Abstract
BACKGROUND
Mesenchymal tumors such as perivascular epithelioid cell neoplasm (PEComa) and inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDC sarcoma) are relatively uncommon in the liver and are particularly rare in the caudate lobe. The clinical manifestations and available imaging tests lack specificity for hepatic mesenchymal tumors. To the best of our knowledge, no caudate PEComa or IPT-like FDC sarcoma has been completely resected by laparoscopy. The standard laparoscopic technique, surgical approaches, and tumor margins for potentially malignant or malignant caudate mesenchymal tumors are still being explored.
AIM
To assess both the safety and feasibility of laparoscopic resection for rare caudate mesenchymal neoplasms.
METHODS
Eleven patients who underwent isolated caudate lobe resection from 2003 to 2017 were identified from a prospective database. Three consecutive patients with rare caudate mesenchymal tumors underwent laparoscopic resection. Patient demographic data, intraoperative parameters, and postoperative outcomes were assessed and compared with the open surgery group.
RESULTS
All procedures for the three resection patients with caudate mesenchymal tumors were completed using a total laparoscopic technique by two different approaches. The average operative time was 226 min, and the estimated blood loss was 133 mL. The average length of postoperative hospital stay was 6.3 ± 0.3 d for the laparoscopy group and 15.5 ± 2.3 d for the open surgery group (P < 0.05). There were no perioperative complications or patient deaths in this series.
CONCLUSION
Laparoscopic isolated caudate lobe resection for rare mesenchymal neoplasms is a feasible and curative surgical option in selected patients.
Keywords: Laparoscopic liver resection, Caudate lobe, Perivascular epithelioid cell neoplasm, Inflammatory pseudotumor-like follicular dendritic cell sarcoma
Core tip: Although laparoscopic liver resection is now considered a standard procedure in peripheral segments, there are a limited number of reports focused on laparoscopic caudate lobe resection. Perivascular epithelioid cell neoplasm and inflammatory pseudotumor-like follicular dendritic cell sarcoma are particularly rare mesenchymal neoplasms in the liver, especially in the caudate lobe. Laparoscopic resection provides better quality of care and shortens hospital stay relative to open surgery. For such rare mesenchymal neoplasms, laparoscopic isolated caudate lobe resection is a feasible and curative surgical option in selected patients.
INTRODUCTION
Hepatic mesenchymal tumors are neoplasms that arise from vascular, fibrous, adipose, and other mesenchymal tissue components[1]. Excluding hemangiomas, mesenchymal tumors such as perivascular epithelioid cell neoplasm (PEComa) and inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDC sarcoma) are relatively uncommon in the liver and are particularly rare in the caudate lobe. The clinical manifestations and available imaging tests lack specificity for hepatic mesenchymal tumors. Therefore, a correct diagnosis is made on the basis of morphologic characteristics and immunohistochemical results after resection or biopsy[2].
The caudate lobe has unique anatomy[3] and is considered the forbidden zone of laparoscopic hepatectomy. However, there have been sporadic attempts to apply the laparoscopic approach to the caudate lobe due to encouraging outcomes of laparoscopic liver resection and improved surgical experience[4-6]. However, to the best of our knowledge, no caudate PEComa or IPT-like FDC sarcoma has been completely resected by laparoscopy. The standard laparoscopic technique, surgical approaches, and tumor margins for potentially malignant or malignant caudate mesenchymal tumors are still being explored. In this study, we present three consecutive cases of caudate mesenchymal tumors (2 cases of PEComa and 1 case of IPT-like FDC sarcoma) completely resected by laparoscopy by two different approaches. We assessed the safety and feasibility of the procedure by comparing the outcomes to those of patients who underwent isolated caudate resection by open surgery.
MATERIALS AND METHODS
All patients who underwent isolated caudate lobe resection at our institution from 2003 to 2017 were identified in a prospective database, and their data were retrospectively reviewed. The patients who underwent isolated caudate lobe resection by laparoscopy were compared with those who underwent open surgery in terms of tumor characteristics, intraoperative characteristics, and postoperative outcomes.
Preoperative evaluation
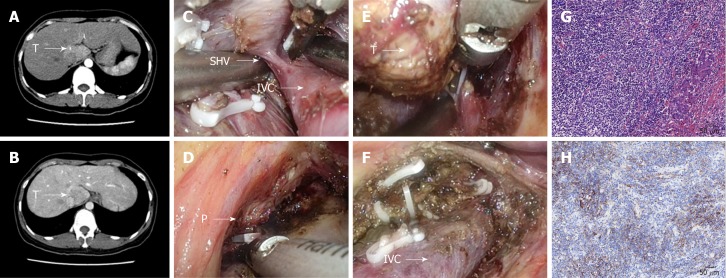
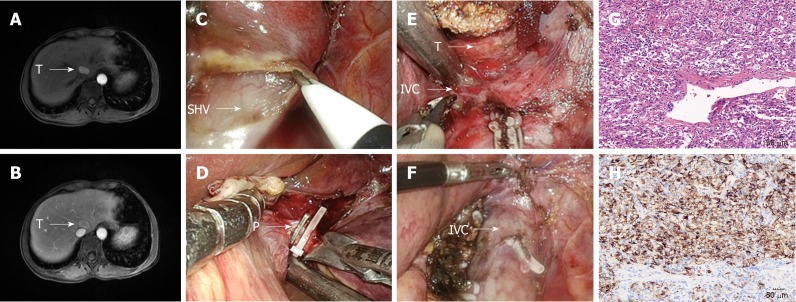
The preoperative investigations included blood and liver function tests and routine cardiorespiratory evaluations. The imaging tests included enhanced computed tomography, magnetic resonance imaging, and ultrasound. There was no specific evaluation required for caudate lobe resection. However, special attention was given to the caudate pedicel and contact between the tumor and inferior vena cava (IVC) (Figure 1A, B and Figure 2A, B).
Figure 1.
Combined approach to laparoscopic caudate inflammatory pseudotumor-like follicular dendritic cell sarcoma lobectomy. A and B: The tumor was located at the junction of the Spiegel’s lobe and the paracaval portion; C: The short hepatic vein was dissected; D: The feeding portal pedicle of the caudate lobe (arrow P); E: Isolation of the caudate lobe from right side; F: Surgical area after the tumor was resected; G: Microscopic appearance of caudate inflammatory pseudotumor-like follicular dendritic cell sarcoma (×200); H: Positive CD21 staining by immunohistochemistry (×200).
Figure 2.
Left approach to laparoscopic caudate perivascular epithelioid cell neoplasm lobectomy. A and B: The tumor was located at the Spiegel’s lobe; C: Isolation of the caudate lobe from left side; D: Dissection of the feeding portal pedicle of the caudate lobe (arrow P); E: Isolation of the tumor (arrow T) and dissection of short hepatic vein (arrow SHV); F: Surgical area after the tumor was resected; G: Microscopic appearance of caudate perivascular epithelioid cell neoplasm (×200); H: Positive HMB-45 staining by immunohistochemistry (×200).
Surgical procedures
The isolated caudate resection in open surgery procedures was performed as previously described[7].
Basic surgical techniques for laparoscopic isolated resection of the caudate lobe
The patient was placed in a supine position. Pneumoperitoneum was established at 13 mmHg. The patient’s central venous pressure was maintained at < 5 mmHg. Five port sites were used during the surgery. A 30-degree laparoscope was inserted through the central 12-mm port site under the umbilicus. Then, a 12-mm trocar was inserted subxiphoid, and two trocars (12 mm) were placed on the right and left midclavicular line subcostally. Two trocars (5 mm) are sited on the right and left anterior axillary line. The patient was tilted to the reverse Trendelenburg position. A complete laparoscopic examination of the abdominal contents, including laparoscopic liver ultrasonography, was performed to exclude undetected lesions or metastases. No inflow or outflow control of the liver was set during the surgery. The liver parenchyma was transected with a Harmonic scalpel.
Right and left combined approach
The liver was mobilized from both sides. The caudate lobe was retracted upward, and the short hepatic veins were identified, dissected, and divided from the right side before the dorsal trunks of the major hepatic veins were exposed (Figure 1C). After the portal pedicles of the caudate lobe were divided, the liver parenchyma was dissected from the caudal to the cranial aspects (Figure 1D). The liver dissection started from both sides and met at the cranial part of the caudate (Figure 1E and F).
Left approach
The left triangular ligament was divided. The liver was lifted upward with the subxiphoid trocar. The gastrohepatic ligament and left vena cava ligament were then divided. The caudate lobe was retracted to the right, and the short hepatic veins were identified, dissected, and divided. The cranial part of the caudate lobe was dissected carefully from the major hepatic veins (Figure 2C, E). The caudate lobe was then retracted anteriorly and caudally, and the portal pedicles going toward the caudate lobe were identified, dissected free, and divided (Figure 2D). The hepatic transection started from the inferior part from the caudal to the cranial aspects along the IVC (Figure 2F).
All intraoperative parameters, including duration of vascular clamping, blood loss with subsequent intraoperative blood transfusion, and duration of surgery, were recorded. The overall surgical policy was to attempt radical anatomic resection while sparing the greatest amount of liver parenchyma feasible.
Statistical analysis
Continuous variables are reported as the mean and standard deviation and were compared by a Student’s t-test. P values < 0.05 were considered significant. Statistical review was performed by a biomedical statistician.
RESULTS
The characteristics of all 11 patients are detailed in Tables 1 and 2. Laparoscopic isolated caudate lobe resection was completed in all three patients without conversion to an open approach. The diagnoses of these three patients were PEComa (patient Nos. 10 and 11) and IPT-like FDC sarcoma (patient No. 9). The pathologic examination of patient No. 9 revealed localized spindle cell proliferation with cellular atypia surrounded by lymphocytes and plasma cells (Figure 1G). The immunohistochemistry staining showed that the spindle cells were positive for CD21 (Figure 1H), CD23, and CD35. Many EBV-positive spindle cells were identified by EBV-encoded small RNA in situ hybridization. The pathologic examination of patient No. 10 showed nodularly and trabecularly distributed epithelioid cells composed of thick-walled blood vessels, smooth muscle cells, and adipose cells (Figure 2G). The tumor cells were positive for HMB-45 (Figure 2H) and melan-A on immuno-histochemical staining.
Table 1.
Clinical data
| Patient No. | Gender | Age | Diagnosis | Tumor size (cm) |
| 1 | Male | 42 | HCC | 1 × 1 |
| 2 | Male | 62 | HCC | 5 × 4 |
| 3 | Male | 52 | HCC | 2 × 1.5 |
| 4 | Male | 51 | HCC | 4 × 3 |
| 5 | Male | 44 | HCC | 8 × 4 |
| 6 | Female | 32 | FNH | 5 × 3 |
| 7 | Female | 52 | HCC | 2 × 2 |
| 8 | Male | 46 | HCC | 1 × 1 |
| 9 | Female | 32 | IPT-like FDC sarcoma | 3 × 3 |
| 10 | Female | 46 | PEComa | 2 × 1.5 |
| 11 | Female | 50 | PEComa | 1.8 × 1.3 |
HCC: Hepatocellular carcinoma; FNH: Focal nodular hyperplasia; PEComa: Perivascular epithelioid cell neoplasm; IPT-like FDC sarcoma: Inflammatory pseudotumor-like follicular dendritic cell sarcoma.
Table 2.
Surgical data
| Patient No. | Surgical procedure | Pringle maneuver | Blood loss (mL) | Operation time (min) | Complications | Time to removal of drainage tube (d) | Postoperative hospital stay (d) |
| 1 | Left | No | 50 | 135 | None | 2 | 12 |
| 2 | Right | No | 300 | 150 | None | 6 | 11 |
| 3 | Combined | No | 500 | 375 | Pleural effusion | 23 | 27 |
| 4 | Left | No | 200 | 270 | None | 9 | 12 |
| 5 | Left | No | 500 | 620 | Diarrheal | 5 | 14 |
| 6 | Left | No | 1000 | 265 | Pneumonia | 6 | 10 |
| 7 | Left | No | 200 | 130 | None | 5 | 13 |
| 8 | Left | No | 200 | 173 | None | 10 | 25 |
| 9 | Left | No | 100 | 193 | None | 3 | 7 |
| 10 | Combined | Yes | 200 | 229 | Pleural effusion | 4 | 6 |
| 11 | Left | No | 100 | 255 | None | 3 | 6 |
The intermittent Pringle’s maneuver was applied in one of three patients. The blood loss ranged from 100 mL to 200 mL, and no blood transfusions were required. The abdominal drainage was removed within 4 d in all cases. There were no signs of hemodynamic instability during surgery, and none of the patients required open conversion.
The postoperative courses were uneventful for all patients. All patients were able to tolerate liquids on the second postoperative day, and no biliary complications were encountered. All surgical margins were negative. The patients were alive and free of disease during a median follow-up period of 8 mo.
The postoperative hospital stay was shorter in the laparoscopy group than in the open surgery group (6.3 ± 0.3 d vs 15.5 ± 2.3 d, P = 0.006). The blood loss (133 ± 33 mL vs 368 ± 105 mL, P = 0.22), operation time (225 ± 18 min vs 264 ± 59 min, P = 0.71), time to removal of drainage tube (3.3 ± 0.3 d vs 8.2 ± 2.3 d, P = 0.23), and occurrence of complications (3/8 vs 1/3, P = 0.72) were comparable between the two groups (Table 3).
Table 3.
Results of statistical analysis
| Open surgery | Laparoscopic surgery | P | |
| Blood loss (mL) | 368 ± 105 | 133 ± 33 | 0.22 |
| Operation time (min) | 264 ± 59 | 225 ± 18 | 0.71 |
| Complication | 3/8 | 1/3 | 0.72 |
| Time to removal of drainage tube (d) | 8.2 ± 2.3 | 3.3 ± 0.3 | 0.23 |
| Postoperative hospital stays (d) | 15.5 ± 2.3 | 6.3 ± 0.3 | 0.006 |
DISCUSSION
PEComa is defined as a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells[8]. ITP-like FDC sarcoma is another rare hepatic mesenchymal tumor characterized by a mixture of chronic inflammatory cells and variable numbers of spindle cells with vesicular nuclei and distinct nucleoli[9]. Both of these tumors are considered either malignant or potentially malignant based on the histology features. Aside from hemangiomas, mesenchymal tumors are relatively uncommon in the liver, especially in the caudate lobe. Surgical treatment remains the mainstay of treatment. However, the optimal surgical procedures have not been defined due to limited experience. Caudate lobe resection is technically challenging due to the unique anatomical location. Local excision of the caudate lobe is technically demanding and requires different surgical strategies for each individual case. There are currently only a limited number of reports on laparoscopic caudate lobe resection.
Our study represents the first series reporting the results of laparoscopic caudate lobe resection for rare hepatic mesenchymal neoplasms and an analysis comparing other lesions in the caudate lobe resected by open surgery. Our results suggest that laparoscopic caudate resection can be safely performed for mesenchymal neoplasms without open conversion or patient death.
Accumulating evidence suggests that, in addition to its role as a staging tool, laparoscopic liver resection provides better quality of care, improves patient outcomes by minimizing blood loss and postoperative pain or morbidity, and shortens hospital stay relative to open surgery[10,11]. These findings are consistent with our results. The major risk for laparoscopic caudate resection is massive bleeding from major vessels. A combination of well-controlled central venous pressure and skilled suture techniques permitted management of major hepatic vein injury while dissecting the attachment in one of our patients, and no conversions or severe complications occurred.
The reported oncologic control of malignant hepatic tumors by laparoscopic hepatectomy in many series has demonstrated comparable results versus the open approach. The 3- and 5-year survival rates reported for hepatocellular carcinoma[12] and colorectal cancer metastases[13] are comparable between laparoscopic hepatectomy and open hepatic resection. However, both PEComa and ITP-like FDC sarcoma are extremely rare mesenchymal tumors reported in only sporadic cases. Several research studies have emphasized that a safe surgical margin is associated with improved survival in patients with malignant hepatic mesenchymal tumors[14,15]. The overall surgical policy for malignant or potentially malignant mesenchymal tumors is to obtain a negative margin. Our study emphasizes several issues that might improve radical laparoscopic caudate resection.
First, laparoscopic ultrasound should be used during the procedure to provide a precise evaluation of tumor location and its relationship with the adjacent vascular structures. Second, a retrohepatic tunnel should be accessed with priority. The laparoscopic surgical field is visualized from the caudal side to the cranial side and has significant advantages because it provides an excellent view and access to the retrohepatic tunnel along the IVC. This allows the short hepatic veins to be meticulously dissected and divided. The dissection of the dorsal part of the caudate lobe provides more space for safely dissecting cranial attachments from the major hepatic veins. Moreover, different surgical approaches should be considered based on tumor location. In the current series, the left approach was used in a patient with tumors located in the Spiegel portions, and the combined approach was used in the other two patients with tumors located in the paracaval portions. It has been widely proposed to use hepatic vascular control in open hepatic caudate lobectomy to reduce hemorrhage and vascular dissemination. However, this might not be necessary in laparoscopic caudate resection. Laparoscopy provides a clear visual image and access to the retrohepatic space, which improves precise dissection and efficient hemostasis without frequent retraction of the liver. In addition, the intra-abdominal pressure can be increased to 15 mmHg to balance the central venous pressure. Laparoscopic caudate resection should be applied in selected patients. Surgeons should consider the risk of massive bleeding from major vessels such as the IVC or middle hepatic vein associated with the anatomical location of the caudate tumor.
The limitations of the present series include the limited number of patients who underwent laparoscopic resection and the relative heterogeneity of the tumors. There were no cases of PEComa or IPT-like FDC sarcoma resected by open surgery in our study. Additionally, the morbidity of these mesenchymal neoplasms makes it difficult to compare the long-term outcomes of the two different surgical approaches. Another limitation of our study is that laparoscopic caudate lobe resection might be indicated for only selected patients with small tumors.
In conclusion, PEComa and ITP-like FDC sarcoma are both extremely rare mesenchymal neoplasms. Our data indicate that laparoscopic isolated caudate lobectomy for mesenchymal tumors is a safe and feasible procedure with acceptable perioperative results.
ARTICLE HIGHLIGHTS
Research background
Mesenchymal tumors such as perivascular epithelioid cell neoplasm (PEComa) and inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDC sarcoma) are very uncommon neoplasms in the liver, especially in the caudate lobe. Laparoscopic resection of such tumors in the caudate lobe has not been reported.
Research motivation
There is no report about laparoscopic resection of the rare hepatic mesenchymal neoplasms in the caudate lobe, and we wanted to explore the feasibility of laparoscopic resection of such tumors in the caudate lobe.
Research objectives
To assess the safety and feasibility of laparoscopic resection of rare caudate neoplasms like PEComa and IPT-like FDC sarcoma compared with open surgery.
Research methods
We analyzed the hospital stay duration, blood loss, operation time, time to removal of drainage tube, and occurrence of complications between the laparoscopic and open surgery groups.
Research results
The postoperative duration of hospital stay was significantly shorter in laparoscopic group, while the operation time, blood loss, time to removal of drainage tube, and occurrence of complications were comparable between the two groups.
Research conclusions
Laparoscopic resection of rare mesenchymal neoplasms like PEComa and IPT-like FDC sarcoma by isolated caudate lobectomy is safe and feasible.
Research perspectives
Our research indicates that laparoscopic caudate lobectomy is a prospective way to handle rare tumors in the caudate lobe.
Footnotes
Institutional review board statement: The research was approved by the ethics committee of The Third Affiliated Hospital of Sun Yat-sen University.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: All authors have nothing to declare.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: June 6, 2019
First decision: July 30, 2019
Article in press: September 9, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mizuguchi T S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
Contributor Information
Yang Li, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Kai-Ning Zeng, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Dan-Yun Ruan, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Jia Yao, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Yang Yang, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Gui-Hua Chen, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China.
Gen-Shu Wang, Department of Liver Surgery and Liver Transplantation, Guangzhou Clinical Research and Translation Center for Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China. wanggshu@mail.sysu.edu.cn.
References
- 1.Thampy R, Elsayes KM, Menias CO, Pickhardt PJ, Kang HC, Deshmukh SP, Ahmed K, Korivi BR. Imaging features of rare mesenychmal liver tumours: beyond haemangiomas. Br J Radiol. 2017;90:20170373. doi: 10.1259/bjr.20170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CH, Chiu NC, Yeh YC, Kuo Y, Yu SS, Weng CY, Liu CA, Chou YH, Chiou YY. Uncommon liver tumors: Case report and literature review. Medicine (Baltimore) 2016;95:e4952. doi: 10.1097/MD.0000000000004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaib E, Ribeiro MA, Jr, Silva Fde S, Saad WA, Cecconello I. Surgical approach for hepatic caudate lobectomy: Review of 401 cases. J Am Coll Surg. 2007;204:118–127. doi: 10.1016/j.jamcollsurg.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Cai X, Zhao J, Wang Y, Yu H, Liang X, Jin R, Meng N, Chen J. A Left-Sided, Purely Laparoscopic Approach for Anatomic Caudate Hepatectomy: A Single-Center Experience. J Laparoendosc Adv Surg Tech A. 2016;26:103–108. doi: 10.1089/lap.2015.0223. [DOI] [PubMed] [Google Scholar]
- 5.Dulucq JL, Wintringer P, Stabilini C, Mahajna A. Isolated laparoscopic resection of the hepatic caudate lobe: surgical technique and a report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2006;16:32–35. doi: 10.1097/01.sle.0000202183.27042.63. [DOI] [PubMed] [Google Scholar]
- 6.Gringeri E, Boetto R, Bassi D, D'Amico FE, Polacco M, Romano M, Barbieri S, Feltracco P, Spampinato M, Zanus G, Cillo U. Totally laparoscopic caudate lobe resection: technical aspects and literature review. Surg Laparosc Endosc Percutan Tech. 2014;24:e233–e236. doi: 10.1097/01.sle.0000442525.26905.6d. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Wang L, Yu YQ, Zhou DE, Liu DR, Yang JJ, Peng SY, Li JT. Anatomic isolated caudate lobectomy: Is it possible to establish a standard surgical flow? World J Gastroenterol. 2017;23:7433–7439. doi: 10.3748/wjg.v23.i41.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patra S, Vij M, Kota V, Kancherla R, Rela M. Pigmented perivascular epithelioid cell tumor of the liver: report of a rare case with brief review of literature. J Cancer Res Ther. 2013;9:305–307. doi: 10.4103/0973-1482.113401. [DOI] [PubMed] [Google Scholar]
- 9.Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005;18:260–266. doi: 10.1038/modpathol.3800294. [DOI] [PubMed] [Google Scholar]
- 10.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–92; discussion 392-4. doi: 10.1097/SLA.0b013e318146996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 13.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 14.Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom Imaging. 2012;37:781–787. doi: 10.1007/s00261-012-9850-1. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang HY, Zhang X, Zhao XM, Su Q. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504–2519. doi: 10.3748/wjg.v16.i20.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]