Abstract
Background
Pleural infection, including parapneumonic effusions and thoracic empyema, may complicate lower respiratory tract infections. Standard treatment of these collections in adults involves antibiotic therapy, effective drainage of infected fluid and surgical intervention if conservative management fails. Intrapleural fibrinolytic agents such as streptokinase and alteplase have been hypothesised to improve fluid drainage in complicated parapneumonic effusions and empyema and therefore improve treatment outcomes and prevent the need for thoracic surgical intervention. Intrapleural fibrinolytic agents have been used in combination with DNase, but this is beyond the scope of this review.
Objectives
To assess the benefits and harms of adding intrapleural fibrinolytic therapy to standard conservative therapy (intercostal catheter drainage and antibiotic therapy) in the treatment of complicated parapneumonic effusions and empyema.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase, ClinicalTrials.gov and the World Health Organization (WHO) trials portal. We contacted trial authors for further information and requested details regarding the possibility of unpublished trials. The most recent search was conducted on 28 August 2019.
Selection criteria
Parallel‐group randomised controlled trials (RCTs) in adult patients with post‐pneumonic empyema or complicated parapneumonic effusions (excluding tuberculous effusions) who had not had prior surgical intervention or trauma comparing an intrapleural fibrinolytic agent (streptokinase, alteplase or urokinase) versus placebo or a comparison of two fibrinolytic agents.
Data collection and analysis
Two review authors independently extracted data. We contacted study authors for further information. We used odds ratios (OR) for dichotomous data and reported 95% confidence intervals (CIs). We used Cochrane's standard methodological procedures of meta‐analysis. We applied the GRADE approach to summarise results and to assess the overall certainty of evidence.
Main results
We included in this review a total of 12 RCTs. Ten studies assessed fibrinolytic agents versus placebo (993 participants); one study compared streptokinase with urokinase (50 participants); and one compared alteplase versus urokinase (99 participants). The primary outcomes were death, requirement for surgical intervention, overall treatment failure and serious adverse effects. All studies were in the inpatient setting. Outcomes were measured at varying time points from hospital discharge to three months. Seven trials were at low or unclear risk of bias and two at high risk of bias due to inadequate randomisation and inappropriate study design respectively.
We found no evidence of difference in overall mortality with fibrinolytic versus placebo (OR 1.16, 95% CI 0.71 to 1.91; 8 studies, 867 participants; I² = 0%; moderate certainty of evidence). We found evidence of a reduction in surgical intervention with fibrinolysis in the same studies (OR 0.37, 95% CI 0.21 to 0.68; 8 studies, 897 participants; I² = 51%; low certainty of evidence); and overall treatment failure (OR 0.16, 95% CI 0.05 to 0.58; 7 studies, 769 participants; I² = 88%; very low certainty of evidence, with evidence of significant heterogeneity). We found no clear evidence of an increase in adverse effects with intrapleural fibrinolysis, although this cannot be excluded (OR 1.28, 95% CI 0.36 to 4.57; low certainty of evidence). In a sensitivity analysis, the reduction in referrals for surgery and overall treatment failure with fibrinolysis disappeared when the analysis was confined to studies at low or unclear risk of bias. In a moderate‐risk population (baseline 14% risk of death, 20% risk of surgery, 27% risk of treatment failure), intra‐pleural fibrinolysis leads to 19 more deaths (36 fewer to 59 more), 115 fewer surgical interventions (150 fewer to 55 fewer) and 214 fewer overall treatment failures (252 fewer to 93 fewer) per 1000 people.
A single study of streptokinase versus urokinase found no clear difference between the treatments for requirement for surgery (OR 1.00, 95% CI 0.13 to 7.72; 50 participants; low‐certainty evidence). A single study of alteplase versus urokinase showed no clear difference in requirement for surgery (OR alteplase versus urokinase 0.46, 95% CI 0.04 to 5.24) but an increased rate of adverse effects, primarily bleeding, with alteplase (OR 5.61, 95% CI 1.16 to 27.11; 99 participants; low‐certainty evidence). This translated into 154 (6 to 499 more) serious adverse events with alteplase compared with urokinase per 1000 people treated.
Authors' conclusions
In patients with complicated infective pleural effusion or empyema, intrapleural fibrinolytic therapy was associated with a reduction in the requirement for surgical intervention and overall treatment failure but with no evidence of change in mortality. Discordance between the negative largest trial of this therapy and other studies is of concern, however, as is an absence of significant effect when analysing low risk of bias trials only. The reasons for this difference are uncertain but may include publication bias. Intrapleural fibrinolytics may increase the rate of serious adverse events, but the evidence is insufficient to confirm or exclude this possibility.
Plain language summary
Clot‐busting drugs for infections of the lining of the lung
Background
Empyema and complicated parapneumonic effusion are conditions involving infected fluid gathering between the lung and the chest wall (the pleural space). They are treated by draining the fluid with a tube inserted through the chest into the fluid (a 'chest tube'), along with antibiotics. If this does not work, then surgery is usually needed to drain the fluid. Fibrinolytic drugs ('clot‐busting drugs') may make the infected pleural fluid thinner, less sticky and easier to drain via a chest tube, meaning that surgery may not be needed.
Review question
We wanted to know if fibrinolytics reduced the need for people with infections in the pleural space to have surgery to fix the infection. We also wanted to see if these medicines reduced the chance of people dying due to these infections; whether the fibrinolytic treatment worked overall; and whether these medicines caused serious side effects. We also wanted to know if one fibrinolytic medicine was more effective than another.
Study characteristics
We searched for studies up to August 2019. We included 10 studies with a total of 993 patients comparing fibrinolytics with a placebo and compared these to look for differences. We also included two studies comparing different fibrinolytics with a total of 149 patients and compared these separately.
Key findings
We found some low‐certainty evidence that fibrinolytics moderately reduced the need for surgery. There was no clear evidence that fibrinolytics changed the risk of death. There was some low‐certainty evidence which showed that there may be a risk of more side effects (mostly bleeding) with fibrinolytics but this is uncertain. We found no clear evidence that any single fibrinolytic was better than another.
Certainty of the evidence
We considered the certainty of the evidence identified comparing fibrinolytic with placebo to vary from moderate (risk of death) to very low (overall treatment failure). This was mostly due to some studies having one or more domains at high risk of bias as well as concerns that not all studies of this treatment appear to have been published. We considered the evidence comparing individual fibrinolytics to be of low certainty due to not enough patients in the studies as well as one study being at a high risk of bias.
Summary of findings
Background
Description of the condition
Thoracic empyema is defined as pus within the pleural space and is usually due to bacterial infection. Empyema may arise in association with pneumonia, as a result of a parapneumonic effusion (a type of pleural effusion, or build‐up of liquid, that arises as a result of a pneumonia) and this may progress from simple to complicated parapneumonic effusion (CPE). Alternately, it may present as a primary pleural infection (i.e. without evidence of pneumonia).
Parapneumonic effusion may occur in up to 57% of pneumonia cases in adults (Sahn 1993), progressing to empyema in 5% to 10% of people (Strange 1999). Mortality rates in empyema are approximately 20% overall (Farjah 2007; Maskell 2005). The incidence of pleural infection has increased markedly in adults aged over 65 years (Grijalva 2011), with a mortality rate greater than 30% in this population (Maskell 2005).
A parapneumonic effusion may constitute an incidental, non‐significant finding or become large and persistent. The formation of parapneumonic effusions can be divided into three stages (Light 1985; Sahn 1993). Simple exudates with no loculations and pH greater than 7.2 do not necessarily require drainage. Fibrino‐purulent pleural fluid (with loculations or a pH less than 7.2) should, however, be drained as should organised empyema (Davies 2010).
Current guidelines from the American Association of Thoracic Surgeons (Shen 2017) and from the British Thoracic Society (Davies 2010) recommend that if chest tube drainage is ineffective then surgical procedures via video‐assisted thoracoscopic surgery (VATS) or thoracotomy should be first‐line management for empyema and CPE. Both guidelines recommend that intrapleural fibrinolysis not be routinely used.
Description of the intervention
For parapneumonic effusions which require clearance, appropriate therapy is effective drainage via an intercostal catheter (ICC) with antibiotic therapy. Frequently, simple ICC drainage is not effective due to the presence of loculations, formed predominantly by fibrinous material deposited in the fibrinopurulent phase of empyema, which prevent free drainage of infected pleural fluid (LeMense 1995). The presence of fibrinous septae in the pleural space, known as loculations, may result in inadequate drainage of effusions and therefore non‐resolution of infection and systemic sepsis. Without effective intercostal catheter drainage, surgical intervention (VATS or open) has usually been required to clear loculations for resolution of infection.
Non‐surgical treatment options to reduce the impact of adhesions and loculae include (in addition to appropriate antibiotic therapy) single and multiple thoracocenteses, or single and multiple intercostal tube thoracostomies, with or without intrapleural fibrinolytic agents. Surgical options include direct‐vision and VATS adhesiolysis, limited and full thoracotomy with adhesiolysis, and decortication for severe pleural thickening.
Although the success rate of surgical intervention remains high (Scarcia 2015), the morbidity and mortality of both VATS and open thoracotomy are of concern, particularly in a cohort of patients who may be older and with significant co‐morbidity. Less invasive therapies which promote pleural space drainage and effective resolution of pleural infection are therefore likely to be of considerable clinical utility.
Intra‐pleural chemical fibrinolysis in the management of complex parapneumonic effusions and thoracic empyema has been employed for over 50 years, with a mixture of agents including streptokinase/streptodornase (Tillett 1951), streptokinase (Bouros 1997; Davies 1997; Diacon 2004; Maskell 2005), urokinase (Bouros 1999), alteplase (Rahman 2011; Thommi 2012), and a combination of streptodornase and alteplase (Rahman 2011). These medications are administered into the pleural space via an ICC.
How the intervention might work
Fibrinolytic agents including streptokinase, urokinase, alteplase and recombinant tissue plasminogen activator (rTPA) have been used safely and effectively intrapleurally for CPE and empyema (Idell 2005; Skeete 2004; Thommi 2000; Walker 2003). During the fibrinopurulent‐purulent stage of empyema, there is an imbalance between fibrin activators and fibrin inhibitors (Idell 1991), with elevated levels of plasminogen activator inhibitor (PAI‐1) resulting from the presence of inflammation‐induced tumour necrosis factor‐alpha, interleukin 8 and transforming growth factor beta, as well as lower levels of endogenous tissue plasminogen activator (TPA) (Chung 2005). This results in a pro‐fibrotic state causing deposition of fibrin‐forming loculations within the infected pleural space (Piccolo 2015). Fibrinolytic agents activate plasmin, lysing fibrinous septations, thereby improving pleural fluid drainage and clearing infection without requiring surgical intervention. Two deoxyribonucleases (DNase) have been used in pleural infection: dornase alfa is a highly purified recombinant human DNase, licensed for usage as a mucolytic (Piccolo 2015); while streptodornase was used in earlier reports (Tillett 1951). Assessing DNase is beyond the scope of this review.
Pus contains large amounts of deoxyribonucleoprotein content from leucocyte degradation which contributes significantly to pus viscosity. DNases in combination with a fibrinolytic agent breaks down this material thereby decreasing its viscosity. The combination of a fibrinolytic and a DNase has been shown to be more effective than either agent alone in a rabbit model (Light 2000). The sole human randomised trial of this combined therapy also showed better pleural fluid clearance and reduced requirement for surgery (Rahman 2011).
Why it is important to do this review
Given the usual alternative therapy of surgical intervention, and a patient group which is frequently older (Grijalva 2011), multi‐morbid and which may not tolerate surgery well, there is clinical equipoise in the usage of intrapleural fibrinolysis as a treatment modality in pleural infection to improve outcomes and reduce requirements for surgery. This review updates a previous Cochrane Review (Cameron 2008)
Objectives
To assess the benefits and harms of adding intrapleural fibrinolytic therapy to standard conservative therapy (intercostal catheter drainage and antibiotic therapy) in the treatment of complicated parapneumonic effusions and empyema.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials.
Types of participants
We included trials with participants older than 14 years presenting with either thoracic empyema or complicated parapneumonic effusions. We excluded studies on known tuberculous effusions and those on participants with malignancy, trauma or prior surgical intervention. We also excluded trials comparing fibrinolytic therapy with surgical therapies.
Types of interventions
-
Intrapleural fibrinolytics versus control
Intrapleural streptokinase versus intrapleural normal saline
Intrapleural urokinase versus intrapleural normal saline
Intrapleural alteplase versus intrapleural normal saline
Intrapleural streptokinase versus intrapleural urokinase
Intrapleural alteplase versus intrapleural urokinase
Types of outcome measures
Primary outcomes
Mortality
Referral for thoracic surgery (open or thorascopic)
Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy
Serious adverse events
Secondary outcomes
None
Search methods for identification of studies
Electronic searches
The Cochrane Airways Information Specialist conducted searches in the following databases and trials registries.
Cochrane Airways Register via the Cochrane Register of Studies (CRS Web) (searched 28 August 2019);
Cochrane Central Register of Controlled Trials (CENTRAL; 2010, Issue 8) via the Cochrane Register of Studies (CRS Web) (searched 28 August 2019);
MEDLINE (Ovid) 1946 to December week 4 2017 (searched 28 August 2019);
Embase (Ovid) 1976 to week 2 2018 (searched 28 August 2019);
ClinicalTrials.gov (searched 28 August 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 28 August 2019).
The full search strategies are detailed in Appendix 1. We searched databases from their inception to the present, with no restriction on language of publication, or publication type. We handsearched conference abstracts via the CENTRAL database. We searched ClinicalTrials.gov and the WHO trials portal for ongoing or unpublished trials.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies.
Data collection and analysis
Selection of studies
EA and IC independently reviewed titles and abstracts to identify all potential RCTs and obtained full‐text versions of these articles. . We reviewed online supplementary data where available. Cochrane language specialists reviewed studies in languages other than English for consideration of inclusion.
Data extraction and management
We extracted data for all included studies using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) and standard templates and methods. Two out of three authors (EA, IC and SW), working independently, updated 'Risk of bias' assessments for all included studies in line with current Cochrane protocols.
Assessment of risk of bias in included studies
Two authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as high, low or unclear and provide a quote from the study report together with a justification for the judgment in the 'Risk of bias' table. We have summarised the 'Risk of bias' judgements across different studies for each of the domains listed in Figure 3. We have noted in the 'Risk of bias' table where information on risk of bias relates to unpublished data or correspondence with a trialist.
3.
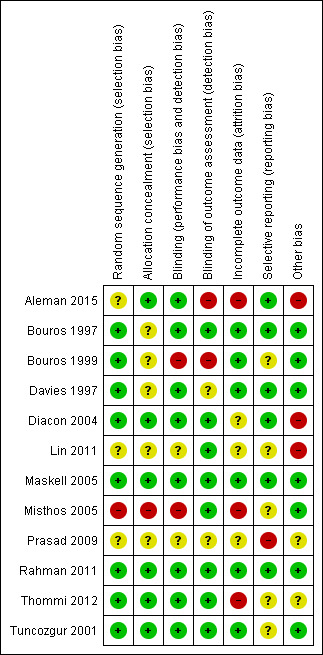
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We assessed trial outcomes for comparability. We analysed dichotomous outcomes as odds ratios with 95% confidence intervals. We analysed all data using Review Manager 5 software (Review Manager 2014).
Unit of analysis issues
Individual patients were used as the unit of analysis in all cases.
Dealing with missing data
We assumed that loss of participants before baseline measurements were obtained had no effect on the eventual outcome data of the study. We analysed remaining studies on an intention‐to‐treat analysis. Where missing data was necessary for study inclusion, we made contact with authors and data were provided in private communication.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. We reported if we identified greater than moderate heterogeneity (I² > 30%).
Assessment of reporting biases
We visually inspected funnel plots for evidence of reporting bias.
Data synthesis
We used random‐effects models to obtain summary statistics for the overall efficacy and safety of fibrinolytics on the studied outcomes.
'Summary of findings' table
We created 'Summary of findings' tables using the following outcomes: death; referral for surgical intervention; overall treatment failure; adverse events. We used the five GRADE considerations (study limitations; consistency of effect; imprecision; indirectness; and publication bias) to assess the certainty of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (Higgins 2011; GRADEpro GDT). We have justified all decisions to downgrade the certainty of evidence in footnotes below the tables.
Subgroup analysis and investigation of heterogeneity
We used the I² statistic to measure heterogeneity amongst the trials for each outcome.
Sensitivity analysis
Outcomes are presented with data from all studies, from those studies at 'low risk of bias', or 'low or unclear risk of bias' and those at 'high risk of bias' (in one or more domains).
Results
Description of studies
Results of the search
The last update of the review included a search run in 2008 (Cameron 2008). Update searches conducted in August 2019 identified 528 references. Following exclusion on the basis of abstracts and the identification of duplicate references, we retrieved 45 studies for full‐text scrutiny. We included five new studies from the search plus seven from the previous update (see Figure 4 for study flow diagram).
4.

Study flow diagram for 2019 update.
Included studies
A total of 12 RCTs met the inclusion criteria of the review. Eight English language full article studies (Bouros 1999; Davies 1997; Diacon 2004; Maskell 2005; Misthos 2005; Rahman 2011; Thommi 2012; Tuncozgur 2001), one English language abstract only publication (Prasad 2009), and one Chinese language full article (Lin 2011) compare intrapleural fibrinolytic with normal saline control in a combined total of 993 participants. This includes one English language article comparing intrapleural fibrinolytics (alteplase) with a DNase (streptodornase alfa) and placebo in a 2 × 2 factorial manner (Rahman 2011). However we only used data from the altepase versus placebo comparison in this review.
One English language full article directly compared streptokinase with urokinase (Bouros 1997). One English language full article directly compared alteplase with urokinase (Aleman 2015).
Details of each study including eligibility criteria and treatment regimens are given in the table 'Characteristics of included studies'.
Study design
All 12 studies were RCTs. Blinding was undertaken in all trials. Bouros 1999 had a treatment algorithm where all patients who failed conservative treatment were given fibrinolysis before consideration for surgery. Referral for fibrinolysis in this group was therefore considered an overall treatment failure but only patients who were referred for surgery following the cross‐over therapy were considered surgical referrals. Thommi 2012 crossed over patients from the placebo to alteplase groups or vice versa if they failed placebo or alteplase treatment respectively: crossing over to fibrinolytic therapy in this study was therefore considered an overall treatment failure but not a surgical referral and the outcome of the cross‐over treatment in this study was not considered for the purpose of this review. Rahman 2011 was a 2 × 2 factorial trial, and we used only data from the relevant groups (i.e. alteplase and placebo groups); Rahman 2011 was therefore treated as standard RCT for the purposes of this review.
Participants
The age range of participants was from 15 to 92 years. Participants were recruited in hospital having presented with parapneumonic effusion only in six studies (Bouros 1997; Bouros 1999; Davies 1997; Lin 2011; Misthos 2005; Tuncozgur 2001), and with parapneumonic effusions and empyema in six studies (Aleman 2015; Diacon 2004; Maskell 2005; Prasad 2009; Rahman 2011; Thommi 2012). Significant comorbidity was common amongst participants, with rates of comorbidity of 38% in Diacon 2004, more than 50% of cases in Bouros 1999, 74% of participants in Maskell 2005 and 62% in Thommi 2012. Rahman 2011 and Aleman 2015 provide data on individual comorbidities which appear to be comparable to other studies but do not provide an overall rate of comorbidity. Davies 1997, Tuncozgur 2001, Prasad 2009 and Lin 2011 do not provide information on underlying patient co‐morbidity,
Interventions
Ten of the studies compared fibrinolytic therapy with saline only; one trial compared fibrinolytic therapy and DNase in a 2 × 2 factorial manner (Rahman 2011); one trial compared streptokinase with urokinase (Bouros 1997); and one compared alteplase with urokinase (Aleman 2015). Full details of which fibrinolytic therapy, dose and delivery mechanism are given in the table 'Characteristics of included studies'. There was some variation between study durations, with Davies 1997 and Tuncozgur 2001 assessing outcome at the end of hospital stay whereas eight studies followed up participants beyond hospital discharge (Aleman 2015; Bouros 1997; Bouros 1999; Diacon 2004; Maskell 2005; Misthos 2005; Rahman 2011; Thommi 2012). Time points of assessment were unclear in Prasad 2009 and Lin 2011. Where data from multiple time points of mortality or requirement for surgical intervention were available, we have taken data from the time point closest to three months following administration of fibrinolytic therapy.
Outcomes
The outcomes of treatment failure resulting in death or referral for surgery were available in seven of the studies assessing fibrinolytics in comparison with placebo (Bouros 1999; Davies 1997; Diacon 2004; Maskell 2005; Misthos 2005; Thommi 2012; Tuncozgur 2001); in the one trial assessing alteplase and urokinase (Aleman 2015); and in the one trial assessing streptokinase and urokinase (Bouros 1997). Eight studies had data for referral for surgery (Bouros 1999, Davies 1997, Diacon 2004, Maskell 2005, Misthos 2005, Prasad 2009, Rahman 2011 and Tuncozgur 2001). One study had neither an overall treatment failure, referral for surgery or death outcome (Lin 2011). The outcome of death was available in 10 of the studies (unavailable in Prasad 2009 and Lin 2011).
In one study, failure of treatment in the saline arm resulted in a subsequent trial of intrapleural fibrinolytic before surgical intervention was considered (Bouros 1999). The need for fibrinolytic therapy in this instance was regarded as a treatment failure. One study crossed over patients who had less than 50% reduction in CT fluid volume to the other group (Thommi 2012). This study had a number of intermediate dropouts between treatments. In this case, group cross‐over was regarded as treatment failure and cross‐over treatment success was not used in this review.
Excluded studies
We excluded 25 studies with reasons: see Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias judgements and the information on which they are predicated are provided in Characteristics of included studies. Overall two of the 12 studies were at low overall risk of bias with no items at unclear or high risk of bias (Maskell 2005; Rahman 2011); three were at unclear risk of bias with no items at high risk of bias (Bouros 1997; Davies 1997; Tuncozgur 2001); and seven were at high risk of bias (Bouros 1999; Diacon 2004; Misthos 2005; Prasad 2009; Lin 2011; Thommi 2012; Aleman 2015).
Allocation
Following correspondence with the study investigators we were able to confirm appropriate generation of randomisation sequence (for example by a computer‐generated system) for six studies (Bouros 1997; Bouros 1999; Davies 1997; Diacon 2004; Rahman 2011; Thommi 2012). Rahman 2011 was the only study to use minimisation in addition to random sequence generation. Maskell 2005 reported that randomisation occurred centrally. Tuncozgur 2001 did not report the method of randomisation, but allocation was concealed with opaque envelopes. Misthos 2005 reported that allocation was performed on the basis of hospital number (odd/even): this is inadequate concealment and we considered that it placed the study at high risk of bias for allocation. Lin 2011 and Prasad 2009 provided no information on sequence generation. Aleman 2015 allocated using a stratified randomised number table technique which has an unclear risk of selection bias.
Allocation concealment was low risk and adequately described in Maskell 2005 and Rahman 2011 via central telephone allocation. Diacon 2004 described a single author not involved in clinical care as the sole holder of allocation, which we thought to be low risk. Tuncozgur 2001 described adequate blinded opaque envelope allocation in private communication. Thommi 2012 had a pharmacist not involved in clinical care holding allocation. No information was available regarding allocation concealment for five studies (Bouros 1997; Bouros 1999; Davies 1997; Lin 2011; Prasad 2009). We considered Misthos 2005 to be at high risk of allocation concealment bias due to the hospital‐number‐based method of allocation.
Blinding
There was a low risk of bias due to participant and treating team blinding (including data from private communication) in eight of the included trials. No information was available for Lin 2011 and Prasad 2009. Misthos 2005 was explicitly non‐blinded to the surgical team and therefore at high risk of bias. In Bouros 1999 nursing staff preparing study drugs for administration were unblinded, placing this study at high risk of bias.
There was a low risk of bias due to blinding of outcome assessor in eight studies (Bouros 1997; Diacon 2004; Lin 2011; Maskell 2005; Misthos 2005; Rahman 2011; Thommi 2012; Tuncozgur 2001). No information was available for Prasad 2009 or Davies 1997. Aleman 2015 broke blinding mid‐way through the trial due to a valid concern of an excess of adverse events (major bleeding) in the alteplase 20 mg arm, putting this study at high risk of bias. We considered Bouros 1999 to be at high risk of bias, with concerns that not all treating staff were blinded.
Incomplete outcome data
Studies reporting no withdrawals were Bouros 1997,Davies 1997,Bouros 1999, and Tuncozgur 2001. No information regarding attrition was available for Lin 2011 and Prasad 2009. Intention‐to‐treat analyses were reported for Diacon 2004 and Maskell 2005. Rahman 2011 analysed data with a modified intention‐to‐treat analysis, with exclusion of six patients who did not receive study medication and 11 who had less than 5% of fluid‐occupied hemithorax at baseline. Misthos 2005 analysed data from those who completed the study. In this study there was a significantly higher dropout rate in the intervention arm (8/65) compared with the control arm (0/70), putting the study at high risk of bias for incomplete outcome data. Thommi 2012 had a high number of post‐randomisation withdrawals for varied reasons, placing the study at high risk of bias. Aleman 2015 had a higher rate of withdrawal in the alteplase arms, particularly the 20 mg arm due to adverse events (predominantly bleeding), placing the study at a higher risk of bias.
Selective reporting
Full study protocols were only available for Maskell 2005 and Rahman 2011. Review of these protocols confirmed that all proposed outcomes (and no additional post hoc results) were reported, placing these studies at low risk of selective reporting. Six studies did not have available protocols; however these studies reported all outcomes documented in the methods of each publication and we have therefore taken these to be at low risk of selective reporting (Bouros 1997; Bouros 1999; Davies 1997; Diacon 2004; Misthos 2005; Tuncozgur 2001). Although Thommi 2012 reported significant variation between planned primary outcome (reduction in surgical intervention between alteplase and placebo groups) and the reported primary outcome, which was proportion of patients with at least 50% reduction in pleural fluid volume on radiologic examination, this did not affect the extracted data as we took data from the cross‐over point of this study. Prasad 2009 reported a single outcome (requirement for surgery) in abstract form only. Given a full report would have been likely to be published in a longer form, we believe this places this study at high risk of selective reporting bias.
Other potential sources of bias
We considered Lin 2011 to be at high risk of bias due to the absence of a number of usual outcome measures (death, referral for surgery, treatment failure) which would have been reported in the vast majority of studies. Aleman 2015 deliberately broke blinding after a number of excess adverse events in the alteplase 20 mg arm, which required a mid‐trial protocol change, placing the study at a high risk of bias. Diacon 2004 had a significant (6/53) number of post‐randomisation exclusions, placing it at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Fibrinolytics compared to placebo in the treatment of complicated parapneumonic effusions and empyema.
| Fibrinolytics compared to placebo in the treatment of complicated parapneumonic effusions and empyema | ||||||
| Patient or population: adult parapneumonic effusions and empyema Setting: hospital inpatients Intervention: fibrinolytics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with fibrinolytics | |||||
| Mortality. Follow‐up: range 3 months to 36 months |
Moderate1 | OR 1.16 (0.71 to 1.91) | 867 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Fibrinolysis does not dramatically alter the risk of death. The confidence intervals are wide so we cannot exclude a modest increase in mortality with fibrinolytics. | |
| 140 per 1000 | 159 per 1000 (104 to 235) | |||||
| Referral for thoracic surgery. Follow‐up: range 3 months to 36 months | Low4 | OR 0.37 (0.21 to 0.68) | 897 (8 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Fibrinolytics probably reduce the rate of surgical intervention for empyema, though the use of surgery varies substantially between centres. | |
| 50 per 1000 | 19 per 1000 (11 to 35) | |||||
| Moderate | ||||||
| 200 per 1000 | 85 per 1000 (50 to 145) | |||||
| High | ||||||
| 500 per 1000 | 270 per 1000 (174 to 405) | |||||
| Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy. Follow‐up: range 3 months to 36 months | Moderate6 | OR 0.16 (0.05 to 0.58) | 769 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | Fibrinolysis may reduce overall treatment failure, perhaps by reducing need for surgical intervention. This result is potentially unreliable as the effect is not seen once the analysis is confined to studies of low risk of bias. | |
| 270 per 1000 | 56 per 1000 (18 to 177) | |||||
| Serious adverse events | Moderate7 | OR 1.28 (0.36 to 4.57) | 935 (9 RCTs) | ⊕⊕⊝⊝ LOW 8 9 | Given the broad confidence intervals, we cannot exclude a modest increase in treatment complications (particularly bleeding) with fibrinolysis | |
| 30 per 1000 | 50 per 1000 (23 to 106) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 14% Mortality rate taken from the placebo arm of the largest study (Maskell 2005).
2 Downgraded by 1 point as 4 of the included studies were deemed at high risk of bias in at least 1 domain.
3 Downgraded by 1 point as funnel plots (Figure 1, Figure 2) suggests significant risk of publication bias.
4 Decision to operate is dependent on many local factors. Example low, moderate and high surgical risk chosen are consistent with the range seen in included trials and authors' practice.
5 Downgraded by 1 point as there is inconsistency between Maskell 2005 (the largest study, deemed at low risk of bias) and many of the other studies.
6 27% placebo failure rate taken from Maskell 2005.
7 3% significant complication rate taken from placebo arm of Maskell 2005.
8 Downgraded by 1 point as 5 of the included studies were deemed at high risk of bias in at least 1 domain.
9 Broad confidence intervals do not rule out a clinically significant increase in treatment complications with fibrinolytics so downgraded 1 point for imprecision.
Summary of findings 2. Streptokinase compared to urokinase in addition to chest drainage in the treatment of adult parapneumonic effusions and empyema.
| Streptokinase compared to urokinase in addition to chest drainage in the treatment of adult parapneumonic effusions and empyema | ||||||
| Patient or population: adult parapneumonic effusions and empyema Setting: hospital inpatients Intervention: streptokinase Comparison: urokinase | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with urokinase | Risk with streptokinase | |||||
| Mortality Follow‐up: 12 months |
Study population | OR 0.32 (0.01 to 8.25) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Based on a single death in the urokinase group which was felt by the study authors to be unrelated to pleural infection. | |
| 40 per 1000 | 13 per 1000 (0 to 256) | |||||
| Referral for thoracic surgery (open or thorascopic) | Study population | OR 1.00 (0.13 to 7.72) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 80 per 1000 | 80 per 1000 (11 to 402) | |||||
| Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy | Study population | OR 1.00 (0.13 to 7.72) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 80 per 1000 | 80 per 1000 (11 to 402) | |||||
| Serious adverse events | Study population | OR 5.43 (0.25 to 118.96) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Based on 1 small RCT, not otherwise felt to be at high risk of bias. Downgraded 2 points for imprecision as very few events so very broad confidence intervals.
Summary of findings 3. Alteplase compared to urokinase in addition to chest drainage in the treatment of adult parapneumonic effusions and empyema.
| Alteplase compared to urokinase in addition to chest drainage in the treatment of adult parapneumonic effusions and empyema | ||||||
| Patient or population: adult parapneumonic effusions and empyema Setting: hospital inpatients Intervention: alteplase Comparison: urokinase | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with urokinase | Risk with alteplase | |||||
| Mortality | Study population | OR 0.94 (0.18 to 4.89) | 99 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 63 per 1000 | 59 per 1000 (12 to 246) | |||||
| Referral for thoracic surgery (open or thorascopic) | Study population | OR 0.46 (0.04 to 5.24) | 99 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 42 per 1000 | 20 per 1000 (2 to 186) | |||||
| Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy | Study population | OR 3.02 (0.89 to 10.27) | 99 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | ||
| 83 per 1000 | 215 per 1000 (75 to 483) | |||||
| Serious adverse events | Study population | OR 5.61 (1.16 to 27.11) | 99 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Substantially more adverse events in the alteplase group compared with urokinase. | |
| 42 per 1000 | 196 per 1000 (48 to 541) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 point as based on 1 small RCT judged at high risk of bias in several domains. Also downgraded 1 point for imprecision ‐ wide confidence intervals.
We performed analysis of studies for the outcomes of mortality, referral for thoracic surgery (open or thorascopic) and overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy and occurrence of serious adverse events. For two studies, an intermediate fibrinolysis step took place prior to referral for surgery for patients administered placebo who experienced treatment failure (Bouros 1999; Thommi 2012). For these studies, we have determined that a patient being referred for intermediate fibrinolysis was an overall treatment failure but only patients who subsequently were referred for surgery after an intermediate fibrinolysis step were counted as referrals for surgery.
We present below the findings by comparison, outcome and then by sensitivity analysis, with removal of studies at high risk of bias.
Fibrinolytic therapy versus placebo (loculation and empyema)
Mortality
Eight studies of fibrinolytics versus placebo reported death as an outcome, although there were no deaths in either group in four of the studies (Bouros 1997; Davies 1997; Thommi 2012; Tuncozgur 2001). There was no clear difference between the groups for this outcome (OR 1.16, 95% CI 0.71 to 1.91; 867 participants; I² = 0%) (Analysis 1.1; Figure 5). In a population with a 14% baseline mortality risk (risk taken from the placebo arm of the largest study (Maskell 2005)), adding intrapleural fibrinolysis to chest tube drainage leads to 19 more deaths per 1000 people (CI 36 fewer to 59 more deaths). However, the confidence intervals in the estimate of the odds ratio are relatively wide and do not exclude clinically significant harm or benefit, reducing our confidence in the estimate of effect. There was no evidence of difference when the sensitivity analysis was restricted to studies at low or unclear risk of bias (OR 1.24, 95% CI 0.74 to 2.07; 4 studies, 598 participants; I² = 0%).
1.1. Analysis.
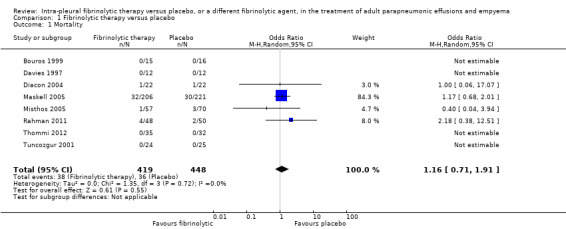
Comparison 1 Fibrinolytic therapy versus placebo, Outcome 1 Mortality.
5.
Forest plot of comparison: 1 Fibrinolytic versus placebo (loculation and empyema), outcome: 1.1 Treatment failure‐ death.
Referral for thoracic surgery (open or thorascopic)
Eight studies of fibrinolytics versus placebo reported rate of surgical referral, of which four were at high risk of bias in one or more domains. Both Bouros 1999 and Thommi 2012 had intermediate steps of fibrinolysis when placebo therapy failed prior to referral for surgery; we treated achieving this stage as a referral for surgery in these studies.
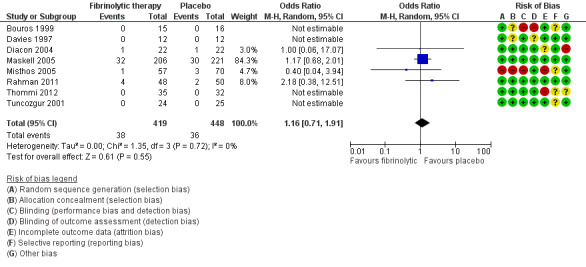
Meta‐analysis of all included studies showed an odds ratio for fibrinolytic therapy reducing referral for surgery of 0.37 (95% CI 0.21 to 0.68; 8 studies, 897 participants) (Analysis 1.2; Figure 6). We observed moderate heterogeneity (I² = 51%). However, there was no evidence of significant reduction in referral for surgery when sensitivity analysis was confined to studies at low or unclear risk of bias (OR 0.48, 95% CI 0.18 to 1.25; 4 studies, 599 participants; I² = 58%).
1.2. Analysis.

Comparison 1 Fibrinolytic therapy versus placebo, Outcome 2 Referral for thoracic surgery (open or thorascopic).
6.
Forest plot of comparison: 1 Fibrinolytic versus placebo (loculation and empyema), outcome: 1.2 Treatment failure‐ surgical intervention.
Rates of surgical referral vary widely from centre to centre. Assuming a 20% baseline referral rate, fibrinolytics lead to 115 fewer (150 to 55 fewer) surgical referrals per 1000 people. Table 1 gives example absolute effects for other baseline referral rates.
Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy
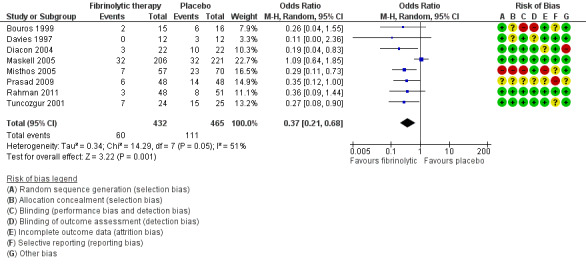
Seven studies of fibrinolytics versus placebo reported sufficient data to either directly state or derive the composite outcome of surgery, death or referral for fibrinolysis. Four studies were at high risk of bias in one or more domains. There was a clear treatment effect of fibrinolysis amongst studies (OR 0.16, 95% CI 0.05 to 0.58; 7 studies, 769 participants; I² = 88%) (Analysis 1.3; Figure 7). A large amount of heterogeneity was present in both the low‐ and unclear‐risk studies (I² = 71%, P= 0.03), as well as when we analysed all studies (I² = 88%, P < 0.00001).
1.3. Analysis.
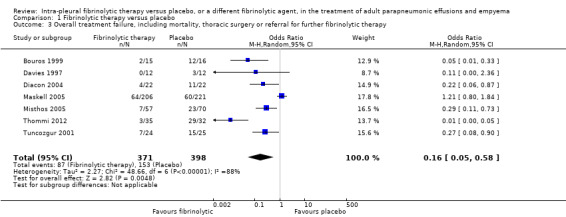
Comparison 1 Fibrinolytic therapy versus placebo, Outcome 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy.
7.
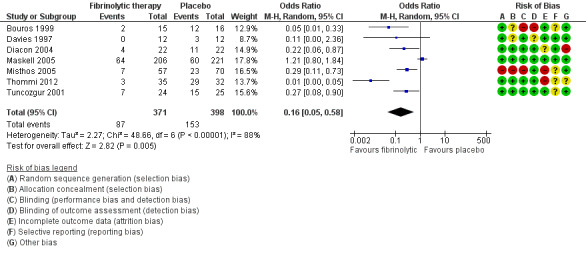
Forest plot of comparison: 1 Fibrinolytic therapy versus placebo, outcome: 1.3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy.
Sensitivity analysis limiting the analysis to studies at low or unclear risk of bias did not show a significant reduction in overall treatment failure (OR 0.50, 95% CI 0.13 to 1.96; 3 studies, 500 participants; I² = 73%).
Compared with a 27% rate of treatment failure with chest tube drainage alone, use of fibrinolytics leads to 214 fewer overall treatment failures per 1000 (252 fewer to 93 fewer).
Serious adverse events
Of the nine studies reporting adverse events comparing fibrinolytics versus placebo, only three reported the occurrence of serious adverse events (Maskell 2005; Rahman 2011; Thommi 2012). These included major bleeding (most common serious adverse event, usually intrapleural), as well as significant chest pain, fever/rash and clinical deterioration. All other studies reported that no adverse events occurred in either group. One study reported a possible excess of serious adverse events in the fibrinolytic group (7% versus 3%, P = 0.08) (Maskell 2005). Overall, we saw no clear difference in the rate of adverse events between fibrinolysis and placebo (OR 1.28, 95% CI 0.36 to 4.57; 9 studies; 935 participants; I2 = 33%), but the confidence intervals are broad enough to conceal a modest increase in adverse events with fibrinolysis (Analysis 1.4; Figure 8). If 30 people per 1000 experience serious adverse events with conservative management alone then 50 (23 to 106) per 1000 will have an adverse event with the addition of fibrinolysis.
1.4. Analysis.
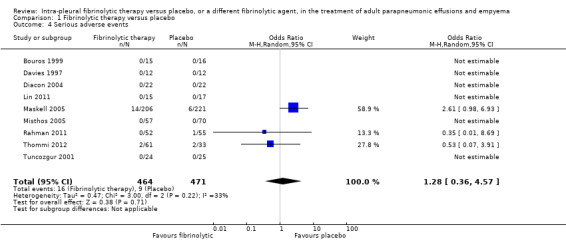
Comparison 1 Fibrinolytic therapy versus placebo, Outcome 4 Serious adverse events.
8.
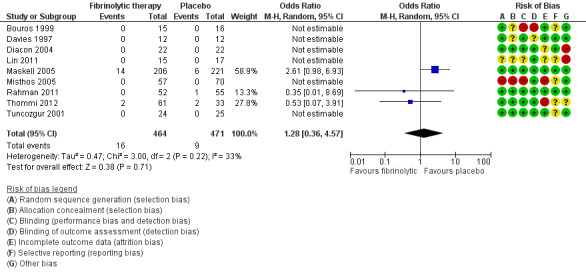
Forest plot of comparison: 1 Fibrinolytic versus placebo (loculation and empyema), outcome: 1.4 Significant treatment complications.
Sensitivity analysis
Table 7 summarises the odds ratios for the various outcomes with fibrinolytics versus placebo, broken down by risk of bias in the included studies. As described above, the benefits of fibrinolytics on the need for surgical intervention and on overall treatment failure disappeared when we excluded from the analysis studies judged to be at high risk of bias in at least one domain or when we confined sensitivity analysis to the two studies with all domains at low risk of bias. Deaths and serious adverse events were reported less frequently in the fibrinolytic arms of studies with at least one domain at high risk of bias, though the analysis of all studies was consistent with that of the low/unclear risk of bias studies for these outcomes.
1. Sensitivity analysis for fibrinolysis versus placebo ‒ low versus high versus low/unclear risk of bias.
| Outcome | All studies | Low Risk of Bias | Low or unclear risk of bias | High risk of bias1 |
| Death | OR 1.16 (0.71 to 1.91) studies = 8, participants = 867 |
OR 1.24 (0.74 to 2.07) studies = 2, participants = 525 | OR 1.24 (0.74 to 2.07) studies = 4, participants = 598 | OR 0.56 (0.10 to 3.41) studies = 4, participants = 269 |
| Need for surgical intervention | OR 0.37 (0.21 to 0.68) studies = 8, participants = 897 |
OR 0.76 (0.27 to 2.10); studies = 2, participants = 526 | OR 0.48 (0.18 to 1.25) studies = 4, participants = 599 | OR 0.28 (0.15 to 0.51) studies = 4, participants = 298 |
| Overall treatment failure | OR 0.16 (0.05 to 0.58) studies = 7, participants = 769 |
OR 1.21 (0.80 to 1.84) studies = 1, participants = 427 | OR 0.50 (0.13 to 1.96) studies = 3 participants = 500 | OR 0.08, (0.02 to 0.37) studies = 4, participants = 269 |
| Serious adverse outcomes | OR 1.28 (0.36 to 4.57) studies = 9, participants = 935 |
OR 1.74 (0.36 to 8.54) studies = 2, participants = 534 | OR 1.74 (0.36 to 8.54) studies = 4, participants = 607 | OR 0.53 (0.07 to 3.91) studies = 5, participants = 328 |
1 "High risk" studies are those with at least 1 domain rated as at high risk of bias.
Streptokinase versus urokinase
One study compared treatment with urokinase versus streptokinase for complicated parapneumonic effusion and empyema (Bouros 1997). This study was at overall unclear risk of bias.
Mortality
One death was reported in this study, which was thought to be unrelated to pleural disease (other malignancy). This person had a clear pleural cavity at autopsy and their death was therefore not considered to be a result of treatment failure. No other deaths were recorded in this study.
Referral for thoracic surgery (open or thorascopic)
An odds ratio of surgical intervention for streptokinase versus urokinase was calculated at 1.00 (95% CI 0.13 to 7.72) (Analysis 2.2).
2.2. Analysis.
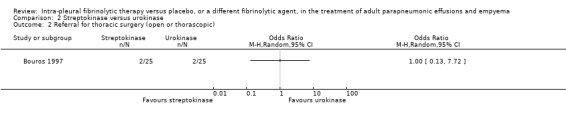
Comparison 2 Streptokinase versus urokinase, Outcome 2 Referral for thoracic surgery (open or thorascopic).
Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy
An odds ratio of surgical intervention or death of streptokinase versus urokinase was calculated at 1.00 (95% CI 0.13 to 7.72) (Analysis 2.3).
2.3. Analysis.
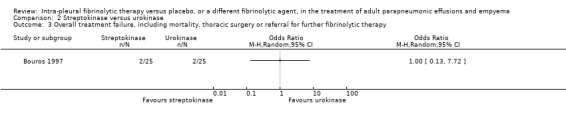
Comparison 2 Streptokinase versus urokinase, Outcome 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy.
Serious adverse events
There was a non‐statistically significant excess of adverse events in the streptokinase group (2/25 versus 0/25 in the urokinase group) with an odds ratio of streptokinase versus urokinase of 5.43 (95% CI 0.25 to 118.96) (Analysis 2.4).
2.4. Analysis.
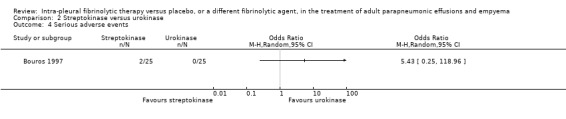
Comparison 2 Streptokinase versus urokinase, Outcome 4 Serious adverse events.
Alteplase versus urokinase
One study compared treatment with urokinase versus alteplase at two different doses (10 mg daily and 20 mg daily) for complicated parapneumonic effusion and empyema (Aleman 2015). This study was at high risk of bias as an excess of adverse events was identified in the 20 mg alteplase arm, which resulted in breaking of the blind and alteration of the protocol with a reduced alteplase dose of 10 mg daily.
Mortality
An odds ratio of death comparing alteplase (combining 10 mg and 20 mg groups) with urokinase at one year was 0.94 (95% CI 0.18 to 4.89) (Analysis 3.1). No deaths were identified during hospitalisation in any group. Of note: all the alteplase deaths were in the 10 mg arm but there are too few people in the study to determine if this is a significant finding.
3.1. Analysis.
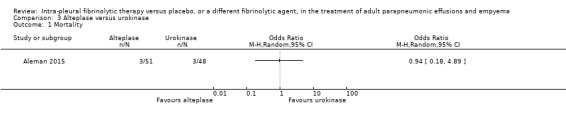
Comparison 3 Alteplase versus urokinase, Outcome 1 Mortality.
Referral for thoracic surgery (open or thorascopic)
We calculated an odds ratio of referral for surgical intervention comparing combined alteplase groups with urokinase to be 0.46 (95% CI 0.04 to 5.24) (Analysis 3.2). The single referral for surgery in the alteplase group was in the 10 mg arm. Again, there are insufficient numbers in this study for this comparison to be of significant value.
3.2. Analysis.
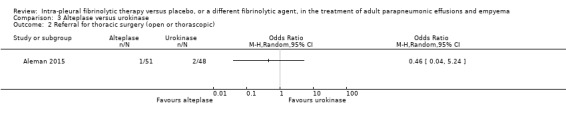
Comparison 3 Alteplase versus urokinase, Outcome 2 Referral for thoracic surgery (open or thorascopic).
Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy
We calculated an odds ratio of overall treatment failure comparing combined alteplase groups with urokinase to be 3.02 (95% CI 0.89 to 10.27) (Analysis 3.3). This effect, although not statistically significant, is in a different direction from the referral for surgical intervention due to the inclusion of serious bleeding as a treatment failure (5/18 in the 20 mg alteplase arm, 4/33 in the 10 mg alteplase arm at day 6) compared with no serious bleeding in the urokinase arm. Of note: requirement for further fibrinolysis was also included in this outcome (1/33 in the 10 mg alteplase arm, 2/48 in the urokinase arm).
3.3. Analysis.
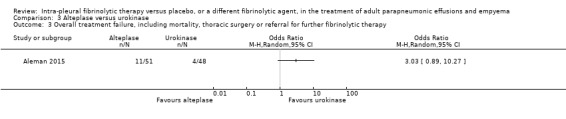
Comparison 3 Alteplase versus urokinase, Outcome 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy.
Serious adverse events
We calculated an odds ratio of serious adverse events comparing combined alteplase groups with urokinase to be 5.61 (95% CI 1.16 to 27.11) (Analysis 3.4). This was predominantly due to an excess of bleeding events in the alteplase arms (as above), particularly in the 20 mg alteplase arm. This was recognised during the study, resulting in breaking blinding and alteration to the study protocol.
3.4. Analysis.
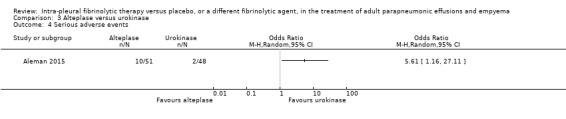
Comparison 3 Alteplase versus urokinase, Outcome 4 Serious adverse events.
Discussion
Summary of main results
This review identified 10 randomised controlled trials comparing intrapleural fibrinolysis with placebo and two further trials comparing different fibrinolytic agents (one trial of streptokinase versus urokinase and one trial of alteplase versus urokinase).
Fibrinolytic therapy did not substantially alter the risk of death in complicated parapneumonic effusion and empyema compared with placebo, based on moderate‐certainty evidence from eight trials including 867 participants (OR 1.16, 95% CI 0.71 to 1.89). These confidence intervals are wide enough that we cannot exclude a modest but clinically significant increase in mortality with fibrinolytic therapy. Although death was a reported outcome in all studies, only four actually reported that deaths had occurred in the study. Time points of death assessment varied from hospital discharge to three months. There was an overall relatively low rate of death (36/448 in participants in the control group) which is lower than reported estimates of mortality in case series (Farjah 2007). The majority of the deaths occurred in Maskell 2005 which also has the highest mortality rate, approaching other case series estimates.
Low‐certainty evidence suggests that, when compared with placebo, fibrinolytic therapies reduce the need for referral for thoracic surgery (OR 0.37, 95% CI 0.21 to 0.68). The use of surgery for empyema varies substantially between centres so the impact of fibrinolysis in terms of absolute numbers of people undergoing thoracic surgery will depend on local thoracic surgery practices. Overall treatment failure (a combined end point of mortality and referral for thoracic surgery or further open‐label fibrinolytic therapy) was reduced by fibrinolytics compared with placebo (OR 0.16, 95% CI 0.05 to 0.58). We consider this to be very low certainty evidence, not least because this is discordant with the findings of Maskell 2005, the largest included study and one judged to be of low risk of bias.
Three studies — Maskell 2005, Rahman 2011 and Thommi 2012 — reported that serious adverse events had occurred, with other studies positively reporting no events. Maskell 2005 had the largest number of significant events, of which the most common was haemorrhage (14/208 in fibrinolytic group versus 6/222 in the control group). We did not detect a clear difference in serious adverse event rates with fibrinolysis compared with placebo (OR 1.70, 95% CI 0.76 to 3.82, low‐certainty evidence). Our analysis is not sufficiently powered to exclude a moderate but clinically important increase in, for example, haemorrhage with fibrinolysis. There is insufficient data to give a precise estimate of the overall risk of significant adverse events (OR 1.70, 95% CI 0.76 to 3.82; 9 studies, 935 participants; I² = 33%), and therefore an increased risk of significant adverse events with fibrinolytics is possible.
The comparison of alteplase with urokinase identified an excess of adverse events with alteplase compared to urokinase (OR 5.61, 95% CI 1.16 to 27.11) which was predominantly due to excess bleeding (Aleman 2015). This is at a much higher rate than seen elsewhere in the review. There is a suggestion of a dose‐dependent relationship in the bleeding rate for Aleman 2015 (5/18 in the 20 mg arm, 4/33 in the 10 mg arm). However, excess rates of bleeding were not seen in either Thommi 2012 which used 25 mg alteplase daily or Rahman 2011 which used 10 mg alteplase twice daily. It is likely that post‐marketing surveillance is needed to confirm or refute the suggestion of excess bleeding with alteplase.
The comparison of streptokinase with urokinase did not show any large differences in a small study (Bouros 1997). It is likely that this study has too few people to make a conclusive determination regarding relative efficacy or safety of these two agents.
Overall completeness and applicability of evidence
We believe these studies are likely to be reflective of real‐world patients in practice given significant rates of comorbidity in all study populations as well as relatively consistent inclusion criteria. The results are therefore likely generalisable to standard hospital practice. Our deliberate exclusion of studies involving people with tuberculous effusions means our analyses are not applicable to these individuals.
Although multiple different fibrinolytic agents were used (streptokinase, urokinase, alteplase and tPA), there appears to be relative consistency in the effect size throughout the studies. The two direct comparisons were consistent with this, with similar rates of success with urokinase and streptokinase in a small comparison (Bouros 1997); and between alteplase and urokinase (Aleman 2015).
The partial cross‐over designs of Bouros 1999 and Thommi 2012 with an intermediate fibrinolysis step in failed placebo therapy may impact upon all outcomes; this is likely to be a small effect, however, given the low number of affected patients. We assessed outcomes either at hospital discharge or at three months, depending on available data. We did not use longer‐term data (e.g. 12‐month data in Maskell 2005) as empyema or complicated parapneumonic effusions are unlikely to recur following successful therapy.
We have not identified any unpublished studies. However, a significant proportion of the identified research predates mandatory trial registration and we therefore believe it is likely there are unpublished studies. Any potential trials are more likely to be negative and would likely reduce the effect size. The funnel plots are consistent with the existence of unpublished small negative trials, although with fewer than 10 included trials these plots should be treated with caution (Figure 1; Figure 2).
1.
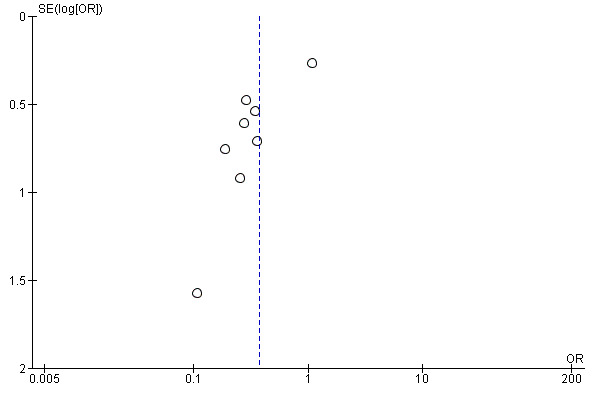
Funnel plot of comparison: 1 Fibrinolytic therapy versus placebo, outcome: 1.2 Referral for thoracic surgery (open or thorascopic).
2.
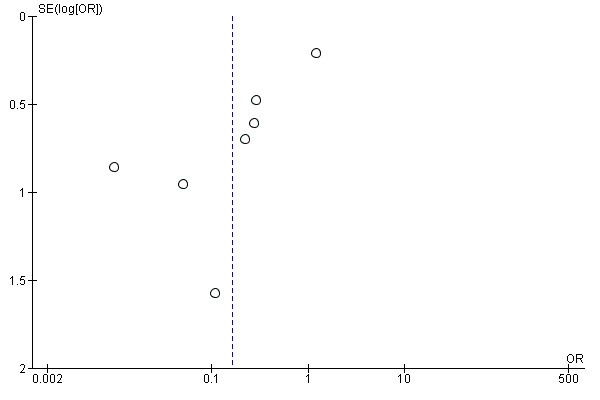
Funnel plot of comparison: 1 Fibrinolytic therapy versus placebo, outcome: 1.3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy.
It is relatively unlikely that further studies of fibrinolysis alone versus placebo will be published as we note that clinical practice has moved away from fibrinolytic monotherapy and towards dual therapy after the publication of Rahman 2011, which showed a significant reduction in referral for surgery with combined fibrinolytic/DNase. The last RCT of fibrinolysis versus placebo in this population was published in 2012 (Thommi 2012) and no other fibrinolysis versus placebo studies for pleural infection appear to be in progress. It is likely that a planned much larger study of combination fibrinolytic/DNase will give more definitive evidence for combined therapy (Najib Rahman, private communication).
Certainty of the evidence
We are moderately confident that fibrinolytics do not affect the occurrence of mortality compared with placebo. Certainty for other outcomes (surgery, treatment failure and treatment complications) was low or very low owing to possible publication bias (need for surgery Figure 1; and overall treatment failure Figure 2). In addition, sensitivity analysis removing studies at high risk of bias in at least one domain did not show evidence of significant effect for surgery or treatment failure (Table 7). We identified significant heterogeneity in the overall treatment failure analysis (I² = 71%) relating to Maskell 2005, which we have discussed above. The risk of treatment complications estimate is imprecise, and whilst the confidence intervals cross unity a clinically significant increase in complications with fibrinolysis is not excluded.
Overall, we believe the studies included in the low and unclear risk of bias group are generally of good methodological quality but the issues identified above mean that we are moderately confident of the result of the mortality analysis but less so of the other outcomes.
Potential biases in the review process
We believe all published studies have been identified using standard Cochrane search methods. It remains possible, however, that unpublished studies significantly affect the analysis. These concerns apply particularly to the outcome of surgical referral for fibrinolytics versus placebo, where the pooled result shows a benefit — i.e. reduction in the number of referrals to thoracic surgery — whereas the largest more robust study did not show a benefit of fibrinolysis (Maskell 2005). Much of the literature predates mandatory trial registration, however, and small unpublished studies may therefore exist. There was no obvious correlation between risk of bias in individual included studies and their size. Although a primary outcome of combined death or referral for surgical intervention was published for all included trials, a combination end point is more heterogenous and individual death or referral for surgery endpoints may be more accurate, although this does not include treatment failures referred for intermediate fibrinolysis.
Sensitivity analysis restricting the meta‐analysis to studies at low or unclear risk of bias does not show a statistically significant positive effect (OR 0.48, 95% CI 0.18 to 1.25; 4 studies, 599 participants; I² = 58%). This assessment is limited by the relatively small number of studies included in the analysis but there may be a degree of small‐study effect on publication bias, and we have therefore downgraded the strength of the summary of findings for all outcomes for fibrinolysis versus placebo to reflect this possibility.
Agreements and disagreements with other studies or reviews
The meta‐analysis of Janda 2012 comparing fibrinolysis versus placebo is in broad agreement with this review. This review found a reduction in combination death and requirement for surgery (RR 0.50, 95% CI 0.45 to 0.92) and requirement for surgical intervention alone (RR 0.61, 95% CI 0.45 to 0.82), which is consistent with the analyses in this review. Of note: Janda 2012 was highly suspicious of publication bias and small‐study effects in their analysis of requirement for surgery (Egger test P = 0.017), as well as presenting a suspicious funnel plot visual inspection.
The meta‐analysis of Nie 2014 included all studies of intrapleural fibrinolysis versus placebo identified in this analysis. However this review additionally included two paediatric studies which do not fit the inclusion criteria of this review (Singh 2004; Thomson 2002). Nie and colleagues found a reduction of requirement for surgery (OR 0.24, 95% CI 0.10 to 0.60) with a mortality odds ratio similar to this review (OR 1.16, 95% CI 0.71 to 1.89). Other discordances between this review and Nie 2014 include the treatment of "rescue" fibrinolysis following administration of placebo: in Nie 2014, those in Thommi 2012 were treated as referrals for surgery but those in Bouros 1999 were not. The possible increase found by Nie 2014 in serious adverse event rate (OR 1.92, 95% CI 0.87 to 4.21) is broadly consistent with this review's findings. The authors of Nie 2014 considered there was publication bias in this group of trials by visual assessment of a funnel plot.
Authors' conclusions
Implications for practice.
Meta‐analysis of the trials included in this review has shown that intrapleural fibrinolytic therapy gave a reduction in requirement for surgical intervention compared to placebo although the certainty of the evidence which we have found is low. Furthermore, there was a reduction in overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy among participants receiving therapy rather than placebo with very low certainty. We identified no clear change to mortality alone (moderate‐certainty evidence). There is discordance between the largest trial (Maskell 2005) and the overall result. The reasons for this are uncertain but may include publication bias, of which we are suspicious.
Intrapleural fibrinolysis may be a reasonable therapy in patients with empyema or complex parapneumonic effusion, particularly in patients in whom surgery is contraindicated. The trials included do suggest a possible increase in serious adverse effects with fibrinolysis (low‐certainty evidence).
Implications for research.
Although we did not consider combined fibrinolytic/DNase therapy in this review, it may be appropriate to consider for an update or a separate systematic review. Any further trials of intrapleural fibrinolysis (with or without DNase) should replicate as closely as possible the demographics of the empyema/complex parapneumonic effusion population with regards to age and comorbidity. Major outcomes should be direct and patient centred (e.g. death, requirement for surgery and serious adverse events) rather than proxy outcomes such as clearance of pleural fluid. We would discourage intermediate fibrinolysis steps or cross‐over studies such as in Bouros 1999 and Thommi 2012. Additionally, more data is required regarding serious adverse events as there is uncertainty if fibrinolytic therapy is associated with morbidity and current treatment numbers are insufficient to make a determination.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2019 | New search has been performed | New literature search run. |
| 28 August 2019 | New citation required but conclusions have not changed | New author team. Review reformatted to reflect current Cochrane headings. We updated the 'Risk of bias' assessment and added a 'Summary of findings' table. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 31 July 2008 | Amended | Converted to new review format. |
| 4 January 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Cochrane Airways Team including Emma Jackson, Emma Dennett and Elizabeth Stovold for assistance with searching the literature. We thank Chris Cates for data and analysis review and advice. We thank Robert Cameron for his work on previous versions of the review. We thank Yue Mingming, Cai Wangyu and Lu Chunli for translation from Chinese of Lin 2011 and Jin 2013.
The authors and Cochrane Airways are grateful to the following peer reviewers for their time and comments: Dr AlaEldin H Ahmed, University of Khartoum and Prof Najib Rahman, University of Oxford.
We are grateful to the authors of Bouros 1997,Davies 1997,Bouros 1999,Tuncozgur 2001,Diacon 2004, Misthos 2005, Bashir 2013 and Rahman 2011 for assisting us in obtaining further information about their studies.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Appendices
Appendix 1. Database search strategies
CENTRAL (including Cochrane Airways Trials Register (via CRS Web)
1. PLEURAL EFFUSION
2. EMPYEMA PLEURAL
3. empyema*
4. (parapneumonic near effusion*)
5. (pleural near effusion*)
6. parapneumonic*
7. (#1 or #2 or #3 or #4 or #5 or #6)
8. FIBRINOLYTIC AGENTS
9. (antithrombotic* or thrombolytic* or antithrombic* or fibrinolytic*)
10. alprostadil or anistreplase or enoxaparin or ancrod or aspirin or batroxobin or brinolase or heparin or hirudin or nadroparin or plasmin or plasminogen or "protein c" or streptokinase or tedelparin or ticlopidine or "tissue plasminogen activator"
11. STREPTOKINASE
12. (avelizin or awelysin or celiase or distreptase or kabikinase or kabivitrum or streptase or streptodecase or apsac)
13. URINARY PLASMINOGEN ACTIVATOR
14. (urokinase or "plasminogen activator*" or "u‐plasminogen activator*" or abbokinase or renokinase or u‐pa)
15. (#8 or #9 or #10 or #11 or #12 or #13 or #14)
MEDLINE (Ovid)
1. Pleural Effusion/ 2. Empyema, Pleural/ 3. empyema$.tw. 4. (parapneumonic adj5 effusion$).tw. 5. (pleural adj5 effusion$).tw. 6. parapneumonic$.tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Fibrinolytic Agents/ 9. (antithrombotic or thrombolytic or antithrombic or fibrinolytic).tw. 10. (Alprostadil or Anistreplase or Enoxaparin or Ancrod or Aspirin or Batroxobin or Brinolase or Heparin or Hirudin or Nadroparin or Plasmin or Plasminogen or Protein C or Streptokinase or Tedelparin or Ticlopidine or Tissue Plasminogen Activator).tw. 11. exp Streptokinase/ 12. (avelizin or awelysin or celiase or distreptase or Kabikinase or kabivitrum or Streptase or streptodecase or apsac or anisoylated plasminogen‐streptokinase activator complex or brl‐26921).tw. 13. exp Urinary Plasminogen Activator/ 14. (Urokinase or plasminogen activator$ or u‐plasminogen activator$ or Abbokinase or renokinase or u‐pa).tw. 15. 8 or 9 or 10 or 11 or 12 or 13 or 14 16. 7 and 15 17. (controlled clinical trial or randomized controlled trial).pt. 18. (randomized or randomised).ab,ti. 19. placebo.ab,ti. 20. dt.fs. 21. randomly.ab,ti. 22. trial.ab,ti. 23. groups.ab,ti. 24. or/17‐23 25. Animals/ 26. Humans/ 27. 25 not (25 and 26) 28. 24 not 27 29. 16 and 28
Embase (Ovid)
1. exp Pleura Effusion/ 2. exp Pleura Empyema/ 3. (Effusion Pleura or Pleural Effusion or Pleurorrhea or Pleurorrhoea).tw. 4. empyema$.tw. 5. (Pleural Suppuration or Pleurisy or Pleuritis or Pleuritis or Pyothorax).tw. 6. (parapneumonic adj5 effusion$).tw. 7. (pleural adj5 effusion$).tw. 8. parapneumonic$.tw. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp Fibrinolytic Agent/ 11. (Benzylidene or Trimethoxybenzylidene or Succinimide or Brinase or Brl or Dimer or Defibrotide or Fibrin$ or Phenylhydrazine or Plasmin$).tw. 12. (antithrombotic or thrombolytic or antithrombic).tw. 13. (Alprostadil or Anistreplase or Enoxaparin or Ancrod or Aspirin or Batroxobin or Brinolase or Heparin or Hirudin or Nadroparin or Plasmin or Plasminogen or Protein C or Streptokinase or Tedelparin or Ticlopidine or Tissue Plasminogen Activator).tw. 14. exp Plasminogen Activator/ 15. (Alteplase or Anistreplase or Duteplase or Lanoteplase or Monteplase or Pamiteplase or Plasminogen or Prourokinase or Reteplase or Saruplase or Staphylokinase or Streptokinase or Tenecteplase or Urokinase).tw. 16. exp STREPTOKINASE/ 17. (Avelizin or Avelysin or Awelysin or Celiase or Kabikinase or Kinalysin or Plasminokinase or Plasmokinase or Streptase or Streptococcal Fibrinolysin or Streptodecase or Streptodekaza).tw. 18. exp Urokinase/ 19. (Actosolv or Alphakinase or Corase or Rheotromb or Ukidan or Urinary Plasminogen Activator or Urokinase).tw. 20. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 9 and 20 22. Randomized Controlled Trial/ 23. randomization/ 24. controlled clinical trial/ 25. Double Blind Procedure/ 26. Single Blind Procedure/ 27. Crossover Procedure/ 28. (clinica$ adj3 trial$).tw. 29. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 30. exp Placebo/ 31. placebo$.ti,ab. 32. random$.ti,ab. 33. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 34. (crossover$ or cross‐over$).ti,ab. 35. or/22‐34 36. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 37. human/ or normal human/ or human cell/ 38. 36 and 37 39. 36 not 38 40. 35 not 39 41. 21 and 40
ClinicalTrials.gov
| Study type: | interventional |
| Condition: | empyema |
Data and analyses
Comparison 1. Fibrinolytic therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | 867 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.71, 1.91] |
| 2 Referral for thoracic surgery (open or thorascopic) | 8 | 897 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.21, 0.68] |
| 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy | 7 | 769 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.05, 0.58] |
| 4 Serious adverse events | 9 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.36, 4.57] |
Comparison 2. Streptokinase versus urokinase.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Referral for thoracic surgery (open or thorascopic) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.1. Analysis.
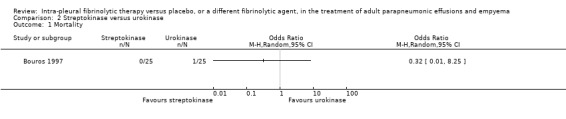
Comparison 2 Streptokinase versus urokinase, Outcome 1 Mortality.
Comparison 3. Alteplase versus urokinase.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Referral for thoracic surgery (open or thorascopic) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Overall treatment failure, including mortality, thoracic surgery or referral for further fibrinolytic therapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aleman 2015.
| Methods | Double‐blind, 2‐armed, parallel group RCT. | |
| Participants | 99 adults with a diagnosis of complex parapneumonic effusion (defined as imaging‐proven loculation and either a pleural fluid pH < 7.2, glucose < 60 mg/dL or positive Gram stain or culture) or empyema (defined as pus in the pleural space). Exclusion criteria were pregnancy or breast feeding, terminal clinical condition, coagulopathy, anti‐coagulant treatment, broncho‐pleural fistula, active haemorrhage, recent puncture of non‐compressible vessel, stroke in previous 6 months, major trauma or surgery in last 6 weeks, allergy to urokinase or streptokinase, renal or hepatic failure, use of agents under research or fertile women not using an effective contraceptive method. |
|
| Interventions | Initially 20 mg alteplase intra‐pleurally daily versus urokinase 100,000 IU daily. Blinding broken after 18 patients received 20 mg alteplase due to high rates of pleural haemorrhage. Remainder of alteplase group patients received 10 mg alteplase (33 patients) with no change to the urokinase arm. Co‐treatment with empirical antibiotics at attending physician's discretion, mostly amoxacillin/clavulanate. Small‐bore (12F) chest tube in the lowest part of the largest loculation. After instillation of agent/placebo, catheter tap turned off for 2 hours then reopened. Treatment daily until pleural fluid collections not visible on CXR for maximum of 6 days. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Blinding was broken after excess of bleeding events in the alteplase group and trial was recommenced after significant delay with a lower dose of alteplase in the alteplase group. Excess bleeding defined as a treatment failure in primary outcome. Study contained patients with both empyema and complex parapneumonic effusion. Funding sources not identified in published manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details lacking but manual process with potential for bias |
| Allocation concealment (selection bias) | Low risk | Block randomisation stratified by institution but blinding mitigates |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind. Identical volumes of drug |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Deliberately broke blinding following adverse events |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some lost to follow‐up in all arms. Higher withdrawals in alteplase 20 mg arm for bleeding — excluded from failure rate |
| Selective reporting (reporting bias) | Low risk | No apparent missing outcomes |
| Other bias | High risk | Mid‐trial protocol change due to excess of adverse events in alteplase 20 mg arm. Gap in recruitment between 2005 and 2008 |
Bouros 1997.
| Methods | Double‐blind, 2‐armed, parallel group RCT | |
| Participants | 33 male, 17 female, age 15 to 92 years Eligibility criteria: parapneumonic effusion as defined by the following criteria: purulent fluid with evidence of bacteria on Gram stain or culture, pH < 7.0, lactate dehydrogenase > 1000 IU/L, pleural fluid glucose < 40 mg/dL with multi‐loculation of pleural fluid on CT or ultrasound, or with an empyema with frank pus on thoracocentesis and multiple locules. Patients had clinical features of systemic sepsis and chest infection. As an inclusion criterion, there must have been failure to drain > 70 mL via thoracostomy tube during the previous 24 hours. Exclusion criteria: empyema as defined in Bouros 1999, florid sepsis, bronchopleural fistula, history of previous thrombolysis with streptokinase (streptokinase group only), known sensitivity to urokinase, bleeding diathesis, and contraindication to thrombolytic therapy such as a history of haemorrhagic stroke, intracranial neoplasm, cranial surgery or head trauma within the previous 14 days, abdominal or thoracic surgery within 10 days, or disease making survival < 2 months unlikely. |
|
| Interventions | Daily intrapleural streptokinase 250,000 IU versus urokinase 100,000 IU infusions Intervention group: intrapleural streptokinase 100,000 IU via a 28 to 32 French intercostal catheter in 100 mL normal saline on 3 consecutive days. Control group: 100,000 IU urokinase in 100 mL normal saline. After instillation, the drain was clamped for 3 hours. Continuous suction of −20 cm water was applied after each instillation. The process was repeated if the amount of pleural fluid was < 50 mL in the preceding 24 hours and a persistent effusion was seen on chest ultrasound or CT scan. The intercostal catheter was removed when the amount of fluid drained was < 50 mL in 24 hours and no persistence of effusion was seen on chest CT or ultrasound. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Withdrawal from the trial by continuing with fibrinolytic therapy or proceeding to surgery was made by the attending physician on the basis of "substantial" (unspecified) residual pleural fluid and persistent sepsis. |
|
| Notes | Treatment success was defined as pleural fluid drainage < 50 mL in 24 hours after 3 days' treatment, without residual pleural fluid collection on chest imaging. Follow‐up by chest radiograph and/or chest ultrasound or thoracic CT ranged from 6 to 30 months (mean 14 months). 2 patients were crossed streptokinase to urokinase due to high fever. Allocation concealment unclear since it is uncertain if allocation protocol revealed at switch to urokinase in these patients. Funding sources not identified in published manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence; identified in previous version of review; additional correspondence from authors. |
| Allocation concealment (selection bias) | Unclear risk | It is unclear from the data whether 2 patients switched from streptokinase to urokinase due to high fever were excluded from the final analysis. Presumably the trial allocation code must have been broken to change treatment arms, but this is unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The participants, treating physicians and outcome assessors were all blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants, treating physicians and outcome assessors were all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed for a mean of 12 months (range 6 to 30 months). No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods fully reported. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | Low risk | Nil identified. |
Bouros 1999.
| Methods | Double‐blind, placebo controlled, parallel group RCT. | |
| Participants | 24 male, 7 female, age 21 to 78 years Participants received antibiotics which were appropriate for the organisms cultured, or broad spectrum antibiotics to cover gram‐positive, gram‐negative and anaerobic organisms. Eligibility criteria: parapneumonic effusion as defined by the following criteria: purulent fluid with evidence of bacteria on Gram stain or culture, pH < 7.0, lactate dehydrogenase > 1000 IU/L, pleural fluid glucose < 40 mg/dL with multi‐loculation of pleural fluid on CT or ultrasound, or with an empyema with frank pus on thoracocentesis and multiple locules. The patients also had clinical features of systemic sepsis and chest infection. Exclusion criteria: bronchopleural fistula, known sensitivity to urokinase, bleeding diathesis, and contraindication to thrombolytic therapy such as a history of haemorrhagic stroke, intracranial neoplasm, cranial surgery or head trauma within the previous 14 days, recent major surgery within 10 days, or disease making survival greater than 2 months unlikely. |
|
| Interventions | Daily intrapleural urokinase 100,000 IU for 3 days versus saline Intervention group: intrapleural urokinase 100,000 IU via a 28 to 32 French intercostal catheter in 100 mL normal saline on 3 consecutive days. Control group: 100 mL normal saline. The drain was then clamped for 3 hours. Continuous suction of −20 cmH₂O was applied for 18 hours after each instillation. The intercostal catheter was removed after the 4th instillation and when the amount of fluid drained was < 50 mL in 24 hours. Follow‐up period: assessment of chest radiograph at between 7 and 28 months (mean 14). |
|
| Outcomes | Primary treatment outcomes:
Secondary and surrogate treatment outcomes:
Withdrawal from the trial by continuing with fibrinolytic therapy or proceeding to surgery was made by the attending physician on the basis of "substantial" (unspecified) residual pleural fluid and persistent sepsis. Treatment success was defined as pleural fluid drainage < 50 mL in 24 hours after 3 days' treatment, without residual pleural fluid collection on chest imaging. Follow‐up by chest radiograph and/or chest ultrasound or thoracic CT ranged from 7 to 28 months (mean 14 months). |
|
| Notes | Parapneumonic effusion and empyema. Funding "Supported in part by a grant from ASTRA Hellas" — i.e. drug company funding. No additional statements made. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The solutions were prepared by the hospital nursing staff, and neither the treating physicians nor the participant were aware of the identity of the treatment. It appears that nursing staff not blinded with potential for unblinding physicians and participant. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Chest radiographs were evaluated independently by 2 experts without knowledge of the randomisation group of the participant. Chest radiographs properly blinded but other measures including observations appear to be made by possibly unblinded nurses or clinicians with high risk of inadvertent unblinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in methods fully reported. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | Low risk | Partial cross‐over design. In the case of treatment failure the codes were broken and further instillation was undertaken with 3 days of instillation of UK in the control group and VATS in case of UK failure in both groups. Usage of UK after breaking codes has been assumed to be treatment failure equivalent to referral for surgery. |
Davies 1997.
| Methods | Double‐blind, placebo‐controlled, parallel group RCT | |
| Participants | 17 male, 7 female, age 18 to 90 years Eligibility criteria: community‐acquired pneumonia, sepsis and a parapneumonic effusion as defined by the following criteria: purulent fluid with evidence of bacteria on microscopy or culture, pH < 7.1, lactate dehydrogenase > 1000 IU/L, pleural fluid/blood glucose ratio < 0.25 with loculation/septation of pleural fluid on CT. Exclusion criteria: previous treatment with streptokinase within the previous 2 years, bleeding diathesis, and significant haemorrhage or stroke within the previous 6 months, or disease making survival < 2 months unlikely. Participants all received antibiotics which were appropriate for the organisms cultured, or broad‐spectrum antibiotics to cover gram‐positive, gram‐negative and anaerobic organisms. |
|
| Interventions | Daily intrapleural streptokinase 250,000 IU for 3 days versus normal saline. Intervention group: intrapleural streptokinase 250,000 IU via a 14 French van Sonnenberg catheter in 20 mL normal saline on 3 consecutive days. Control group: 20 mL normal saline in the control group. The drain was then clamped for 2 hours. Both groups received a background of 6‐hourly 20 mL normal saline flushes until the intercostal catheter was removed. The catheters were inserted into the most dependent portion of the effusion or into the largest loculation. Continuous suction of −20 cmH₂O was applied. The intercostal catheter was removed after the 5th day and when the amount of fluid drained was < 150 mL for 2 consecutive days. Further aspiration or catheter drainage was at the discretion of the admitting physician. Follow‐up: 3 years. |
|
| Outcomes |
|
|
| Notes | Parapneumonic effusion only. Funding sources not identified in published manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence (author correspondence). |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind author correspondence. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Radiographs scored by blinded radiologists. Not specified for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Withdrawals" were referrals for surgery — an outcome. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods fully reported. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | Low risk | Nil identified. |
Diacon 2004.
| Methods | Double‐blind parallel RCT. The results were analysed on an intention‐to‐treat basis, with all participants who received at least 1 treatment in either arm being entered into the final analysis of primary outcomes. | |
| Participants | 44 total: 33 male, 11 female, age 16 and older All participants received broad‐spectrum antibiotic therapy, tailored to organisms grown thereafter. Eligibility criteria: adults with a lung infection and concomitant pleural effusion were included if they had an empyema or complicated parapneumonic effusion with pH < 7.0, or pH < 7.2 with evidence of fluid loculation on chest radiograph or ultrasound. Exclusion criteria: less than 16 years of age, recent severe trauma, haemorrhage or stroke, bleeding disorder or anticoagulation therapy, administration of streptokinase within 2 previous years, likely survival of less than 6 months or with preceding thoracic drainage procedures. |
|
| Interventions | Streptokinase 250,000 IU once daily for up to 7 days or until net drainage less than 100 mL per day. A 24 or 28 French Argyle chest tube was inserted. Intervention group: 100 mL normal saline with streptokinase 250,000 IU. Control group: 100 mL of normal saline. The drain was clamped for 2 hours. The therapy was administered once daily for up to 7 days or until net drainage was less than 100 mL per day. |
|
| Outcomes |
|
|
| Notes | Parapneumonic effusions and empyema. Treatment success was defined as a study in point at Day 3 and Day 7; and at discharge, which required the following.
Criteria for referral to surgery were:
Referral for surgery required agreement of at least 2 physicians involved in the study. Funding: "Supported by a grant from the Voluntary Academic Association Basel, Switzerland, and by a research fellowship grant of the University of Stellenbosch, South Africa (A.H.D.). Aventis South Africa provided the streptokinase free of charge; the company had no further involvement in the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence (stratified by blocks of 4). |
| Allocation concealment (selection bias) | Low risk | Only 1 author was aware of group assignment throughout the trial and did not participate in clinical decision making. Randomisation was concealed to all other participants throughout the trial. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were randomised by MMS in blocks‐of‐four using a computer generated table. MMS also made the rinse solutions, which were identical in appearance. MMS was the only person aware of group assignment throughout the trial and did not participate in clinical decision making. Randomization was concealed to all other participants throughout the trial and the code was broken only after all data had been entered into the database" ‒ supplemental material |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 excluded post‐randomisation prior to therapy. 1/22 lost post therapy in control, 2/22 loss post therapy in treatment group |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods fully reported. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | High risk | Post‐randomisation exclusions |
Lin 2011.
| Methods | Parallel RCT. SIngle centre study. | |
| Participants | 32 participants with loculated pleural effusions from infective pleurisy. No further information reported. | |
| Interventions | Intervention group: intrapleural tPA 10 mg and saline 50 mL intrapleurally after each thoracocentesis. Control group: intrapleural saline 5 mL after each thoracocentesis was injected with 50 mL saline. Number of thoracocenteses not reported for either group. |
|
| Outcomes |
|
|
| Notes | Chinese language paper (Abstract in English): 2 separate translations by non‐authors. Funding: 2007 Huangpu District Government‐funded projects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised, no further information available. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate with placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | Multiple uncovered outcomes (referral for surgery, death). |
| Other bias | High risk | Subjective outcome measures. |
Maskell 2005.
| Methods | Double‐blind parallel RCT | |
| Participants | 299 male, 131 female aged 18 years or more All participants received broad‐spectrum antibiotic therapy, subsequently tailored to microbiological culture and sensitivity as well as intercostal catheter drainage. Eligibility criteria: presence of pleural fluid which was macroscopically purulent, positive on culture for bacterial infection, positive for bacteria on Gram stain or pH < 7.2 in participants with clinical and laboratory indicators of infection such as fever, raised white cell count and raised C reactive protein. Exclusion criteria: < 18 years, coincidental serious illness with survival at 3 months unlikely, previous intrapleural fibrinolytic or surgical therapy, sensitivity to streptokinase, coincidental stroke or major haemorrhage, major surgery within 5 days previous, previous pneumonectomy of same side as pleural infection, known pleural malignancy and females who were pregnant or lactating. |
|
| Interventions | Daily intrapleural streptokinase 250,000 IU every 12 hours for 6 doses versus placebo. Control group: 30 mL of normal saline delivered by an intercostal chest tube (median size 12 French, interquartile range 12 to 20) installed every 12 hours for 6 doses. Intervention group: streptokinase 250,000 IU in 30 mL of normal saline using the same chest tube regimen. |
|
| Outcomes | Primary outcomes
Secondary endpoints
|
|
| Notes | Parapneumonic effusions and empyema Funding: "Aventis UK provided the streptokinase and placebo for the trial. The United Kingdom Medical Research Council provided funding for the trial. The British Thoracic Society promoted the trial. Neither the company nor these organizations had any influence on the design or execution of the trial or on decisions relating to publication". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At randomisation, the groups were balanced by minimisation for the presence of frankly purulent pleural fluid and the availability of thoracic surgery in the recruiting centre. |
| Allocation concealment (selection bias) | Low risk | Centrally prepared allocation schedule by telephone from the study centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both the participants and treating physicians were blind to treatment group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the primary outcomes and adverse events were reviewed by 2 blinded observers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up (2 in streptokinase group, 1 in placebo group), not high enough to impact outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes in primary study protocol reported. Nil added. |
| Other bias | Low risk | Nil identified. |
Misthos 2005.
| Methods | Double‐blind parallel RCT. | |
| Participants | 96 men, 37 women, aged 19 to 77 years Decision to continue with chest tube thoracostomy with or without fibrinolytic therapy, or to proceed to surgery was made by an independent chest physician who was blinded to the mode of treatment. All participants were assessed with CT or ultrasound scans or both for the presence of loculations. All participants were managed with antibiotic treatment and thoracocentesis. Eligibility criteria: primary bacterial post‐pneumonic thoracic empyema, parapneumonic effusion defined as purulent pleural fluid or positive effusion Gram stain or culture for bacteria, or pH < 7.2, glucose < 40 mg/dL and LDH greater than 1000 IU/L. Exclusion criteria: participants who had a contraindication to surgery or with end‐stage underlying disease or tuberculous empyema, bronchopleural fistula, the presence of a lung abscess, a known sensitivity to streptokinase or a contraindication to thrombolytic therapy. |
|
| Interventions | Intervention group: intercostal catheter drainage and daily intrapleural streptokinase 250,000 units. Control group: intercostal catheter drainage only (no placebo). All participants had an Argyle intercostal drain size 28 to 32 French attached to suction at −20 cmH₂O. Intervention group: streptokinase 250,000 IU in 60 mL normal saline intrapleurally daily for 3 days. Control group: 60 mL normal saline daily intrapleurally for 3 days. The tube was clamped for 4 hours then re‐opened. Streptokinase or control was given daily for 3 successive days and treatment result assessed after 3 days. Drainage was left in place until daily output fluid was less than 50 mL and the radiographic image remained unchanged. Absence of fluid at the posterior costophrenic angle at lateral chest radiograph or at CT scan was considered as adequate pleural fluid evacuation (author communication) The decision to proceed to surgery (VATS or open thoracotomy decortication) was indicated by progressive or persistent sepsis and the presence of substantial residual pleural fluid. Criteria for surgery included assessment of the amount of pleural fluid that could not be drained with chest tube thoracostomy because of trapped lung or chronicity; and more specifically if the fluid filled the posterior costophrenic angle and led to atelectasis of the posterior basal segments of the lower lobes (author communication). |
|
| Outcomes |
|
|
| Notes | Parapneumonic effusions only. Funding sources not identified in published manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pariticipants allocated to placebo or treatment arms on the basis of their hospital admission number. |
| Allocation concealment (selection bias) | High risk | Allocation concealment potentially threatened by allocation procedure (hospital admission number). |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participant and treating physicians do not appear to have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment by a single‐blinded respiratory physician. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher dropout rate in intervention arm (8/65) cf control arm (0/70) may have biased results. |
| Selective reporting (reporting bias) | Unclear risk | Major outcomes in methods prospectively (mortality, clinical success, length of stay, chest imaging) all reported. Surgery frequency reported but not pre‐specified. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | Low risk | Nil identified. |
Prasad 2009.
| Methods | Parallel RCT. Conference abstract only. | |
| Participants | 96 participants total (48 in streptokinase group and 48 in control group) Inclusion criteria: loculated empyema thoracis secondary to bacterial pneumonia. Exclusion criteria unstated. Nil further information available. |
|
| Interventions | Intervention group: intrapleural pigtail catheter with 2.5 million units streptokinase daily for 5 days. Control group: normal saline intrapleural instillation, volume not stated. Both arms used intrapleural pigtail catheters and twice‐daily manual aspiration of pus. |
|
| Outcomes |
All participants who did not recover were treated with surgical decortication. |
|
| Notes | Conference abstract only. Authors contacted but did not reply with further information. All participants who did not completely recover were referred for surgical intervention. Manuscript identifies no sources of funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for determination. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for determination. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information for determination. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for determination. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information for determination. |
| Selective reporting (reporting bias) | High risk | Only a single conference report to go on. It is likely that the authors had other data they would have included had they written a full paper. |
| Other bias | Unclear risk | Limited information, no study protocol. |
Rahman 2011.
| Methods | Double‐blind placebo controlled 2 × 2 parallel factorial design ‒ alteplase versus placebo and alfa dornase (DNase) versus control. Participants allocated by minimization with randomised component. | |
| Participants | 210 total, 151 male, 59 female with empyema or complicated pleural effusion recruited from normal clinical practice. Broad spectrum antibiotic therapy for at least 2 weeks. Inclusion criteria: clinical evidence of infection and pleural fluid that was macroscopically purulent OR positive on culture for bacterial infection OR positive for bacteria on Gram staining; or pleural fluid that had a pH < 7.2 (measured by means of a blood‐gas analyser). Evidence of infection, which was assessed by the recruiting physician, included the presence of fever and elevated serum levels of inflammatory markers such as CRP or an elevated white cell count. Exclusion criteria: an age of less than 18 years, previous treatment with intrapleural fibrinolytic agents, DNase, or both for empyema; known sensitivity to DNase or tPA; coincidental stroke; major haemorrhage or major trauma; major surgery in the previous 5 days; previous pneumonectomy on the infected side; pregnancy or lactation; expected survival of less than 3 months owing to a pathologic condition other than that responsible for the pleural abnormalities |
|
| Interventions | 4 arms. Medications delivered via intercostal catheter.
|
|
| Outcomes |
|
|
| Notes | Parapneumonic effusion and empyema. Funding: "Roche UK (DNase) and Boehringer Ingelheim (tPA) provided the trial medication and placebos for the trial. The trial was funded through an unrestricted educational grant provided by Roche UK to the University of Oxford. The British Thoracic Society promoted the trial. None of these organizations had any influence on the design, conduct or analysis of the trial or on decisions relating to publication" (appendix). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned to study group by central telephone service with minimisation. |
| Allocation concealment (selection bias) | Low risk | Central telephone service randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐dummy, double‐blind and placebo controlled — all investigators, participants and outcome assessors were blind to treatment allocation (confirmed in private communication). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All computed tomography and chest radiograph assessments were conducted blind of each other." A single clinician (an experienced respiratory physician not involved in trial recruitment), blind to treatment allocation and participant identity, was provided with clinical information from the trial case report forms for deaths, surgical events and serious adverse events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on the primary outcome were available for all 210 participants; although 7 participants (3%) died before day 7, radiographs were available from the day of death for all these participants. Data regarding survival were available for 209 of the 210 participants (99.5%) at 3 months and for 203 participants (97%) at 12 months. Data on referral for surgery were available for 209 of the 210 participants (99.5%) at 3 months and for 203 participants (97%) at 12 months. No data loss for primary outcome. Minimal data loss on referral for surgery and 3/12 surgery. |
| Selective reporting (reporting bias) | Low risk | All outcomes identified. Protocol reviewed, no non‐measured outcomes in protocol. |
| Other bias | Low risk | Nil identified. |
Thommi 2012.
| Methods | Double‐blind, placebo‐controlled, cross‐over design inappropriate for outcome. Truncated prior to crossover for meta‐analysis. | |
| Participants | 37 male, 21 female, age range 21 to 90 years. All received broad‐spectrum antibiotics. | |
| Interventions | Intervention group: daily alteplase 25 mg in 100 mL normal saline intrapleurally. Control group: 100 mL normal saline intrapleurally Most participants received 28F intercostal catheters, some with 14‐16F catheters attached to suction. The tube was clamped for 1 hour then placed on suction. Treatment result assessed on day 4. Treatment was deemed successful if the CT pleural fluid volume decreased by at least 50% compared with pre‐alteplase CT. Participants who failed treatment were then crossed over to the alternative group and administered the other therapy (alteplase 25 mg daily for 3 days or placebo). Participants who failed both arms of the trial were then offered surgical decortication. Further follow‐up CT was performed as 6 weeks. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Parapneumonic effusions and empyema. Inappropriate trial design, truncated for meta‐analysis prior to cross‐over. Funding: A restricted grant was sponsored by Genetech, Inc. for this trial. Monies was distributed to the Institutional Review Board at Methodist hospital and to the Pharmacy department per participant. Monies were also distributed per participant to the physician co‐investigators, nursing staff and Physicians Assitsant(s) that were involved in following the participants' care and collecting the data. Genetech did not have any input in any part of the protocol, in participant care or collection of data. Genetech was informed whenever a participant entered the trial and the drug alteplase was supplied free of charge to the participant. Genetech was not involved in writing up the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospital pharmacist randomised participants by a fixed‐allocation randomisation process using a computer‐based random sequence generator. |
| Allocation concealment (selection bias) | Low risk | Central control (pharmacist with no participant contact). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All investigators were blinded and the pharmacist had no contact with the participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators were blinded, specifics not mentioned. Principal investigator sole assessor of pleural fluid outcomes (only reported outcomes). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant number of post‐randomisation withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Planned primary outcome described as a 40% reduction in surgical intervention between alteplase and placebo groups. Note: all participants refused surgery. All "failures" (i.e. participants without 50% reduction in fluid volume) were offered surgery but no more details available. The secondary end point was a 50% difference in resolution of dyspnoea, sepsis syndrome and pneumonia between the 2 groups. Note: all participants refused surgery. Success instead reported as proportion of participants with at least 50% reduction in pleural fluid volume on radiologic examination. No description of proportion of participants with resolution of sepsis syndrome documented. Breathlessness mentioned as secondary outcome measure in protocol; not reported. However these outcomes (except treatment failure) were not included in the review, and so they have no effect on the review results. |
| Other bias | Unclear risk | Cross‐over study design inappropriate for a binary outcome such as requirement for surgery or death. This had no effect on the review, however, as we only used data from the first phase (i.e. before participants were crossed over). |
Tuncozgur 2001.
| Methods | Double‐blind, placebo‐controlled, parallel. There were no dropouts. The study methods and randomisation procedure were well accounted for (personal communication with author). | |
| Participants | 38 male,11 female, age 15 to 85 years. The participants all received antibiotics appropriate for the organisms cultured, or empirically selected broad‐spectrum antibiotics. Exclusion criteria: tuberculosis or hospital‐acquired, post‐traumatic or post‐operative pneumonia were excluded. Those older than 85 and younger than 15 were also excluded. |
|
| Interventions | Intervention group: daily intrapleural urokinase 100,000 IU in 100 mL normal saline for 5 days in addition to intercostal catheter drainage. Control group: 100 mL intrapleural normal saline for 5 days in addition to intercostal catheter drainage. |
|
| Outcomes |
|
|
| Notes | Parapneumonic effusions only. Funding sources not identified in published manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was appropriately described by the author in private communication. |
| Allocation concealment (selection bias) | Low risk | Blinded opaque envelope allocation described in private communication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The participants, treating physicians and outcome assessors were all blinded; described in private communication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants, treating physicians and outcome assessors were all blinded; described in private communication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts during study. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not prospectively mentioned in Methods section other than decortication rate and reasons for decortication. Original protocol unavailable to confirm original primary outcomes. |
| Other bias | Low risk | Nil identified. |
Abbreviations cmH₂O: centimetres of water ‐ a unit of pressure; CRP: C‐reative protein; CT: computerised tomography; CXR: chest X‐ray; IU: international units; LDH: lactate dehydrogenase; RCT: randomised controlled trial; tPA: Tissue Plasminogen Activator; UK: urokinase; VATS: video‐assisted thoracoscopic surgery.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2016 | No randomised studies in a set of conference abstracts. |
| Anonymous 2019 | No randomised studies in a set of conference abstracts. |
| Ayed 2013 | Not a randomised study. |
| Bachmayer 2011 | Not an original study, but a letter to the editor. |
| Bashir 2013 | This study contained a majority of tuberculous effusions/empyemas (37 of 66 participants) with no separate data available for parapneumonic effusions only. Published and unpublished data. |
| Beckert 2019 | Not a randomised study. |
| Bilaçeroglu 1997 | Study results found in abstract form only. Too many missing details regarding all aspects of study to consider inclusion. |
| Bouros 2006 | Not an original study, but an editorial. |
| Cases Viedma 2006 | The study has participants with only tuberculous effusions. |
| Chin 1997 | Not a randomised controlled trial. This was sequential study (effectively a cohort study with historical controls) in which the participants received either intrapleural saline or intrapleural streptokinase 250,000 IU. The participants were allocated to each of the treatment arms according to temporal sequence. |
| Chung 2003 | Non‐randomised study. |
| Chung 2008 | The study has participants with tuberculous effusions only. |
| CTRI/2018/08/015361 2018 | This ongoing study contains large number of participants with tuberculous effusions. |
| Fernandez 2007 | This study contained exclusively paediatric participants. |
| Fester 2012 | Commentary, not a randomised study. |
| Froudarakis 2008 | Not a randomised study. |
| ISRCTN12852177 2008 | Not a study of empyema/CPE; study of malignant effusions. |
| Jin 2013 | Not a randomised study. |
| Komissarov 2013 | No relevant outcomes‐in vitro evaluation of pleural fluid collected during Rahman 2011. |
| Light 2010 | Narrative review; not a randomised study. |
| Lim 1999 | Not a randomised study. |
| Maiga 2017 | Not a randomised study; retrospective cohort study. |
| Maskell 2006 | Letter to the editor; not a randomised study. |
| Masood 2006 | The study has participants with tuberculous effusions only. No placebo group. |
| Mathur 2011 | Journal Club discussion, not a randomised study. |
| Muers 1997 | Commentary; not a randomised study. |
| NCT00103766 2004 | This trial was abandoned due to lack of participant enrolment; direct communication from Dr Jeana O'Brien, lead investigator. |
| NCT01862458 2013 | Wrong population; paediatric study. |
| NCT02973139 2016 | Wrong comparator; medical pleuroscopy versus fibrinolysis |
| NCT03172052 2017 | Wrong comparator; medical pleuroscopy versus fibrinolysis |
| Ruiz 2006 | Not a randomised study; case report. |
| Shah 2006 | This study has participants with predominantly (12/18 intervention group, 12/18 control group) tuberculous effusions. |
| Skeete 2004 | Retrospective chart review; not a randomised study. |
| Talib 2003 | The study has participants with predominantly tuberculous effusions. |
| Tsang 2007 | Retrospective review; not a randomised study. |
| Wong 2006 | Editorial accompanying a paediatric trial. |
| Zuckerman 2009 | No control/comparator arm. |
Differences between protocol and review
We used the headings currently recommended by Cochrane. We added a 'Summary of findings' table and PRISMA diagram; and updated the 'Risk of bias' assessment criteria.
We removed the subgroup analysis of effusions with confirmed loculation, which was originally contained in the protocol as well as previous versions of the review. This is due to concerns regarding variation in the subgroup of patients which would be included in this subgroup, which is likely to vary significantly between studies. Additionally, the authors believe that the clinical utility of such a subgroup is limited when most inclusion criteria of contained studies require an empyema or CPE which does not resolve with an ICC and antibiotic therapy.
Earlier versions of this review presented analysis of low versus high risk of bias trials as subgroups. Here we have chosen to present low versus low/unclear versus high risk of bias outcomes as sensitivity analyses.
We chose to use random‐effects models rather than fixed‐effect models. The included trials are clearly in a wide range of populations with differing health systems and a broad group of interventions. We believe it is reasonable to assume the true effect varies across these populations.
Contributions of authors
ESA: study assessment, data extraction and analysis, interpretation and discussion.
IC: study assessment, data extraction and analysis, interpretation and discussion.
SW: data extraction, interpretation and discussion.
HRD: protocol development; study assessment, interpretation and discussion.
Contributions of editorial team
Rebecca Fortescue (Coordinating Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to publication. Chris Cates (Coordinating Editor, Contact Editor) checked the data entry prior to the full write‐up of the review; edited the review; advised on methodology, interpretation and content. Emma Dennett (Managing Editor): coordinated the editorial process; advised on interpretation and content; edited the review. Emma Jackson (Assistant Managing Editor): conducted peer review; edited the Plain Language Summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
The review authors declare that no such funding was received for this systematic review., Other.
External sources
The review authors declare that no such funding was received for this systematic review,, Other.
Declarations of interest
ESA: none known.
IC: received travel and training expenses from Hamilton Medical that are not connected to the topic of this review.
SW: none known.
HRD: is a member of Astra Zeneca COPD Advisory Board Interaction and has given lectures for and on behalf of Astra Zeneca, GSK and Boehringer‐Ingelheim in areas of COPD, ILD and asthma. No direct conflict of interest with work on fibrinolytic agents.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aleman 2015 {published data only}
- Aleman C, Porcel JM, Alegre J, Ruiz E, Bielsa S, Andreu J, et al. Intrapleural fibrinolysis with urokinase versus alteplase in complicated parapneumonic pleural effusions and empyemas: a prospective randomized study. Lung 2015;193(6):993‐1000. [DOI] [PubMed] [Google Scholar]
Bouros 1997 {published data only}
- Bouros D, Schiza S, Patsourakis G, Chalkiadakis G, Panagou P, Siafakas N. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions. American Journal of Respiratory and Critical Care Medicine 1997;155(1):291‐5. [DOI] [PubMed] [Google Scholar]
Bouros 1999 {published data only}
- Bouros D, Schiza S, Tzanakis N, Chalkiadakis G, Drositis J, Siafakas N. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. American Journal for Respiratory and Critical Care Medicine 1999;159(1):37‐42. [DOI] [PubMed] [Google Scholar]
- Bouros D, Schiza SE, Drositis J, Tzanakis N, Papalexatos S, Mitrouska I, et al. Control study of intrapleural instillation of urokinase in the treatment of multi‐loculated pleural effusion and empyema. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A65. [Google Scholar]
- Schiza S, Bouros D, Maltezakis G, Drositis J, Tzanakis N, Siafakas NM. Intrapleural urokinase vs normal saline in the treatment of thoracic empyema: a randomized double blind study. European Respiratory Journal 1997;10(Suppl 25):455s. [Google Scholar]
Davies 1997 {published and unpublished data}
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52(5):416‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diacon 2004 {published data only}
- Diacon AH, Theron J, Schuurmans MM, Wal BW, Bolliger CT. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. American Journal of Respiratory and Critical Care Medicine 2004;170(1):49‐53. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin J‐C, Zhang C‐R, Li M, Zhou H, Niu Y‐Y, Liu X‐Y. Effectiveness and safety of intrapleural tissue plasminogen activator in the prevention of pleural thickening and loculated pleural effusions. Chinese Journal of Clinicians 2011;5(9):2610‐14. [Google Scholar]
- Lin J‐C, Zhang C‐R, Xu W‐M, Li M, Cui W‐L. Effectiveness and safety of intrapleural tissue plasminogen activator in the prevention of pleural thickening and loculated effusions by infective pleurisy. International Journal of Infectious Diseases 2011;15(suppl 1):S104‐5. [Google Scholar]
Maskell 2005 {published data only}
- Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, et al. U.K. controlled trial of intrapleural streptokinase for pleural infection. New England Journal of Medicine 2005;352(9):865‐74. [DOI] [PubMed] [Google Scholar]
Misthos 2005 {published data only}
- Misthos P, Sepsas E, Konstantinou M, Athanassiadi K, Skottis I, Lioulias A. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. European Journal of Cardio‐thoracic Surgery 2005;28(4):599‐603. [DOI] [PubMed] [Google Scholar]
Prasad 2009 {published data only}
- Prasad BNBM, Bhattacharyya D, Luthra M, Mathur AD. Management of empyema thoracis with pleural pigtail drainage and intrapleural thrombolytic therapy [Abstract]. American Journal of Respiratory and Critical Care Medicine 2009;179:A4486 [Poster #G115]. [Google Scholar]
Rahman 2011 {published and unpublished data}
- Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. New England Journal of Medicine 2011;365(6):518‐26. [DOI] [PubMed] [Google Scholar]
Thommi 2012 {published data only}
- Thommi G, Shehan JC, Robison KL, Christensen M, Backemeyer LA, McLeay MT. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respiratory Medicine 2012;106(5):716‐23. [DOI] [PubMed] [Google Scholar]
Tuncozgur 2001 {published and unpublished data}
- Tuncozgur B, Ustunsoy H, Dikensoy O, Topal M, Sanli M, Elbeyli L. Intrapleural urokinase in the management of parapneumonic empyema: a randomised controlled trial. International Journal of Clinical Practice 2001;55(10):658‐60. [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2016 {published data only}
- Anonymous. 21st Congress of the Asian Pacific Society of Respirology; 2016 Nov 12‐15; Bangkok. 2016; Vol. 21:S3.
Anonymous 2019 {published data only}
- Anonymous. CHEST 2019 Regional Congress Abstracts. Chest 2019;Conference: CHEST 2019 Regional Congress. Greece. 155(6 Supplement):A327‐88. [Google Scholar]
Ayed 2013 {published data only}
- Ayed A, Shehab D. Role of intrapleural tissue plasminogen in management of complicated pleural effusion. Cardiothoracic Surgery 2013;144(4):108A. [Google Scholar]
Bachmayer 2011 {published data only}
- Bachmayer K. Tissue plasminogen activator and DNase in empyema. New England Journal of Medicine 2011;365(20):1936; author reply 1936‐7. [DOI] [PubMed] [Google Scholar]
Bashir 2013 {published and unpublished data}
- Bashir M, Mousbah H, Gdeedo A. Effectiveness and safety of tissue plasminogen activators (t‐PA) in complicated pleural effusion (CPE), what is the correlation with time it stays in the pleural space?. European Respiratory Journal 2013;42:5040. [Google Scholar]
Beckert 2019 {published data only}
- Beckert L, Brockway B, Simpson G, Southcott AM, Lee YCG, Rahman N, et al. Phase 1 trial of intrapleural LTI‐01; single chain urokinase in complicated parapneumonic effusions or empyema. JCI Insight 2019;4(10):e127470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilaçeroglu 1997 {published data only}
- Bilaçeroglu S, Cagirici U, Cakan A, Kumcuoglu Z, Perim K. Management of complicated parapneumonic pleural effusions with image‐guided drainage and intrapleural urokinase or streptokinase: a controlled randomized trial. European Respiratory Journal 10;Suppl 25:325s. [Google Scholar]
Bouros 2006 {published data only}
- Bouros D, Antoniou KM, Light RW. Intrapleural streptokinase for pleural infection. BMJ 2006;332(7534):133‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cases Viedma 2006 {published data only}
- Cases Viedma E, Lorenzo Dus MJ, Gonzalez‐Molina A, Sanchis Aldas JL. A study of loculated tuberculous pleural effusions treated with intrapleural urokinase. Respiratory Medicine 2006;100(11):2037‐42. [DOI] [PubMed] [Google Scholar]
Chin 1997 {published data only}
- Chin NK, Lim T. Controlled trial of intrapleural streptokinase in the treatment of pleural empyema and complicated parapneumonic effusions. Chest 1997;111(2):275‐9. [DOI] [PubMed] [Google Scholar]
- Lim TK, Chin NK. Empirical treatment with fibrinolysis and early surgery reduces the duration of hospitalization in pleural sepsis. European Respiratory Journal 1999;13(3):514‐8. [DOI] [PubMed] [Google Scholar]
Chung 2003 {published data only}
- Chung CL, Chen YC, Chang SC. Effect of repeated thoracenteses on fluid characteristics, cytokines, and fibrinolytic activity in malignant pleural effusion. Chest 2003;123(4):1188‐95. [DOI] [PubMed] [Google Scholar]
Chung 2008 {published data only}
- Chung CL, Chen CH, Yeh CY, Sheu JR, Chang SC. Early effective drainage in the treatment of loculated tuberculous pleurisy. European Respiratory Journal 2008;31(6):1261‐7. [DOI] [PubMed] [Google Scholar]
CTRI/2018/08/015361 2018 {published data only}
- CTRI/2018/08/015361. Empyema of lung treatment. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/08/015361 2018.
Fernandez 2007 {published data only}
- Fernández Fernández A, Giachetto Larraz G, Giannini Fernandez G, Garat Gomez MC, Vero Acevedo MA, Pastorini Correa J, et al. Intrapleural streptokinase in the treatment of complicated parapneumonic empyema. Anales de Pediatria (Barcelona, Spain : 2003) 2007;66(6):585‐90. [DOI] [PubMed] [Google Scholar]
Fester 2012 {published data only}
- Fester A. Revisiting intrapleural fibrinolysis in empyema. Thorax 2012;67(4):308. [Google Scholar]
Froudarakis 2008 {published data only}
- Froudarakis ME, Kouliatsis G, Steiropoulos P, Anevlavis S, Pataka A, Popidou M, et al. Recombinant tissue plasminogen activator in the treatment of pleural infections in adults. Respiratory Medicine 2008;102(12):1694‐700. [DOI] [PubMed] [Google Scholar]
ISRCTN12852177 2008 {published data only}
- ISRCTN12852177. A randomised controlled trial to evaluate whether use of intrapleural urokinase aids the drainage of multi‐septated pleural effusion compared to placebo. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN12852177 2008.
Jin 2013 {published data only}
- Jin JM, Sun YC. [Evaluation of febrinolytic therapy for treatment of pleural infection]. [Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2013;36(5):327‐9. [PubMed] [Google Scholar]
Komissarov 2013 {published data only}
- Komissarov AA, Florova G, Schaefer C, Rahman NM, Lee YCG, Idell S. Pleural fluids collected during the second multicenter intrapleural sepsis trial (MIST2) demonstrate highly variable fibrinolytic potential prior to the treatment and endogenous fibrinolytic activity depletion during intrapleural fibrinolytic therapy. American Journal of Respiratory and Critical Care Medicine 2013;187:A4313. [Google Scholar]
Light 2010 {published data only}
- Light RW. Management of parapneumonic effusions. Journal of Clinical and Analytical Medicine 2010;1(2):48‐51. [Google Scholar]
Lim 1999 {published data only}
- Lim TK, Chin NK. Empirical treatment with fibrinolysis and early surgery reduces the duration of hospitalization in pleural sepsis. European Respiratory Journal 1999;13(3):514‐8. [PUBMED: 10232418] [DOI] [PubMed] [Google Scholar]
Maiga 2017 {published data only}
- Maiga A, Pinkerman R, Deppen SA, Scruggs J, Mcguire P, Tarpley JL, et al. tPA/DNase for Complicated Parapneumonic Effusions and Empyemas. American Surgeon 2017;83(12):1458‐9. [PMC free article] [PubMed] [Google Scholar]
Maskell 2006 {published data only}
- Maskell N, Nunn A, Davies RJ. Intrapleural streptokinase for pleural infection. BMJ 2006;332(7540):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masood 2006 {published data only}
- Masood I, Bhargava R, Ahmad Z, Pandey DK, Ahmad S. Role of intrapleural streptokinase in empyema. Journal, Indian Academy of Clinical Medicine : JIACM 2006;7(4):313‐5. [Google Scholar]
Mathur 2011 {published data only}
- Mathur PN. Intrapleural t‐PA plus DNase improved clinical outcomes in patients with pleural infection. Annals of Internal Medicine 2011;155(12):JC6‐9. [DOI] [PubMed] [Google Scholar]
Muers 1997 {published data only}
- Muers MF. Streptokinase for empyema. Lancet 1997;349(9064):1491‐2. [DOI] [PubMed] [Google Scholar]
NCT00103766 2004 {published data only}
- NCT00103766. Randomized comparison of two dose and frequency regimens of alteplase for treatment of empyema and complicated parapneumonic effusion. clinicaltrials.gov/ct2/show/NCT00103766 (First received February 15 2005).
NCT01862458 2013 {published data only}
- NCT01862458. Empyema Treated With tPA & DNAse. clinicaltrials.gov/show/NCT01862458 2013.
NCT02973139 2016 {published data only}
- NCT02973139. Fibrinolysis Compared to Thoracoscopy for Pleural Infection. clinicaltrials.gov/show/NCT02973139 2016.
NCT03172052 2017 {published data only}
- NCT03172052. Evaluating Different Modalities for Pleural Adhesiolysis at Assuit University Hospital. clinicaltrials.gov/show/nct03172052 2017.
Ruiz 2006 {published data only}
- Ruiz A, Porcel JM, Madronero AB, Galindo C. Hemothorax following administration of intrapleural alteplase. Respiration 2006;73(5):715. [DOI] [PubMed] [Google Scholar]
Shah 2006 {published data only}
- Shah NN, Bachh AA, Bhargava R, Ahmad Z, Panday DK, Shameem Mohd. Role of intrapleural streptokinase in management of multiloculated thoracic empyemas. JK Practitioner 2006;13(2):91‐4. [Google Scholar]
Skeete 2004 {published data only}
- Skeete DA, Rutherford EJ, Schlidt SA, Abrams JE, Parker LA, Rich PB. Intrapleural tissue plasminogen activator for complicated pleural effusions. Journal of Trauma 2004;57(6):1178‐83. [DOI] [PubMed] [Google Scholar]
Talib 2003 {published data only}
- Talib SH, Verma GR, Arshad M, Tayade BO, Rafeeque A. Utility of intrapleural streptokinase in management of chronic empyemas. Journal of the Association of Physicians of India 2003;51:464‐8. [PubMed] [Google Scholar]
Tsang 2007 {published data only}
- Tsang KY, Leung WS, Chan VL, Lin AW, Chu CM. Complicated parapneumonic effusion and empyema thoracis: microbiology and predictors of adverse outcomes. Hong Kong Medical Journal 2007;13(3):178‐86. [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong K‐S. Is intrapleural streptokinase instillation a routine for parapneumonic empyema?. Acta Paediatrica Taiwanica 2006;47(2):59‐60. [PubMed] [Google Scholar]
Zuckerman 2009 {published data only}
- Zuckerman DA, Reed MF, Howington JA, Moulton JS. Efficacy of intrapleural tissue‐type plasminogen activator in the treatment of loculated parapneumonic effusions. Journal of Vascular and Interventional Radiology 2009;20(8):1066‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Chung 2005
- Chung CL, Chen CH, Sheu JR, Chen YC, Chuang SC. Proinflammatory cytokines, transforming growth factor‐beta1, and fibrinolytic enzymes in loculated and free‐flowing pleural exudates. Chest 2005;128(2):690‐7. [DOI] [PubMed] [Google Scholar]
Davies 2010
- Davies HE, Davies RJO, Davies CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65(Suppl 2):ii41‐ii53. [DOI] [PubMed] [Google Scholar]
Farjah 2007
- Farjah F, Symons RG, Krishnadasan B, Wood DE, Flum DR. Management of pleural space infections: a population‐based analysis. Journal of Thoracic and Cardiovascular Surgery 2007;133(2):346‐51. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed before 18 September 2019).
Grijalva 2011
- Grijalva CG, Zhu Y, Nuorti JP, Griffin MR. Emergence of parapneumonic empyema in the USA. Thorax 2011;66(8):663‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Idell 1991
- Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. American Review of Respiratory Disease 1991;144(1):187‐94. [DOI] [PubMed] [Google Scholar]
Idell 2005
- Idell S. Update on the use of fibrinolysins in pleural disease. Clinical Pulmonary Medicine 2005;12(3):184‐90. [Google Scholar]
Janda 2012
- Janda S, Swiston J. Intrapleural fibrinolytic therapy for treatment of adult parapneumonic effusions and empyemas: a systematic review and meta‐analysis. Chest 2012;142(2):401‐11. [DOI] [PubMed] [Google Scholar]
LeMense 1995
- LeMense PG, Strange C, Sahn SA. Empyema thoracis. Therapeutic management and outcome. Chest 1995;107(6):1532‐7. [DOI] [PubMed] [Google Scholar]
Light 1985
- Light RW. Parapneumonic effusion and empyema. Clinical Chest Medicine 1985;6(1):55‐62. [PubMed] [Google Scholar]
Light 2000
- Light RW, Nguyen T, Mulligan ME, Sasse SA. The in vitro efficacy of varidase versus streptokinase or urokinase for liquefying thick purulent exudative material from loculated empyema. Lung 2000;178(1):13‐8. [DOI] [PubMed] [Google Scholar]
Nie 2014
- Nie W, Liu Y, Ye J, Shi L, Shao F, Ying K, Zhang R. Efficacy of intrapleural instillation of fibrinolytics for treating pleural empyema and parapneumonic effusion: a meta‐analysis of randomized control trials.. Clinical Respiratory Journal 2014;8(3):281‐91. [DOI] [PubMed] [Google Scholar]
Piccolo 2015
- Piccolo F, Popowicz N, Wong D, Lee YCG. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. Journal of Thoracic Diseases 2015;7(6):999‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sahn 1993
- Sahn SA. Management of complicated parapneumonic effusions. American Review of Respiratory Disease 1993;148(3):813‐7. [DOI] [PubMed] [Google Scholar]
Scarcia 2015
- Scarcia M, Abaha U, Piergiorgio S, Pagea A, Wallerb D, Schilc P, et al. EACTS expert consensus statement for surgical management of pleural empyema. European Journal of Cardiothoracic Surgery 2015;48(5):642‐53. [DOI] [PubMed] [Google Scholar]
Shen 2017
- Shen KR, Bribiesco A, Crabtree T, Denlinger C, Eby J, Eiken, P, et al. The American Association of Thoracic Surgery consensus guidelines for the management of empyema. Journal of Thoracic and Cardiovascular Surgery 2017;153(6):e129‐e146. [DOI] [PubMed] [Google Scholar]
Singh 2004
- Singh M, Mathew JL, Chandra S, Katariya S, Kumar L. Randomised controlled trial of intrapleural streptokinase in empyema thoracis in children. Acta Paediatrica 2004;93(11):1443‐5. [DOI] [PubMed] [Google Scholar]
Strange 1999
- Strange C, Sahn SA. The definitions and epidemiology of pleural space infection. Seminars in Respiratory Infections 1999;14(1):3‐8. [PUBMED: 10197392] [PubMed] [Google Scholar]
Thommi 2000
- Thommi G, Shehan C, Bell A, Coughlin N, McLeay M. Intrapleural instillation of TPA in the management of complicated pleural effusions. Chest 2000;118(Suppl 4):S164. [Google Scholar]
Thomson 2002
- Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Randomised controlled trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 2002;57(4):343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tillett 1951
- Tillett WS, Sherry S, Read CT. The use of streptokinase‐streptodornase in the treatment of post‐pneumonic empyema. Journal of Thoracic Surgery 1951;21(3):275‐97. [PubMed] [Google Scholar]
Walker 2003
- Walker CA, Shirki MB, Marva MT, Visconti J. Intrapleural alteplase in a patient with complicated pleural effusion. Annals of Pharmacotherapy 2003;37(3):376‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cameron 1999
- Cameron R, Davies H. Intra‐pleural fibrinolytic therapy for parapneumonic effusions and empyema. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD002312] [DOI] [PubMed] [Google Scholar]
Cameron 2004
- Cameron R, Davies HR. Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of parapneumonic effusions and empyema. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002312.pub2] [DOI] [PubMed] [Google Scholar]
Cameron 2008
- Cameron R, Davies HR. Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database of Systematic Reviews 2008;16(2):CD002312. [DOI] [PubMed] [Google Scholar]


