Abstract
Background and Purpose
In Alzheimer's continuum (a comprehensive of preclinical Alzheimer's disease [AD], mild cognitive impairment [MCI] due to AD, and AD dementia), cognitive dysfunctions are often related to cortical atrophy in specific brain regions. The purpose of this study was to investigate the association between anatomical pattern of cortical atrophy and specific neuropsychological deficits.
Methods
A total of 249 participants with Alzheimer's continuum (125 AD dementia, 103 MCI due to AD, and 21 preclinical AD) who were confirmed to be positive for amyloid deposits were collected from the memory disorder clinic in the department of neurology at Samsung Medical Center in Korea between September 2013 and March 2018. To analyze neuropsychological test-specific neural correlates representing the relationship between cortical atrophy measured by cortical thickness and performance in specific neuropsychological tests, a linear regression analysis was performed. Two neural correlates acquired by 2 different standardized scores in neuropsychological tests were also compared.
Results
Cortical atrophy in several specific brain regions was associated with most neuropsychological deficits, including digit span backward, naming, drawing-copying, verbal and visual recall, semantic fluency, phonemic fluency, and response inhibition. There were a few differences between 2 neural correlates obtained by different z-scores.
Conclusions
The poor performance of most neuropsychological tests is closely related to cortical thinning in specific brain areas in Alzheimer's continuum. Therefore, the brain atrophy pattern in patients with Alzheimer's continuum can be predict by an accurate analysis of neuropsychological tests in clinical practice.
Keywords: Neuropsychological Tests, Cortical Atrophy, Alzheimer's Disease, Alzheimer's Continuum, Cognition, Neural Correlates
INTRODUCTION
Neuropsychological-anatomical correlations are important for clinical practice. They are not only important for understanding a patient's current clinical symptoms, but also important for predicting the progression of symptoms. In this regard, researchers have tried to find out whether specific forms of cognitive deficits are directly correlated with certain brain regions using several methods including injury-lesion and functional neuroimaging studies. Initially, case studies of patients with strokes or head injuries have shown brain-behavior relationships. Functional neuroimaging studies have also been performed to determine the function of a specific brain region.
With the advent of neuroimaging techniques, it is possible to detect subtle changes of cortical structures in neurodegenerative diseases. Increasing evidence showed that various kinds of cognitive dysfunctions were often related to cortical atrophy in neurodegenerative diseases including Alzheimer's disease (AD).1,2,3,4 A previous study from our group investigated the correlation between neuropsychological tests of the Seoul Neuropsychological Screening Battery (SNSB) and the cortical thickness in a number of patients with AD or amnestic mild cognitive impairment (aMCI).4 Results showed that poor performances in most neuropsychological tests were correlated to cortical thinning in specific brain areas. Thus, cortical thinning was useful for understanding neural correlates of cognitive deficits.
The diagnosis of AD and aMCI patients in the prior study only relied on clinical consequences including symptoms and signs. However, the importance of biomarkers for AD has been on the rise because the National Institute on Aging and Alzheimer's Association has shifted the definition of AD in living people from a syndrome to a biological construct.5 According to this paradigm shift, Alzheimer's pathologic change without clinical syndrome and AD are not regarded as separate entities, but earlier and later phases of “Alzheimer's continuum” including preclinical AD, mild cognitive impairment (MCI) due to AD and AD dementia. However, neural correlates targeting all participants with Alzheimer's pathology have not been reported yet. Thus, it is necessary to investigate neural correlates of Alzheimer's continuum including preclinical AD.
The SNSB was modified and complemented to produce the SNSB-II in 2012.6 The norms by which raw scores could be converted to z-scores were changed and new forms of assessment were added to reflect the latest trends. The Flynn effect, which is the general trend of increased intelligence quotients (IQs) over time, is estimated to contribute to an increase of 0.3 IQ points per year.7 During periodic revision and restandardization of the Wechsler intelligence scales, subjects in validation samples who were administered by both older and newer versions of the same test consistently obtained higher IQ scores on the older version.8,9,10 This meant that norms for the newer tests were more stringent. This phenomenon may also apply to restandardization of the SNSB-II.
The present study aimed to explore neuropsychological test-specific neuroanatomical correlates representing the relationship between cortical atrophy measured by cortical thickness and performance in specific neuropsychological tests measured by SNSB-II in a large sample of subjects with Alzheimer's continuum. This study also investigated whether there were discrepancies between neuroanatomical correlates of z-scores which was converted from raw scores based on the criteria in the SNSB-I and those of z-scores using the criteria in the SNSB-II. We hypothesized that statistic maps of SNSB-II z-scores were broader than those of SNSB-I z-scores.
METHODS
Participants
A total of 249 participants with Alzheimer's continuum (125 participants with AD dementia, 103 participants with MCI due to AD, and 21 participants with preclinical AD) were collected from the memory disorder clinic in the department of neurology at Samsung Medical Center in Seoul, Korea between September 2013 and March 2018. Each participant received neuropsychological battery, high-resolution T1-weighted magnetic resonance imaging (MRI) scan, and 18F-flutemetamol positron emission tomography (PET) to assess amyloid-β (Aβ) deposition. The time interval between assessments was less than 6 months. According to the National Institute on Aging-Alzheimer's Association criteria,11,12,13 Aβ (+) cognitive normal or subjective memory concerns, Aβ (+) MCI, and Aβ (+) clinically diagnosed AD type dementia were defined as preclinical AD, MCI due to AD, and AD dementia, respectively. We excluded secondary causes of cognitive impairment by laboratory tests, including complete blood count, blood chemistry, vitamin B12/folate, syphilis serology, and thyroid function tests. All participants had no significant whiter matter hyperintensities (cap or band <5 mm and the longest diameter of deep white matter lesion <10 mm), cerebral infarctions, intracranial hemorrhages, brain tumors, hydrocephalus, or other structure lesions.
Our study protocol was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB file No. 2013-07-073). All participants provided informed consent for research according to the guidelines outlined in the Declaration of Helsinki.
Neuropsychological tests
SNSB-II, a neuropsychological battery including standardized and validated tests of a variety of cognitive functions,6 was used to access all participants, although a small number of participants could not complete all these tests. The SNSB-II evaluates many cognitive factors, including verbal and visual memory, visuoconstructive function, language, praxis, components of Gerstmann syndrome (acalculia, agraphia, right/left disorientation, finger agnosia), and frontal/executive functions. We used tests that provided numeric scores, such as digit span (forward and backward), the Korean version of the Boston Naming Test (K-BNT), the Seoul Verbal Learning Test (SVLT) (immediate and 20-minutes delayed recall and recognition), the Rey-Osterrieth Complex Figure Test (RCFT) (copying, immediate and 20-minutes delayed recall and recognition), the Clock Drawing Test (CDT), the Controlled Oral Word Association Test (COWAT), the Stroop test, the Digit Symbol Coding (DSC), and part B of the Trail Making Test (TMT-B) for our study. Results with numeric continuous values were converted to z-scores using age, sex, and education criteria presented in the SNSB-II except for the DSC. We obtained 3 types of scores including raw scores, SNSB-II z-scores, and SNSB-I z-scores. SNSB-II z-scores represented standardized z-scores corrected by norms presented in the SNSB-II. SNSB-I z-score meant standardized z-scores based on norms of the SNSB-I except for the CDT, the DSC, and the TMT-B.
Acquisition of 3-dimensional MRI images
Three-dimensional T1 Turbo Field Echo MRI images for 249 participants (125 participants with AD dementia, 103 participants with MCI due to AD, and 21 participants with preclinical AD) were acquired using a 3.0T MRI scanner (Philips 3.0T Achieva; Philips Medical Systems, Best, the Nederland) with the following imaging parameters: sagittal slice thickness, 1.0 mm with 50% overlap; no gap; repetition time of 9.9 ms; echo time of 4.6 ms; flip angle of 8°; and matrix size of 240×240 pixels reconstructed to 480×480 over a field view of 240 mm.
MRI data processing for cortical thickness measurements
Images were processed using the CIVET anatomical pipeline.14 Native MRI images were registered to the Montreal Neurological Institute -152 template by a linear transformation15 and corrected for intensity non-uniformities using the N3 algorithm.16 Registered and corrected images were divided into white matter, gray matter, cerebrospinal fluid, and background. Using the Constrained Laplacian-based Automated Segmentation with Proximities algorithm,17,18 surfaces of inner and outer cortices were extracted automatically. Inner and outer surfaces had the same numbers of vertices. There were close correspondences between counterpart vertices of inner and outer cortical surfaces. Cortical thickness defined as the Euclidean distance between linked vertices of inner and outer surfaces19 was not calculated in Talairach spaces, but in native brain spaces due to the limit to linear stereotaxic normalization. As expected, there was a significant positive correlation between cortical thickness and intracranial volume (ICV) in native space.20 Controlling for ICV reflecting brain size effect was necessary to compare cortical thickness among participants. In the previous study,20 our group proposed that the measurement of native space cortical thickness followed by analyses that include brain size as a covariate is an efficient method to explain the relationship between cortical thickness and brain size in depth. ICV was defined as the total volume of gray matter, white matter, and cerebrospinal fluid. It was calculated by measuring total volumes of voxels within the brain mask made by Functional Magnetic Resonance Imaging of the Brain Software Library using BET algorithm.21 As we extracted cortical surface models from MRI volumes transformed into stereotaxic space, cortical thickness was measured in the native space by applying an inverse transformation matrix to cortical surfaces and reconstructing them in native space.22
We applied surface-based 2-dimensional registration with a sphere-to-sphere warping algorithm and normalized cortical thicknesses spatially to compare thicknesses of corresponding regions among subjects. We used an improved surface registration algorithm and an unbiased iterative group template showing enhanced anatomic detail23 to transform thickness information for vertices into an unbiased iterative group template. Surface-based diffusion smoothing with a full-width at half-maximum of 20 mm was used to blur each map of cortical thickness to increase signal-to-noise ratio and statistical power.19,22,24
Asymmetric index (AI)
To measure an asymmetric degrees of neuroanatomical correlates for neuropsychological tests, we obtained an AI which was calculated with the following formula: (R−L/R+L), where R was the number of vertices with significant correlations in the right hemisphere and L was the number of vertices with significant correlations in the left hemisphere.4 After obtaining the AI, we divided the extent of asymmetry into 3 groups according to absolute value of the AI. |AI|<0.1, 0.1≤|AI|<0.5, and |AI|≥0.5 were classified as no hemispheric dominance, weak hemispheric dominance, and strong hemispheric dominance, respectively.
Statistical analyses
Chi-square test and analysis of variance with Bonferroni post-hoc tests were used to compare demographic and clinical characteristics of groups. For cortical thickness analyses of MRI data from Alzheimer's continuum patients, a MATLAB-based toolbox available free online at the University of Chicago website (http://galton.uchicago.edu/faculty/InMemoriam/worsley/research/surfstat/) was used. We entered score of each neuropsychological test as a predictor and vertex-by-vertex cortical thickness as an outcome to analyze the relationship between cortical thickness and neuropsychological performance in the surface model. A linear regression was then performed after controlling for sex, education years, Mini Mental State Examination (MMSE) score, and ICV as covariates. Statistical maps were thresholded using the random field theory at p<0.05. SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) was used and 2-sided p-value<0.05 was regarded as significant in our study to analyze statistical data.
RESULTS
Clinical characteristics of our participants
Demographic and clinical data of the participants are presented in Table 1. There were no significant differences in age, sex, or educational level among groups.
Table 1. Demographic variables, MMSE scores of normal cognition, aMCI, and AD groups.
| Variable | Preclinical AD (n=21) | MCI due to AD (n=103) | AD dementia (n=125) | Total | p-value |
|---|---|---|---|---|---|
| Age (yr) | 69.5±6.9 | 70.4±6.4 | 68.3±7.6 | 69.3±7.1 | 0.092 |
| Sex (M:F) | 10:11 | 44:59 | 52:73 | 106:143 | 0.875 |
| Education | 10.2±4.9 | 11.5±4.5 | 11.8±4.1 | 11.5±4.3 | 0.310 |
| MMSE (score) | 27.9±1.6 | 25.4±3.2 | 19.0±6.1 | 22.4±5.9 | <0.001 |
Values are presented as mean±standard deviation. ‘n’ represents number of patients whose data were available for analysis. The p-value was obtained by analysis of variance model and χ2 test.
AD: Alzheimer's disease, MCI: mild cognitive impairment, aMCI: amnestic mild cognitive impairment, MMSE: Mini Mental State Examination.
Correlation between neuropsychological tests and cortical thickness
Table 2 presents results of neuropsychological tests for participants with AD dementia, participants with MCI due to AD, and participants with preclinical AD.
Table 2. Results of neuropsychological tests for AD dementia group, MCI due to AD group, normal preclinical AD group, and total participants.
| Neuropsychological test | AD dementia | MCI due to AD | Preclinical AD | Total | ||
|---|---|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | Number | Mean±SD | ||
| Attention | ||||||
| Forward digit span | 5.2±1.6 | 6.0±1.4 | 6.1±1.8 | 249 | 5.6±1.6 | |
| Backward digit span | 3.0±1.4 | 3.8±1.1 | 4.1±1.5 | 246 | 3.4±1.3 | |
| Language | ||||||
| K-BNT | 34.0±13.5 | 41.9±10.6 | 48.5±6.3 | 238 | 38.5±12.8 | |
| Visuospatial function | ||||||
| RCFT: copying | 19.8±12.0 | 29.1±7.4 | 31.8±6.0 | 245 | 24.7±11.0 | |
| CDT | 2.0±0.9 | 2.5±0.8 | 2.8±0.5 | 193 | 2.3±0.9 | |
| Memory | ||||||
| SVLT: immediate recall | 10.9±5.3 | 14.4±4.2 | 20.9±5.0 | 249 | 13.2±5.6 | |
| SVLT: delayed recall | 0.7±1.3 | 1.6±2.1 | 7.2±2.1 | 249 | 1.6±2.5 | |
| SVLT: recognition | 15.5±2.9 | 18.1±2.6 | 21.2±1.5 | 249 | 17.1±3.2 | |
| RCFT: immediate recall | 3.0±3.4 | 6.2±4.9 | 14.4±6.2 | 244 | 5.3±5.4 | |
| RCFT: delayed recall | 1.8±3.0 | 5.5±4.9 | 14.0±5.9 | 244 | 4.4±5.4 | |
| RCFT: recognition | 15.4±2.8 | 17.8±2.5 | 19.4±2.8 | 244 | 16.7±3.0 | |
| Frontal/executive function | ||||||
| COWAT: animal | 8.4±4.5 | 12.0±4.5 | 14.4±5.1 | 249 | 10.4±5.0 | |
| COWAT: supermarket | 8.0±5.1 | 12.5±5.5 | 17.0±6.1 | 234 | 10.6±6.1 | |
| COWAT: phonemic | 15.0±10.9 | 22.1±10.9 | 24.2±13.5 | 220 | 18.8±11.7 | |
| Stroop test: color reading | 36.7±26.8 | 63.9±29.0 | 82.2±25.3 | 229 | 52.5±31.8 | |
| TMT-B | 188.6±107.1 | 92.5±86.9 | 66.9±39.0 | 184 | 134.2±106.4 | |
| DSC | 30.6±17.1 | 63.9±29.0 | 82.2±25.3 | 183 | 37.2±17.9 | |
Number represents number of patients whose data were available for analysis.
AD: Alzheimer's disease, MCI: mild cognitive impairment, SD: standard deviation, K-BNT: Korean version of the Boston Naming Test, RCFT: Rey-Osterrieth Complex Figure Test, CDT: Clock Drawing Test, SVLT: Seoul Verbal Learning Test, COWAT: Controlled Oral Word Association Test, TMT-B: part B of the Trail Making Test, DSC: Digit Symbol Coding.
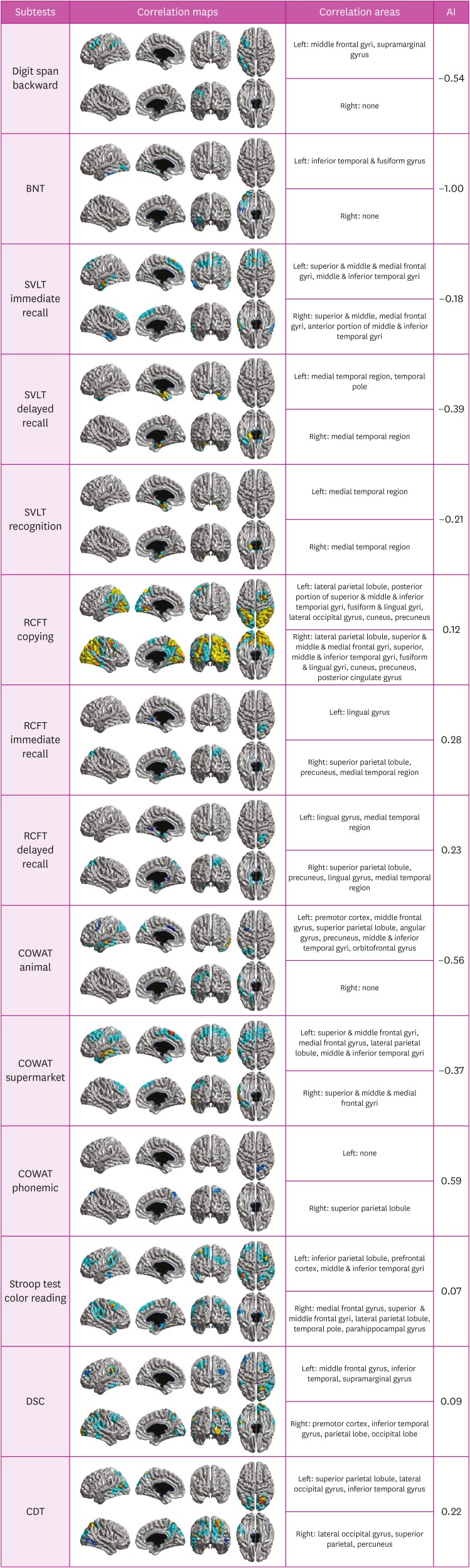
The statistical map showed that cortical thinnings in specific brain regions were associated with raw scores of all neuropsychological tests except for forward digit span, recognition in the RCFT, and the TMT-B (Fig. 1 and Table 3). Specifically, scores in the backward digit span test which reflected attention and working memory were positively associated with cortical thicknesses in the left middle frontal and supramarginal gyri. Scores in the Boston Naming Test (BNT) reflecting language function were positively associated with cortical thicknesses in the left inferior temporal and fusiform gyri. Scores in the immediate recall in the SVLT which was included in the memory domain were positively associated with cortical thicknesses in the bilateral superior & middle frontal gyri and middle & inferior temporal gyri. Scores in delayed recall and recognition of the SVLT were positively associated with cortical thicknesses in bilateral medial temporal regions. Scores in the copying test of the RCFT composing visuospatial domain were positively correlated with cortical thicknesses in widespread regions including bilateral parieto-occipital area and fusiform & lingual gyri. Scores in the immediate recall in the RCFT which comprised the memory domain were positively correlated with cortical thicknesses in the right medial temporal region, superior parietal lobule, precuneus, and left lingual gyrus. Scores in the delayed recall of the RCFT were positively correlated with cortical thicknesses in bilateral medial temporal regions and right superior parietal lobule. Scores in the semantic component of the COWAT (animal) were positively correlated with cortical thicknesses in the left medial frontal gyrus, middle & inferior temporal gyri, superior parietal lobule angular gyrus, and precuneus. Score in the semantic component of the COWAT (supermarket) were positively correlated with cortical thicknesses in the bilateral superior& middle & medial frontal gyri, middle& inferior temporal gyri, and left lateral parietal lobule. Scores in the phonemic component of the COWAT were positively correlated with cortical thicknesses of the right superior parietal lobule. Scores in the color reading portion of the Stroop test were positively associated with cortical thicknesses in the right prefrontal, right superior & middle & medial frontal gyri, left premotor cortex, and bilateral lateral parieto-temporal areas.
Fig. 1. Correlation maps demonstrating associations between cortical thickness and neuropsychological tests in patients with Alzheimer's continuum (AI >0 means right-sided correlated areas > left-sided correlated areas, and vice versa for AI <0).
AI: asymmetric index, BNT: Boston Naming Test, SVLT: Seoul Verbal Learning Test, RCFT: Rey-Osterrieth Complex Figure Test, COWAT: Controlled Oral Word Association Test, DSC: Digit Symbol Coding, CDT: Clock Drawing Test.
Table 3. Correlations among neuropsychological tests, cortical thickness, and hemispheric dominance.
| Neuropsychological test | Correlation areas | Hemispheric dominance |
|---|---|---|
| Digit span backward | Left: frontal (mid, inf), parietal (inf) | Left strong dominance |
| Right: none | ||
| BNT | Left: temporal (mid, inf) | Left strong dominance |
| Right: none | ||
| SVLT immediate recall | Left: frontal (sup, mid, med), temporal (mid, inf) | Left weak dominance |
| Right: frontal (sup, mid, med), temporal (mid, inf) | ||
| SVLT delayed recall | Left: temporal (med, inf) | Left weak dominance |
| Right: temporal (med) | ||
| SVLT recognition | Left: temporal (med) | Left weak dominance |
| Right: temporal (med) | ||
| RCFT copying | Left: parietal (sup, inf), temporal (post), occipital | Right weak dominance |
| Right: frontal (sup, mid), parietal (sup, inf) temporal (mid, inf), occipital | ||
| RCFT immediate recall | Left: temporal (inf) | Right weak dominance |
| Right: parietal (sup, med), temporal (med), occipital | ||
| RCFT delayed recall | Left: temporal (inf, med) | Right weak dominance |
| Right: parietal (sup, med), temporal (inf, med) | ||
| COWAT animal | Left: frontal (med, inf), parietal (sup, inf), temporal (mid, inf) | Left strong dominance |
| Right: none | ||
| COWAT supermarket items | Left: frontal (sup, med), parietal (sup, inf), temporal (mid, inf) | Left weak dominance |
| Right: frontal (sup, mid) | ||
| COWAT phonemic fluency | Left: parietal (inf) | Right weak dominance |
| Right: parietal (sup) | ||
| Stroop test color reading | Left: frontal (sup, mid), parietal (inf), temporal (mid, inf) | None |
| Right: frontal (sup, mid, med), parietal (sup, inf), temporal (inf) | ||
| DSC | Left: frontal (mid), parietal (inf), temporal (inf) | None |
| Right: frontal (mid), parietal (sup, inf), temporal (inf), occipital | ||
| CDT | Left: parietal (sup), temporal (inf), occipital | Right weak dominance |
| Right: parietal (sup, med), occipital |
BNT: Boston Naming Test, SVLT: Seoul Verbal Learning Test, RCFT: Rey-Osterrieth Complex Figure Test, COWAT: Controlled Oral Word Association Test, DSC: Digit Symbol Coding, CDT: Clock Drawing Test.
Hemispheric dominance of cortical thickness related to neuropsychological results
We also investigated hemispheric dominance of neural correlates by using AI (Table 3). Neural correlates for backward digit span test, the BNT, and semantic component of the COWAT (animal) showed strong left hemispheric dominance. Neural correlates for all components of the SVLT and semantic component of the COWAT (supermarket) had weak left hemispheric dominance. In contrast, neural correlates for phonemic component of the COWAT showed strong right hemispheric dominance, and neural correlates for copying test of the RCFT, immediate and delayed recall of the RCFT, and the CDT showed weak right hemispheric dominance.
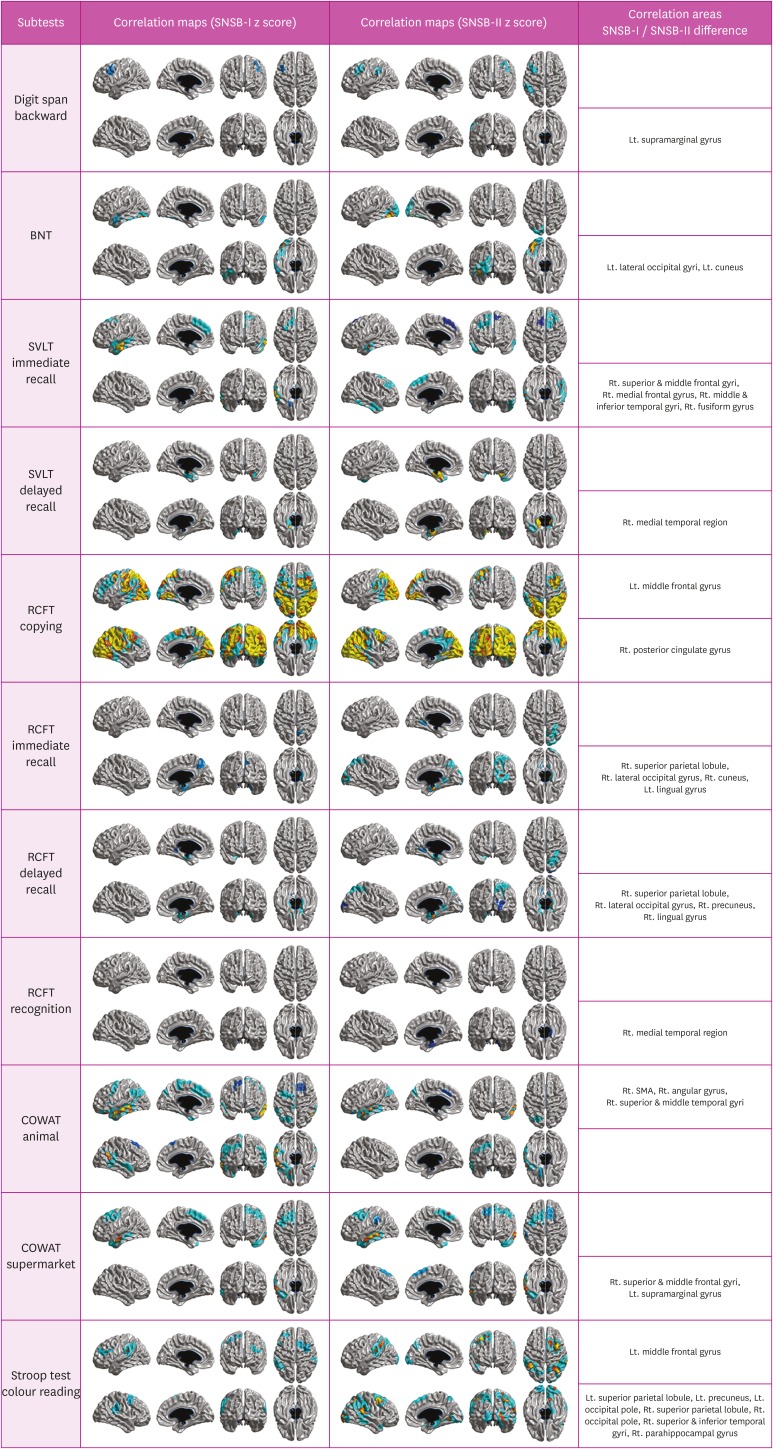
Comparison with 2 different statistical maps of z-scores
Results of our study showed differences between statistical maps of SNSB-II z-scores and statistical maps of SNSB-I z-scores except for forward digit span and recognition in the SVLT and RCFT (Fig. 2). Statistical maps of SNSB-II z-scores were wider than those of SNSB-I z-scores in backward digit span test, the BNT, immediate recall and delayed recall of the SVLT, immediate recall, delayed recall and recognition of the RCFT, and semantic component of the COWAT (animal). Opposite results were shown in semantic component of the COWAT (supermarket) and phonemic component of the COWAT. Two kinds of statistic maps had non-overlapping areas in copying test of the RCFT and color reading portion of the Stroop test. Non-overlapping areas in copying test of the RCFT were left middle frontal gyrus (which only correlated with the SNSB-I z-score) and right posterior cingulate gyrus (which only correlated with the SNSB-II z-score). Non-overlapping areas in the color reading portion of the Stroop test were left middle frontal gyrus (which was only associated with the SNSB-I z-score), bilateral superior parietal lobule, occipital pole, left precuneus, right superior & inferior temporal gyri, and parahippocampal gyrus, all of which were only associated with the SNSB-II z-score.
Fig. 2. Difference in cortical atrophy pattern between correlation maps with SNSB-II z-score and those with SNSB-I z-score.
BNT: Boston Naming Test, SVLT: Seoul Verbal Learning Test, RCFT: Rey-Osterrieth Complex Figure Test, COWAT: Controlled Oral Word Association Test, SNSB: Seoul Neuropsychological Screening Battery, SMA: supplementary motor area.
DISCUSSION
The present study investigated relationships between cortical thickness and neuropsychological results in Alzheimer's continuum. We found that most of neuropsychological tests were associated with specific cortical regions. Especially, we obtained AI to demonstrate laterality representing impaired performances of certain neuropsychological tests was more likely to be associated with dysfunction in one hemisphere than the other. Finally, comparison with 2 different statistical maps of z-scores showed the degree of discrepancy between neural correlates of z-scores converted from raw scores based on the criteria in the SNSB-I and those of z-scores using the criteria in the SNSB-II.
Deficit in the backward digit span was correlated with decreased cortical thickness in the left middle frontal gyri and supramarginal gyrus. Considering that to perform backward digit span, temporarily store of digits which were presented was needed additionally and this process required working memory for temporal order, it would be reasonable to expect that backward digit span might be related to regions responsible for working memory. Convergent evidence from lesions25,26,27 and functional MRI (fMRI)28,29,30,31,32 have suggested that prefrontal areas could play a critical role in working memory. A few fMRI studies have also shown that activation posterior parietal cortex is increased during a temporal order working memory task.33,34 In fact, fMRI studies have revealed that the backward digit span test is associated with activation in the right and left dorsolateral prefrontal cortex, inferior parietal lobule, Broca's area, and anterior cingulate gyrus.35,36,37
In the present study, poor performances in BNT were associated with decreased cortical thicknesses in the left inferior temporal and fusiform gyri. BNT reflects semantic representation which lies in the inferior temporal lobe38 or the left temporal and parietal cortices.39 In fact, previous neuroimaging studies have shown that bad performances in BNT are linked to cortical thinning in the left middle temporal, superior temporal gyri, inferior parietal cortex,40,41 and hypometabolism in the left middle temporal and fusiform.
We found that the low performance of RCFT was associated with cortical atrophy in extensive brain regions including bilateral lateral parietal lobule, precuneus, posterior cingulate gyrus, fusiform gyrus, lingual gyrus, occipital lobe, and left prefrontal cortex in the present study. Previous studies have shown that poor performance in the RCFT is related to lesions in the right frontal lobe, superior temporal gyrus, lateral parietal lobule, and middle occipital gyrus.42 The RCFT copying test represents visuo-perceptive and visuo-constructive functions related to parietal and frontal regions, respectively. CDT also represents visuo-perceptive and visuo-constructive functions like the RCFT copying test. However, its deficits were related to decreased cortical thickness in the parietal region. In fact, a previous study has demonstrated that CDT performance is related to temporo-parietal regions.43,44 It might be related to the fact that recent studies have shown that visuo-constructive task is mainly related to parieto-temporal regions.3,45 Alternatively, the RCFT might be more complex than the CDT. Thus, it necessitates executive function more than the CDT.
Poor performance of the SVLT immediate recall was related to cortical thinning in the frontal region as well as lateral temporal region while poor performances of the SVLT delayed recall and recognition were associated with cortical thinning primarily in the medial temporal areas. The immediate recall task of the SVLT was used to access verbal learning ability while the delayed recall task of the SVLT was used to evaluate ability of memory consolidation. Verbal learning ability was correlated with medial frontal area, prefrontal area, and lateral temporal area in prior studies.46,47,48,49 The essential role of medial temporal lobe structures for the consolidation of new information has been known for a long time.50 As expected, poor performance on the delayed recall task was related to only bilateral medial temporal area because the task reflected long-term memory. These facts might lead to differences in neural correlates between immediate recall and delayed recall.
Recognition of the SVLT is closely related to retrieval ability. Recognition memory poses less demand on retrieval processes than free recall. Low scores of delayed recall and recognition of the SVLT meant retention deficit caused by the problem of memory consolidation. Therefore, the neural correlate for recognition of the SVLT had the same pattern as the neural correlate for delayed recall of the SVLT.
Low score in the RCFT immediate recall was related to cortical thinning in the right posterior cortical region as well as the medial temporal region while low score in the RCFT delayed recall was associated with cortical thinning in bilateral medial temporal regions and the right superior parietal lobule. The reason why posterior cortical regions are involved in tasks is that visual memory encoding needs non-spatial aspects of visual information processing. Support of this fact comes from several studies reporting primate parietal cortex's major involvement in visuospatial-related processing.51,52,53
Our study showed that semantic component of the COWAT was mainly associated with left temporal, lateral parietal, and frontal regions while phonemic component of the COWAT was mainly associated with lateral parietal regions. Semantic component of the COWAT has been used as standard tests to evaluate language function as well as frontal executive function.54 However, phonemic component of the test is regarded as a measure of frontal executive dysfunction because generating words on the basis of orthographic criteria is unusual, requiring the creation of non-habitual strategies primarily based on lexical representations.55 In a meta-analysis, deficits in semantic verbal fluency were associated predominantly with temporal and frontal cortex, whereas phonemic verbal fluency depended on frontal cortex.56 Contrary to expectations, impaired performance on phonemic fluency was only associated with parietal area. It might be explained by the fact that our participants were Alzheimer's continuum which had profound cortical thinning in parietal regions. Because frontal region has profound connections with parietal regions, deficits in phonemic fluency observed in Alzheimer's continuum might be due to lesions in the parietal region which in turn can lead to frontal dysfunction through secondary degeneration of front-parietal connections. However, the reason why phonemic fluency is associated with parietal cortex needs to be elucidated in the future.
We examined neural correlates of the Stroop test, the DSC, and the TMT-B to investigate brain areas associated with executive function tests. Stroop color reading and DSC showed decreased cortical thicknesses prominently in prefrontal and parietal regions while no cortical thinning was associated with the TMT in our study.
Color reading portion of the Stroop test was developed as a neuropsychological test to measure selective attention and cognitive flexibility.57 Stroop-related activations have been observed in the dorsolateral and ventrolateral prefrontal cortex, predominantly in the left hemisphere by functional neuroimaging studies.58,59,60 However, our results showed that the poor performance on the Stroop test was related to not only cortical thinning of prefrontal cortex, but also the thinning of parieto-temporal areas, different from results of prior studies. Luciano has also shown that right superior parietal gyrus and middle temporal gyrus are correlated negatively with scores of the Stroop test in patients with AD.61 In fact, previous studies using different neuroimaging methods have correlated parietal and temporal structures with executive functions.62,63 Although neural correlates of the DSC were similar to those of the Stroop test, the cortical atrophy related to poor performance on the DSC was more occipital and parietal dominant than neural correlates associated with decrements of the Stroop test. This difference may be explained by the fact that DSC tasks require not only executive functions, but also visual imagery. TMT is a tool measuring the ability of psychomotor speed, visuospatial searching, target-directed motor tracking, and set-shifting. Slower TMT-B completion time was associated with widespread cortical areas and white matter microstructures including left anterior thalamic radiation and right uncinate fasciculus.1 These deficits might not be directly related to cortical atrophy, but rather to subcortical dysfunction.
As expected, the BNT and semantic portion of the COWAT related to language function were strongly correlated with the left hemisphere. Several 18F-fluorodeoxyglucose (FDG) PET studies have shown that the BNT is closely related to hypometabolism in the left hemisphere.39,46 Some fMRI studies regarding semantic fluency have stressed the involvement of the left hemisphere.64,65 Unlike previous studies showing that deficit in backward digit span is related to the involvement in the bilateral prefrontal cortex, decline in backward digit span is only correlated with left hemisphere atrophy. Stimulation studies have revealed that left prefrontal cortex plays a crucial role in at least one type of working memory (sequential-letter working memory task).66 The backward digit span test is included in the verbal working memory tasks, not spatial working memory tasks. Therefore, the poor performance on the backward digit span might be related to decreased ability of verbal working memory. It might be associated with the left hemisphere more than with the right hemisphere.
In the present study, neural correlates for copying test of the RCFT and the CDT were a little lateralized to the right hemisphere. Traditionally, visuospatial functions evaluated by the 2 tests have been primarily attributed to the right hemisphere, which is more specialized in processing nonverbal information including spatial orientation, complex visual patterns, and visuospatial transformation.67 Structural neuroimaging studies have suggested that a poor performance on the CDT is mainly associated with regional volume loss of the right hemisphere.68 A FDG PET study has indicated that CDT performance is related to the right hemisphere, especially the parietal area.69 A previous study has also shown that the poor performance in the RCFT is related to right hemispheric lesions by lesion-symptom mapping.42 However, single photon emission computed tomography (SPECT) studies showed the CDT task had a close relationship with the function of the left hemisphere.70,71 Another fMRI study has reported the RCFT task is related to activation in bilateral hemispheres.72 Our results support that the right hemisphere is mostly involved in the visuo-spatial function, although the left hemisphere also has a role in visuo-spatial processing.
Verbal and visual memory tests showed material specificity, although their lateralities were modest. Investigation of patients with unilateral lesions to the medial temporal lobectomy demonstrated material-specificity of these memory impairment.73 In fMRI study, lateralization of activation associated with memory was determined by the nature of materials. The left hemisphere was active during encoding of words, while the right hemisphere was active during encoding of faces.74
Although the statistical map of SNSB-II z-score was generally similar to that of SNSB-I z-score, there were a few differences between the 2 maps. Neural correlates of SNSB-II z-scores were wider than those of SNSB-I z-scores in backward digit span test, BNT, immediate recall and delayed recall of the SVLT, immediate recall, delayed recall and recognition of the RCFT, and semantic component of the COWAT (animal). It might be related to the fact that the ability to perform tasks is increased over time. For this reason, norms made for newer tests are more stringent. In contrast, opposite results were shown in semantic component of the COWAT (supermarket) and phonemic component of the COWAT. Both kinds of neural correlates had non-overlapping area in copying test of the RCFT and color reading portion of the Stroop test. However, the exact reason why results were different in these tests was unknown. Further study is needed to confirm how much norms have potential effects on major consequence for neuropsychological assessment. Our findings suggest that the selection of appropriate norms is very important for neuropsychological tests. It alters cutoffs used for determination of certain disease and results of neuropsychological test completely. Some neuropsychological tests are old and standardized by norms made several decades ago. It causes score inflation which is secondary to the Flynn effect. It also induces higher functioning individuals to reach ceiling point on the test.
Our study was the first research investigating corresponding regions in cortical thinning associated with impaired performance on the SNSB-II in participants with Alzheimer's continuum who had amyloid pathology measured by amyloid PET. However, this study also has some limitations. First, although we showed cortical atrophy patterns associated with poor performance on a lot of neuropsychological tests, whether all neural correlates represented test-specific brain areas was doubtful. Rather, neural correlates for some tests might have relevance to basic cognitive processes sharing a variety of neuropsychological tests. Second, we could not consider effects of other pathologies including other AD (soluble Aβ and neurofibrillary tangles), microinfarcts, or possible combined degenerative dementia (dementia with lewy bodies and frontotemporal dementia) pathologies known to be associated with cognitive impairments. Finally, since our study population was only comprehensive of patients with Alzheimer's continuum, our results might be founded by anatomical noise meaning that areas might be irrelevant to specific signs and symptoms being studied. Thus, it is hard to generalize results to subjects with other neurodegenerative disease such as Parkinson's disease and frontotemporal dementia. Further study using participants with other neurodegenerative disease is needed to solve the second limitation of our study. Despite these limitations, our study is note-worthy because it is useful for knowing neuropsychological test-anatomical associations in Alzheimer's continuum, helping the diagnosis of these diseases by understanding neural correlates of cognitive deficits, and informing us that appropriate norms of neuropsychological test are important.
In conclusion, poor performance of most neuropsychological tests is closely related to cortical thinning in specific brain areas of patients with Alzheimer's continuum. Thus, we can predict brain atrophy patterns in patients with Alzheimer's continuum by accurately analyzing SNSB-II in clinical practice.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kang SH, Na DL, Kim YJ, Seo SW.
- Data curation: Park YH, Lee D, Kim JP, Chin J, Ahn Y, Park SB, Kim HJ, Jang H, Jung YH, Kim J, Lee J, Kim JS, Cheon BK, Hahn A, Lee H, Na DL, Kim YJ, Seo SW.
- Formal analysis: Park YH.
- Investigation: Kang SH, Park YH, Na DL, Seo SW.
- Methodology: Kang SH, Park YH, Kim YJ, Seo SW.
- Writing - original draft: Kang SH.
- Writing - review & editing: Kang SH, Seo SW.
References
- 1.MacPherson SE, Cox SR, Dickie DA, Karama S, Starr JM, Evans AC, et al. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex. 2017;95:92–103. doi: 10.1016/j.cortex.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallesi A, Mazzonetto I, Ambrosini E, Babcock L, Capizzi M, Arbula S, et al. Structural hemispheric asymmetries underlie verbal Stroop performance. Behav Brain Res. 2017;335:167–173. doi: 10.1016/j.bbr.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Zink DN, Miller JB, Caldwell JZ, Bird C, Banks SJ. The relationship between neuropsychological tests of visuospatial function and lobar cortical thickness. J Clin Exp Neuropsychol. 2018;40:518–527. doi: 10.1080/13803395.2017.1384799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn HJ, Seo SW, Chin J, Suh MK, Lee BH, Kim ST, et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49:3931–3945. doi: 10.1016/j.neuropsychologia.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang YJ, Na DL. Seoul Neuropsychological Screening Battery (SNSB-II) 2nd ed. Seoul: Human Brain Research & Consulting Co.; 2012. [Google Scholar]
- 7.Flynn JR. The mean IQ of Americans: massive gains 1932 to 1978. Psychol Bull. 1984;95:29–51. [Google Scholar]
- 8.Wechsler D. Wechsler Intelligence Scale for Children-Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 9.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 10.Wechsler D. Wechsler Adult Intelligence Scale-III. New York: Psychological Corporation; 1997. [Google Scholar]
- 11.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 15.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 16.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 19.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31:31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 25.Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 26.McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- 27.Chiba AA, Kesner RP, Reynolds AM. Memory for spatial location as a function of temporal lag in rats: role of hippocampus and medial prefrontal cortex. Behav Neural Biol. 1994;61:123–131. doi: 10.1016/s0163-1047(05)80065-2. [DOI] [PubMed] [Google Scholar]
- 28.Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, et al. Brain regions differentially involved in remembering what and when: a PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 30.Majerus S, D'Argembeau A, Martinez Perez T, Belayachi S, Van der Linden M, Collette F, et al. The commonality of neural networks for verbal and visual short-term memory. J Cogn Neurosci. 2010;22:2570–2593. doi: 10.1162/jocn.2009.21378. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshuetz C, Smith EE. Working memory for order information: multiple cognitive and neural mechanisms. Neuroscience. 2006;139:195–200. doi: 10.1016/j.neuroscience.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. J Cogn Neurosci. 2000;12(Suppl 2):130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- 35.Canavan AG, Passingham RE, Marsden CD, Quinn N, Wyke M, Polkey CE. Sequence ability in parkinsonians, patients with frontal lobe lesions and patients who have undergone unilateral temporal lobectomies. Neuropsychologia. 1989;27:787–798. doi: 10.1016/0028-3932(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 36.Gerton BK, Brown TT, Meyer-Lindenberg A, Kohn P, Holt JL, Olsen RK, et al. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia. 2004;42:1781–1787. doi: 10.1016/j.neuropsychologia.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Hoshi Y, Oda I, Wada Y, Ito Y, Yamashita Y, Oda M, et al. Visuospatial imagery is a fruitful strategy for the digit span backward task: a study with near-infrared optical tomography. Brain Res Cogn Brain Res. 2000;9:339–342. doi: 10.1016/s0926-6410(00)00006-9. [DOI] [PubMed] [Google Scholar]
- 38.Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 39.Apostolova LG, Lu P, Rogers S, Dutton RA, Hayashi KM, Toga AW, et al. 3D mapping of language networks in clinical and pre-clinical Alzheimer's disease. Brain Lang. 2008;104:33–41. doi: 10.1016/j.bandl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldo JV, Arévalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49:658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau JK, Humphreys GW, Douis H, Balani A, Bickerton WL, Rotshtein P. The relation of object naming and other visual speech production tasks: a large scale voxel-based morphometric study. Neuroimage Clin. 2015;7:463–475. doi: 10.1016/j.nicl.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biesbroek JM, van Zandvoort MJ, Kuijf HJ, Weaver NA, Kappelle LJ, Vos PC, et al. The anatomy of visuospatial construction revealed by lesion-symptom mapping. Neuropsychologia. 2014;62:68–76. doi: 10.1016/j.neuropsychologia.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Tranel D, Rudrauf D, Vianna EP, Damasio H. Does the Clock Drawing Test have focal neuroanatomical correlates? Neuropsychology. 2008;22:553–562. doi: 10.1037/0894-4105.22.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuoka T, Narumoto J, Okamura A, Taniguchi S, Kato Y, Shibata K, et al. Neural correlates of the components of the clock drawing test. Int Psychogeriatr. 2013;25:1317–1323. doi: 10.1017/S1041610213000690. [DOI] [PubMed] [Google Scholar]
- 45.Hirjak D, Wolf RC, Pfeifer B, Kubera KM, Thomann AK, Seidl U, et al. Cortical signature of clock drawing performance in Alzheimer's disease and mild cognitive impairment. J Psychiatr Res. 2017;90:133–142. doi: 10.1016/j.jpsychires.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Teipel SJ, Willoch F, Ishii K, Bürger K, Drzezga A, Engel R, et al. Resting state glucose utilization and the CERAD cognitive battery in patients with Alzheimer's disease. Neurobiol Aging. 2006;27:681–690. doi: 10.1016/j.neurobiolaging.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- 48.Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baddeley A. The central executive: a concept and some misconceptions. J Int Neuropsychol Soc. 1998;4:523–526. doi: 10.1017/s135561779800513x. [DOI] [PubMed] [Google Scholar]
- 50.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong SK, Xu Y. Task-context-dependent linear representation of multiple visual objects in human parietal cortex. J Cogn Neurosci. 2017;29:1778–1789. doi: 10.1162/jocn_a_01156. [DOI] [PubMed] [Google Scholar]
- 52.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 54.Lam LC, Ho P, Lui VW, Tam CW. Reduced semantic fluency as an additional screening tool for subjects with questionable dementia. Dement Geriatr Cogn Disord. 2006;22:159–164. doi: 10.1159/000094543. [DOI] [PubMed] [Google Scholar]
- 55.Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 56.Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- 57.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 58.Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, et al. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cipolotti L, Spanò B, Healy C, Tudor-Sfetea C, Chan E, White M, et al. Inhibition processes are dissociable and lateralized in human prefrontal cortex. Neuropsychologia. 2016;93:1–12. doi: 10.1016/j.neuropsychologia.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Vasconcelos LG, Jackowski AP, Oliveira MO, Flor YM, Souza AA, Bueno OF, et al. The thickness of posterior cortical areas is related to executive dysfunction in Alzheimer's disease. Clinics (Sao Paulo) 2014;69:28–37. doi: 10.6061/clinics/2014(01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolk DA, Dickerson BC Alzheimer's Disease Neuroimaging Initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickerson BC, Wolk DA Alzheimer's Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82:45–51. doi: 10.1136/jnnp.2009.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- 66.Mull BR, Seyal M. Transcranial magnetic stimulation of left prefrontal cortex impairs working memory. Clin Neurophysiol. 2001;112:1672–1675. doi: 10.1016/s1388-2457(01)00606-x. [DOI] [PubMed] [Google Scholar]
- 67.Lezak MD, Loring DW. Neuropsychological Assessment. 5th ed. Oxford: Oxford University Press; 2012. [Google Scholar]
- 68.Cahn-Weiner DA, Sullivan EV, Shear PK, Fama R, Lim KO, Yesavage JA, et al. Brain structural and cognitive correlates of clock drawing performance in Alzheimer's disease. J Int Neuropsychol Soc. 1999;5:502–509. doi: 10.1017/s1355617799566034. [DOI] [PubMed] [Google Scholar]
- 69.Lee DY, Seo EH, Choo IH, Kim SG, Lee JS, Lee DS, et al. Neural correlates of the Clock Drawing Test performance in Alzheimer's disease: a FDG-PET study. Dement Geriatr Cogn Disord. 2008;26:306–313. doi: 10.1159/000161055. [DOI] [PubMed] [Google Scholar]
- 70.Nagahama Y, Okina T, Suzuki N, Nabatame H, Matsuda M. Neural correlates of impaired performance on the clock drawing test in Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:390–396. doi: 10.1159/000084710. [DOI] [PubMed] [Google Scholar]
- 71.Ueda H, Kitabayashi Y, Narumoto J, Nakamura K, Kita H, Kishikawa Y, et al. Relationship between clock drawing test performance and regional cerebral blood flow in Alzheimer's disease: a single photon emission computed tomography study. Psychiatry Clin Neurosci. 2002;56:25–29. doi: 10.1046/j.1440-1819.2002.00940.x. [DOI] [PubMed] [Google Scholar]
- 72.Makuuchi M, Kaminaga T, Sugishita M. Both parietal lobes are involved in drawing: a functional MRI study and implications for constructional apraxia. Brain Res Cogn Brain Res. 2003;16:338–347. doi: 10.1016/s0926-6410(02)00302-6. [DOI] [PubMed] [Google Scholar]
- 73.Milner B. Biology of Memory. New York: Academic Press; 1970. pp. 29–50. [Google Scholar]
- 74.Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]