Abstract
Reduced high-frequency heart rate variability (HF-HRV) is associated with a greater risk for cardiovascular disease (CVD). Although African Americans (AA) are at greater risk for CVD, they show greater HF-HRV compared to European Americans (EA). Previous studies suggest that differences in the association between regional cerebral blood flow (CBF) and HF-HRV in AA and EA may explain this surprising pattern of findings, termed the Cardiovascular Conundrum. Here pooled data from a total of n = 452 EA and n = 102 AA, investigating differences in the association between CBF in 8 regions of interest (ROI), including the cingulate (anterior, mid, posterior), insula (anterior, posterior), and amygdala (basolateral, centromedial, superfical), with HF-HRV, mean heart rate (HR) and their Coefficient of Variation (CoV). Bayesian statistics illustrate that CBF – in particular in the anterior cingulate cortex (ACC) - is positively associated with HF-HRV and CoV in EA, but negatively associated in AA. Exploring the association between HF-HRV and CBF with self-reports of affect and affect regulation showed some differences as a function of ethnicity. The association between greater habitual use of reappraisal only showed a positive correlation with HF-HRV in AA. Similar, greater suppression or non-expression of angry emotions was associated with greater HF-HRV whereas greater outward direction of anger was associated with lower HF-HRV in AA only. Given the importance of the ACC in emotion and emotion regulation, we suggest that increased HF-HRV may serve a compensatory role in AA. Implications from these findings and suggestions for future studies are discussed.
Introduction
Individual differences in cardiac autonomic control, as reflected by metrics of heart rate variability (HRV), have been associated with diverse aspects of physical and mental health. In particular, lower parasympathetic cardiac control, as indexed by reduced high-frequency HRV (HF-HRV), has been associated with a greater risk for morbidity and premature mortality, including risks attributable to cardiovascular disease (CVD) in primarily European American (EA) populations (Thayer et al., 2010). Recent cumulative evidence summarized by meta-analysis, however, suggests a surprising pattern of findings with respect to HF-HRV and health disparities (Hill et al., 2015); namely, that African Americans (AA) reliably exhibit higher levels of HF-HRV compared to EA. The association between HF-HRV and CVD risk in AAs is largely unknown at this time. Precisely, it is not known whether variations in HF-HRV are associated with variations in CVD risk in AA to a similar degree as in EA. Thus this finding of higher HF-HRV in AA is arguably surprising in view of longstanding evidence that AA are at greater risk for a host of adverse cardiovascular and related health outcomes that are otherwise linked to impaired cardiac autonomic control (Lockwood et al., 2018). Accordingly, it is no longer reasonable to presuppose that AA might exhibit lower, rather than elevated HF-HRV, in association with corresponding disease risks. The reasons for this Cardiovascular Conundrum – wherein AA exhibit both elevated HF-HRV and elevated disease risk - remain unclear, as do its neural and psychosocial correlates. Explicating these correlates may further our understanding of the pathways and mechanisms linking ethnicity to physical and mental health. In this regard, one speculative possibility is that brain systems jointly implicated in regulating emotions and autonomic function (Mather and Thayer, 2018; Thayer and Lane, 2000), may help to understand the pathways linking HF-HRV to such health disparities.
To elaborate, the structural (Carnevali et al., 2018) and functional (Thayer et al., 2012) cortical and subcortical neural concomitants of individual differences in HF-HRV are becoming increasingly clearer, and recent studies have shown that differences in HF-HRV are associated with differences in neural activity during implicit (Lane et al., 2013) and explicit emotion regulation (Steinfurth et al., 2018). It seems possible, therefore, that HF-HRV might differentially associate with neural activity in brain regions dually implicated in affective processes and autonomic control among AA compared to EA. Indeed, previous studies suggest differential neural correlates of HF-HRV in AA: asides structural and functional MRI studies, research addressed the association between resting regional cerebral blood flow (rCBF) and HF-HRV. Allen et al (Allen et al., 2015) reported that global CBF was positively associated with HF-HRV in EA but non-significant negatively associated with HF-HRV in AA. Whereas both greater gCBF and greater HF-HRV have been associated with better cardiovascular health in EA (Jennings et al., 2013), as noted above AA have been shown to have greater cardiovascular risk despite having greater HF-HRV. Allen et al (Allen et al., 2015) speculated that the differential association between CBF and HF-HRV particularly in areas linked with both sympathetic regulation and affective salience might have relevance for the known health disparities between EA and AA. Jennings et al., for example, found HF-HRV to be inversely correlated with rCBF in several brain regions of interest (ROIs) with respect to affective and autonomic processes, including the left amygdala and left insula (Jennings et al., 2015). Most interestingly, the study showed significant differences in the association between HF-HRV and rCBF comparing AA and EA for rCBF in the dorsal anterior cingulate (ACC), the rostral medial frontal gyrus, and the ventral ACC (Jennings et al., 2015). However, ethnic differences in the association between rCBF and HF-HRV in the context of affect were beyond the scope of that investigation, focusing on the association between HF-HRV, rCBF and cognitive function. Importantly, the ACC is a key region implicated in regulating emotion (Etkin et al., 2011; Stevens et al., 2011) and controlling autonomic cardiac outflow.
In addressing potential psychosocial factors associated with disparities in HF-HRV, previous studies have shown that greater self-reported discrimination is associated with lower HF-HRV within AA (Hill et al., 2017). Moreover, this relationship is moderated by rumination (Williams et al., 2017). Yet, while discrimination and rumination may partly explain within-group differences in HF-HRV among AA, it remains unclear why AA show greater HF-HRV compared to EA. One possibility that could partly account for ethnic differences in HF-HRV pertains to differences in negative affect and its regulation. More specifically, studies have shown an inverse relationship between measures of HF-HRV and negative affect (e.g., Bleil et al., 2008; Sloan et al., 2017). It is also suggested that lower HF-HRV is related to greater difficulties in emotion regulation as assessed via self-reports (Williams et al., 2015). Trait anger and emotion inhibition are specific affective processes linked to adverse health outcomes.
However, these patterns of association show considerable ethnic variation (Consedine et al., 2005). Further, it has been shown that there are ethnic differences in the use of emotion regulation strategies (Chang et al., 2010; Kwon et al., 2013). Importantly, in a study that examined physiological responses to anger provocation, AA that could express their anger had the lowest HF-HRV whereas both AA and EA that had to inhibit their anger had higher HF-HRV (Dorr et al., 2007). In addition, anger expression was associated with higher systolic blood pressure (SBP) in AA but lower SBP in EA whereas anger inhibition was associated with greater total peripheral resistance (TPR) in both EA and AA participants. The higher HF-HRV during anger inhibition is consistent with research showing that successful emotion regulation via either suppression or reappraisal was associated with increases in HF-HRV (Butler, Wilhelm, & Gross, 2006). The greater SBP and TPR in AA suggests that both anger expression and anger inhibition may have adverse health consequences for AA. Accordingly, the aforementioned association between greater negative affect, difficulties in affect regulation and HF-HRV might not generalize to AA. In the present study measures of trait anger expression and emotion regulation were examined in exploratory analyses to determine if these prior associations of differential associations in EA and AA between HF-HRV and anger expression and emotion regulation might also be present.
Despite years of neuroimaging research few studies have explicitly examined potential ethnic differences in cerebral blood flow or its autonomic concomitants. Here we thus aimed to investigate potential ethnic differences in rCBF associated with increased resting state HF-HRV in AA, attempting to replicate and extend prior findings from our group. Further, we aimed to assess whether differences in affect and self-report indicators of emotion regulation are associated with altered rCBF and HRV in AA. In line with meta-analytical evidence, it was hypothesized that AA would show greater HF-HRV (Hill et al., 2015). Focusing on rCBF in cortical and subcortical ROIs also associated with emotion regulation and autonomic control from prior work (i.e., the amygdala, insula and cingulate), as well as aiming to replicate previous findings (Jennings et al., 2015), it was hypothesized that rCBF in these ROIs would associate with HF-HRV and that associations would be moderated by ethnicity. In exploratory analyses, we tested for further associations of rCBF and HF-HRV with indicators of affect and emotion regulation, also examining a potential moderation by ethnicity status. We focused on self-reports on the use of different emotion regulation strategies and anger expression that have previously shown to differ as a function of HF-HRV (Denson et al., 2011; Steinfurth et al., 2018) and ethnicity (Dorr et al., 2007).
Methods
Archival data for the present secondary analyses were pooled from two studies: the Pittsburgh Imaging Project (PIP) and the Adult Health and Behavior (AHAB) study. Details on the recruitment of participants and assessments involved in PIP (Gianaros et al., 2012; Ryan et al., 2013) and AHAB (Allen et al., 2015; Jennings et al., 2015) have been reported previously. Both studies were approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent. rCBF data on the pooled sample were previously reported (Jennings et al., 2013). Focusing on ethnic differences, only data from AA and EA from both studies were included in the final sample for analyses.
Recording of Resting Cerebral Blood Flow
Details on the recording of CBF in both studies were previously reported. In brief, neuroimaging data were acquired on a Siemens 3T Trio TIM whole-body scanner (Erlangen, Germany), equipped with a 12-channel phased-array head coil. T1-weighted 3-dimensional magnetization-prepared rapid gradient echo neuroanatomical images were acquired for the purposes of anatomical normalization and co-registration of CBF images (time to repetition/time to echo=2100/3.29 ms; time to inversion=1100 ms; matrix resolution=256×208; field of view=256×208 mm; slice thickness=1 mm (no gap); flip angle=8°.) For perfusion imaging, interleaved perfusion images with and without arterial spin labeling (PASL) were obtained over a period of 328 sec using gradient-echo echo-planar imaging (EPI). The PASL sequence employed a modified version of the flow-sensitive alternating inversion recovery method (Kim, 1995), applying a saturation pulse 700 ms after an inversion pulse. To reduce transit artifact, a 1,000-ms delay separated the end of the labeling pulse and the time of image acquisition. Resting perfusion image acquisition parameters were: field of view (FOV) = 240 × 240 mm, matrix = 64 × 64, repetition time (TR) = 4,000 ms, echo time (TE) = 18 ms, and flip angle (FA) = 90°. Twenty-one slices (5 mm thick, 1-mm gap) were acquired sequentially in an inferior-to-superior direction, yielding 80 total perfusion images (40 labeled, 40 unlabeled, 2 initial discarded images allowing for magnetic equilibration), and the acquisition time of each slice was 45 ms. A 24-s equilibrium magnetization of brain (two sets of 21 slices; TR = 8,000 ms; all other parameters are described as above) provided two images for CBF baseline quantification. Details on the preprocessing of data in SPM8 are provided in previous publications (Allen et al., 2015; Gianaros et al., 2012; Jennings et al., 2015; Ryan et al., 2013). Beyond global CBF, the present analyses focus on rCBF in 8 ROI, including the cingulate (anterior, mid, posterior), insula (anterior, posterior), and amygdala (basolateral, centromedial, superfical). The anatomical masks used to create these ROIs were drawn from the AAL atlas, SPM’s anatomy toolbox, and archival ROIs described previously (Biol Psychiatry. 2014 May 1;75(9):738-45). All are available on request. A schematic illustration of the ROI is provided in Figure 1.
Figure 1: Schematic Illustration of the Regions of Interest;
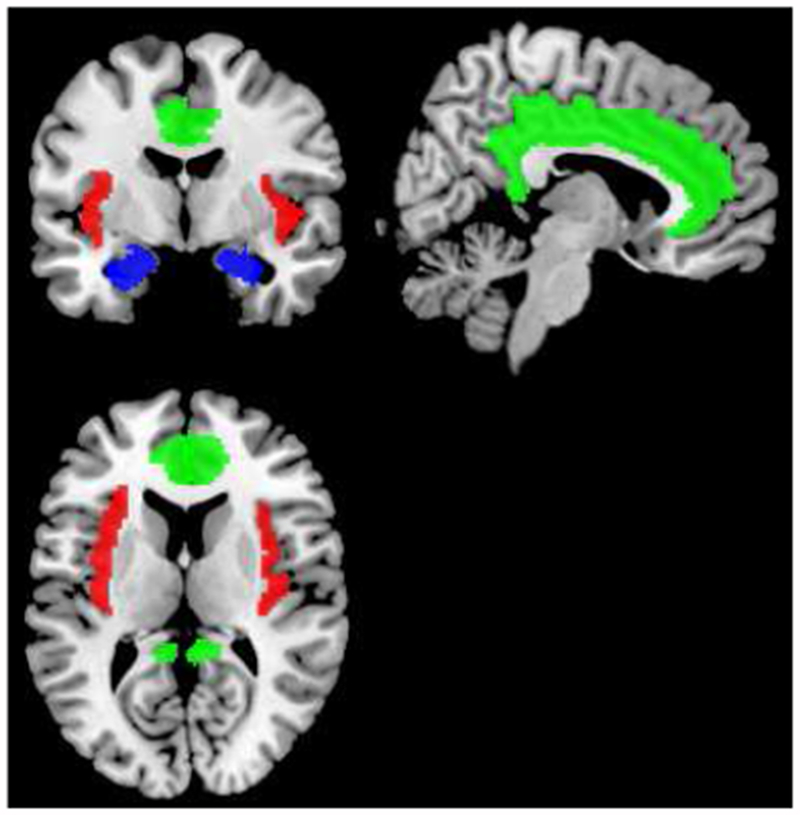
green: cingulate (anterior, mid, posterior); red: insula (anterior, posterior); blue: amygdala (basolateral, centromedial, superfical)
Recording of Autonomic Nervous System Function
In both study cohorts, assessments of resting cardiac activity were done on a separate visit from brain imaging. In AHAB2, HR and HF-HRV were derived from a continuous recording of a two-lead electrocardiogram (ECG) attached bilaterally to the wrists throughout a 5-min period. The ECG was collected in the morning, and participants were asked to not drink caffeine for 4 h, avoid alcohol or exercise for 12 h, and abstain from over-the-counter medications for 24 h preceding the recording. ECG signals were digitized at a sampling rate of 1000 Hz (LabView acquisition software, cNational Instruments Corporation, Austin, TX). An inter-beat-interval time (IBI) series was derived from the ECG, corrected for artifacts in the R-wave detection process, and the band-limited variance within the HF (0.12-0.40 Hz) was extracted using PhysioScripts (Christie and Gianaros, 2013). Mean HR (bpm) and HF-HRV (log) were used for analyses. Comparable data from PIP were likewise processed to derive HF-HRV with the same software. In PIP, however, beat-to-beat pulse intervals (in ms) were collected while participants were inside a mock MRI scanner using a Finometer® PRO (FMS, Finapres Measurement Systems, Arnhem, Netherlands). Estimates of HF-HRV were averaged over the last 5min of an 8-min resting baseline. Further, in accordance with recent recommendations, the coefficient of variation (CoV), adjusting HF-HRV for HR was determined as described elsewhere (de Geus et al., 2019). HF-HRV data below the 1st (n = 5) and above the 99th (n = 5) percentile were excluded from analyses. There were no differences on HR (t(552) = −1.537, p = .125), HF-HRV (t(552) = −0.851, p = .395), HF-HRV (log) (t(552) = −1.785, p = .075), but CoV (log) (t(552) = −2.441, p = .015), comparing data from the two studies.
Self-Reports on Emotion Regulation
Anger expression was assessed using the State-Trait Anger Expression Inventory (STAXI) (Spielberger, 1988). The three sub-scales (anger in: suppression or non-expression of angry emotions; and anger out: outward direction of anger; anger-control: use of a restrained approach in the face of anger) were used for analyses. Further, the Emotion Regulation Questionnaire (ERQ) (Gross and John, 2003) was used to assess the habitual use of cognitive reappraisal and expressive suppression.
Data Analysis
Data analyses were conducted in the full-sample and, utilizing a conservative approach for secondary analyses, in a sub-sample using propensity score matching (PSM). PSM was used as secondary statistical approach, given unbalanced sample size in EA and AA. PSM was conducted matching one nearest neighbor for each subject in the AA group, based on age, sex, BMI and dataset. The maximum distance at which two observations were considered a potential match (caliper) was set at .20. Group differences in the full- and PSM sample on sociodemographic variables, ANS function (HR/HF-HRV), emotion regulation and rCBF were assessed using Chi-Square tests (χ2) for categorical variables and t-tests for continuous variables, comparing AA and EA. Analyses on group differences in rCBF P were Bonferroni corrected for the number of ROI (p*8) to control for multiple comparisons. In the full sample, ethnic differences in the association between rCBF by ROI and ANS function were assessed using regression models predicting HR/HF-HRV as a function of the interaction between ethnicity and rCBF. First, frequentist analyses were conducted using mixed-models. For each ROI and dependent variable (HR, HF-HRV, CoV) three models were computed. Model 1 (M1) included ethnicity (AA/EA) and the following covariates: dataset (AHAB/PIP), age (continuous), sex (dichotomous), BMI (continuous), as well as global CBF. Analyses were controlled for dataset, to rule out systematic differences between studies. Analyses were adjusted for sex, age and BMI, given their well-known influence on cardiac activity (Antelmi et al., 2004; Koenig and Thayer, 2016). Global CBF was included as covariate to investigate the effect of ROI specific rCBF. Model 2 (M2) include rCBF of the respective ROI (total of 8). Model 3 (M3) included ethnicity and rCBF of the respective ROI as interaction term. Second, models were compared using the Bayes factor (BF). The interpretation of the BF by Raftery (Raftery, 1995) was used to interpret the level of evidence (i.e., a BF ≥ 3 was considered as positive evidence). In the PSM sample, simple correlations between ANS function (HR/HRV) and rCBF were calculated and compared among EA and AA. ROIs showing a significant interaction with ethnicity in predicting HR or HF-HRV and showing superior model fit as indicated by the BF (full sample) of differences in the PSM sample were subjected to further exploratory analyses. Exploratory analyses focused on the association between self-reports of anger and emotion regulation and rCBF as well as HR/HF-HRV respectively, using simple zero-order correlations. Differences between correlations coefficients were calculated with 1-sided Z statistics (Steiger, 1980) comparing the strength of associations in AA and EA. All analyses were performed using Stata (Version 15.1; StataCorp LP, College Station, TX, USA).
Results
Sociodemographic Characteristics and Autonomic Function
In the full sample, groups by ethnicity showed significant sex differences, with more females among AA (χ2(1) = 8.448, p = .004). AA were older compared to EA (t(552) = −2.565, p = .011) and showed greater BMI (t(552) = −4.899, p < .0001). EA reported more school years (t(552) = 7.139, p < .0001). There were no differences on HR (t(552) = −0.272, p = .786), HF-HRV (t(552) = −0.072, p = .943) or the CoV (t(552) = −0.017, p = .987). Analyses in the matched sample following PSM, showed significant differences by ethnicity on HF-HRV (t(180) = −2.442, p = .016) and the CoV (t(180) = −2.386, p = .018), but not HR (t(180) = 1.325, p = .187), as illustrated in Figure 2. Groups showed no significant differences in global CBF or rCBF in selected ROIs when correcting for multiple testing across 8 ROIs in the full- or PSM-sample. Descriptive statistics are provided in Table 1 and Table 2, respectively.
Figure 2: Heart Rate and Heart Rate Variability by Ethnicity;
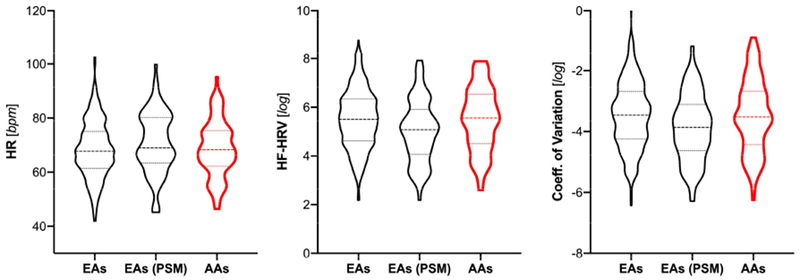
HR: heart rate; HF-HRV: high-frequency heart rate variability; bpm: beats per minute: EA: European Americans: AA: African Americans; PSM: EA sample after propensity score matching\
Table 1:
Sociodemographic Characteristics by Ethnicity
| Full Sample | PSM Sample | ||||
|---|---|---|---|---|---|
| European Americans | African Americans | p | European Americans | p | |
| N (%) | 452 (81.58) | 102 (18.41) | 80 (43.96) | ||
| Female N (%) | 225 (49.78)* | 67 (62.75)* | .004 | 48 (60.00) | .430 |
| Years School | 17.20 (2.74) [10/24]* | 15.10 (2.44) [9/24]* | <.0001 | 16.48 (2.34) [10/22] * | <.001 |
| Age | 42.18 (7.26) [30/54]* | 44.18 (6.49) [30/54]* | .010 | 44.79 (6.34) [30/54] | .532 |
| BMI | 26.65 (.4.96) [17.5/49.6]* | 29.36 (5.44) [18.8/45.1]* | <.0001 | 29.03 (5.28) [18.76/46.5] | .680 |
| HR (bpm) | 68.35 (10.40) [41.99/102.47] | 68.66 (10.36) [46.42/95.20] | .786 | 70.80 (11.33) [45.15/99.81] | .187 |
| HF-HRV (log) | 5.51 (1.21) [2.19/8.78] | 5.50 (1.30) [2.60/7.89] | .964 | 5.03 (1.28) [2.19/7.92] * | .016 |
| CoV (log) | −3.47 (1.09) [−6.42/−0.01] | −3.47 (1.20) [−6.25/−0.88] | .987 | −3.88 (1.09) [−6.29/−1.16] * | .018 |
| STAXI: Anger Control | 23.20 (4.75) [11/32] | 22.54 (4.85) [13/32] | .208 | 22.65 (5.18) [11/32] | .887 |
| STAXI: Anger In | 16.25 (3.77) [9/29] | 15.72 (3.37) [9/26] | .194 | 15.61 (3.57) [9/25] | .829 |
| STAXI: Anger Out | 14.14 (3.40) [8/29] | 13.80 (3.19) [8/23] | .359 | 14.03 (3.26) [8/29] | .647 |
| ERQ: Reappraisal | 4.97 (0.98) [1.17/7] | 5.14 (1.00) [1.50/7] | .101 | 4.99 (0.97) [1.17/7] | .292 |
| ERQ: Suppression | 3.65 (1.24) [1/6.50] | 3.47 (1.27) [1/6.75] | .173 | 3.68 (1.15) [1/6.50] | .236 |
PSM Sample: sample following propensity score matching; BMI: body mass index; HR: heart rate in beats per minute (bpm); HF-HRV: high frequency heart rate variability; CoV: coefficient of variation; STAXI: State-Trait Anger Expression Inventory; ERQ: Emotion Regulation Questionnaires; P: referring to p-values from test on group differences, t-test for continuous variables, chi-square tests for categorical variables; all values represent means and standard deviations, range [min/max];
significant difference
Table 2:
Resting Cerebral Blood Flow by Ethnicity and Region of Interest
| Full Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| European Americans | African Americans | p | pcor | ES | European Americans | p | pcor | ES | |
| Global CBF | 57.98 (10.16) [33.00/100.84] | 59.46 (10.57) [35.18/94.07] | .229 | −.15 [−.38; .09] | 57.92 (10.02) [35.42/78.82] | .353 | −.15 [−.46; .17] | ||
| Amygdala | |||||||||
| basolateral | 46.50 (10.20) [19.80/80.54] | 47.63 (10.43) [15.45/76.66] | .361 | > 1 | −.11 [−.35; .13] | 46.60 (10.50) [19.80/73.30] | .537 | > 1 | −.10 [−.41; .22] |
| centromedial | 52.42 (9.40) [25.28/87.04] | 54.62 (9.44) [27.21/82.39] | .053 | .424 | −.23 [−.47; .00] | 52.53 (9.67) [28.90/77.08] | .174 | > 1 | −.22 [−.53; .10] |
| superficial | 51.03 (9.78) [23.98/85.32] | 53.24 (9.50) [27.49/79.34] | .061 | .488 | −.23 [−.46; .01] | 51.00(10.03) [23.98/74.05] | .155 | > 1 | −.23 [−.54; .09] |
| Insula | |||||||||
| anterior | 59.76 (10.69) [34.73/98.46] | 62.12 (10.82) [40.90/89.77] | .069 | .552 | −.22 [−.46; .02] | 59.66 (10.76) [37.27/85.57] | .157 | > 1 | −.23 [−.54; .09] |
| posterior | 60.34 (10.79) [32.58/98.21] | 62.72 (11.60) [39.12/95.22] | .072 | .576 | −.22 [−.46; .02] | 60.65 (11.13) [33.07/88.18] | .259 | > 1 | −.18 [−.50; .13] |
| Cingulate | |||||||||
| anterior | 58.71 (10.87) [33.98/107.07] | 59.50 (10.05) [36.62/88.94] | .543 | > 1 | −.07 [−.31; .16] | 57.77 (9.95) [39.97/81.77] | .281 | > 1 | −.17 [−.49; .14] |
| mid | 65.55 (12.31) [33.47/105.30] | 68.79 (12.63) [46.09/103.59] | .031 | .248 | −.26 [−.50; −.02] | 65.93 (12.64) [40.13/98.76] | .160 | > 1 | −.23 [−.54; .09] |
| posterior | 57.84 (14.42) [19.66/113.88] | 60.33 (13.94) [11.79/97.80] | .152 | > 1 | −.17 [−.41; .06] | 58.03 (14.74) [22.44/88.50] | .318 | > 1 | −.16 [−.48; .15] |
PSM Sample: sample following propensity score matching; pcor: p-value after correcting for multiple testing (Bonferroni); missing rCBF data for n = 35 EA and n = 20 AA in the full-sample, n = 6 EA and n = 20 in the matched sample; ES: Cohen’s d [95% Confidence Interval]
In the full sample, gCBF showed a significant positive correlation with HF-HRV (full sample: r(417) = .100, p = .041) and CoV (r(417) = .123, p = .012) in EA, the correlation was negative in AA for HF-HRV (r(82) = −.232, p = .036) and CoV (r(82) = .231, p = .037). There was no significant correlation between gCBF and HR in EA (r17) = .036, p = .464) or AA (r(82) = .081, p = .469). In the PSM sample of EA, none of the positive correlations between HF-HRV/CoV and gCBF remained significant. Zero-Order correlations between rCBF in all ROI and cardiac activity by sample are provided in the Supplementary Table 1.
Analyses by ROI adjusted for dataset, age, sex, BMI, and gCBF in the full sample showed a significant main effect of ethnicity in predicting HF-HRV (log) and the CoV (log) but not HR, such that HF-HRV was greater in AA compared to EA – in line with analyses in the PSM sample. There were no significant main effects of rCBF in predicting cardiac activity. Frequentist analyses, showed a significant ethnicity by rCBF interaction in predicting HF-HRV and the CoV across all ROIs, with the exception of rCBF in the posterior cingulate not predicting HF-HRV and the CoV. Only rCBF in the superficial amygdala, insula (anterior and posterior) and cingulate (anterior and mid) predicting HF-HRV and the CoV, survived correction for multiple testing. Bayesian statistics showed best evidence for rCBF in the ACC predicting HRV and the CoV in interaction with ethnicity (full reporting in Supplementary Table 2). As illustrated in Figure 3, rCBF in the ACC was positively correlated with HF-HRV/CoV in EA but negatively correlated in AA.
Figure 3: Resting Cerebral Blood Flow in the Anterior Cingulate Cortex Predicting Heart Rate and Heart Rate Variability as a Function of Ethnicity;
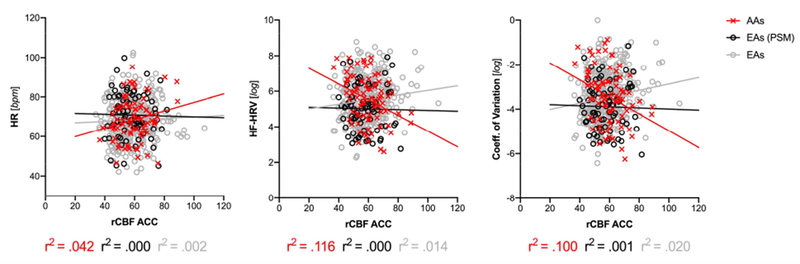
HF-HRV: high-frequency heart rate variability; PSM: EA sample after propensity score matching
Links to Affect and Affect Regulation
Exploring the association between HF-HRV and self-reports of affect and affect regulation, greater suppression or non-expression of angry emotions was significantly correlated with greater HF-HRV in the full sample (r(551) = .113, p = .008). Full reporting of correlation coefficients by sample is provided in Table 3. Correlation coefficients for reappraisal and HF-HRV showed significant differences in EA (full sample) and AA, suggesting that greater habitual use of cognitive reappraisal is associated with greater HF-HRV in AA but lower HF-HRV in EA. Greater suppression of emotions was only negatively associated with rCBF in the ACC in EA (PSM sample). In addition, anger-in was associated with greater HF-HRV (r = .101) and anger-out was associated with lower HF-HRV (r = −.128) assessing within group differences in AA (Zdiff=1.63, p1=.049).
Table 3: Correlations with Self-Reports;
| European Americans | European Americans (PSM Sample) | African Americans | Difference (Full Sample) | Difference (PSM Sample) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF (log) r [95%CI] | ACC rCBF r [95%CI] | HF (log) r [95%CI] | ACC rCBF r [95%CI] | HF (log) r [95%CI] | ACC rCBF r [95%CI] | HF (log) | ACC rCBF | HF (log) | ACC rCBF | |||||
| z | p | z | p | z | p | z | p | |||||||
| Anger Control | .004 [−.088; .097] | −.065 [−.160; .032] | −.042 [−.261;.181] | −.004 [−.232;.225] | .127 [−.069; .314] | −.081 [−.293; .138] | −1.11 | .134 | 0.13 | .448 | −1.11 | .134 | 0.47 | .319 |
| Anger In | .116 [.024; .207] | .016 [−.081; .112] | .210 [−.012;.412] | −.040 [−.266;.191] | .101 [−.095; .290] | −.057 [−.271; .162] | 0.14 | .444 | 0.46 | .323 | 0.73 | .233 | 0.10 | .460 |
| Anger Out | .022 [−.071; .114] | .024 [−.073; .120] | −.003 [−.224;.219] | .069 [−.162:.293] | −.128 [−.315; .068] | −.045 [−.260; .173] | 1.36 | .087 | 0.56 | .289 | 0.82 | .206 | 0.70 | .242 |
| Reappraisal | −.041* [−.133; .051] | .071 [−.026; .166] | −.089 [−.303; .133] | .010 [−.219;.238] | .147* [−.050; .333] | −.091 [−.302; .129] | −1.70 | .045 | 1.32 | .093 | −1.56 | .059 | 0.62 | .268 |
| Suppression | .018 [−.074; .111] | −.085 [−.180; .011] | −.076 [−.291;.146] | −.245 [−.448;−.017] | .110 [−.087; .299] | −.048 [−.263; .170] | −0.83 | .203 | −.30 | .382 | −1.22 | .111 | −1.24 | .108 |
r: Pearson correlation coefficients; 95%CI: 95% confidence interval; Difference: one-sided test for differences between correlation coefficients;
significant between group differences; significant within group correlations are bold.
Discussion
Historically few neuroimaging studies have explicitly investigated ethnic differences. The present study aimed to investigate differences in the neural correlates of resting ANS activity in relation to ethnic differences in HF-HRV. In line with meta-analytical findings (Hill et al., 2015), AA from the present sample showed greater HF-HRV in adjusted analyses, controlling for dataset, age, sex, BMI, and global CBF, and analyses on matched samples. These differences were not evident in unadjusted analyses. However, the present findings are in line with prior meta-regression, suggesting that ethnic differences might differ as a function of age, with lower effect sizes reported in midlife adults as included in the present sample (Hill et al., 2015). In addition, whereas greater HF-HRV was associated with greater global CBF in EA, HRV and global CBF were negatively associated in AA. Both greater HF-HRV and greater global CBF have been shown to be associated with lower cardiovascular disease risk in EA (Jennings et al., 2013; Thayer et al., 2010). That these brain-body relationships may not hold among different ethnicities raises important issues about the generalizability of various risk factors in different populations. Addressing potential differences in the association between rCBF and HF-HRV, analyses showed that rCBF in the ACC in particular is differentially associated with HRV (HF-HRV and CoV) across AA and EA. In EA we found a positive or no (PSM sample) correlation between HF-HRV and rCBF in the ACC, whereas the correlation was negative in AA. Analyses replicate and extend findings from previous studies (Jennings et al., 2015; Allen et al 2015), including almost twice the number of AA. The present study adds that these patterns are stable in a large sample of pooled data, robust when correcting HRV for the influence of HR and applying Bayesian models of statistical testing. In exploratory analyses, aiming to address how different facets of emotion regulation may relate to differences in the association between HF-HRV and rCBF, we found greater suppression of anger related to increased HF-HRV in the full sample. Investigating differences in the association between self-reports of affect and HF-HRV, we found that greater habitual use of reappraisal only showed a positive correlation with HF-HRV in AA (in the absence of a correlation or tendency towards a negative correlation in EA). In addition, consistent with prior research (Dorr et al., 2007), anger-in was associated with greater HF-HRV whereas anger-out was associated with lower HF-HRV in AA only.
The differential association of HF-HRV with global CBF found in the present study adds to the mystery of the Cardiovascular Conundrum in particular and the differential association of risk factors in different populations in general. For example, global CBF has been found to be inversely related to different components of the metabolic syndrome (MetS) in a sample of primarily EA (Jennings et al., 2013). However, the different MetS components have also been shown to be differentially associated with risk in AA compared to EA (Gaillard et al., 2009) with AA showing lower rates of MetS as well as better lipid profiles despite greater cardiometabolic disease burden. Similarly, HF-HRV has been found to be inversely related to all MetS components in a German sample (Jarczok et al., 2013). But as noted, HF-HRV has been shown to be higher in AA despite their increased cardiovascular risk. Thus not all risk factors have the same risk in different populations. Furthermore, several pathways have been suggested to account for the association between global CBF and HF-HRV including nitric oxide mediated vasodilation and related increased cardiac vagal activity, autonomic innervation of the cerebral vasculature, or common third variables linking CBF and HF-HRV. Interestingly, AA have been shown to have reduced nitric oxide mediated vasodilation among several vascular differences compared with EA, and it has been suggested that the increased HF-HRV may serve a compensatory role (Hill & Thayer, 2019; Taherzadeh et al., 2010). However, this does not explain the inverse relationship between global CBF and HF-HRV in AA in the present study. Clearly, additional research is needed to further explicate the factors involved in these health disparities.
Based on the present findings, we speculate that increased HF-HRV in AA may be associated with the increased effort in consciously regulating emotions. Better emotion regulation has been associated with higher resting HF-HRV as well as increased HF-HRV during emotion regulation (Butler et al., 2006; Williams et al., 2015). Of particular relevance to the present study, we reported increased HF-HRV in both EA and AA when they had to inhibit their anger is response to provocation whereas the AA that expressed their anger had the lowest HF-HRV in response to the provocation (Dorr et al., 2007). Consistent with this idea, greater anger-in was associated with greater HF-HRV and increased anger-out was associated with decreased HF-HRV but only in AA. One potential mechanism underlying the inversed association between rCBF in the ACC and HF-HRV in AA, that we were not able to address in the present analyses, involves affect regulation processes linked to the prefrontal cortex (PFC). Greater HF-HRV, for example, has been proposed to be associated with prefrontal neural function (Thayer et al., 2009). In line with this proposal, recent evidence suggests that individuals with greater resting HF-HRV exhibit a greater engagement of prefrontal brain regions relative to reduced sub-cortical neural activity during explicit emotion regulation (Steinfurth et al., 2018). More specifically, in participants with high HF-HRV, right amygdala activity was decreased and right dorsomedial PFC activity was increased when using reappraisal only (not response modification) to downregulate unpleasant emotions – possibly suggesting that habitual conscious, cognitive emotion regulation (i.e., reappraisal), is associated with greater HF-HRV. However, in light of the relatively weak correlations between self-reports of affect and affect regulation with HF-HRV and rCBF in the present study, there is limited evidence suggesting that the habitual use of conscious emotion regulation in AA corresponds to differences in the neural correlates of HF-HRV. Moreover, participants in the present cohorts did not engage in effortful emotion regulation concurrent with HF-HRV recording, as assessments were solely based on self-reports. Further, in the absence of longitudinal data, we can only speculate on the causal relationship between rCBF and HF-HF-HRV and their mediation (or moderator) by psychological factors such as emotion regulation and affect. An inverse association between rCBF and HF-HRV in AA, compared to EA, might be explained by other factors not assessed in the present set of studies. Whereas lower rCBF in the ACC in AA might result in insufficient regulation of emotion, leading to increased physiological arousal, that in turn triggers greater vagal regulation, an inverse or bi-directional pathway is possible. Findings from the study should therefore be considered preliminary, outlining hypotheses and a research agenda to be tested in rigorous experimental trials. Existing studies on HF-HRV in association with neural activity during emotion regulation (Steinfurth et al., 2018) should be replicated, including ethnic minorities to test mechanisms, potentially underlying differences in association between rCBF and HF-HRV. From a developmental perspective, studies trying to replicate the present findings in subjects of younger age or in other ethnic minorities and cultural contexts, might inform the generation of theories, regarding the temporal course of effects. It is possible that an inverse association between rCBF and HF-HRV is a consequence of long-term adaption, depending on the cultural context and the everyday need to regulate emotions, resulting in an increased risk for CVD despite greater HF-HRV. In this regard, HF-HRV may be one factor associated with differences in CVD risk.
The present study has several limitations that need to be addressed. First, we pooled data from two different studies. Methods in the recording of HF-HRV and rCBF were relatively comparable between studies, however, there were some differences. These include recording methodologies and posture differences across the assessment paradigms. Potentially, these differences account for the statistical difference found in CoV data. Given these differences between studies, we controlled for data heritage in all analyses and conducted supplementary PSM analyses. Another limitation is that HF-HRV was not assessed concurrently with perfusion imaging to derive CBF. However, HF-HRV has been shown to be stable over time within individuals. Prior studies on the association between self-report indicators of negative affect and affect regulation have typically revealed small effect sizes, which were also evident in the present study. Few prior studies, however, have tested for ethnic differences in the magnitude or direction of these associations, as was done in the present study. Accordingly, the present findings suggest the importance of examining the modifying role of ethnicity status in studies of the autonomic correlates of affective processes. A strength of the present study is that both participant cohorts were free of confounding medical conditions and medication uses that could have affected estimates of HF-HRV. However, as a result, it is unknown whether the present findings in midlife adults would generalize to a broader population with a greater range of clinical conditions. Further, we only addressed resting HF-HRV and CBF. Measures of reactivity (HF-HRV and CBF) to standardized tasks (e.g. on emotion regulation) as discussed above, may help to illuminate mechanisms underlying the present findings that only capture some of the complexity of ANS-CNS coregulation. Whereas we were able to draw on a relatively large pooled-sample of participants, the relatively small number of AA in comparison to EA remains critical when testing interactions. We tried to compensate for this, reporting model fit characteristics and Bayesian statistics, beyond the interpretation of p-values from frequentist analysis. Further, while we controlled for sex, there were relative few males among AA. Given important sex differences in HF-HRV (Koenig and Thayer, 2016), larger studies are necessary to further address a potential sex by ethnicity interaction. Finally, we predominantly focused on measures of cardiac vagal activity. Future research would do well to disentangle the contributions of sympathetic and parasympathetic systems into ethnic differences in CVD risk., brain activity and their association with emotion and emotion regulation (Shokri-Kojori et al., 2018; Taylor et al., 2016).
To conclude, findings from the present study suggest that whereas in EA greater rCBF in the ACC is associated with greater HF-HRV (full sample), the opposite is observed in AA, such that lower rCBF in the ACC is associated with greater HF-HRV. In a well-matched sub-sample of EA and AA, HF-HRV only showed a negative association with rCBF in the ACC in AA in the absence of an association in EA. We suggest that this difference in association might partly explain the Cardiovascular Conundrum of greater HF-HRV in AA compared to EA. Testing for the association between HF-HRV and self-reports of emotion regulation, we found differences between EA and AA, such that in AA only greater HF-HRV was associated with more habitual use of reappraisal. Based on the limited evidence from self-reports, we speculate that differences in habitual emotion regulation may be associated with altered ANS-CNS coupling. However, future studies are necessary to test these claims in experimental trials, including behavioral tasks of explicit emotion regulation.
Supplementary Material
Acknowledgements:
The authors acknowledge the tremendous support and contribution by Stephen B. Manuck and Peter J. Gianaros from the Department of Psychology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, providing access to their data. The AHAB and PIP studies were founded by the National Heart, Lung, and Blood Institute (NHLBI) NHLBI R01 089850 and NHLBI P01 040962.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB, 2015. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology 52, 277–287. 10.1111/psyp.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ, 2004. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol 93, 381–385. 10.1016/j.amjcard.2003.09.065 [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ, 2006. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology 43, 612–622. 10.1111/j.1469-8986.2006.00467.x [DOI] [PubMed] [Google Scholar]
- Carnevali L, Koenig J, Sgoifo A, Ottaviani C, 2018. Autonomic and Brain Morphological Predictors of Stress Resilience. Front. Neurosci 12 10.3389/fnins.2018.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Tsai W, Sanna LJ, 2010. Examining the relations between rumination and adjustment: Do ethnic differences exist between Asian and European Americans? Asian Am. J. Psychol 1, 46–56. 10.1037/a0018821 [DOI] [Google Scholar]
- Christie IC, Gianaros PJ, 2013. PhysioScripts: an extensible, open source platform for the processing of physiological data. Behav. Res. Methods 45, 125–131. 10.3758/s13428-012-0233-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consedine NS, Magai C, Horton D, 2005. Ethnic Variation in the Impact of Emotion and Emotion Regulation on Health: A Replication and Extension. J. Gerontol. Ser. B 60, P165–P173. 10.1093/geronb/60.4.P165 [DOI] [PubMed] [Google Scholar]
- de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG, 2019. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 56, e13287 10.1111/psyp.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Grisham JR, Moulds ML, 2011. Cognitive reappraisal increases heart rate variability in response to an anger provocation. Motiv. Emot 35, 14–22. 10.1007/s11031-011-9201-5 [DOI] [Google Scholar]
- Dorr N, Brosschot JF, Sollers JJ, Thayer JF, 2007. Damned if you do, damned if you don’t: the differential effect of expression and inhibition of anger on cardiovascular recovery in black and white males. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 66, 125–134. 10.1016/j.ijpsycho.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard T, Schuster D, Osei K, 2009. Independent role of blood pressure on cardiovascular risk factors in nondiabetic, obese African-American women with family history of type 2 diabetes: Implications for metabolic syndrome components. J. Am. Soc. Hypertens. JASH 3, 25–34. 10.1016/j.jash.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD, 2012. Brain systems for baroreflex suppression during stress in humans. Hum. Brain Mapp 33, 1700–1716. 10.1002/hbm.21315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP, 2003. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Hill LK, Hoggard LS, Richmond AS, Gray DL, Williams DP, Thayer JF, 2017. Examining the association between perceived discrimination and heart rate variability in African Americans. Cultur. Divers. Ethnic Minor. Psychol 23, 5–14. 10.1037/cdp0000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Hu DD, Koenig J, Sollers JJ, Kapuku G, Wang X, Snieder H, Thayer JF, 2015. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom. Med 77, 16–25. 10.1097/PSY.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok MN, Li J, Mauss D, Fischer JE, Thayer JF, 2013. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int. J. Cardiol 167, 855–861. 10.1016/j.ijcard.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Jennings J. Richard, Heim Alicia F., Kuan Dora Chieh-Hsin, Gianaros Peter J., Muldoon Matthew F., Manuck Stephen B., 2013. Use of Total Cerebral Blood Flow as an Imaging Biomarker of Known Cardiovascular Risks. Stroke 44, 2480–2485. 10.1161/STROKEAHA.113.001716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Allen B, Gianaros PJ, Thayer JF, Manuck SB, 2015. Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology 52, 214–224. 10.1111/psyp.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, 1995. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn. Reson. Med 34, 293–301. [DOI] [PubMed] [Google Scholar]
- Koenig J, Thayer JF, 2016. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev 64, 288–310. 10.1016/j.neubiorev.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Kwon H, Yoon KL, Joormann J, Kwon J-H, 2013. Cultural and gender differences in emotion regulation: Relation to depression. Cogn. Emot 27, 769–782. 10.1080/02699931.2013.792244 [DOI] [PubMed] [Google Scholar]
- Lane RD, Weidenbacher H, Smith R, Fort C, Thayer JF, Allen JJB, 2013. Subgenual anterior cingulate cortex activity covariation with cardiac vagal control is altered in depression. J. Affect. Disord 150, 565–570. 10.1016/j.jad.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Lockwood KG, Marsland AL, Matthews KA, Gianaros PJ, 2018. Perceived discrimination and cardiovascular health disparities: a multisystem review and health neuroscience perspective. Ann. N. Y. Acad. Sci 1428, 170–207. 10.1111/nyas.13939 [DOI] [PubMed] [Google Scholar]
- Mather M, Thayer JF, 2018. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci., Emotion-cognition interactions 19, 98–104. 10.1016/j.cobeha.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery AE, 1995. Bayesian Model Selection in Social Research. Sociol. Methodol 25, 111–163. 10.2307/271063 [DOI] [Google Scholar]
- Ryan JP, Sheu LK, Verstynen TD, Onyewuenyi IC, Gianaros PJ, 2013. Cerebral blood flow links insulin resistance and baroreflex sensitivity. PloS One 8, e83288 10.1371/journal.pone.0083288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi D, Volkow ND, 2018. An Autonomic Network: Synchrony Between Slow Rhythms of Pulse and Brain Resting State Is Associated with Personality and Emotions. Cereb. Cortex N. Y. N 1991 28, 3356–3371. 10.1093/cercor/bhy144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, Schwarz E, McKinley PS, Weinstein M, Love G, Ryff C, Mroczek D, Choo T, Lee S, Seeman T, 2017. Vagally-Mediated Heart Rate Variability and Indices of Wellbeing: Results of a Nationally Representative Study. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc 36, 73–81. 10.1037/hea0000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, 1988. Manual for the State-Trait Anger Expression Inventory (STAXI). Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Steiger JH, 1980. Tests for comparing elements of a correlation matrix. Psychol. Bull 87, 245–251. 10.1037/0033-2909.87.2.245 [DOI] [Google Scholar]
- Steinfurth ECK, Wendt J, Geisler F, Hamm AO, Thayer JF, Koenig J, 2018. Resting State Vagally-Mediated Heart Rate Variability Is Associated With Neural Activity During Explicit Emotion Regulation. Front. Neurosci 12 10.3389/fnins.2018.00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH, 2011. Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci 23, 121–125. 10.1176/jnp.23.2.jnp121 [DOI] [PubMed] [Google Scholar]
- Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E, 2010. Function and structure of resistance vessels in black and white people. J. Clin. Hypertens. Greenwich Conn 12, 431–438. 10.1111/j.1751-7176.2010.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Kucyi A, Millar PJ, Murai H, Kimmerly DS, Morris BL, Bradley TD, Floras JS, 2016. Association between resting-state brain functional connectivity and muscle sympathetic burst incidence. J. Neurophysiol 115, 662–673. 10.1152/jn.00675.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ III, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev 36, 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. Publ. Soc. Behav. Med 37, 141–153. 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord., Arousal in Anxiety 61, 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF, 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol 141, 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF, 2015. Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Front. Psychol 6, 261 10.3389/fpsyg.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Pandya KD, Hill LK, Kemp AH, Way BM, Thayer JF, Koenig J, 2017. Rumination Moderates the Association Between Resting High-Frequency Heart Rate Variability and Perceived Ethnic Discrimination. J. Psychophysiol 1–9. 10.1027/0269-8803/a000201 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


