 | 0.874 |
| Stroke Risk % | 9.78 | 6.77 | 9.78 | 6.84 | 0.00 | 114 | 0.999 |
| Systolic Blood Pressure | 130.2 | 15.9 | 124.5 | 11.6 | 2.20 | 112 | 0.029* |
| Diastolic Blood Pressure | 76.97 | 8.51 | 71.66 | 9.23 | 2.97 | 109 | 0.004** |
| DRS Total Score | 140.95 | 2.82 | 140.66 | 3.03 | 0.52 | 115 | 0.608 |
| Memory Composite | 0.148 | 0.79 | −0.275 | 0.89 | 2.53 | 115 | 0.013* |
| WMS-R LM Immediate Recall+ | 31.92 | 6.27 | 28.63 | 5.83 | 2.77 | 115 | 0.007** |
| WMS-R LM Delayed Recall+ | 29.12 | 7.13 | 25.78 | 7.45 | 2.38 | 115 | 0.019* |
| CVLT-II List 1–5 total+ | 51.59 | 9.49 | 46.415 | 11.09 | 2.65 | 115 | 0.009** |
| CVLT-II SD Free Recall+ | 10.56 | 3.05 | 9.78 | 3.1 | 1.32 | 115 | 0.189 |
| CVLT-II LD Free Recall+ | 11.57 | 2.89 | 10.41 | 3.09 | 2.03 | 115 | 0.045* |
| DKEFS CW Inhibition | 58.55 | 10.78 | 67.68 | 14.29 | 3.87 | 114 | <0.001*** |
| DKEFS CW Inhibition Switch | 64.27 | 14.98 | 72.90 | 21.92 | 2.50 | 113 | 0.014* |
| Trail Making Test-A | 32.56 | 9.16 | 32.39 | 10.26 | 0.09 | 114 | 0.927 |
| Trail Making Test-B | 76.80 | 28.84 | 81.46 | 27.21 | 0.84 | 114 | 0.398 |
| DKEFS Letter Fluency | 46.12 | 13.83 | 49.12 | 12.13 | 1.16 | 115 | 0.245 |
| WISC-R Block Design | 47.07 | 7.93 | 45.22 | 6.901 | 1.25 | 114 | 0.213 |
| R Hippocampal CBF | 47.6 | 11.9 | 50.0 | 9.98 | 1.09 | 105 | 0.306 |
| R Hippocampal Vo | 3719 | 548 | 3670 | 474 | 0.47 | 115 | 0.633 |
| L Hippocampal CBF | 49.37 | 12.6 | 52.31 | 10.6 | 1.20 | 104 | 0.232 |
| L Hippocampal Vo | 3616 | 469 | 3529 | 492 | 0.93 | 114 | 0.350 |
| R Entorhinal CBF | 45.22 | 16.7 | 44.33 | 20.5 | 0.23 | 96 | 0.815 |
| R Entorhinal CT | 3.39 | 0.38 | 3.40 | 0.36 | 0.19 | 115 | 0.847 |
| L Entorhinal CBF | 45.69 | 13.6 | 39.55 | 15.8 | 2.01 | 94 | 0.047* |
| L Entorhinal CT | 3.20 | 0.32 | 3.21 | 0.34 | 0.08 | 114 | 0.934 |
| R Caudal Anterior Cingulate CBF | 54.8 | 13.6 | 57.88 | 11.1 | 1.16 | 102 | 0.246 |
| R Caudal Anterior Cingulate CT | 2.67 | 0.29 | 2.70 | 0.32 | 0.62 | 115 | 0.530 |
| L Caudal Anterior Cingulate CBF | 55.31 | 15.7 | 58.53 | 11.9 | 1.08 | 104 | 0.282 |
| L Caudal Anterior Cingulate CT | 2.49 | 0.25 | 2.63 | 0.36 | 2.53 | 115 | 0.012* |
| R Rostral Middle Frontal CBF | 61.11 | 14.7 | 62.78 | 10.1 | 0.61 | 106 | 0.541 |
| R Rostral Middle Frontal CT | 2.18 | 0.09 | 2.21 | 0.13 | 1.14 | 115 | 0.256 |
| L Rostral Middle Frontal CBF | 63.69 | 14.9 | 64.48 | 10.8 | 0.28 | 106 | 0.779 |
| L Rostral Middle Frontal CT | 2.25 | 0.13 | 2.22 | 0.14 | 1.11 | 115 | 0.266 |
2.2. Verbal memory composite
All participants were administered a full neuropsychological battery. A verbal memory composite score was created using trials 1–5, short delay free-recall, and long delay free-recall raw scores from the California Verbal Learning Test – Second Edition (CVLT-II), measuring word list recall, and the Logical Memory immediate and delayed recall subtests of the Wechsler Memory Scale-Revised (WMS-R), measuring story recall. These tests were selected based on results from a principal component analysis previously reported by our group on a similar sample of older adults (Wierenga et al., 2012). Verbal memory composite scores were derived by averaging the z-scores for each of the tests within the composite for the entire sample.
2.3. Vascular risk
All participants were administered the Framingham Stroke Risk Profile (FSRP), a common vascular risk index (D’Agostino, Wolf, Belanger, & Kannel, 1994; Wolf, D’Agostino, Belanger, & Kannel, 1991) that is based on the following risk factors: age, systolic blood pressure, antihypertensive medication, diabetes, cigarette smoking status, history of cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy. Risk of stroke (stroke risk %) is defined as the 10-year risk or predicted probability of incident stroke expressed as a percentage.
2.4. Apolipoprotein E genotyping
Genotyping was performed by the ADCS Biomarker Core at UCSD using real time PCR Restriction Fragment Length Polymorphism analysis. Genomic DNA was collected from participants using buccal swab and extracted using Qiamp DNA blood mini kit (Qiagen) followed by PCR amplification. Those with at least one ε4 allele (i.e., ε2/ε4, ε3/ε4, ε4/ε4) were classified as APOE e4 carriers (ε4+) and those without an ε4 allele (i.e., ε2/ε2, ε3/ε3, ε3/ε3) were classified as non-carriers (ε4-). Given that the APOE ε2 allele is thought to have protective effects (Suri, Heise, Trachtenberg, & Mackay, 2013), we ran all analyses including and excluding ε2 carriers and results did not differ. Therefore, results from the entire sample are presented. An exact test of Hardy-Weinberg Equilibrium was not significant (p= 0.443), suggesting that the distribution of APOE genotype in the sample included in this manuscript does not differ significantly from the expected distribution in the general population.
2.5. MRI acquisition
Imaging data were acquired on a GE Discovery MR750 3T whole body system with a body transmit coil and an 8-channel receive-only head coil at the University of California San Diego Center for Functional MRI. The structural brain sequence consisted of a high-resolution T1-weighted Fast Spoiled Gradient Recall (3D FSPGR) scan: 172 1 mm contiguous sagittal slices, FOV = 25 cm, TR = 8 ms, TE = 3.1 ms, flip angle = 12, T1 = 600 ms, 256 × 192 matrix, Bandwidth = 31.25 kHz, frequency direction = S-I, NEX = 1, scan time = 8 min and 13 sec. Resting CBF was acquired with the Multiphase Pseudocontinuous Arterial Spin Labeling (MPPCASL) sequence, which is optimized for robust CBF quantification (Jung, Wong, & Liu, 2010): tagging duration = 2 sec, post-labeling delay = 1.6 sec, TR = 4.2 sec, TE = 3 ms, reps = 64, FOV = 22 × 22 cm, 20 5 mm axial slices with a single shot spiral acquisition, collecting 8 cycles where each cycle consists of 8 images acquired with unique phase offsets, acquisition time = 4:46 minutes. A CSF calibration scan was also obtained using a spiral readout with TR = 4 sec and TE = 3.4 ms and comprised nine 90◦ excitation pulses which were turned off for the first eight repetitions to generate PD-weighted contrast (scan time: 36 sec) to obtain an estimate of the equilibrium magnetization of cerebral spinal fluid, which is used to convert the perfusion signal into calibrated CBF units (mL blood/100g tissue/min). Finally, a minimum contrast image was acquired to adjust for transmit and receive coil inhomogeneities. Two field map scans were also acquired and used for off-line field map correction to help correct for signal bunching and dropouts in the frontal/medial temporal lobes.
2.6. MRI pre-processing
2.6.1. CBF
ASL image processing was performed with Analysis of Functional NeuroImages (AFNI, afni.nimh.nih.gov)(Cox, 1996), FMRIB Software Library (FSL, Oxford, United Kingdom), and locally created Matlab scripts. Field map correction was applied to the ASL time series prior to co-registration to the middle time point to minimize the effects of participant motion. For each participant, a mean ASL difference image was formed from the average difference of the control and tag images and slice timing delays were accounted for in order to make the post-labeling delay time slice specific (T. T. Liu & Wong, 2005). This mean ASL image was then converted to absolute units of CBF (mL/100g tissue/min) using an estimate of the equilibrium magnetization of CSF as a reference signal (Chalela et al., 2000). This procedure resulted in a calibrated perfusion value for each voxel. Skull stripping of the high-resolution T1-weighted image was performed using AFNI’s 3dSkullStrip. Tissue segmentation was performed using FSL’s Automated Segmentation Tool (FAST) algorithm (http://fsl.fmrib.ox.ac.uk/fsl/) to define cerebrospinal fluid (CSF), gray matter (GM) and white matter (WM) regions. The high-resolution T1-weighted image and partial volume segmentations were registered to ASL space, and the CBF data were resampled to the same resolution as the T1-weighted image. The partial volume estimates were used to perform partial volume correction of the high-resolution CBF data using a linear regression approach with kernel size of 3 (Asllani et al., 2008; Zhao et al., 2017) as implemented by the ASL_file function in the BASIL toolset of FSL (Chappell, Groves, Whitcher, & Woolrich, 2009). The partial volume corrected gray matter CBF data were then resampled back to their native resolution and registered to FreeSurfer space. CBF values were extracted from each anatomically defined FreeSurfer ROI (see below for FreeSurfer methods). Quality assurance of ASL data was performed prior to analysis using outlier detection, inspection of CBF histograms, and visual checks of the CBF maps, with removal of values for regions with poor CBF map coverage. This resulted in removal of 4% of the data.
2.6.2. CT and Vo
Cortical thickness and volume analysis were performed using FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu), with the technical details of these procedures described in prior publications (Dale, Fischl, & Sereno, 1999; Dale & Sereno, 1993; B. Fischl & Dale, 2000; B. Fischl, Liu, & Dale, 2001; B. Fischl, Sereno, Tootell, & Dale, 1999; Bruce Fischl et al., 2002; Bruce Fischl, Salat, et al., 2004; Bruce Fischl, van der Kouwe, et al., 2004; Reuter, Rosas, & Fischl, 2010; Reuter, Schmansky, Rosas, & Fischl, 2012). Of note, FreeSurfer derives measures of cortical thickness for cortical/surface regions, whereas volume is derived for subcortical regions. As such, cortical thickness was extracted from the left and right EC, cACC, and rMFC and volume was extracted from the left and right Hc. A quality assurance protocol was performed before analysis using the ENIGMA guidelines (http://enigma.usc.edu/protocols/imaging-protocols) and included visual checks of the cortical segmentations and region-by-region removal of values for segmentations found to be incorrect. Histograms of all regions’ values were also computed for visual inspection. This resulted in removal of 1% of the data.
2.7. Statistical analyses
t-tests were used to compare groups on age, years of education, continuous vascular risk factors, brain variables (CBF, CT/Vo), and cognitive variables. χ2-tests were used to compare groups on sex and categorical vascular risk factors. CBF and CT/Vo were extracted from FreeSurfer-derived regions of interest (i.e., CT of the EC, cACC, and rMFC; Vo of the Hc) and multiple linear regression models were employed in R with the memory composite score as the dependent variable and APOE genotype and brain variables (CBF, CT/Vo) as independent variables. To test our a priori hypotheses, we explored the following 3-way interactions: 1) APOE genotype × EC CBF × EC CT, and 2) APOE genotype × Hc CBF × Hc Vo. A Bonferroni correction was applied to correct for multiple comparisons, and correlations were only considered significant at p<0.0125. Exploratory analyses also examined these same 3-way interactions in frontal regions: 1) APOE genotype × cACC CBF × cACC CT, and 2) APOE genotype × rMFC CBF × rMFC CT. Simples slopes analysis was utilized to determine the magnitude and direction of relationships at arbitrary values (+/− 1SD), whereas the Johnson-Neyman regions of significance method was applied to determine the magnitude and direction of relationships across a full range of values. Post hoc analyses explored two-way interaction models in regions that did not show a significant three-way interaction. Correlations between CBF and CT by region of interest (e.g., right EC CBF and right EC CT) were also examined post hoc. To assess model fit, post hoc analyses compared complex models including three-way interactions to simpler models including only two-way interactions.
All analyses statistically adjusted for the effects of age, sex, and education. Due to observed group differences, all analyses also adjusted for blood pressure (systolic blood pressure), and executive functioning performance (DKEFS color-word interference). Although groups did not differ on other measures of vascular risk (i.e., antihypertensive medication, diabetes, cigarette smoking status, history of cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy), we ran additional models that included these variables given their potential impacts on brain structure and CBF. When added to the models, none of these additional variables predicted significant variance nor did any model including these variables demonstrate better fit than simpler models that did not include these variables (all p > 0.05). Moreover, all results remained the same whether these variables were included in the models or not. Thus, aiming at parsimony, we present results from simpler models that did not include these additional variables. Analyses including measures of Hc Vo adjusted for the effects of total intracranial volume.
Non-multicollinearity between all independent variables was confirmed by application of the multicollinearity index VIF (all VIF<2), linearity was confirmed by residuals versus fits plots, normality was confirmed by Q-Q plots, non-heteroskedasticity was confirmed by scale location plots, and no influential cases were identified through examinations of residuals versus leverage plots.
3. Results
3.1. Group differences in demographic and assessment variables
Groups (ε4+, ε4−) did not differ significantly on age, sex, or years of education, nor did they differ significantly in the time interval between neuropsychological testing and neuroimaging (all ps > 0.05; see Table 1). With regard to cardiovascular health, groups did not differ significantly on stroke risk percent based on the FSRP, nor did they differ on any of the variables that comprise this measure (i.e., history of smoking, cardiovascular disease, diabetes, stroke, atrial fibrillation, left ventricular hypertrophy, antihypertensive therapy), aside from significant differences in systolic and diastolic blood pressure (t = 2.20, p = 0.029; t = 2.97, p = 0.004; respectively; see Table 1). With regard to cognition, APOE ε4 carriers demonstrated worse performance on the memory composite than did non-carriers (t = 2.53, p = 0.013). They also performed worse on four of the five subtests that were used to create the memory composite (i.e., CVLT-II trials 1–5, long delay free recall; WMS Logical Memory Immediate and Delayed Recall; see Table 1). With regard to tests in other cognitive domains, ε4 carriers performed worse on two subtests of the DKEFS Color Word Interference test (i.e., DKEFS Color Word Interference Inhibition and Inhibition Switching; all ps < 0.01; see Table 1), measuring cognitive flexibility and inhibitory control. No other group differences in cognitive performance were found (all ps > 0.05). With regard to measures of brain structure and function, APOE ε4 carriers exhibited lower left EC CBF than did non-carriers (t = 2.01, p = 0.047, see Table 1). No other significant group differences in resting CBF were found in brain regions of interest (i.e., Hc, cACC, rMFC; all ps > .05). APOE ε4 carriers demonstrated greater left cACC CT than did non-carriers (t = 2.53, p = 0.012; see Table 1). No other significant group differences in CT were found in brain regions of interest (i.e., EC, Hc, rMFC).
3.2. Three-way interaction of CBF, CT/Vo, and APOE, on memory performance
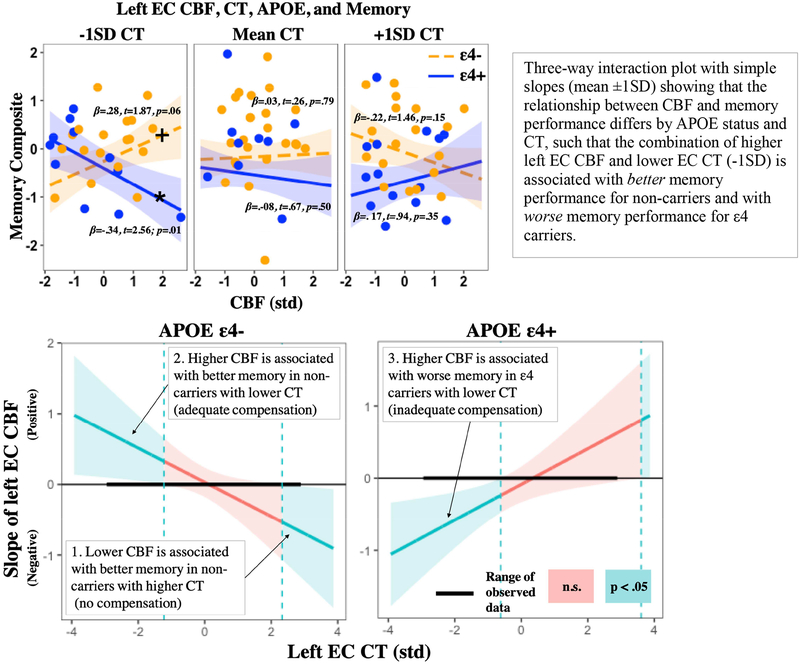
A priori analyses revealed a significant three-way interaction in the left EC, such that the interaction of left EC CBF and left EC CT on memory, differs by APOE genotype (see Table 2). Simples slopes revealed that the association between left EC CBF and memory was positive (for non-carriers with lower CT (-1SD) and negative for ε4 carriers with lower CT (-1SD see Figure 1). Johnson-Neyman intervals revealed that the relationship between entorhinal CBF and memory was negative (lower CBF was associated with better memory) in non-carriers with high EC CT (≥ 2.3SD), positive (higher CBF was associated with better memory) in non-carriers with low EC CT (≤ -1.2SD), and negative (higher CBF was associated with worse memory) in ε4 carriers with low EC CT (≤-1.0SD see Figure 1). Three-way interactions were not statistically significant in the right EC, left Hc, or right Hc (see Table 2); however, in the right EC and right Hc, there appeared to be slight trends toward the same three-way interaction (including directionality) found in the left EC (see Figures S1 and Figure S2). Exploratory analysis of frontal regions (i.e., cACC, rMFC) revealed no statistically significant three-way interactions (see Table 3).
Table 2. CBF, CT/Vo, APOE, and Memory.
Note: Only variables of interest are included in table; All continuous independent variables were standardized; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; Vo= Volume; EC= Entorhinal Cortex; Hc= Hippocampus; β= Standardized regression coefficient; CI= confidence interval; bs= Bootstrapped with 5000 replications; t= t-statistic. The 95% confidence interval of the coefficient was derived using the bootstrap bias-adjusted and accelerated bootstrap interval; +Denotes significant at p<0.10; *Denotes significance at p<0.05; **Denotes significance at p<0.01
| Independent Variable | β | 95% CI (bs) | s.e. (bs) | t | p-value |
|---|---|---|---|---|---|
| APOE | −0.375 | (−0.749, −0.019) | 0.167 | 2.176 | 0.032* |
| Left EC CT | 0.092 | (−0.093, 0.280) | 0.184 | 0.935 | 0.352 |
| Left EC CBF | 0.029 | (−0.183, 0.197) | 0.092 | 0.282 | 0.778 |
| APOE*Left EC CT | −0.228 | (−0.566, 0.084) | 0.165 | 1.345 | 0.182 |
| APOE*Left EC CBF | −0.115 | (−0.482, 0.176) | 0.156 | 0.705 | 0.482 |
| Left EC CT*Left EC CBF | −0.241 | (−0.423, −0.050) | 0.092 | 2.302 | 0.023* |
| APOE*Left EC CT*Left EC CBF | 0.488 | (0.220, 0.761) | 0.148 | 3.338 | 0.001** |
| APOE | −0.416 | (−0.793, −0.064) | 0.182 | 2.426 | 0.017* |
| Right EC CT | −0.039 | (−0.219, 0.149) | 0.092 | 0.409 | 0.683 |
| Right EC CBF | 0.038 | (−0.166, 0.277) | 0.114 | 0.357 | 0.722 |
| APOE*Right EC CT | 0.085 | (−0.303, 0.467) | 0.195 | 0.454 | 0.650 |
| APOE*Right EC CBF | −0.266 | (−0.590, 0.064) | 0.168 | 1.557 | 0.123 |
| Right EC CT*Right EC CBF | −0.117 | (−0.346, 0.145) | 0.124 | 0.970 | 0.334 |
| APOE*Right EC CT*Right EC | (−0.270, 0.526) | ||||
| CBF | 0.169 | 0.206 | 1.050 | 0.296 | |
| APOE | −0.303 | (−0.645, 0.062) | 0.178 | 1.821 | 0.071 + |
| Left Hc Vo | −0.007 | (−0.196, 0.171) | 0.094 | 0.071 | 0.943 |
| Left Hc CBF | 0.024 | (−0.172, 0.200) | 0.094 | 0.260 | 0.795 |
| APOE*Left Hc Vo | 0.011 | (−0.330, 0.371) | 0.178 | 0.070 | 0.943 |
| APOE*Left Hc CBF | −0.231 | (−0.711, 0.158) | 0.218 | 1.280 | 0.203 |
| Left Hc Vo*Left Hc CBF | 0.037 | (−0.151, 0.214) | 0.092 | 0.384 | 0.702 |
| APOE*Left Hc Vo*Left Hc CBF | 0.076 | (−0.354, 0.552) | 0.228 | 0.405 | 0.686 |
| APOE | −0.290 | (−0.786, 0.002) | 0.182 | 1.717 | 0.089+ |
| Right Hc Vo | −0.088 | (−0.097, 0.268) | 0.102 | 0.904 | 0.368 |
| Right Hc CBF | 0.079 | (−0.101, 0.263) | 0.092 | 0.864 | 0.390 |
| APOE*Right Hc Vo | 0.049 | (−0.307, 0.415) | 0.180 | 0.270 | 0.787 |
| APOE*Right Hc CBF | −0.343 | (−0.797, 0.032) | 0.205 | 1.844 | 0.068+ |
| Right HC Vo*Right Hc CBF | −0.152 | (−0.369, 0.018) | 0.096 | 1.784 | 0.077+ |
| APOE*Right Hc Vo*Right Hc CBF | 0.220 | (−0.154, 0.669) | 0.214 | 1.432 | 0.155 |
Figure 1. Left EC CBF, CT, APOE, and memory.
Note: EC= Entorhinal cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; SD= standard deviation; t= t-statistic; p= p-value; n.s.= not significant; +Denotes significance at p<0.10; *Denotes simple slope significance at p<0.05
Table 3. cACC CBF, CT, APOE, and memory.
Note: Only variables of interest are included in table; All continuous independent variables were standardized; cACC= Caudal anterior cingulate cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; L= Left; R= Right; β= Standardized regression coefficient; CI= confidence interval; bs= Bootstrapped with 5000 replications; t= t-statistic. The 95% confidence interval of the coefficient was derived using the bootstrap bias-adjusted and accelerated bootstrap interval; +Denotes significant at p<0.10; *Denotes significance at p<0.05
| Independent Variable | β | 95% CI (bs) | s.e. (bs) | t | p-value |
|---|---|---|---|---|---|
| APOE | 0.371 | (−0.762, 0.016) | 0.201 | 2.136 | 0.035* |
| R cACC CT | −0.236 | (−0.168, 0.393) | 0.141 | 2.039 | 0.044* |
| R cACC CBF | −0.088 | (−0.211, 0.292) | 0.126 | 0.461 | 0.646 |
| APOE* R cACC CT | 0.369 | (−0.739, −0.019) | 0.184 | 3.655 | 0.031* |
| APOE* R cACC CBF | 0.118 | (−0.558, 0.299) | 0.220 | 2.312 | 0.586 |
| R cACC CT* R cACC CBF | −0.062 | (−0.560, 0.103) | 0.166 | 1.443 | 0.599 |
| APOE* R cACC CT* R cACC CBF | 0.041 | (−0.468, 0.338) | 0.207 | 2.178 | 0.804 |
| APOE | −0.305 | (−0.786, 0.002) | 0.193 | 1.745 | 0.084+ |
| L cACC CT | −0.089 | (−0.294, 0.101) | 0.098 | 0.884 | 0.378 |
| Right EC CBF | −0.003 | (−0.235, 0.218) | 0.114 | 0.032 | 0.974 |
| APOE* L cACC CT | −0.017 | (−0.330, 0.345) | 0.175 | 0.110 | 0.912 |
| APOE* L cACC CBF | −0.149 | (−0.668, 0.230) | 0.220 | 0.713 | 0.477 |
| L cACC CT* L cACC CBF | −0.112 | (−0.322, 0.086) | 0.104 | 1.153 | 0.251 |
| APOE* L cACC CT* L cACC CBF | 0.130 | (−0.288, 0.612) | 0.237 | 0.647 | 0.519 |
3.3. Post hoc analyses
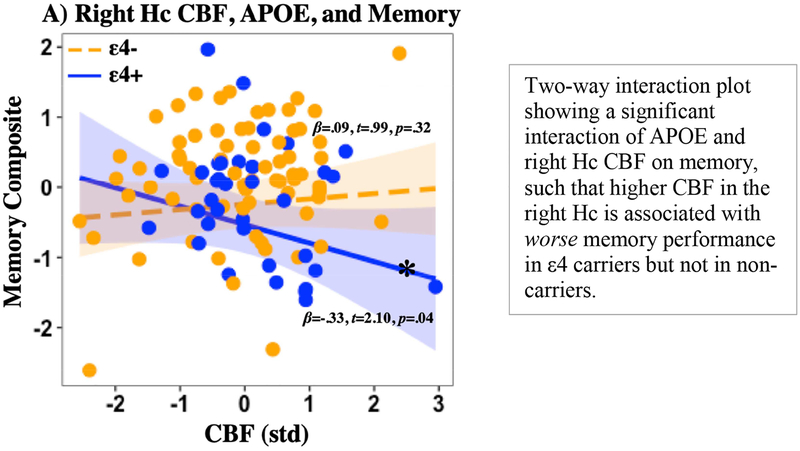
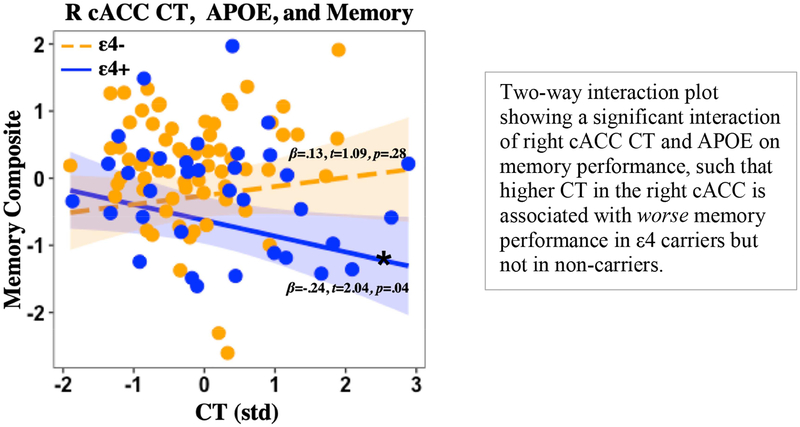
Post hoc analyses of two-way interaction models in regions that did not show a significant three-way interaction revealed an interaction of APOE genotype and right Hc CBF on memory performance (t = 2.365, p = 0.020), such that increased CBF in the right Hc was associated with worse memory in ε4 carriers but not in non-carriers (see Figure 2). A significant two-way interaction of APOE and right cACC CT on memory performance was found (t = 2.192, p = 0.030), such that increased CT in the right cACC was associated with worse memory in ε4 carriers but not in non-carriers (see Figure 3). No other significant two-way interactions were found (all p> 0.05). Post hoc analyses of correlations between regional CBF and CT showed no significant or marginal correlations by group (ε4-, ε4+) or when collapsing across the whole sample (all ps > 0.2). Post hoc model comparison of three-way and two-way interaction models showed that in the left EC, a three-way interaction model best fit the data (Adjusted R2=0.2298, p = 0.0006) and demonstrated significantly better fit than a two-way interaction model (F = 11.14, p = 0.0012). In the right Hc, a three-way interaction model best fit the data (Adjusted R2=0.1926, p = 0.0006) and demonstrated marginally better fit (F = 2.05, p = 0.1556) than a two-way interaction model (Adjusted R2=0.1833, p = 0.001). Two-way interaction models best fit the data in the right EC (Adjusted R2= 0.1342, p = 0.0145) and the left Hc (Adjusted R2= 0.1279, p = 0.0155).
Figure 2. Right Hc CBF, APOE, and memory.
Note: Hc= Hippocampus; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; p= p-value; *Denotes simple slope significance at p<0.05.
Figure 3. Right cACC CT, APOE, and memory.
Note: cACC= Caudal anterior cingulate cortex; APOE= Apolipoprotein E gene; CBF= Cerebral blood flow; CT= Cortical thickness; std= Z-score standardization; p= p-value; *Denotes simple slope significance at p<0.05.
4. Discussion
Results showed that APOE genotype modified the interaction of MTL CBF and CT on cognitive performance among a sample of cognitively normal older adults. More specifically, relationships between left EC CBF and memory were negative in ε4 carriers with lower CT, positive in non-carriers with lower CT, and negative in non-carriers with higher CT. Moreover, the magnitude of the relationship between CBF and memory was increased in the context of high and low, versus intermediate, values of CT. Although not statistically significant, there were slight trends toward this same three-way interaction in the right EC and the right Hc. Moreover, post hoc analyses demonstrated a modifying role of APOE genotype on the independent effects of both CBF and CT on memory, such that higher CBF in the right Hc predicted worse verbal memory performance in ε4 carriers, but not in non-carriers and higher CT in the right cACC predicted worse verbal memory performance in ε4 carriers, but not in non-carriers. APOE ε4 carriers also exhibited worse verbal memory performance, worse executive functioning performance, lower left EC CBF, and higher right cACC CT. These findings are consistent with other APOE-related aging studies, supporting the notion that regionally specific perfusion and brain structure differences exist between ε4 carriers and non-carriers in areas that have been implicated in normal aging and AD-risk and that these differences are associated with worse cognitive function (Bangen et al., 2012; Dai et al., 2009; Hays et al., 2016; Meltzer et al., 2000; Wierenga et al., 2012; Zlatar et al., 2016). However, this is the first study, to our knowledge, to show that APOE genotype modifies the interactive relationship between MTL CBF and CT on cognitive function, providing a more complete accounting of the relationships among these variables.
The complex three-way interaction of left EC CBF, CT, and APOE on verbal memory performance may be appreciated in the context of regional compensatory brain activity, reflecting efforts to maintain adequate brain oxygenation in the face of vascular aging and/or neuropathological damage. For example, the observed negative relationship between EC CBF and cognition (lower CBF being associated with better memory) in non-carriers with higher CT may reflect neural efficiency, such that non-carriers with higher cortical reserve have no need to invoke compensatory increases in CBF. In contrast, the observed positive relationship between EC CBF and cognition (higher CBF being associated with better memory) in non-carriers with lower CT may reflect successful cerebrovascular compensation, such that non-carriers with lower cortical reserve are invoking compensatory increases in CBF that are adequately supporting current memory function. In this same context, the observed negative relationship between EC CBF and cognition (higher CBF being associated with worse memory) in ε4 carriers with lower CT may reflect a relative breakdown of cerebrovascular compensation, such that compensatory increases in CBF among ε4 carriers with lower cortical reserve are maximally invoked and not fully supportive of current memory function (see the Johnson-Neyman plot in Figure 1 for a visual depiction), though future research is needed to determine if they adequately support memory function over time (e.g., memory stability). Overall, these findings are largely consistent with the Capillary Dysfunction Hypothesis of Alzheimer’s disease, which posits that ε4 carriers experience increased heterogeneity of capillary blood flow, which reduces the amount of oxygen that can diffuse into tissue. This reduction in oxygen diffusion necessitates a compensatory increase in CBF to maintain adequate brain oxygenation (Østergaard et al., 2013). However, progressive increases in this heterogeneity of flow is thought to result in low tissue oxygen tension, a state which paradoxically benefits (due to increased blood-tissue oxygen concentration gradients) from suppression of CBF (Jespersen & Østergaard, 2012; Østergaard et al., 2013). Although essential for the maintenance of oxygen availability, this compensatory reduction in cerebral perfusion may ultimately lead to oxidative stress, activation of inflammatory pathways, and increased amyloid levels in the brain (Ostergaard et al., 2013). Taken together, this suggests that cognitively normal ε4 carriers with higher EC CBF and lower EC CT may be at elevated risk for cognitive decline due to inadequate/maximally invoked cerebrovascular compensatory mechanisms. It is also possible, that ε4 carriers with lower cortical reserve who do not demonstrate compensatory increases in CBF could be at even greater risk for cognitive decline, though future longitudinal research is needed to test this hypothesis. Moreover, non-carriers showing this same compensatory pattern (higher CBF and lower CT) may also be at elevated risk for cognitive decline, as this may signal some degree of vascular aging and/or or neuropathological damage. Interestingly, the results of the current study may help explain mixed findings in the literature with regard to relationships between MTL CBF and cognition (findings of both positive and negative associations), as the direction and magnitude of this relationship appears to vary as a function of both CT and APOE genotype. As such, future studies exploring the relationship between CBF and cognition among older adults should consider accounting for underlying cortical thickness.
More broadly, observation of an interaction between CBF, CT, APOE, and memory performance in the MTL among cognitively normal adults supports the hypothesis that the APOE ε4 isoform confers risk for cognitive decline through altered MTL perfusion, and that concomitant altered cortical morphology in the same region may further exacerbate the detrimental effects of CBF alteration on cognition. The concept of APOE-related alterations in MTL CBF (rather than MTL CT) being more tightly linked to cognitive function is further supported by our observation of an independent association between CBF and cognitive performance in ε4 carriers in MTL regions (i.e., right hippocampus), but no independent association between CT and cognition in MTL regions. Moreover, the localization of the interaction between APOE, CBF and CT on verbal memory in the left entorhinal cortex suggests the presence of AD-related neuropathological processes, rather than an exacerbation or speeding up of normal aging processes, as the EC is one of the first regions to be affected by AD pathology (Braak & Braak, 1991), but is relatively spared in normal aging (Fjell et al., 2009; Good et al., 2001; Raz et al., 2005; Raz, Rodrigue, & Haacke, 2007). It is also important to note that the three-way interaction of APOE, CBF, and CT on memory in the entorhinal cortex was observed in the context of uncorrelated regional CBF and CT (e.g., no correlation between left EC CBF and left EC CT). The notion that alterations in regional CBF may be independent from alteration in brain structure is supported by previous literature (Chen, Rosas, & Salat, 2011) and taken together with the current results, suggests that although perhaps dissociable (e.g., not occurring concurrently; reflecting different underlying pathological processes), these brain indicators demonstrate associations in the MTL when accounting for APOE genotype and cognitive performance. While the current study did not test laterality directly, it may be important to note that we found a statistically significant three-way interaction of CBF, CT, APOE, and memory in the left, but not the right, EC. Although our observation of a slight non-significant trend toward this same interaction in the right EC suggests that statistical power may have played a role, prior studies have also shown that ε4 carriers demonstrate lower left than right EC CT (Donix et al., 2013), suggesting that the left EC may be more vulnerable to APOE-related neuropathological damage compared to the right EC. Similarly, our finding of a significant two-way interaction of CBF and APOE on memory in the right, but not the left, Hc could reflect low power or could suggest that APOE-related neurovascular alteration in MTL regions may be less left-localized than structural alteration.
Exploratory analyses of prefrontal regions and memory function revealed a rather unexpected finding: higher right cACC CT among ε4 carriers compared to non-carriers was associated with worse verbal memory performance. Although counterintuitive, given that most studies report widespread cortical thinning in ε4 carriers, this finding may be better understood in the context of the normal aging cortical morphology literature. Against the backdrop of widespread age-related cortical thinning and volume reduction, there is accumulating evidence suggesting that there may be isolated areas of age-related cortical thickening in the prefrontal cortex, including the ACC . As such, it is possible that the APOE ε4 isoform may lead to an exaggeration or speeding up of normal age-related morphological changes in the ACC. This concept is supported by recent studies showing that ε4 carriers do indeed exhibit higher CT and/or volume in prefrontal regions (including the ACC) compared to non-carriers (Dowell et al., 2016; Espeseth et al., 2008, 2012). The current results are similar to those reported on an event related potential study of attention and cortical thickness by APOE status. This 2012 study found that higher prefrontal CT in APOE ε4 carriers was associated with worse selective attention. Our results extend these prior findings to other cognitive domains, showing that higher CT in prefrontal regions (i.e., cACC) in ε4 carriers, compared to non-carriers is also associated with worse verbal memory performance. The observed negative association between CT and cognition lends support to the concept of higher relative thickness of the prefrontal cortex as part of a dysfunctional process associated with the ε4 allele, perhaps due to impaired repair mechanisms (Sundstrom et al., 2004; Teter et al., 2002).
Limitations of the current study include the use of a cross-sectional design, which restricted our ability to draw causal conclusions and limited our ability to determine whether associations among APOE, CBF, and CT, and cognition represent normal aging or pathologic processes. It is also important to note that our sample had relatively high levels of education, and although this demographic factor was not associated with APOE genotype, its limited range may reduce the generalizability of these findings. Although groups (ε4+/ ε4-) did not differ significantly in the average time interval between cognitive testing and fMRI scanning (53 and 51 days, respectively), decreasing the time interval between cognitive testing and MRI may improve accuracy of brain-behavior associations. Moreover, our sample of 117 only included 41 APOE ε4 carriers, which may have limited our ability to detect statistically significant three-way interactions in other regions. It may also be important to note that the confidence bands for the intervals of significance in the Johnson-Neyman plot approached (but did not include) zero, particularly with respect to non-carriers. Future investigations should include longitudinal designs with larger, more diverse samples to replicate and extend the current findings. Furthermore, the inclusion of additional markers of AD, such as CSF biomarkers may help better characterize APOE ε4 carriers.
The strengths of the current study include the use of non-invasive ASL MRI to measure partial volume corrected CBF, and the use of a high-resolution structural MRI scan to examine CT and Vo. Furthermore, use of FreeSurfer offers advantages over traditional voxel-based morphometry methods, as it also allows for examination of the components of volume separately (thickness and surface area), as it has been found that these two do not necessarily track with one another. Moreover, the extraction of CBF from FreeSurfer-derived brain regions represents a strength, allowing us to directly investigate CBF (and brain structure) in regions that are defined by each individual’s anatomy, rather than atlas-defined regions which are less sensitive to individual anatomical differences because they require that data are first aligned and warped to a generic anatomic template. Lastly, the current study benefited from the inclusion of a well-controlled and well-characterized sample of cognitive normal older adults, which included the use of several cognitive test performances to characterize cognitive status.
5. Conclusions
In conclusion, our findings support the notion that APOE ε4 carriers may be experiencing vascular dysregulation and concomitant morphological alteration in the MTL that interact to negatively affect cognition prior to the onset of overt clinical symptoms, providing potential insight into the mechanistic link between APOE ε4 and detriments in cognition. Although future longitudinal research is needed to determine whether these relationships also predict future cognitive decline, the current observed pattern of higher CBF and lower CT in the EC predicting worse memory performance suggests a novel multimodal neural signature with the potential to detect ε4 carriers who are at elevated risk for cognitive impairment. Such early detection could inform candidate selection and study design for future clinical trials. On a broader scale, the current results add to accumulating evidence supporting the early role of vascular dysregulation in AD risk and could lead to the identification of vasoprotective treatments with the potential to delay or prevent the onset of age-related cognitive decline and/or AD.
Supplementary Material
Johnson-Neyman plot showing that the direction and magnitude of the relationship between left EC CBF and memory performance differs across different values of CT (x-axis) and by APOE status. Intervals of left EC CT for which the relationship between CBF and memory is statistically significant are depicted in blue and non-significant intervals are depicted in red. Positive relationships between CBF and memory are depicted above the horizontal midline with greater positive values on the y-axis corresponding to increasing magnitude of the relationship in standard deviation units, whereas negative relationships are depicted below the horizontal line with greater negative values on the y-axis corresponding to increasing magnitude of the relationship. The thick aspect of the horizonal midline represents the range of observed data, whereas the thinner aspect represents the range of data that has been extrapolated beyond the observed data. Curved lines represent 95% confidence bands. 1. There is a negative relationship between EC CBF and memory in non-carriers with higher EC CT (≥ 2.3SD) that increases in magnitude as CT increases, perhaps reflecting neural efficiency and no current need for compensation; 2. There is a positive relationship between EC CBF and memory in non-carriers with lower EC CT (≤1.2SD) that increases in magnitude as CT decreases, perhaps reflecting compensatory increases in CBF that are supportive of memory function; 3. There is a negative relationship between CBF and memory in ε4 carriers with lower EC CT (≤ −.62SD) that increases in magnitude as CT decreases, perhaps reflecting maximally invoked compensatory increases in CBF that are not fully supportive of memory function. Relationships between CBF and memory were non-significant in non-carriers with intermediate levels of EC CT and in ε4 carriers with intermediate or higher EC CT.
Acknowledgements:
We would like to acknowledge Dr. Robert Rissman, Associate Professor of Neurosciences at the University of California San Diego for his contributions to this project.
Funding: This work was supported by VA CSR&D Merit Award [5I01CX000565 C.E.W.], the National Science Foundation Graduate Research Fellowship Program [2015207525 C.C.H.], and the National Institute on Aging of the National Institutes of Health [K23AG049906 Z.Z.Z.], [K24AG026431 M.W.B], [R01AG054049 M.W.B]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the VA, National Science Foundation, or National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, … Ohtomo K (2008). Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging, 29(1), 102–116. 10.1016/j.neurobiolaging.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Adamson MM, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, … Taylor JL (2010). Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiology of Aging, 31(6), 1059–1063. 10.1016/j.neurobiolaging.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Bergfield KL, Chen K, Reiman EM, Hanson KD, Lin L, … Moeller JR (2012). Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiology of Aging, 33(12), 2723–2732. 10.1016/j.neurobiolaging.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, & Stern Y (2008). Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 28(4), 725–736. 10.1038/sj.jcbfm.9600570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Jak AJ, Wierenga CE, Salmon DP, & Bondi MW (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: Associations with cognition and stroke risk. Neurobiology of Aging, 30(8), 1276–1287. 10.1016/j.neurobiolaging.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, & Bondi MW (2012). Assessment of Alzheimer’s disease risk with functional magnetic resonance imaging: an arterial spin labeling study. Journal of Alzheimer’s Disease: JAD, 31 Suppl 3, S59–74. 10.3233/JAD-2012-120292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, & Naumann E (2009). Resting cerebral blood flow, attention, and aging. Brain Research, 1267, 77–88. 10.1016/j.brainres.2009.02.053 [DOI] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathology (Zurich, Switzerland), 1(3), 213–216. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE, & MacArthur Studies of Successful Aging. (2003). The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology, 60(7), 1077–1081. [DOI] [PubMed] [Google Scholar]
- Buxton RB (2009). Introduction to functional magnetic resonance imaging: Principles and techniques (Second). New York: Cambridge University Press. [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, … Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. The New England Journal of Medicine, 361(3), 255–263. 10.1056/NEJMoa0809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, & Alexander GG (2004). Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology, 62(11), 1990–1995. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, & Detre JA (2000). Magnetic Resonance Perfusion Imaging in Acute Ischemic Stroke Using Continuous Arterial Spin Labeling. Stroke, 31(3), 680–687. 10.1161/01.STR.31.3.680 [DOI] [PubMed] [Google Scholar]
- Chappell MA, Groves AR, Whitcher B, & Woolrich MW (2009). Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Transactions on Signal Processing, 57(1), 223–236. 10.1109/TSP.2008.2005752 [DOI] [Google Scholar]
- Chen JJ, Rosas HD, & Salat DH (2011). Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage, 55(2), 468–478. 10.1016/j.neuroimage.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, & Sunderland T (2001). Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology, 57(12), 2223–2228. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, & Kannel WB (1994). Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke, 25(1), 40–43. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, & Gach HM (2009). Mild Cognitive Impairment and Alzheimer Disease: Patterns of Altered Cerebral Blood Flow at MR Imaging. Radiology, 250(3), 856–866. 10.1148/radiol.2503080751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dale AM, & Sereno MI (1993). Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. Journal of Cognitive Neuroscience, 5(2), 162–176. 10.1162/jocn.1993.5.2.162 [DOI] [PubMed] [Google Scholar]
- De Blasi S, Montesanto A, Martino C, Dato S, De Rango F, Bruni AC, … Passarino G (2009). APOE polymorphism affects episodic memory among non demented elderly subjects. Experimental Gerontology, 44(3), 224–227. 10.1016/j.exger.2008.11.005 [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, & Breteler MMB (2002). Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology, 59(5), 746–748. [DOI] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Scharf M, Marschner K, Suthana NA, Siddarth P, … Bookheimer SY (2013). APOE associated hemispheric asymmetry of entorhinal cortical thickness in aging and Alzheimer’s disease. Psychiatry Research, 214(3). 10.1016/j.pscychresns.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Evans SL, Tofts PS, King SL, Tabet N, & Rusted JM (2016). Structural and resting-state MRI detects regional brain differences in young and mid-age healthy APOE-e4 carriers compared with non-APOE-e4 carriers. NMR in Biomedicine, 29(5), 614–624. 10.1002/nbm.3502 [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, & Reinvang I (2008). Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ɛ4. Neurobiology of Aging, 29(3), 329–340. 10.1016/j.neurobiolaging.2006.10.030 [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Walhovd KB, Fjell AM, Endestad T, Rootwelt H, & Reinvang I (2012). Apolipoprotein E ε4-related thickening of the cerebral cortex modulates selective attention. Neurobiology of Aging, 33(2), 304–322.e1. 10.1016/j.neurobiolaging.2009.12.027 [DOI] [PubMed] [Google Scholar]
- Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, … Mackay CE (2011). Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage, 54(1), 602–610. 10.1016/j.neuroimage.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, & Dale AM (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, & Dale AM (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23 Suppl 1, S69–84. 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, & Dale AM (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y.: 1991), 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, … Dale AM (2009). One year brain atrophy evident in healthy aging. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(48), 15223–15231. 10.1523/JNEUROSCI.3252-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 Pt 1), 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, West NA, Tschanz JT, Norton MC, Corcoran C, … Welsh-Bohmer KA (2009). Effects of Family History and Apolipoprotein E ε4 Status on Cognitive Decline in the Absence of Alzheimer Dementia: The Cache County Study. Archives of Neurology, 66(11), 1378–1383. 10.1001/archneurol.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, & Wierenga CE (2016). The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cellular and Molecular Neurobiology, 36(2), 167–179. 10.1007/s10571-015-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, & Kramer AF (2010). Resting hippocampal blood flow, spatial memory and aging. Brain Research, 1315, 119–127. 10.1016/j.brainres.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Vidoni E, Harsha A, & Burns JM (2009). Impact of APOE on the Healthy Aging Brain: A Voxel-Based MRI and DTI Study. Journal of Alzheimer’s Disease : JAD, 18(3), 553–564. 10.3233/JAD-2009-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, & Delis DC (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 17(5), 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, & Bondi MW (2007). Differential Cross-Sectional and Longitudinal Impact of APOE Genotype on Hippocampal Volumes in Nondemented Older Adults. Dementia and Geriatric Cognitive Disorders, 23(6), 382–389. 10.1159/000101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen SN, & Østergaard L (2012). The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. Journal of Cerebral Blood Flow & Metabolism, 32(2), 264–277. 10.1038/jcbfm.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen HM, Muller M, van der Graaf Y, & Geerlings MI (2012). APOE ε4 differentially influences change in memory performance depending on age. The SMART-MR study. Neurobiology of Aging, 33(4), 832.e15–22. 10.1016/j.neurobiolaging.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Jung Y, Wong EC, & Liu TT (2010). Multiphase pseudocontinuous arterial spin labeling (MPPCASL) for robust quantification of cerebral blood flow. Magnetic Resonance in Medicine, 64(3), 799–810. 10.1002/mrm.22465 [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Xu H, & Bu G (2014). ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron, 81(4), 740–754. 10.1016/j.neuron.2014.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, … Iadecola C (2018). Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nature Communications, 9(1), 3816 10.1038/s41467-018-06301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, & Fink GR (2010). Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE ε4 carriers. Neuroscience, 168(2), 487–497. 10.1016/j.neuroscience.2010.03.044 [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson L-G, Bäckman L, … Nyberg L (2006). Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ɛ4: Relation to chronological age and recognition memory. Neuroscience Letters, 396(1), 23–27. 10.1016/j.neulet.2005.11.070 [DOI] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, & Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nature Reviews. Neurology, 9(2), 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, & Wong EC (2005). A signal processing model for arterial spin labeling functional MRI. NeuroImage, 24(1), 207–215. 10.1016/j.neuroimage.2004.09.047 [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Flüß MO, Wittsack H-J, Grass-Kapanke B, Jänner M, Khalili-Amiri R, … Cohnen M (2008). Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. NeuroImage, 40(2), 495–503. 10.1016/j.neuroimage.2007.11.053 [DOI] [PubMed] [Google Scholar]
- Mahley RW, & Rall SC (2000). Apolipoprotein E: far more than a lipid transport protein. Annual Review of Genomics and Human Genetics, 1, 507–537. 10.1146/annurev.genom.1.1.507 [DOI] [PubMed] [Google Scholar]
- Marchant NL, King SL, Tabet N, & Rusted JM (2010). Positive Effects of Cholinergic Stimulation Favor Young APOE ɛ4 Carriers. Neuropsychopharmacology, 35(5), 1090–1096. 10.1038/npp.2009.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Cantwell MN, Greer PJ, Ben-Eliezer D, Smith G, Frank G, … Price JC (2000). Does Cerebral Blood Flow Decline in Healthy Aging? A PET Study with Partial-Volume Correction. Journal of Nuclear Medicine, 41(11), 1842–1848. [PubMed] [Google Scholar]
- Mondadori CRA, de Quervain DJ-F, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, … Henke K (2007). Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cerebral Cortex (New York, N.Y.: 1991), 17(8), 1934–1947. 10.1093/cercor/bhl103 [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, … Johnson SC (2014). Cerebral Blood Flow is Diminished in Asymptomatic Middle-Aged Adults with Maternal History of Alzheimer’s Disease. Cerebral Cortex (New York, N.Y.: 1991), 24(4), 978–988. 10.1093/cercor/bhs381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, … West MJ (2013). The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging, 34(4), 1018–1031. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Aamand R, Gutiérrez-Jiménez E, Ho Y-CL, Blicher JU, Madsen SM, … West MJ (2013). The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiology of Aging, 34(4), 1018–1031. 10.1016/j.neurobiolaging.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD (2005). Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, & Haacke EM (2007). Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences, 1097, 84–93. 10.1196/annals.1379.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, & Fischl B (2010). Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage, 53(4), 1181–1196. 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–1418. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Pope W, & Salamon N (2010). Insensitivity of visual assessment of hippocampal atrophy in familial Alzheimer’s disease. Journal of Neurology, 257(5), 839–842. 10.1007/s00415-009-5436-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Evans SL, King SL, Dowell N, Tabet N, & Tofts PS (2013). APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. NeuroImage, 65, 364–373. 10.1016/j.neuroimage.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, … Fischl B (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex (New York, N.Y.: 1991), 14(7), 721–730. 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, & Deary IJ (2012). APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Molecular Psychiatry, 17(3), 315–324. 10.1038/mp.2010.137 [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, … Giedd JN (2007). Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. The Lancet. Neurology, 6(6), 494–500. 10.1016/S1474-4422(07)70106-0 [DOI] [PubMed] [Google Scholar]
- Sundstrom A, Marklund P, Nilsson L-G, Cruts M, Adolfsson R, Van Broeckhoven C, & Nyberg L (2004). APOE influences on neuropsychological function after mild head injury: Within-person comparisons. Neurology, 62(11), 1963–1966. 10.1212/01.WNL.0000129268.83927.A8 [DOI] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, & Mackay CE (2013). The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neuroscience & Biobehavioral Reviews, 37(10, Part 2), 2878–2886. 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, & Bu G (2016). The Role of APOE in Cerebrovascular Dysfunction. Acta Neuropathologica, 131(5), 709–723. 10.1007/s00401-016-1547-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Holland D, Dale AM, & Miller MI (2015). APOE affects the volume and shape of the amygdala and the hippocampus in mild cognitive impairment and Alzheimer’s disease: Age matters. Journal of Alzheimer’s Disease: JAD, 47(3), 645–660. 10.3233/JAD-150262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B, Xu P-T, Gilbert JR, Roses AD, Galasko D, & Cole GM (2002). Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. Journal of Neuroscience Research, 68(3), 331–336. 10.1002/jnr.10221 [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held L, An Y, Kraut MA, & Resnick SM (2010). APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Archives of Neurology, 67(1), 93–98. 10.1001/archneurol.2009.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, … Sasaki M (1997). Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neuroscience Letters, 236(1), 21–24. [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, … Zabetian CP (2013). APOE ϵ4 Increases Risk for Dementia in Pure Synucleinopathies. JAMA Neurology, 70(2), 223–228. 10.1001/jamaneurol.2013.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuminello ER, & Han SD (2011). The Apolipoprotein E Antagonistic Pleiotropy Hypothesis: Review and Recommendations [Research article]. 10.4061/2011/726197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehair DC, Sherzai A, Emond J, Raman R, Aisen PS, Petersen RC, … Alzheimer’s Disease Cooperative Study. (2010). Influence of apolipoprotein E varepsilon4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 6(5), 412–419. 10.1016/j.jalz.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, … Bondi MW (2013). Interaction of age and APOE genotype on cerebral blood flow at rest. Journal of Alzheimer’s Disease: JAD, 34(4), 921–935. 10.3233/JAD-121897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, … Bondi MW (2012). Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. Journal of Cerebral Blood Flow & Metabolism, 32(8), 1589–1599. 10.1038/jcbfm.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, & Zlatar ZZ (2014). Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 42 Suppl 4, S411–419. 10.3233/JAD-141467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, & Kannel WB (1991). Probability of stroke: a risk profile from the Framingham Study. Stroke, 22(3), 312–318. [DOI] [PubMed] [Google Scholar]
- Zhao MY, Mezue M, Segerdahl AR, Okell TW, Tracey I, Xiao Y, & Chappell MA (2017). A systematic study of the sensitivity of partial volume correction methods for the quantification of perfusion from pseudo-continuous arterial spin labeling MRI. NeuroImage, 162, 384–397. 10.1016/j.neuroimage.2017.08.072 [DOI] [PubMed] [Google Scholar]
- Zhong N, & Weisgraber KH (2009). Understanding the Basis for the Association of apoE4 with Alzheimer’s Disease: Opening the Door for Therapeutic Approaches. Current Alzheimer Research, 6(5), 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, Bischoff-Grethe A, Hays CC, Liu TT, Meloy MJ, Rissman RA, … Wierenga CE (2016). Higher Brain Perfusion May Not Support Memory Functions in Cognitively Normal Carriers of the ApoE ε4 Allele Compared to Non-Carriers. Frontiers in Aging Neuroscience, 151 10.3389/fnagi.2016.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.