Abstract
The Christensenellaceae, a recently described family in the phylum Firmicutes, is emerging as an important player in human health. The relative abundance of Christensenellaceae in the human gut is inversely related to host body mass index (BMI) in different populations and multiple studies, making its relationship with BMI the most robust and reproducible link between the microbial ecology of the human gut and metabolic disease reported to date. The family is also related to a healthy status in a number of other different disease contexts, including obesity and inflammatory bowel disease. In addition, Christensenellaceae is highly heritable across multiple populations, although specific human genes underlying its heritability have so far been elusive. Further research into the microbial ecology and metabolism of these bacteria should reveal mechanistic underpinnings of their host-health associations and enable their development as therapeutics.
Introduction
The composition of the human gut microbiome is now well established as a factor important to human health conditions, including metabolic, pathogen, and immune-related diseases [1]. Its composition varies substantially between individuals and populations due to local, personal, and stochastic factors. The high inter-individual variability of the gut microbiome has challenged efforts to define what constitutes a healthy versus an unhealthy microbiome. Indeed, community composition alone is generally not a good predictor of disease state [2]. The contribution of specific taxa, their metabolic pathways, and their interactions to human health is a new priority for microbiome research [3], and this deeper understanding of the microbiome will be necessary for the development of evidence-based microbial therapeutics [4–6]. Given that thousands of microbial species and strains live in the gut, one challenge is to identify targets for further investigation and development.
Here, we focus on the family Christensenellaceae, within the Firmicutes phylum of Bacteria, due to its emergence as a health-related group. First encountered from 16S rRNA gene sequences alone, the family was named in 2012 after an isolate named Christensenella minuta (pictured in Fig. 1), cultivated from the feces of a healthy Japanese male [7]. Members of this family of Firmicutes are, with a few exceptions, increasingly revealing themselves as associated with a healthy phenotype in humans. Because of the relatively recent naming and phylogenetic placement of the Christensenellaceae family (Box 1), it was not discussed in the literature prior to a few years ago. And since representatives of this family were only recently isolated (Box 2), little is known about its ecology outside of what can be inferred from its associations with host factors and other microbiota (Box 3). Here, we review the literature to date, focusing on consistent trends that associate Christensenellaceae with parameters of human health. Taken together, these various observations strongly argue for further investigation into the Christensenellaceae.
Fig. 1.
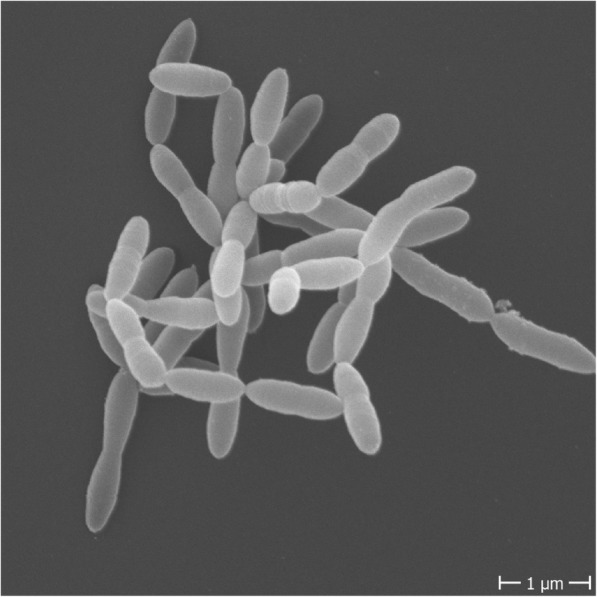
Cell morphology of Christensenella minuta. C. minuta (DSM22607) was grown in supplemented brain heart infusion to reach full turbidity, approximately 72 h. Cells were washed twice and subsequently resuspended in phosphate buffered saline prior to submission to the electron microscopy facility at the Max Planck Institute for Developmental Biology
Box 1.
Discovery and phylogenetic classification of the Christensenellaceae
|
The family Christensenellaceae belongs to the bacterial phylum Firmicutes, the phylogenetically diverse and predominant phylum of the human gut microbiome. The name Christensenellaceae is derived from the isolate named Christensenella minuta (pictured in Fig. 1), which was first cultivated from the feces of a healthy Japanese male by Morotomi and colleagues and published in 2012 [7]. This isolate was named to honor the Danish microbiologist Henrik Christensen, and the species designated “minuta”, due to the small size of the cell (0.8–1.9 μM) and the colonies it forms on agar plates (only 0.1 mm in diameter). In their species description, Morotomi et al. compared C. minuta’s full length 16S rRNA against publicly available databases and identified Caldicoprobacter oshimai, a bacterium in the family Caldicoprobactereaceae (Clostridiales), as the closest relative, with 86.9% pairwise ID. Other related taxa included Tindallia californiensis (86.3% ID) and Clostridium ganghwense (86.1% ID), both of which are in the family Clostridiaceae in the phylum Firmicutes. They did note that other sequences were identified with matches greater than 98% ID; however, these were unclassified taxa from other 16S rRNA gene diversity surveys. C. minuta was designated to represent a novel family, Christensenellaceae, in the order Clostridiales in the phylum Firmicutes [7]. A closely related bacterium, Catabacter hongkongensis, was described in 2007 [8]. The 16S rRNA genes of C. minuta and Catabacter hongkongensis share 96.5% sequence identity, suggesting the two should be in the same family, and possibly the same genus [9] (Fig. 2). As a result, some databases use the family name Catabacteriaceae, some use Christensenellaceae, and some studies include both as two distinct families. The family name Christensenellaceae, however, is now considered with standing in nomenclature [10]. The Genome Taxonomy Database, a recent taxonomy developed by Phil Hugenholtz and colleagues that is based on whole genome comparisons rather than 16S rRNA gene sequences for reconstructing phylogeny, supports that Christensenella and Catabacter are separate genera in the family Christensenellaceae, within a new order Christensenellales [11]. |
Box 2.
Cultured isolates of the family Christensenellaceae (2019)
|
The first isolate, Christensenella minuta (DSM 22607), was isolated from the feces of a healthy Japanese male. It is strictly anaerobic, non-sporulating, non-motile, and described as Gram-negative [7]. Intriguingly, others have described it as Gram-positive [12], which is also consistent with our unpublished observations. A Gram-positive cell wall is consistent with its classification as belonging to the phylum Firmicutes, which includes predominantly Gram-positive bacteria. However, C. minuta is able to produce small amounts of lipopolysaccharide, an attribute that is more typical of, but not exclusive to, Gram-negative bacteria [13]. Morotomi and colleagues demonstrated that C. minuta produces the short chain fatty acids acetate and butyrate, and is saccharolytic, with the ability to utilize arabinose, glucose, mannose, rhamnose, salicin, and xylose. C. minuta was negative for many of the standard biochemical assays used for characterization, which included catalase, oxidase, esculin and gelatin hydrolysis, indole production, and nitrate reduction [7]. The genome was published in 2017 [14], and is estimated as 2.94 Mb with 51.5% G + C content. Catabacter hongkongensis (DSM 18959), first described in 2007, was isolated from the blood of patients who developed bacteremia in Canada and Hong Kong. Catabacter hongkongensis is described as strictly anaerobic, non-sporulating, and Gram-positive [8]. In contrast to the other Christensenella isolates, Catabacter hongkongensis is in fact motile. Catabacter has been associated with bacteremia in at least 12 additional instances, and there may be more due to the difficulty in many chemical-based methods of accurately identifying Catabacter hongkongensis [15–17]. Catabacter hongkongensis has a similar saccharolytic profile to C. minuta, with the exception of glycerol and rhamnose utilization depending on the isolate, and it was not able to utilize salicin. Catabacter hongkongensis differs from C. minuta in that it is catalase positive. Like C. minuta, it was negative for oxidase, esculin and gelatin hydrolysis, indole production, and nitrate reduction [8]. No short chain fatty acid production has been reported for Catabacter. The genome for this bacterium was published in 2015, and is 3.2 Mb with 48.5% G + C content. Annotation of the genome supported that Catabacter hongkongensis is motile, and the authors identified a number of antibiotic resistance genes, which may contribute to its pathogenicity [18]. Christensenella massiliensis (DSM 102344) and Christensenella timonensis (DSM 102800), both isolated from the feces of a diabetic patient in Marseilles, France, are described as strictly anaerobic, non-motile, non-sporulating, and Gram-negative, similar to C. minuta [19, 20]. Although 16S rRNA gene sequence comparisons place C. timonensis within the Christensenella genus (> 97% identity to C. minuta), whole genome taxonomy indicates it belongs to a genus distinct from both Christensenella and Catabacter [11]. No characterization of these isolates has been reported. |
Box 3.
Ecological role of the Christensenellaceae in the human gut
| Based on Morotomi’s observations, C. minuta ferments glucose to acetate and butyrate under anaerobic conditions [7], which indicates it ferments sugars in the gut to short chain fatty acids and other fermentation products such as H2 and CO2. Goodrich et al. reported that the Christensenellaceae form the hub of a co-occurrence network with other microbiota, including methanogens (archaea of the family Methanobacteriaceae) [21]. Co-occurrence of Christensenellaceae and Methanobacteriaceae across individuals has been reported elsewhere [22, 23]. The Methanobacteriaceae include Methanobrevibacter smithii, the predominant methanogen in the human gut. Given that M. smithii uses fermentation products (e.g., H2 and CO2) to produce methane, the co-occurrence with Christensenellaceae may represent a H2-based syntrophy. |
Christensenellaceae is ubiquitous among humans and other animals
Most of what is known about the family Christensenellaceae comes from 16S rRNA gene surveys of the microbiome obtained from feces of humans and other animals. Given that Christensenellaceae 16S rRNA gene sequences were relatively recently included in reference databases, only microbiome studies published since 2013 report this taxon. Two cultured isolates, Christensenella minuta and Catabacter hongkongensis, have published genomes [14, 18], and genomes constructed during metagenomic assemblies are increasingly available. At the time of writing this review, there are 11 Christensenellaceae genomes in the Genome Taxonomy Database and 89 genomes for the order Christensenellales (Box 1) [11]. A phylogeny of 9 members of the Christensenellaceae, based on full length 16S rRNA gene sequences available in NCBI, is shown in Fig. 2. Surveying the post-2013 literature, it is evident that members of the Christensenellaceae are cosmopolitan inhabitants of the animal gut (Table 1), with a likely preference for the distal colon [44], which is consistent with its fermentative activities (detailed in Box 3) [7].
Fig. 2.
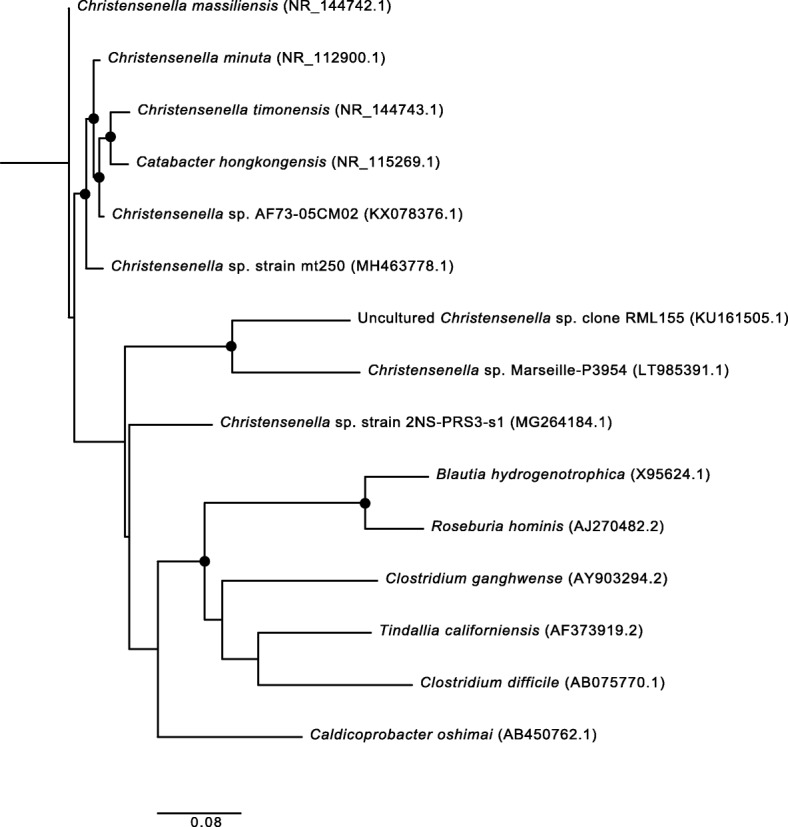
Phylogenetic relatedness of Christensenellaceae. Full length 16S rRNA gene sequences were obtained from NCBI and aligned using MAFFT. Accession numbers for each sequence are provided in parentheses. Bootstrap values (> 50%) are expressed as a percentage for 100 iterations. A maximum likelihood tree was built using RaxML with a general time reversible evolutionary model, and B. thetaiotaomicron was selected as the outgroup for rooting the tree. The scale bar represents substitutions per site
Table 1.
Christensenellaceae has a wide range of hosts in the animal kingdom
| Phylum | Class | Order | Family | Genus | Species | Common name | Reference(s) |
|---|---|---|---|---|---|---|---|
| Chordata | Aves | Casuariiformes | Casuariidae | Dromaius | novaehollandiae | Emu | [24] |
| Galliformes | Phasianidae | Gallus | gallus | Chicken | [25] | ||
| Coturnix | japonica | Japanese quail | [26] | ||||
| Struthioniformes | Struthionidae | Struthio | camelus | Ostrich | [27] | ||
| Mammalia | Artiodactyla | Bovidae | Bos | frontalis | Gayal | [28] | |
| taurus | Cow | [28, 29] | |||||
| Capra | aegagrus hircus | Goat | [30] | ||||
| Syncerus | caffer | African Buffalo | [28] | ||||
| Ovis | aries | Sheep | [31] | ||||
| Camelidae | Camelus | bactrianus | Bactrian camel | [32] | |||
| dromedarius | Dromedary camel | [33] | |||||
| Cervidae | Cervus | nippon | Sika Deer | [34] | |||
| elaphus | Red deer | [28] | |||||
| Giraffidae | Giraffa | camelopardalis | Giraffe | [28] | |||
| Suidae | Sus | scrofa | Pig | [35, 36] | |||
| Carnivora | Canidae | Canis | lupus | Dog | [37] | ||
| Felidae | Felis | catus | Cat | [38] | |||
| Diprotodontia | Vombatidae | Lasiorhinus | latifrons | Southern hairy-nosed wombat | [39] | ||
| Lagomorpha | Leporidae | Oryctolagus | cuniculus | Rex rabbit | [40] | ||
| Perissodactyla | Equida | Equus | caballus | Horse | [28, 41] | ||
| Equus | quagga | Zebra | [28] | ||||
| Primates | Cercopithecidae | Cercopithecus | ascanius a | Red-tailed monkey | [42] | ||
| wolfi a | Wolf’s mona monkey | [42] | |||||
| neglectus a | De Brazza’s monkey | [42] | |||||
| Macaca | mulatta | Rhesus Macaque | [43] | ||||
| papio | anubis | Baboon | [44] | ||||
| Rodentia | Cricetidae | Cricetus | cricetus | European hamster | [28] | ||
| Microtus | californicus scirpensis | Amargosa vole | [45] | ||||
| Muridae | Mus | musculus | Mouse | [46] | |||
| Rattus | norvegicus | Rat | [47] | ||||
| Sirenia | Dugongidae | Dugong | dugon | Dugong | [48] | ||
| Trichechidae | Trichechus | Manatus manatus | Antillean manatee | [49] | |||
| Reptilia | Squamata | Lacertidae | Podarcis | lilfordi | Lilford’s wall lizard | [50] | |
| Liolaemidae | Liolaemus | parvus | Lesser smooth-throated lizard | [51] | |||
| ruibali | Ruibal’s tree iguana | [51] | |||||
| Testudines | Testudinidae | Gopherus | polyphemus | Gopher tortoise | [52] | ||
| Anthropoda | Insecta | Coleoptera | Scarabaeidae | Holotrichia | parallela | Large black chafer | [53] |
| Blattodea | Blaberidae | Diploptera | punctata | Pacific beetle cockroach | [54] | ||
| Pycnoscelus | surinamensis | Surinam cockroach | [55] |
aChristensenellaceae is listed as detected in the Cercopithecus genus, without further species detail. The three species listed were studied in McKenzie et al. [42]
In humans, the family comprises on average 0.01% of the fecal microbiota [21]. Its fine-scale distribution along the human gastrointestinal tract remains to be clarified; but in addition to feces, Christensenellaceae has been detected in human colonic mucosa, ileum, and appendix, and there is also suggestive evidence of airway colonization [21, 56–59]. The family Christensenellaceae is widespread across human populations, and is reported from subjects inhabiting North America [60–62], South America [63, 64], Europe [21, 65], Asia [66, 67], Africa [68–70], and Australia [71].
Within human populations, traits associated with different relative abundances of Christensenellaceae include ethnicity and sex. For instance, a recent study of > 2000 individuals with various ethnicities residing in Amsterdam, Deschasaux et al. reported that Dutch subjects harbored the greatest relative abundances of Christensenellaceae [72]. Similarly, Brooks et al. compared microbiome variation between ethnicities in 1673 people residing in the USA and reported that Christensenellaceae was overall less represented in fecal samples of Asian-Pacific Islanders relative to other ethnicities [60]. A greater relative abundance of Christensenellaceae in women compared to men was also observed [60], and similar observations have been reported in animals [26, 73, 74]. The underlying causes of these ethnic and sex differences are unclear.
Christensenellaceae has been associated with human longevity, based on the observation that the relative abundance of Christensenellaceae is greater in centenarians and supercentenarians in comparison to younger individuals in populations in China [75, 76], Italy [77], and Korea [78]. Positive associations of Christensenellaceae with age have also emerged from studies with relatively young individuals across multiple geographic locations [60, 68, 79–82] (Table 2). Given that none of these studies followed the same individuals over time, the association with age could reflect a cohort effect rather than an age effect. For example, dietary patterns that vary by age may influence this association (see below), or individuals born earlier may have always harbored greater levels of Christensenellaceae compared to those born later.
Table 2.
The relative abundance of Christensenellaceae increases with age
| Country | Sample size of cohort | Age | Sex | Reference |
|---|---|---|---|---|
| (mean ± std. dev.) *, # | (% male/% female) | |||
| China | 168 | 93.3 (90-102) Long-living people# | 37/63 | [75] |
| 61.6 (24-83) Young# | 52/48 | |||
| China | 24 | 104 (100-108) Centenarians* | 38/62 | [76] |
| 92 (85-89) Bama elderly* | 38/62 | |||
| 83 (80-92) Nanning elderly* | 50/50 | |||
| Italy | 69 | 106.2 (105-109) Semi-supercentenarians# | 25/75 | [77] |
| 100.4 (99-104) Centenarians# | 7/93 | |||
| 72.5 (65-75) Elderly# | 47/53 | |||
| 30.5 (22-48) Adults# | 47/53 | |||
| Korea | 47 | 98.9 ± 3.4 Centenarians | 33,147 | [78] |
| 73.6 ± 3.6 Elderly | 59/41 | |||
| 34.3 ± 6.5 Adults | 67/33 | |||
| Korea | 57 | 25-65 (no other participant info or table) | 54/46 | [82] |
| USA | 1673 | 40.2 ± 9.7a | 52/48a | [60] |
| USA | 28 | 49.5 (20-82)* | 54/46 | [79] |
| Nigeria | 30b | Infant-85c | NA | [68] |
| United Kingdom | 2764d | 59.5 ± 12.3 | 32,813 | [81] |
| Canada | 41 | 24.3 ± 3.7e | 54/46 | [80] |
* In these studies age is reported as median (age range)
# In these studies age is reported as average (age range)
a Metadata were only reported for the American Gut Participants (n = 1375) as participant data for the Human Microbiome Project is restricted access
b These findings only pertain to the urban dwelling Nigerians from this study
c A median or average for age groups was not provided. Infants were defined as < 3 years of age (n = 12) and adults were 5-85 (n = 18)
d These values only pertain to the analysis in the TwinsUK cohort in this paper
e These values are reported for the AVG cohort with regard to cardiorespiratory fitness, but is reflective of all study participants. Total age range for all participants is between 18 and 35 years
The Christensenellaceae are linked to host genetic variation
Host genotype is estimated to influence 30–60% of the variation in the relative abundance of Christensenellaceae across individuals [21, 62, 66, 83]. Of the hundreds of taxa in the gut, the family Christensenellaceae is consistently identified as among the most highly heritable. This means that a significant proportion of the variance in the relative abundance of the family across a population can be attributed to genetic factors. Heritability refers to the genetic predisposition of a quantitative trait: for example, height is heritable, because this trait is largely genetically determined. Heritability calculations take into account quantitative measures of the trait (such as relative abundance) and should not be confused with whether the Christensenellaceae are inherited (i.e., vertically transmitted) from family members, which is not known.
Goodrich et al. first identified the Christensenellaceae as heritable in a well-powered (n = 977) study of monozygotic and dizygotic twins from the UK [21]. A remarkable 40% of the variation between individuals in the relative abundance of the family Christensenellaceae could be attributed to host genetic factors. A more fine-grained analysis of species-level operational taxonomic units (OTUs) showed that just a few Christensenellaceae OTUs were driving the heritability of the family [21]. Other studies of heritability employing the same population have observed similar results, whether the analysis was specific to species-level OTUs or when analyzing modules of co-occurring microbes [81, 84]. So far, the Christensenellaceae have not been included in the analysis of heritability based on shotgun metagenome data, due to the absence of genomes for this family in the reference databases used [85].
The high heritability of the Christensenellaceae has been corroborated in other human populations. Goodrich et al. had confirmed its heritability in two previous studies involving twin pairs from the USA [21, 61, 86]. Additionally, Lim et al. evaluated microbiome heritability in a Korean cohort of 655 individuals and identified Christensenellaceae as heritable. In a Canadian cohort (n = 270), it was again identified as among the most highly heritable taxa [62]. Together, these observations across multiple populations indicate that the heritability of the Christensenellaceae is a widely shared trait. That individuals are genetically predisposed to harbor a high or low relative abundance of these bacteria may be a generalizable human trait.
So far, attempts to identify the genetic factors that account for the high heritability of Christensenellaceae by genome-wide association (GWA) have not succeeded [83]. These studies are generally underpowered, given the millions of tests conducted simultaneously (i.e., testing all genetic variants against all microbiome traits), and the necessity to correct for false positives [87]. An alternative to GWA is to take a candidate gene approach, restricting the analysis to genes with interesting functions. For instance, Zakrzewski et al. examined the relationship between a SNP in the interleukin 23 receptor (ILR23) gene and the microbiome of mucosal biopsies from the ileum and rectum. The A allele of this variant has been associated with a reduced risk of ileal Crohn’s disease (CD). Within a population of individuals with no signs of CD or other gastrointestinal disorders, a significantly greater relative abundance of Christensenellaceae was detected in the feces of individuals harboring the protective allele (AG genotype) compared to the population with the GG genotype [56]. How the IL23R genotype may affect members of the gut microbiota remains to be clarified.
Christensenellaceae has also been associated with the fucosyltransferase 2 (FUT2) gene, which encodes an enzyme responsible for ABO blood group antigens that are expressed on the intestinal surface as well as secreted. Non-secretors (AA genotype) have an elevated risk for CD, while secretors (AG or GG) are less likely to develop CD [88]. A re-analysis of healthy individuals studied in [88] showed that secretors harbored relatively more of this family compared to non-secretors (n = 24) [21]. It is important to note that in this case a targeted approach was used, and subsequent studies associating the microbiome with FUT2 do not reach this same conclusion. When Davenport et al. also did this analysis in UK twins (n = 1503), where heritability of Christensenellaceae was first reported, no link between Christensenellaceae and secretor status was found [89], which is consistent with the results of Turpin et al. in a cohort of 1190 healthy individuals [90].
The Christensenellaceae may interact with host genetic status to affect risk of colorectal cancer (CRC). Le Gall et al. reported elevated Christensenellaceae in healthy controls relative to individuals with CRC (n = 50 age- and sex-matched individuals per group) [91], yet Yazici et al. observed that the relative abundance of Christensenellaceae in stool was higher on average in African-American CRC patients compared to controls [92]. Furthermore, using tumor and healthy mucosal tissue biopsies from 44 patients with five different loss-of-function mutations in CRC, Burns et al. observed that the association of Christensenellaceae with CRC was dependent on the type of mutation present [58]. These findings may offer an explanation for the inconsistent patterns of Christensenellaceae abundance with respect to CRC status. However, whether the Christensenellaceae participate in CRC pathology remains to be ascertained. While associations between Christensenellaceae and host genotypes remain to be reproduced, they suggest that health/disease promotion by these genotypes may be mediated in part through promotion of the Christensenellaceae.
The Christensenellaceae are linked to metabolic health
Body composition and metabolic health
Body mass index (BMI) was the first host phenotype associated with the relative abundance of Christensenellaceae in the gut. Goodrich et al. observed that Christensenellaceae was significantly enriched in individuals with a normal BMI (18.5–24.9) compared to obese individuals (BMI ≥ 30) [21]. Since this initial observation, the association of Christensenellaceae with a normal BMI has been corroborated repeatedly in populations from a number of countries that included adult men and women of various ages (Table 3). Consistent with its association with leanness, Christensenellaceae have been shown to increase after diet-induced weight loss [100]. Although obese and lean subjects can often be differentiated using aspects of microbial ecology of the gut, these aspects (e.g., alpha-diversity, or abundances of phyla) have differed between studies [101]: the link between Christensenellaceae and BMI therefore stands as the strongest corroborated association between the gut microbiome and BMI.
Table 3.
Global associations of Christensenellaceae with a healthy body mass index
| Country | Sample size of cohort | Age (mean ± std. dev.)* | Sex (% male/% female) | Reference |
|---|---|---|---|---|
| USA | 154 | 15 (21-32)*,a | 0/100 | [61] |
| USA | 599 | 62.7 ± 7.7b | 54/46 | [93] |
| USA | 1673 | 40.2 ± 9.7c | 52/48 c | [60] |
| Mexico | 138 | 9.9 ± 1.72b | 58/42 | [94] |
| United Kingdom | 977 | 60.6 ± 0.3 | 2/98 | [21] |
| United Kingdom | 2764d | 59.5 ± 12.3 | 11/89 | [81] |
| Spain | 39 | 14.8 (13-16)* | 49/51 | [95] |
| Netherlands | 893 | 44.7 ± 12.9 | 43/57 | [96] |
| Norway | 384 | 48 (23-82)* | 42/58 | [97] |
| Norway | 169 | 30 (27-34)* | 0/100 | [98] |
| Korea | 655 | 47.0 ± 12.2 | 42/58 | [66] |
| Korea | 1274 | 45.7 ± 9.0 | 64/36 | [99] |
| Japan | 516 | 52.4 ± 13.4 | 37/63 | [67] |
* In these studies age is reported as median (range)
a 49 participants are mothers of the twins, for which no age is reported
b These values are reported for the healthy weight cohort, but is reflective of all study participants
c Metadata were only reported for the American Gut Participants (n = 1375) as participant data for the Human Microbiome Project is restricted access
d These values only pertain to the analysis in the TwinsUK cohort in this paper. Other studies were included, but Christensenellaceae was not reported
BMI is a proxy for adiposity, and consistent with reports linking levels of Christensenellaceae with BMI, studies in which adiposity is more directly measured have also noted strong associations with the abundance of Christensenellaceae in the gut. For instance, Beaumont et al. correlated adiposity measures, determined using dual x-ray absorptiometry (DEXA), with the microbiome in a study of 1313 UK twins. At the family level, the most significant association was with Christensenellaceae, which negatively correlated with visceral fat mass [84], a type of fat that is considered a cardiometabolic risk factor. A similar observation was made by Hibberd et al., who reported significant negative correlations of Christensenellaceae with trunk fat and android fat [102]. Additionally, Christensenellaceae has been negatively correlated with waist circumference and waist to hip ratio, which are direct markers of central adiposity [66, 102–104].
In addition to its association with body fat measures, Christensenellaceae is negatively correlated with serum lipids in several studies. In the Dutch LifeLines DEEP cohort (n = 893), Fu et al. reported a negative correlation of Christensenellaceae with BMI, together with a strong association with low triglyceride levels and elevated levels of high density lipoprotein (HDL, or “good cholesterol”) [96]. Other groups have also reported that Christensenellaceae is associated with reduced serum triglycerides [66, 102, 104]. Similarly, this family is also negatively associated with total cholesterol, low density lipoprotein (LDL; or “bad cholesterol”), and apolipoprotein B, a component of LDL particles [94, 102].
Christensenellaceae is reported as depleted in individuals with metabolic syndrome (MetS) compared to healthy controls [66, 104]. In addition to excess visceral fat, MetS includes other risk factors such as dyslipidemia and impaired glucose metabolism, and is a risk factor for type 2 diabetes and cardiovascular disease. Christensenellaceae was identified in a cohort of 441 Colombians as positively associated with a lower cardiometabolic risk score [103], and others report it is negatively correlated with blood pressure [66, 104, 105], which is often elevated in MetS [106]. Christensenellaceae has also been associated with healthy glucose metabolism [66, 107] and Christensenellaceae OTUs are reduced in individuals with pre-type 2 diabetes [65]. Given that a high BMI, impaired glucose metabolism, dyslipidemia, and other aspects of MetS are comorbidities, it is not surprising that Christensenellaceae inversely tracks with many of these conditions. The mechanism underlying its negative association with MetS remains to be elucidated.
Metabolic disorders are often linked to dietary patterns. The Christensenellaceae appear to be responsive to diet, and evidence points to a role in protein and fiber fermentation. On a coarse level, large-scale diet studies have associated Christensenellaceae with healthy dietary habits low in refined sugar and high in consumption of fruit and vegetables [108–110]. Christensenellaceae is reported higher in relative abundance in humans with an omnivorous diet, relative to vegetarians [71, 111], and has also been associated with dairy consumption [112]. In a more direct link, Christensenellaceae has been shown to respond rapidly to an increase in animal products in the diet [113]. Furthermore, Christensenellaceae has been positively associated with gut metabolites typical of protein catabolism and dietary animal protein [114–116]. Christensenellaceae has also been reported to increase in human dietary interventions involving prebiotic fibers such as resistant starch 4, galacto-oligosaccharide, and polydextrose [22, 102, 112]. Similar observations have also been made in rodent models [117–119]. Taken together, these studies indicate that the association of Christensenellaceae with health parameters may in part be due to its association with a diet high in protein and fiber.
To test for a causal role for Christensenellaceae in metabolic disease while controlling for diet, Goodrich et al. selected an obese human donor based on almost undetectable levels of Christensenellaceae in the microbiome, and performed fecal transfers to germfree mice that were fed the same fiber-rich chow, but otherwise only differed by whether or not the obese human microbiome inoculum was amended with C. minuta. These experiments showed that amendment with C. minuta reduced the adiposity gains of mice compared to those that received unamended stool (or stool amended with heat-killed C. minuta) [21]. The mechanism underlying the protective effect of C. minuta against excess adiposity gain remains to be elucidated, but may involve re-modeling the microbial community, as a shift in diversity was observed when C. minuta was added. These experiments demonstrated that the activity of C. minuta in the gut microbiome can affect host body composition even when diet is controlled for, possibly via interactions with other members of the microbiota. Indeed, the ecological role of members of the Christensenellaceae and their function in the gut in general remains to be better understood (Box 3).
Inflammation and transit time
In a meta-analysis of inflammatory bowel disease (IBD) that included over 3000 individuals, Mancabelli et al. reported Christensenellaceae as one of five taxa considered a signature of a healthy gut [120]. Indeed, Christensenellaceae were consistently depleted in individuals with Crohn’s disease [121–129] and ulcerative colitis [97, 122, 125, 129, 130], the two major sub-types of IBD. In irritable bowel syndrome (IBS), a gastrointestinal disorder characterized by abdominal pain and abnormal bowel movements, a higher relative abundance of Christensenellaceae in healthy controls relative to individuals with IBS has been reported in several studies [131–134]. Several studies have also noted a positive correlation of Christensenellaceae and longer transit time or even constipation [67, 114, 133, 135, 136]. Thus, the Christensenellaceae appear to be depleted in conditions associated with inflammation and fast transit time.
Given Christensenellaceae’s link with transit time, it is perhaps not surprising that the family has been linked to affective disorders that impact gut motility. For instance, gastric dysfunction, particularly constipation, affects approximately two-thirds of patients with Parkinson’s disease (PD) and multiple sclerosis (MS) [137, 138]. Studies have noted a greater relative abundance of Christensenellaceae in PD and MS patients relative to healthy controls [139–142]. Since diet is also related to gut transit time, the effects of diet, host status, and host genetics remain to be carefully disentangled to better understand how levels of the Christensenellaceae are controlled.
Prospectus
The family Christensenellaceae is a relatively recently described bacterial family that is highly heritable and shows compelling associations with host health. Its strong ties to host health have warranted the suggestion that cultured representatives of the Christensenellaceae, such as C. minuta, should be considered for use as a therapeutic probiotic for the improvement of human health [143]. However, the functional role of Christensenellaceae in the gut remains to be understood. While the collection of associations between Christensenellaceae and host health parameters continues to grow, allowing inferences about the role of these bacteria, they remain to be studied experimentally. Genomes offer a powerful platform for generating hypotheses regarding the metabolic capacity of the Christensenellaceae, but further functional characterization in vitro and in vivo will be necessary to fully characterize the role of Christensenellaceae in the gut. The ecological role of members of the Christensenellaceae, their interactions with other members of the microbiome and with the host and host diet, all remain to be better understood if these intriguing microbes are to be harnessed fully to improve human health.
Acknowledgements
We thank Tanja Schoen, Taichi Suzuki, Nicholas Youngblut, and Tony Walters for input on earlier drafts of this manuscript, in addition to the edits and suggestions from two anonymous reviewers. Funding was provided by the Max Planck Society.
Authors’ contributions
JLW and REL wrote this article and read and approved the final version.
Availability of data and materials
16S rRNA gene sequences used to construct the phylogenetic tree were obtained from NCBI (https://www.ncbi.nlm.nih.gov/). Accession numbers for each sequence are in parentheses in Fig. 2.
Competing interests
R.E.L. and J.L.W. are co-inventors on patent number US10206958B2, “Modulation of fat storage in a subject by altering population levels of christensenellaceae in the GI tract”.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Integrative HMP. (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Integrative HMP. (iHMP) Research Network Consortium. After the Integrative Human Microbiome Project, what’s next for the microbiome community? Nature. 2019;569:599. [DOI] [PubMed]
- 3.Proctor L. Priorities for the next 10 years of human microbiome research. Nature. 2019;569:623–625. doi: 10.1038/d41586-019-01654-0. [DOI] [PubMed] [Google Scholar]
- 4.Douillard François P., de Vos Willem M. Biotechnology of health-promoting bacteria. Biotechnology Advances. 2019;37(6):107369. doi: 10.1016/j.biotechadv.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Stenman LK, Burcelin R. Establishing a causal link between gut microbes, body weight gain and glucose metabolism in humans–towards treatment with probiotics. Benef Microbes. 2016; http://www.wageningenacademic.com/doi/abs/10.3920/BM2015.0069. [DOI] [PubMed]
- 6.Brunkwall Louise, Orho-Melander Marju. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60(6):943–951. doi: 10.1007/s00125-017-4278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morotomi M, Nagai F, Watanabe Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol. 2011;62:144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 8.Lau SKP, McNabb A, Woo GKS, Hoang L, Fung AMY, Chung LMW, et al. Catabacter hongkongensis gen. nov., sp. nov., isolated from blood cultures of patients from Hong Kong and Canada. J Clin Microbiol. 2007;45:395–401. doi: 10.1128/JCM.01831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parte AC. LPSN - List of Prokaryotic names with Standing in Nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol. 2018;68:1825–1829. doi: 10.1099/ijsem.0.002786. [DOI] [PubMed] [Google Scholar]
- 11.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 12.Alonso BL. Irigoyen von Sierakowski A, Sáez Nieto JA, Rosel AB. First report of human infection by Christensenella minuta, a Gram-negative, strickly anaerobic rod that inhabits the human intestine. Anaerobe. 2017;44:124–125. doi: 10.1016/j.anaerobe.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang Yingyin, Gu Huawei, Sun Qiuli, Wang Jufang. Effects of Christensenella minuta lipopolysaccharide on RAW 264.7 macrophages activation. Microbial Pathogenesis. 2018;125:411–417. doi: 10.1016/j.micpath.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Rosa BA, Hallsworth-Pepin K, Martin J, Wollam A, Mitreva M. Genome sequence of Christensenella minuta DSM 22607T. Genome Announc. 2017;5. 10.1128/genomeA.01451-16. [DOI] [PMC free article] [PubMed]
- 15.Choi YJ, Won EJ, Kim SH, Shin MG, Shin JH, Suh SP. First case report of bacteremia due to Catabacter hongkongensis in a Korean patient. Ann Lab Med. 2017;37:84–87. doi: 10.3343/alm.2017.37.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau SKP, Fan RYY, Lo H-W, Ng RHY, Wong SSY, Li IWS, et al. High mortality associated with Catabacter hongkongensis bacteremia. J Clin Microbiol. 2012;50:2239–2243. doi: 10.1128/JCM.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsendoorn A, Robert R, Culos A, Roblot F, Burucoa C. Catabacter hongkongensis Bacteremia with fatal septic shock. Emerg Infect Dis. 2011;17:1330–1331. doi: 10.3201/eid1707.101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau SKP, Teng JLL, Huang Y, Curreem SOT, Tsui SKW, Woo PCY. Draft genome sequence of Catabacter hongkongensis type strain HKU16T, isolated from a patient with bacteremia and intestinal obstruction. Genome Announc. 2015;3. 10.1128/genomeA.00531-15. [DOI] [PMC free article] [PubMed]
- 19.Ndongo S, Khelaifia S, Fournier P-E, Raoult D. Christensenella massiliensis, a new bacterial species isolated from the human gut. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndongo S, Dubourg G, Khelaifia S, Fournier PE, Raoult D. Christensenella timonensis, a new bacterial species isolated from the human gut. New Microbes New Infect. 2016;13:32–33. doi: 10.1016/j.nmni.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrich Julia K., Waters Jillian L., Poole Angela C., Sutter Jessica L., Koren Omry, Blekhman Ran, Beaumont Michelle, Van Treuren William, Knight Rob, Bell Jordana T., Spector Timothy D., Clark Andrew G., Ley Ruth E. Human Genetics Shape the Gut Microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyaya B, McCormack L, Fardin-Kia AR, Juenemann R, Nichenametla S, Clapper J, et al. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci Rep. 2016;6:28797. doi: 10.1038/srep28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A, et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett DC, Tun HM, Kim JE, Leung FC, Cheng KM. Characterization of cecal microbiota of the emu (Dromaius novaehollandiae) Vet Microbiol. 2013;166:304–310. doi: 10.1016/j.vetmic.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Crisol-Martínez E, Stanley D, Geier MS, Hughes RJ, Moore RJ. Sorghum and wheat differentially affect caecal microbiota and associated performance characteristics of meat chickens. PeerJ. 2017;5:e3071. doi: 10.7717/peerj.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson N, Hughes RJ, Aspden WJ, Chapman J, Moore RJ, Stanley D. The gastrointestinal tract microbiota of the Japanese quail. Coturnix japonica. Appl Microbiol Biotechnol. 2016;100:4201–4209. doi: 10.1007/s00253-015-7280-z. [DOI] [PubMed] [Google Scholar]
- 27.Videvall E, Song SJ, Bensch HM, Strandh M, Engelbrecht A, Serfontein N, et al. The development of gut microbiota in ostriches and its association with juvenile growth. bioRxiv. 2018:270017. 10.1101/270017.
- 28.Youngblut ND, Reischer GH, Walters W, Schuster N, Walzer C, Stalder G, et al. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat Commun. 2019;10:2200. doi: 10.1038/s41467-019-10191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Shi H, Wang Y, Li S, Cao Z, Ji S, et al. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front Microbiol. 2017;8:2206. doi: 10.3389/fmicb.2017.02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Xue, Martin Graeme B, Wen Qi, Liu Shulin, Zhang Juan, Yu Yang, Shi Binlin, Guo Xiaoyu, Zhao Yanli, Yan Sumei. Linseed oil and heated linseed grain supplements have different effects on rumen bacterial community structures and fatty acid profiles in cashmere kids1. Journal of Animal Science. 2019;97(5):2099–2113. doi: 10.1093/jas/skz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamke J, Kittelmann S, Soni P, Li Y, Tavendale M, Ganesh S, et al. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome. 2016;4:56. doi: 10.1186/s40168-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Yi L, Hai L, Ming L, Gao W, Ji R. Characterizing the bacterial microbiota in different gastrointestinal tract segments of the Bactrian camel. Sci Rep. 2018;8:654. doi: 10.1038/s41598-017-18298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samsudin AA, Evans PN, Wright A-DG, Al JR. Molecular diversity of the foregut bacteria community in the dromedary camel (Camelus dromedarius) Environ Microbiol. 2011;13:3024–3035. doi: 10.1111/j.1462-2920.2011.02579.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Si H, Nan W, Wang X, Zhang T, Li G. Bacterial community and metabolome shifts in the cecum and colon of captive sika deer (Cervus nippon) from birth to post weaning. FEMS Microbiol Lett. 2019. 10.1093/femsle/fnz010. [DOI] [PubMed]
- 35.Quan J, Cai G, Ye J, Yang M, Ding R, Wang X, et al. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci Rep. 2018;8:4536. doi: 10.1038/s41598-018-22692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Chenyang, Zhou Jun, Li Yanyan, Zhang Dijun, Wang Zuzhong, Li Ye, Cheong Lingzhi, Zhang Chundan, Su Xiurong. Structural modulation of gut microbiota in Bama minipigs in response to treatment with a “growth-promoting agent”, salbutamol. Applied Microbiology and Biotechnology. 2017;101(14):5809–5818. doi: 10.1007/s00253-017-8329-y. [DOI] [PubMed] [Google Scholar]
- 37.Gebreselassie EE, Jackson MI, Yerramilli M, Jewell DE. Anti-aging food that improves markers of health in senior dogs by modulating gut microbiota and metabolite profiles. bioRxiv. 2018:324327. 10.1101/324327.
- 38.Ramadan Z, Xu H, Laflamme D, Czarnecki-Maulden G, Li QJ, Labuda J, et al. Fecal microbiota of cats with naturally occurring chronic diarrhea assessed using 16S rRNA gene 454-pyrosequencing before and after dietary treatment. J Vet Intern Med. 2014;28:59–65. doi: 10.1111/jvim.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiffman ME, Soo RM, Dennis PG, Morrison M, Tyson GW, Hugenholtz P. Gene and genome-centric analyses of koala and wombat fecal microbiomes point to metabolic specialization for Eucalyptus digestion. PeerJ. 2017;5:e4075. doi: 10.7717/peerj.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C., Zhu Y., Li F., Huang L. The Effect of Lactobacillus isolates on growth performance, immune response, intestinal bacterial community composition of growing Rex Rabbits. Journal of Animal Physiology and Animal Nutrition. 2017;101(5):e1–e13. doi: 10.1111/jpn.12629. [DOI] [PubMed] [Google Scholar]
- 41.Hansen NCK, Avershina E, Mydland LT, Næsset JA, Austbø D, Moen B, et al. High nutrient availability reduces the diversity and stability of the equine caecal microbiota. Microb Ecol Health Dis. 2015;26:27216. doi: 10.3402/mehd.v26.27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenzie Valerie J., Song Se Jin, Delsuc Frédéric, Prest Tiffany L., Oliverio Angela M., Korpita Timothy M., Alexiev Alexandra, Amato Katherine R., Metcalf Jessica L., Kowalewski Martin, Avenant Nico L., Link Andres, Di Fiore Anthony, Seguin-Orlando Andaine, Feh Claudia, Orlando Ludovic, Mendelson Joseph R., Sanders Jon, Knight Rob. The Effects of Captivity on the Mammalian Gut Microbiome. Integrative and Comparative Biology. 2017;57(4):690–704. doi: 10.1093/icb/icx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Xiao, Yasuda Koji, Gilmore Robert A., Westmoreland Susan V., Platt Donna M., Miller Gregory M., Vallender Eric J. Alcohol-induced changes in the gut microbiome and metabolome of rhesus macaques. Psychopharmacology. 2019;236(5):1531–1544. doi: 10.1007/s00213-019-05217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan C, Graham M, Subramanian S. Microbiota-metabolites interactions in non-human primate gastrointestinal tract. bioRxiv. 2018:454496. 10.1101/454496.
- 45.Allan Nora, Knotts Trina, Pesapane Risa, Ramsey Jon, Castle Stephanie, Clifford Deana, Foley Janet. Conservation Implications of Shifting Gut Microbiomes in Captive-Reared Endangered Voles Intended for Reintroduction into the Wild. Microorganisms. 2018;6(3):94. doi: 10.3390/microorganisms6030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connor KL, Chehoud C, Altrichter A, Chan L, DeSantis TZ, Lye SJ. Maternal metabolic, immune, and microbial systems in late pregnancy vary with malnutrition in mice. Biol Reprod. 2018;98:579–592. doi: 10.1093/biolre/ioy002. [DOI] [PubMed] [Google Scholar]
- 47.Tillmann Sandra, Abildgaard Anders, Winther Gudrun, Wegener Gregers. Altered fecal microbiota composition in the Flinders sensitive line rat model of depression. Psychopharmacology. 2018;236(5):1445–1457. doi: 10.1007/s00213-018-5094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukinowa E, Karita S, Asano S, Wakai Y, Oka Y, Furuta M, et al. Fecal microbiota of a dugong (Dugong dugong) in captivity at Toba Aquarium. J Gen Appl Microbiol. 2008;54:25–38. doi: 10.2323/jgam.54.25. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki A, Ueda K, Segawa T, Suzuki M. Fecal microbiota of captive Antillean manatee Trichechus manatus manatus. FEMS Microbiol Lett. 2019. 10.1093/femsle/fnz134. [DOI] [PubMed]
- 50.Baldo L, Riera JL, Mitsi K, Pretus JL. Processes shaping gut microbiota diversity in allopatric populations of the endemic lizard Podarcis lilfordi from Menorcan islets (Balearic Islands). FEMS Microbiol Ecol. 2018;94. 10.1093/femsec/fix186. [DOI] [PubMed]
- 51.Kohl Kevin D., Brun Antonio, Magallanes Melisa, Brinkerhoff Joshua, Laspiur Alejandro, Acosta Juan Carlos, Caviedes-Vidal Enrique, Bordenstein Seth R. Gut microbial ecology of lizards: insights into diversity in the wild, effects of captivity, variation across gut regions and transmission. Molecular Ecology. 2016;26(4):1175–1189. doi: 10.1111/mec.13921. [DOI] [PubMed] [Google Scholar]
- 52.Yuan ML, Dean SH, Longo AV, Rothermel BB, Tuberville TD, Zamudio KR. Kinship, inbreeding and fine-scale spatial structure influence gut microbiota in a hindgut-fermenting tortoise. Mol Ecol. 2015;24:2521–2536. doi: 10.1111/mec.13169. [DOI] [PubMed] [Google Scholar]
- 53.Huang S, Zhang H. The impact of environmental heterogeneity and life stage on the hindgut microbiota of Holotrichia parallela larvae (Coleoptera: Scarabaeidae) PLoS One. 2013;8:e57169. doi: 10.1371/journal.pone.0057169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayayee PA, Keeney G, Sabree ZL, Muñoz-Garcia A. Compositional differences among female-associated and embryo-associated microbiota of the viviparous Pacific Beetle cockroach. Diploptera punctata. FEMS Microbiol Ecol. 2017;93. 10.1093/femsec/fix052. [DOI] [PubMed]
- 55.Richards C, Otani S, Mikaelyan A, Poulsen M. Pycnoscelus surinamensis cockroach gut microbiota respond consistently to a fungal diet without mirroring those of fungus-farming termites. PLoS One. 2017;12:e0185745. doi: 10.1371/journal.pone.0185745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakrzewski Martha, Simms Lisa A, Brown Allison, Appleyard Mark, Irwin James, Waddell Nicola, Radford-Smith Graham L. IL23R-Protective Coding Variant Promotes Beneficial Bacteria and Diversity in the Ileal Microbiome in Healthy Individuals Without Inflammatory Bowel Disease. Journal of Crohn's and Colitis. 2018;13(4):451–461. doi: 10.1093/ecco-jcc/jjy188. [DOI] [PubMed] [Google Scholar]
- 57.Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14:9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burns MB, Montassier E, Abrahante J, Priya S, Niccum DE, Khoruts A, et al. Colorectal cancer mutational profiles correlate with defined microbial communities in the tumor microenvironment. PLoS Genet. 2018;14:e1007376. doi: 10.1371/journal.pgen.1007376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuņo MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res. 2016;8:5672–5684. [PMC free article] [PubMed] [Google Scholar]
- 60.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16:e2006842. doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. 2016;48:1413–1417. doi: 10.1038/ng.3693. [DOI] [PubMed] [Google Scholar]
- 63.Obregon-Tito AJ, Tito RY, Metcalf J, Sankaranarayanan K, Clemente JC, Ursell LK, et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat Commun. 2015;6:6505. doi: 10.1038/ncomms7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014;14:311. doi: 10.1186/s12866-014-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017;18:70. doi: 10.1186/s13059-017-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim Mi Young, You Hyun Ju, Yoon Hyo Shin, Kwon Bomi, Lee Jae Yoon, Lee Sunghee, Song Yun-Mi, Lee Kayoung, Sung Joohon, Ko GwangPyo. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2016;66(6):1031–1038. doi: 10.1136/gutjnl-2015-311326. [DOI] [PubMed] [Google Scholar]
- 67.Oki K, Toyama M, Banno T, Chonan O, Benno Y, Watanabe K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016;16:284. doi: 10.1186/s12866-016-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayeni FA, Biagi E, Rampelli S, Fiori J, Soverini M, Audu HJ, et al. Infant and adult gut microbiome and metabolome in rural Bassa and urban settlers from Nigeria. Cell Rep. 2018;23:3056–3067. doi: 10.1016/j.celrep.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Gomez A, Petrzelkova KJ, Burns MB, Yeoman CJ, Amato KR, Vlckova K, et al. Gut microbiome of coexisting BaAka Pygmies and Bantu reflects gradients of traditional subsistence patterns. Cell Rep. 2016;14:2142–2153. doi: 10.1016/j.celrep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Morton ER, Lynch J, Froment A, Lafosse S, Heyer E, Przeworski M, et al. Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and Subsistence. PLoS Genet. 2015;11:e1005658. doi: 10.1371/journal.pgen.1005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrett Helen, Gomez-Arango Luisa, Wilkinson Shelley, McIntyre H., Callaway Leonie, Morrison Mark, Dekker Nitert Marloes. A Vegetarian Diet Is a Major Determinant of Gut Microbiota Composition in Early Pregnancy. Nutrients. 2018;10(7):890. doi: 10.3390/nu10070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deschasaux Mélanie, Bouter Kristien E., Prodan Andrei, Levin Evgeni, Groen Albert K., Herrema Hilde, Tremaroli Valentina, Bakker Guido J., Attaye Ilias, Pinto-Sietsma Sara-Joan, van Raalte Daniel H., Snijder Marieke B., Nicolaou Mary, Peters Ron, Zwinderman Aeilko H., Bäckhed Fredrik, Nieuwdorp Max. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nature Medicine. 2018;24(10):1526–1531. doi: 10.1038/s41591-018-0160-1. [DOI] [PubMed] [Google Scholar]
- 73.Chi L, Mahbub R, Gao B, Bian X, Tu P, Ru H, et al. Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem Res Toxicol. 2017;30:2110–2119. doi: 10.1021/acs.chemrestox.7b00162. [DOI] [PubMed] [Google Scholar]
- 74.Davis Daniel J., Hecht Patrick M., Jasarevic Eldin, Beversdorf David Q., Will Matthew J., Fritsche Kevin, Gillespie Catherine H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain, Behavior, and Immunity. 2017;59:38–48. doi: 10.1016/j.bbi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Kong F, Hua Y, Zeng B, Ning R, Li Y, Zhao J. Gut microbiota signatures of longevity. Curr Biol. 2016;26:R832–R833. doi: 10.1016/j.cub.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 76.Wang F, Yu T, Huang G, Cai D, Liang X, Su H, et al. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians. J Microbiol Biotechnol. 2015;25:1195–1204. doi: 10.4014/jmb.1410.10014. [DOI] [PubMed] [Google Scholar]
- 77.Biagi Elena, Franceschi Claudio, Rampelli Simone, Severgnini Marco, Ostan Rita, Turroni Silvia, Consolandi Clarissa, Quercia Sara, Scurti Maria, Monti Daniela, Capri Miriam, Brigidi Patrizia, Candela Marco. Gut Microbiota and Extreme Longevity. Current Biology. 2016;26(11):1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Kim Bong-Soo, Choi Chong Won, Shin Hyoseung, Jin Seon-Pil, Bae Jung-Soo, Han Mira, Seo Eun Young, Chun Jongsik, Chung Jin Ho. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. Journal of Microbiology and Biotechnology. 2019;29(3):429–440. doi: 10.4014/jmb.1811.11023. [DOI] [PubMed] [Google Scholar]
- 79.Anand Rohit, Song Yang, Garg Shashank, Girotra Mohit, Sinha Amitasha, Sivaraman Anita, Phillips Laila, Dutta Sudhir K. Effect of Aging on the Composition of Fecal Microbiota in Donors for FMT and Its Impact on Clinical Outcomes. Digestive Diseases and Sciences. 2017;62(4):1002–1008. doi: 10.1007/s10620-017-4449-6. [DOI] [PubMed] [Google Scholar]
- 80.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson MA, Bonder MJ, Kuncheva Z, Zierer J, Fu J, Kurilshikov A, et al. Detection of stable community structures within gut microbiota co-occurrence networks from different human populations. PeerJ. 2018;6:e4303. doi: 10.7717/peerj.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin Ji-Hee, Park Young-Hee, Sim Minju, Kim Seong-Ah, Joung Hyojee, Shin Dong-Mi. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Research in Microbiology. 2019;170(4-5):192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016;19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beaumont M, Goodrich JK, Jackson MA, Yet I, Davenport ER, Vieira-Silva S, et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 2016;17:189. doi: 10.1186/s13059-016-1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Hailiang, Guo Ruijin, Zhong Huanzi, Feng Qiang, Lan Zhou, Qin Bingcai, Ward Kirsten J., Jackson Matthew A., Xia Yan, Chen Xu, Chen Bing, Xia Huihua, Xu Changlu, Li Fei, Xu Xun, Al-Aama Jumana Yousuf, Yang Huanming, Wang Jian, Kristiansen Karsten, Wang Jun, Steves Claire J., Bell Jordana T., Li Junhua, Spector Timothy D., Jia Huijue. Shotgun Metagenomics of 250 Adult Twins Reveals Genetic and Environmental Impacts on the Gut Microbiome. Cell Systems. 2016;3(6):572-584.e3. doi: 10.1016/j.cels.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodrich JK, Davenport ER, Waters JL, Clark AG, Ley RE. Cross-species comparisons of host genetic associations with the microbiome. Science. 2016;352:532–535. doi: 10.1126/science.aad9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wacklin P, Tuimala J, Nikkilä J, Tims S, Mäkivuokko H, Alakulppi N, et al. Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS One. 2014;9:e94863. doi: 10.1371/journal.pone.0094863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davenport ER, Goodrich JK, Bell JT, Spector TD, Ley RE, Clark AG. ABO antigen and secretor statuses are not associated with gut microbiota composition in 1,500 twins. BMC Genomics. 2016;17:941. doi: 10.1186/s12864-016-3290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turpin W, Bedrani L, Espin-Garcia O, Xu W, Silverberg MS, Smith MI, et al. FUT2 genotype and secretory status are not associated with fecal microbial composition and inferred function in healthy subjects. Gut Microbes. 2018;9:357–368. doi: 10.1080/19490976.2018.1445956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Le Gall G, Guttula K, Kellingray L, Tett AJ, Ten Hoopen R, Kemsley KE, et al. Metabolite quantification of faecal extracts from colorectal cancer patients and healthy controls. Oncotarget. 2018;9:33278–33289. doi: 10.18632/oncotarget.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yazici Cemal, Wolf Patricia G, Kim Hajwa, Cross Tzu-Wen L, Vermillion Karin, Carroll Timothy, Augustus Gaius J, Mutlu Ece, Tussing-Humphreys Lisa, Braunschweig Carol, Xicola Rosa M, Jung Barbara, Llor Xavier, Ellis Nathan A, Gaskins H Rex. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8:9749. doi: 10.1038/s41598-018-28126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.López-Contreras BE, Morán-Ramos S, Villarruel-Vázquez R, Macías-Kauffer L, Villamil-Ramírez H, León-Mimila P, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes. 2018;13:381–388. doi: 10.1111/ijpo.12262. [DOI] [PubMed] [Google Scholar]
- 95.Ferrer M, Ruiz A, Lanza F, Haange S-B, Oberbach A, Till H, et al. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol. 2013;15:211–226. doi: 10.1111/j.1462-2920.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 96.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JAM, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kummen Martin, Holm Kristian, Anmarkrud Jarl Andreas, Nygård Ståle, Vesterhus Mette, Høivik Marte L, Trøseid Marius, Marschall Hanns-Ulrich, Schrumpf Erik, Moum Bjørn, Røsjø Helge, Aukrust Pål, Karlsen Tom H, Hov Johannes R. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2016;66(4):611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 98.Stanislawski MA, Dabelea D, Wagner BD, Sontag MK, Lozupone CA, Eggesbø M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5:113. doi: 10.1186/s40168-017-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yun Y, Kim H-N, Kim SE, Heo SG, Chang Y, Ryu S, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17:151. doi: 10.1186/s12866-017-1052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alemán JO, Bokulich NA, Swann JR, Walker JM, De Rosa JC, Battaglia T, et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J Transl Med. 2018;16:244. doi: 10.1186/s12967-018-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hibberd AA, Yde CC, Ziegler ML, Honoré AH, Saarinen MT, Lahtinen S, et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes. 2019;10(2):121–135. doi: 10.3920/BM2018.0028. [DOI] [PubMed] [Google Scholar]
- 103.Guzman-Castaneda SJ, Ortega-Vega EL, de la Cuesta-Zuluaga J, Velasquez-Mejia EP, Rojas W, Bedoya G, et al. Gut microbiota composition explains more variance in the host cardiometabolic risk than genetic ancestry. bioRxiv. 2018:394726. 10.1101/394726. [DOI] [PMC free article] [PubMed]
- 104.He Y, Wu W, Wu S, Zheng H-M, Li P, Sheng H-F, et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome. 2018;6:172. doi: 10.1186/s40168-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, et al. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 106.Yanai H, Tomono Y, Ito K, Furutani N, Yoshida H, Tada N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr J. 2008;7:10. doi: 10.1186/1475-2891-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8(4):545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- 108.Bowyer RCE, Jackson MA, Pallister T, Skinner J, Spector TD, Welch AA, et al. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome. 2018;6:77. doi: 10.1186/s40168-018-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maskarinec Gertraud, Hullar Meredith A J, Monroe Kristine R, Shepherd John A, Hunt Jeani, Randolph Timothy W, Wilkens Lynne R, Boushey Carol J, Le Marchand Loïc, Lim Unhee, Lampe Johanna W. Fecal Microbial Diversity and Structure Are Associated with Diet Quality in the Multiethnic Cohort Adiposity Phenotype Study. The Journal of Nutrition. 2019;149(9):1575–1584. doi: 10.1093/jn/nxz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klimenko Natalia, Tyakht Alexander, Popenko Anna, Vasiliev Anatoly, Altukhov Ilya, Ischenko Dmitry, Shashkova Tatiana, Efimova Daria, Nikogosov Dmitri, Osipenko Dmitrii, Musienko Sergey, Selezneva Kseniya, Baranova Ancha, Kurilshikov Alexander, Toshchakov Stepan, Korzhenkov Aleksei, Samarov Nazar, Shevchenko Margarita, Tepliuk Alina, Alexeev Dmitry. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients. 2018;10(5):576. doi: 10.3390/nu10050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 112.Azcarate-Peril M. Andrea, Ritter Andrew J., Savaiano Dennis, Monteagudo-Mera Andrea, Anderson Carlton, Magness Scott T., Klaenhammer Todd R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proceedings of the National Academy of Sciences. 2017;114(3):E367–E375. doi: 10.1073/pnas.1606722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roager HM, Hansen LBS, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093. doi: 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- 115.Beaumont M, Portune KJ, Steuer N, Lan A, Cerrudo V, Audebert M, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am J Clin Nutr. 2017;106:1005–1019. doi: 10.3945/ajcn.117.158816. [DOI] [PubMed] [Google Scholar]
- 116.Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, et al. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24:935–946. doi: 10.1016/j.celrep.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 117.Jiminez JA, Uwiera TC, Abbott DW, Uwiera RRE, Inglis GD. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016;8:67. doi: 10.1186/s13099-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng Junping, Cheng Gong, Li Qiongyu, Jiao Siming, Feng Cui, Zhao Xiaoming, Yin Heng, Du Yuguang, Liu Hongtao. Chitin Oligosaccharide Modulates Gut Microbiota and Attenuates High-Fat-Diet-Induced Metabolic Syndrome in Mice. Marine Drugs. 2018;16(2):66. doi: 10.3390/md16020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, et al. How to feed the mammalian gut microbiota: bacterial and metabolic modulation by dietary fibers. Front Microbiol. 2017;8:1749. doi: 10.3389/fmicb.2017.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mancabelli L, Milani C, Lugli GA, Turroni F, Cocconi D, van Sinderen D, et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol Ecol. 2017. 10.1093/femsec/fix153. [DOI] [PubMed]
- 121.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Imhann Floris, Vich Vila Arnau, Bonder Marc Jan, Fu Jingyuan, Gevers Dirk, Visschedijk Marijn C, Spekhorst Lieke M, Alberts Rudi, Franke Lude, van Dullemen Hendrik M, Ter Steege Rinze W F, Huttenhower Curtis, Dijkstra Gerard, Xavier Ramnik J, Festen Eleonora A M, Wijmenga Cisca, Zhernakova Alexandra, Weersma Rinse K. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2016;67(1):108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pascal Victoria, Pozuelo Marta, Borruel Natalia, Casellas Francesc, Campos David, Santiago Alba, Martinez Xavier, Varela Encarna, Sarrabayrouse Guillaume, Machiels Kathleen, Vermeire Severine, Sokol Harry, Guarner Francisco, Manichanh Chaysavanh. A microbial signature for Crohn's disease. Gut. 2017;66(5):813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66:863–871. doi: 10.1136/gutjnl-2015-309940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wright EK, Kamm MA, Wagner J, Teo S-M, Cruz PD, Hamilton AL, et al. Microbial factors associated with postoperative Crohn’s disease recurrence. J Crohns Colitis. 2017;11:191–203. doi: 10.1093/ecco-jcc/jjw136. [DOI] [PubMed] [Google Scholar]
- 127.Kennedy NA, Lamb CA, Berry SH, Walker AW, Mansfield J, Parkes M, et al. The impact of NOD2 variants on fecal microbiota in Crohn’s disease and controls without gastrointestinal disease. Inflamm Bowel Dis. 2018;24:583–592. doi: 10.1093/ibd/izx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pérez-Brocal V, García-López R, Nos P, Beltrán B, Moret I, Moya A. Metagenomic analysis of Crohn’s disease patients identifies changes in the virome and microbiome related to disease status and therapy, and detects potential interactions and biomarkers. Inflamm Bowel Dis. 2015;21:2515–2532. doi: 10.1097/MIB.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 129.Papa E, Docktor M, Smillie C, Weber S, Preheim SP, Gevers D, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One. 2012;7:e39242. doi: 10.1371/journal.pone.0039242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajilic-Stojanovic M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19:481. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 131.Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 132.De Palma Giada, Lynch Michael D. J., Lu Jun, Dang Vi T., Deng Yikang, Jury Jennifer, Umeh Genevieve, Miranda Pedro M., Pigrau Pastor Marc, Sidani Sacha, Pinto-Sanchez Maria Ines, Philip Vivek, McLean Peter G., Hagelsieb Moreno-Gabriel, Surette Michael G., Bergonzelli Gabriela E., Verdu Elena F., Britz-McKibbin Philip, Neufeld Josh D., Collins Stephen M., Bercik Premysl. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Science Translational Medicine. 2017;9(379):eaaf6397. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 133.Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, et al. Reduction of butyrate- and methane-producing microorganisms in patients with irritable bowel syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hollister EB, Cain KC, Shulman RJ, Jarrett ME, Burr RL, Ko C, et al. Relationships of microbiome markers with extraintestinal, psychological distress and gastrointestinal symptoms, and quality of life in women with irritable bowel syndrome. J Clin Gastroenterol. 2018. 10.1097/MCG.0000000000001107. [DOI] [PMC free article] [PubMed]
- 135.Tigchelaar E F, Bonder M J, Jankipersadsing S A, Fu J, Wijmenga C, Zhernakova A. Gut microbiota composition associated with stool consistency. Gut. 2015;65(3):540–542. doi: 10.1136/gutjnl-2015-310328. [DOI] [PubMed] [Google Scholar]
- 136.Jalanka Jonna, Major Giles, Murray Kathryn, Singh Gulzar, Nowak Adam, Kurtz Caroline, Silos-Santiago Inmaculada, Johnston Jeffrey, de Vos Willem, Spiller Robin. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. International Journal of Molecular Sciences. 2019;20(2):433. doi: 10.3390/ijms20020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pedrosa Carrasco AJ, Timmermann L, Pedrosa DJ. Management of constipation in patients with Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:6. doi: 10.1038/s41531-018-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wiesel PH, Norton C, Glickman S, Kamm MA. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol. 2001;13:441–448. doi: 10.1097/00042737-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 139.Barichella Michela, Severgnini Marco, Cilia Roberto, Cassani Erica, Bolliri Carlotta, Caronni Serena, Ferri Valentina, Cancello Raffaella, Ceccarani Camilla, Faierman Samanta, Pinelli Giovanna, Bellis Gianluca, Zecca Luigi, Cereda Emanuele, Consolandi Clarissa, Pezzoli Gianni. Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Movement Disorders. 2018;34(3):396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 140.Hill-Burns Erin M., Debelius Justine W., Morton James T., Wissemann William T., Lewis Matthew R., Wallen Zachary D., Peddada Shyamal D., Factor Stewart A., Molho Eric, Zabetian Cyrus P., Knight Rob, Payami Haydeh. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Movement Disorders. 2017;32(5):739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petrov V. A., Saltykova I. V., Zhukova I. A., Alifirova V. M., Zhukova N. G., Dorofeeva Yu. B., Tyakht A. V., Kovarsky B. A., Alekseev D. G., Kostryukova E. S., Mironova Yu. S., Izhboldina O. P., Nikitina M. A., Perevozchikova T. V., Fait E. A., Babenko V. V., Vakhitova M. T., Govorun V. M., Sazonov A. E. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bulletin of Experimental Biology and Medicine. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 142.Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23:1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang Chih-Jung, Lin Tzu-Lung, Tsai Yu-Ling, Wu Tsung-Ru, Lai Wei-Fan, Lu Chia-Chen, Lai Hsin-Chih. Next generation probiotics in disease amelioration. Journal of Food and Drug Analysis. 2019;27(3):615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
16S rRNA gene sequences used to construct the phylogenetic tree were obtained from NCBI (https://www.ncbi.nlm.nih.gov/). Accession numbers for each sequence are in parentheses in Fig. 2.


