Abstract
Purpose
Direct application of atmospheric-pressure plasma jets (APPJs) has been established as an effective method of microbial decontamination. This study aimed to investigate the bactericidal effect of direct application of an APPJ using helium gas (He-APPJ) on Porphyromonas gingivalis biofilms on sandblasted and acid-etched (SLA) titanium discs.
Methods
On the SLA discs covered by P. gingivalis biofilms, an APPJ with helium (He) as a discharge gas was applied at 3 different time intervals (0, 3, and 5 minutes). To evaluate the effect of the plasma itself, the He gas–only group was used as the control group. The bactericidal effect of the He-APPJ was determined by the number of colony-forming units. Bacterial viability was observed by confocal laser scanning microscopy (CLSM), and bacterial morphology was examined by scanning electron microscopy (SEM).
Results
As the plasma treatment time increased, the amount of P. gingivalis decreased, and the difference was statistically significant. In the SEM images, compared to the control group, the bacterial biofilm structure on SLA discs treated by the He-APPJ for more than 3 minutes was destroyed. In addition, the CLSM images showed consistent results. Even in sites distant from the area of direct He-APPJ exposure, decontamination effects were observed in both SEM and CLSM images.
Conclusions
He-APPJ application was effective in removing P. gingivalis biofilm on SLA titanium discs in an in vitro experiment.
Keywords: Bacterial Load, Confocal Microscopy, Plasma Gases, Porphyromonas gingivalis, Scanning Electron Microscopy, Titanium
Graphical Abstract
INTRODUCTION
Since their introduction in 1965, dental implants have been widely used to replace missing teeth. However, the accumulation of bacteria around dental implants results in inflammatory disease, such as peri-implant mucositis and peri-implantitis [1]. Moreover, peri-implantitis shows refractory features, and one of the reasons is the difficulties in decontaminating the implant surface after it is covered by bacterial deposits [2].
No gold-standard decontamination procedure has yet emerged for implant surfaces. In several in vitro and animal experiments, mechanical debridement using ultrasonic scalers, curettes, lasers, and air-powder abrasion has been applied, with or without antiseptics or antibiotics [3,4,5]. However, mechanical debridement of implants with microthreads using conventional curettes is difficult because of the narrow interthread space. Air-powder abrasive systems are effective in microthreaded implants, although alterations of the implant surface have been observed in some cases [6,7].
Atmospheric-pressure plasma jets (APPJs) are a candidate for decontaminating implant surfaces. Plasma is one of the 4 fundamental states of matter and can be artificially generated by adding energy to a gas in an electromagnetic field. If the gas is electrically charged, it radiates radicals, charged species and metastable species, which induce bactericidal effects in vitro [8,9]. Generally, because the energy heating and energy transition processes increase the temperature of the target, the applicability of plasma under in vivo conditions is limited. However, since APPJs generate ions and neutral species at cold temperatures, while electrons are only hot along a short mean free path, APPJs do not cause any thermal damage to their surroundings [10]. Due to this feature, APPJs have been applied to sterilize materials in biological research and medical fields [11]. In dentistry, APPJs are used to control endodontic infections, remove dental decay, increase the bonding efficiency of composite resin, and even for bleaching [12,13,14,15]. In the periodontal field, argon (Ar) plasma treatment on implant abutments was found to improve tissue adaptation in patients with periodontal disease and a thin biotype [16]. For implant treatment, Koban et al. [17] applied an APPJ to machined titanium discs contaminated by Streptococcus mutans or multiple species from human saliva and observed a superior antiseptic effect of the APPJ compared to that of Chlorhexidine rinse. Recently, Carreiro et al. [18] evaluated an Ar gas–based APPJ treatment on sandblasted and acid-etched (SLA) surfaces, which are among the most widely used surfaces of currently available commercial dental implants. They showed the possibility of using APPJs on SLA surfaces as a decontamination method for P. gingivalis biofilms.
Previously, we revealed that in APPJs, helium (He) gas is superior to Ar gas because the energy of metastable He is higher than that of Ar, so He gas-based APPJ (He-APPJ) should be investigated for clinical applications [19]. This study aimed to evaluate a He-APPJ as a potential decontamination method for peri-implantitis treatment. For this purpose, the bactericidal effect of the He-APPJ was tested using P. gingivalis biofilms on SLA titanium discs.
MATERIALS AND METHODS
Disc preparation
SLA titanium discs of 10 mm in diameter and 1 mm in thickness (Osstem Implant Co., Ltd., Busan, Korea) were used. Each SLA disc was cut evenly into 4 quarter circles for the experiments. Before cell culture or bacterial biofilm formation, 1-quarter of a SLA disc was placed into each well. The discs were rinsed with ethanol and sterilized with ethylene oxide gas.
Bacterial strain and biofilm formation
P. gingivalis (American Type Culture Collection 33277) was used for biofilm formation. Bacteria were grown in brain heart infusion growth medium (BHI medium) supplemented with hemin and menadione and incubated in an anaerobic environment (GasPak-EZ anaerobic container system; Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) for 3 days. The bacterial concentration was adjusted by spectrophotometry in BHI medium containing 1×106 colony-forming units (CFU)/mL. For the development of biofilms, the sterilized discs were placed in 4-well polystyrene plates. The bacteria were inoculated and incubated in an anaerobic environment for 6 days until confluency was attained, with a change to fresh medium at 48-hour intervals after 72 hours of incubation.
He-APPJ application
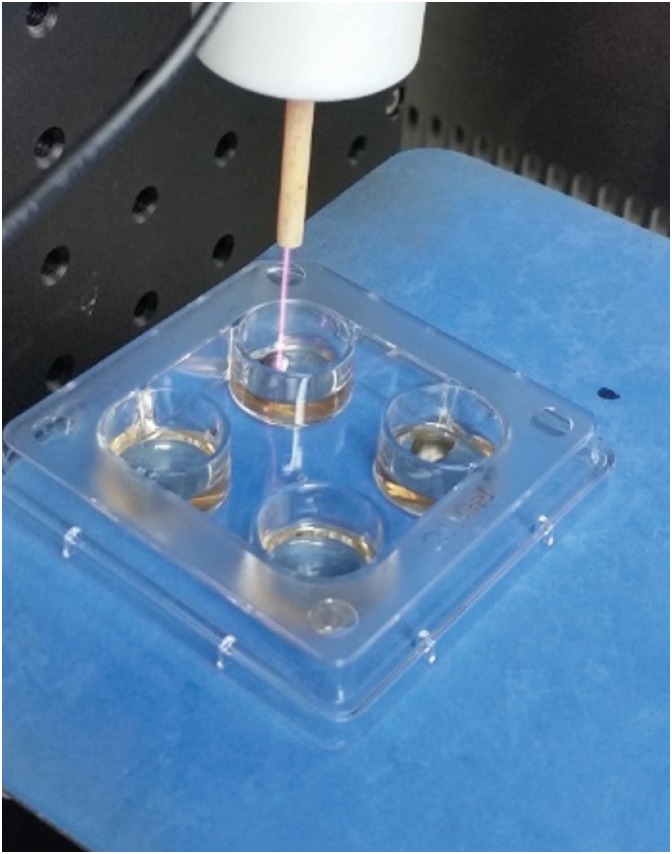
The design of the apparatus for generating an APPJ was described by Yoon et al. [19]. The APPJ source consists of a very-low-frequency (VLF)-driven 0.2 mm tungsten electrode surrounded by an alumina tube with an outer diameter of 0.6 mm and a coaxial quartz gas guiding tube with an inner diameter of 1 mm and a thickness of 1 mm. He was used as the discharge gas, which was injected into the gap between the alumina and quartz tubes and then released into the open air. The gas flow rate was maintained at 5 standard liters per minute and controlled by a mass flow controller (TN 2900, Brooks Instrument, Hatfield, PA, USA). The VLF frequency was generated using a function generator connected to a voltage amplifier and set to 7 kV under a pulse of 10 kHz (MF plasma power supply, Dawonsys, Ansan, Korea). The plasma device was fixed on a stand to prevent the device from moving and to maintain the distance between the plasma tip and specimens (Figure 1). The distance from the plasma tip to the specimens was 30 mm.
Figure 1. Photograph of the apparatus for He-APPJ generation and He-APPJ application to titanium discs in this study.
He-APPJ: helium atmospheric-pressure plasma jet.
The experimental groups were divided as follows: 1) the untreated control group, 2) the He without plasma generation group, and 3) the He-APPJ group.
Bacterial count assay
After the plasma treatment, the bacteria on each disc (n=5 per group) were transferred to 0.5 mL of a dispersion solution and then dispersed by vortexing for 1 minute to remove the remaining bacteria. The solutions were serially diluted and plated on Brucella agar plates (Hanil Komed Co., Seongnam, Korea). The bacteria were incubated anaerobically at 37°C for 7 days, and the number of CFUs was counted. All experiments were performed in triplicate.
Confocal laser scanning microscopy (CLSM) examinations
To evaluate cell viability, the bacteria on each disc after the abovementioned plasma treatment (n=2 per group) were stained with LIVE/DEAD® BacLight™ bacterial viability kits (Thermo Fisher Scientific, Waltham, MA, USA) and examined using CLSM (Carl Zeiss MicroImaging GmbH, Jena, Germany). Viable bacteria with intact membranes showed green fluorescence, and dead bacteria with damaged membranes showed red fluorescence.
Scanning electron microscopy (SEM) examinations
The structural and morphological changes of the bacteria after the abovementioned plasma treatment (n=2 per group) were evaluated using SEM (HITACHI S-4700, Tokyo, Japan). The plasma-treated discs were fixed with 2.5% glutaraldehyde for 2 hours and rinsed in a secondary fixative of osmium tetroxide and cacodylate solution, followed by rinsing and dehydration in ethanol. The discs were then dried and coated with a thin layer of platinum. Each specimen was examined with SEM.
Statistical analysis
The data are presented as the mean±standard deviation. Statistical analysis was performed using 1-way analysis of variance for comparison of the control, 3-minute, and 5-minute treatment groups and the Student t-test for comparisons between treatment groups. P values <0.05 were considered to indicate statistical significance.
RESULTS
Changes in the number of P. gingivalis CFUs after He-APPJ treatment
As the SLA surface treatment time increased, the number of P. gingivalis CFUs prominently decreased in both the He gas and He-APPJ groups, and the changes were statistically significant (Table 1). After 5 minutes of treatment, the number of P. gingivalis CFUs in the He-APPJ group was below the threshold level (1×103) and was significantly different from that in the He gas group (P=0.033).
Table 1. Number of Porphyromonas gingivalis colonies (CFU/mL) in the biofilm according to the He-APPJ treatment time.
| Group | SLA surface | P value | |||
|---|---|---|---|---|---|
| He gas | P value | He-APPJ | P value | ||
| Untreated control group | 14,392±5,215 | - | 13,520±3,790 | - | 0.829b) |
| 3-min treatment group | 1,476±269 | <0.001a) | 754±1,299 | <0.001a) | 0.426b) |
| 5-min treatment group | 1,773±946 | <0.001a) | 0 | <0.001a) | 0.033b) |
Data are shown as mean±standard deviation.
CFU: colony-forming unit, SLA: sandblasted and acid-etched, He: helium, He-APPJ: helium atmospheric-pressure plasma jet.
a)Statistically significant difference compared to the control group (1-way analysis of variance); b)Statistically significant difference between the He group and the He-APPJ group (Student t-test).
P. gingivalis biofilm and bacterial viability after He-APPJ treatment
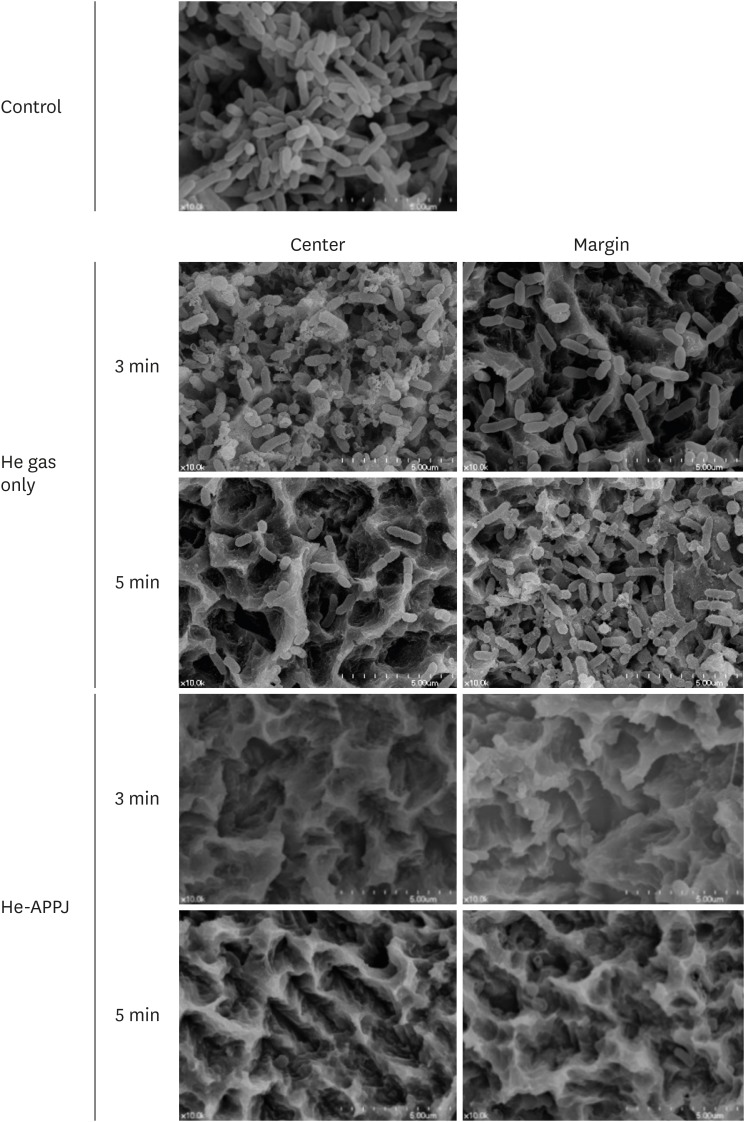
In the SEM images, no treatment group showed multiple, thick layers of clustered bacterial biofilm on the titanium disc surface (Figure 2). In the He gas–only treatment group, remaining bacterial biofilm was observed in both the central and marginal areas in the 3-minute treatment group, while bacterial rods had been eliminated from the central area, but not the marginal area, of the 5-minute treatment group. However, the He-APPJ group showed a clear SLA surface and destroyed bacterial biofilm structure, and no intact bacteria were detected in the central or marginal areas of both the 3-minute and 5-minute treatment groups.
Figure 2. Scanning electron microscopy images of the central and marginal areas after He-APPJ treatment. The control group (no treatment) showed heavy bacterial accumulation on the discs. In the He gas–only treatment group, remaining bacterial biofilm was observed in both the central and marginal areas in the 3-minute treatment group, while bacterial rods had been eliminated from the central area of the 5-minute treatment group, but not in the marginal area. However, the He-APPJ group showed a clear titanium surface without a bacterial biofilm in both the 3-minute and 5-minute treatment groups.
He-APPJ: helium atmospheric-pressure plasma jet.
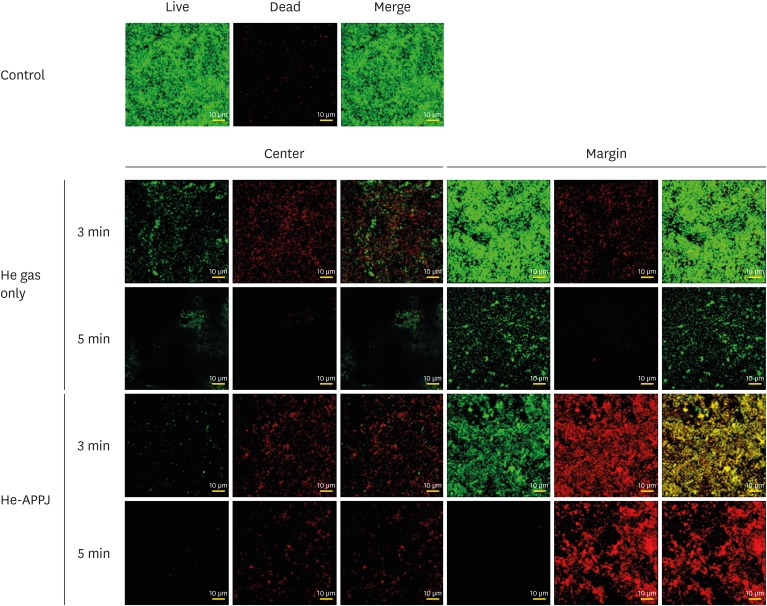
In the CLSM examinations, heavy live bacterial accumulation on the discs was observed in the control group (Figure 3). In the He gas–only group, the amount of bacteria dramatically decreased in the central area. However, in the marginal area, 3 minutes of treatment did not induce a reduction in the amount of bacteria. The amount of bacteria decreased in the 5-minute treatment group, although a moderate amount of live bacteria were still observed. Among the He gas–only treatment groups, the amount of bacteria also remarkably decreased in the central area compared to the marginal area in both the 3-minute and 5-minute treatment groups. Only a few viable bacteria were observed in the central area of the 3-minute treatment group. However, in the marginal area, a moderate amount of P. gingivalis remained; a mixture of live and dead bacteria was observed in the marginal area of the 3-minute treatment group, whereas most of the remaining bacteria were dead in the 5-minute treatment group.
Figure 3. Confocal laser scanning microscopy images of the central and marginal areas after He-APPJ application. Heavy live bacterial accumulation on the discs was observed in the control group. In the He gas–only treatment group, the amount of bacteria was higher in the central area than in the marginal area in the 3-minute treatment group. In the 5-minute treatment group, the amount of bacteria decreased in both the central and marginal areas, although more live bacteria were observed in the marginal area. in the He-APPJ treatment group, the amount of bacteria was lower in the central area than in the marginal area in both the 3-minute and 5-minute treatment groups. Only a few viable bacteria were observed in the central area of the 3-minute treatment group. In the marginal area, a mixture of live and dead bacteria was found, whereas most bacteria were dead in the 5-minute treatment group.
He: helium, He-APPJ: helium atmospheric-pressure plasma jet.
DISCUSSION
The treatment of peri-implantitis is mainly focused on the removal of bacterial biofilms. However, peri-implant biofilms are more difficult to remove than periodontal biofilms, because both the implant design and rough surface texture impede the removal of the biofilm. The decontamination of rough surfaces, such as SLA surfaces, is more difficult than that of machined surfaces, which is one of the main reasons why special care is needed to treat the disease, as shown in the spontaneous peri-implantitis model proposed by Berglundh et al. [20]. In this study, a He-APPJ was used to decontaminate the SLA surface of titanium discs because blowing He gas, with or without plasma, can be done without altering the surface texture. The bactericidal effect of the He-APPJ on P. gingivalis biofilms on titanium discs was confirmed using several methods.
Microbial decontamination by APPJs is mediated by radicals that destroy the bacterial cell wall [21]. The mechanism of plasma-induced cell membrane rupture is mediated by the action of charged particles and reactive oxygen and nitrogen species (RONS). Charged particles and RONS produced by plasma can damage some small surface irregularities of the bacterial membrane and cause the membrane to rupture [8,22,23]. Because RONS are radicals produced by APPJs [24,25], and this investigation was performed in ambient air which is a good source of oxygen and nitrogen, the bactericidal effect of RONS cannot be excluded. These RONS attack membrane lipids, which are vulnerable macromolecules of the cell, resulting in the destruction of the membrane [26]. This APPJ system has additional benefits for the treatment of peri-implantitis, as APPJ has shown the capacity for high levels of radical generation in humid environments and with fixed targets [27]. However, the generation of RONS is sensitive to the discharge conditions [21]. Unlike the temperature of Ar gas, which reaches 45°C during the plasma treatment, that of He gas does not exceed 25°C [17]. Previously, we conducted experiments to compare Ar-APPJ to He-APPJ, and observed that He-APPJ yielded more metastable species, such as NO, O, and O3, than Ar did and achieved bacterial inactivation in significantly less time than Ar-APPJ did (1.63 minutes for He vs. 12.1 minutes for Ar) [19]. In addition, the surface temperature increase during APPJ application was also significantly lower for He-APPJ than for Ar-APPJ. Therefore, Ar-APPJ and He-APPJ show significantly different bactericidal effects, and He-APPJ is considered to be an appropriate application for the decontamination of titanium surfaces.
To determine the proper treatment time of He-APPJ, we designed an experimental group with 3- and 5-minute treatments and observed a significant decrease in the number of bacteria as the treatment time increased. The treatment time required to completely eradicate bacteria depends on the power setting of the plasma, discharge gas, bacterial type, type of medium, and the distance between the cell and plasma tip [24,28]. In addition to this study, Miura et al. [28] also tested the bactericidal effect of APPJs on P. gingivalis with different plasma durations and distances on machined surfaces in an in vitro setting. However, they did not suggest a proper treatment time for P. gingivalis. Carreiro et al. [18] applied an Ar-APPJ to an SLA surface for 1 and 3 minutes and reported a reduction in P. gingivalis colonization. However, the plasma treatment was ineffective compared to Chlorhexidine irrigation. Shi et al. [29] performed plasma treatment on contaminated implants in an experimental peri-implantitis model with beagle dogs and observed an increase in bone formation and re-osseointegration in the group treated by plasma for 3 minutes compared to that in the control group, which was irrigated with Chlorhexidine and saline for 3 minutes. The authors demonstrated a decrease in periodontal pathogenic bacterial load after plasma treatment that was significantly different for 3 months after treatment. In this study, the 3-minute He-APPJ treatment was effective for debridement of the SLA surface, but the 5-minute treatment completely killed P. gingivalis, including both directly plasma-exposed and unexposed sites. However, there is no consensus on the most suitable plasma treatment time for achieving bactericidal effects because of limited evidence on the plasma type, settings, and treatment parameters. P. gingivalis is a representative periodontal pathogen that contributes to destructive periodontitis and peri-implantitis. P. gingivalis has a unique cell membrane structure, with polysaccharide capsular materials that generally show higher resistance than those of other Gram-negative bacteria [30,31]. Furthermore, dental microorganisms usually exist in the form of biofilm in the periodontal pocket, and bacteria in biofilms are usually stable and resistant to outer attack because they produce a polymeric matrix [30,32]. These features make P. gingivalis very resistant to antibacterial treatment. Considering those conditions, the relatively longer killing time of P. gingivalis in this study can be compared to that of other Gram-negative bacteria [25].
In this study, the efficacy of plasma treatment was determined by evaluating the number of bacteria after treatment. The changes in bacterial viability after plasma treatment were evaluated using live/dead staining and CLSM. The morphological changes in the bacterial biofilm after plasma treatment were evaluated by SEM. In the cell assay, bacteria were not detected after direct plasma exposure for more than 3 minutes. The CLSM results were in accordance with those of the cell assay, and either the bacteria were undetected or most of the residual bacteria were dead in the 3- and 5-minute plasma groups. Other studies have shown a similar bactericidal effectiveness of plasma treatment by live/dead staining and CLSM and reported that plasma could diffuse on surfaces and even penetrate surface irregularities and porous structures, thereby efficiently disinfecting the implants [33,34]. In contrast, the unexposed area of the 3-minute plasma group showed yellow fluorescence, rather than green or red fluorescence, in the CLSM observations (red fluorescence indicates dying or dead bacteria). The expression of yellow fluorescence might have meant that propidium iodide was in the middle of the penetrated and ruptured cells; therefore, the mixture of green and red colors could have appeared yellow. One of the limitations of CLSM is that staining of bacterial cells does not always produce distinct red and green fluorescence, leading to difficulty in the interpretation of results [35].
The SEM images clearly show that bacteria were not found in the plasma-exposed area of the SLA titanium discs in this study. Intriguingly, most bacteria were removed, except a few ruptured bacteria in the adjacent, unexposed area. The effect of plasma seemed to gradually decrease as the distance from the plasma area increased. This phenomenon is in contrast with the localization of plasma in a previous low-resolution study, in which high-resolution observations showed that the influence of plasma on adjacent, unexposed areas was prominent [31]. A possible mechanism is that bacterial death in unexposed areas was caused by the displacement of the covering medium, resulting in the desiccation of bacteria. Those desiccated bacteria were observed by SEM and were called “dead frozen bacteria”; similar observations were made in our study [36]. Displacement of the covering medium is unavoidable if the plasma treatment time is over 3 minutes; therefore, desiccation may be one of the ways in which the bacteria were killed.
We found it interesting that the bacterial counts of the He gas group were considerably lower than those of the untreated control group in this study. Simple gas blowing without plasma generation seemed to attenuate bacterial biofilms to some degree. Plasma radicals can hardly penetrate a thick biofilm; thus, removal of the superficial layer of biofilm can be clinically attractive [34,37]. In addition, He gas blowing is thought to spread charged plasma particles over a more extensive area, thereby widening the plasma-affected region and resulting in a shift in the sterilization or disinfection area [38]. In the SEM investigation, the He group also showed disruption of the superficial biofilm and a lower bacterial count. However, the remaining bacteria exhibited intact morphology, and most of them expressed green fluorescence in the CLSM images, indicating a viable state. These viable residual bacteria can recover rapidly and cause the recurrence of peri-implantitis. Thus, He gas blowing without plasma can temporarily reduce the bacterial count, but the effect might not be long-lasting due to rapid bacterial regrowth.
This study has several limitations that need to be addressed in further studies. P. gingivalis exists as a complex biofilm in the peri-implant pocket, not as a monoculture or as planktonic cells. P. gingivalis shows different phenotypes in biofilms of in vitro cultures [32]. Therefore, the response of P. gingivalis to the plasma treatment may not be the same in the mixed-species subgingival environment. Further research is needed to determine the response of P. gingivalis in an in vivo environment. Several mechanisms of action have been suggested previously, and further investigation is necessary to determine which mechanism contributes more to our plasma system. Additionally, the safety of plasma application in the mouth in contact with living cells should be evaluated in future studies.
In conclusion, a He-APPJ was effective for the decontamination of P. gingivalis biofilms on SLA surfaces without surface alterations. Additionally, the He-APPJ resulted in removal of bacteria from both direct plasma-exposed and unexposed sites. Based on these results, further research should investigate the clinical applicability of He-AAPJs as a decontamination technique for peri-implantitis treatment.
ACKNOWLEDGMENTS
The authors thank Ms. Mi-Jung Kang for her excellent technical assistance.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2009296 and NRF-2017R1D1A1B03033244).
- Conceptualization: Gon-Ho Kim, Yang-Jo Seol, Kyoung-Hwa Kim.
- Formal analysis: Ji-Yoon Lee.
- Investigation: Ji-Yoon Lee, Kyoung-Hwa Kim.
- Methodology: Sung-Young Yoon, Kyoung-Hwa Kim, Yang-Jo Seol.
- Project administration: Gon-Ho Kim, Yang-Jo Seol.
- Writing - original draft: Ji-Yoon Lee, Shin-Young Park, Yang-Jo Seol.
- Writing - review & editing: Yong-Moo Lee, In-Chul Rhyu.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–189. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz F, Sculean A, Romanos G, Herten M, Horn N, Scherbaum W, et al. Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig. 2005;9:111–117. doi: 10.1007/s00784-005-0305-8. [DOI] [PubMed] [Google Scholar]
- 3.Persson LG, Mouhyi J, Berglundh T, Sennerby L, Lindhe J. Carbon dioxide laser and hydrogen peroxide conditioning in the treatment of periimplantitis: an experimental study in the dog. Clin Implant Dent Relat Res. 2004;6:230–238. doi: 10.1111/j.1708-8208.2004.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz F, Nuesry E, Bieling K, Herten M, Becker J. Influence of an erbium, chromium-doped yttrium, scandium, gallium, and garnet (Er,Cr:YSGG) laser on the reestablishment of the biocompatibility of contaminated titanium implant surfaces. J Periodontol. 2006;77:1820–1827. doi: 10.1902/jop.2006.050456. [DOI] [PubMed] [Google Scholar]
- 5.Schou S, Holmstrup P, Jørgensen T, Skovgaard LT, Stoltze K, Hjørting-Hansen E, et al. Implant surface preparation in the surgical treatment of experimental peri-implantitis with autogenous bone graft and ePTFE membrane in cynomolgus monkeys. Clin Oral Implants Res. 2003;14:412–422. doi: 10.1034/j.1600-0501.2003.00912.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009;88:83–91. doi: 10.1002/jbm.b.31154. [DOI] [PubMed] [Google Scholar]
- 7.Kreisler M, Kohnen W, Christoffers AB, Götz H, Jansen B, Duschner H, et al. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er : YAG laser and an air powder system. Clin Oral Implants Res. 2005;16:36–43. doi: 10.1111/j.1600-0501.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 8.Mendis DA, Rosenberg M, Azam F. A note on the possible electrostatic disruption of bacteria. IEEE Trans Plasma Sci. 2000;28:1304–1306. [Google Scholar]
- 9.Laroussi M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. IEEE Trans Plasma Sci. 2002;30:1409–1415. [Google Scholar]
- 10.Kim JH, Lee MA, Han GJ, Cho BH. Plasma in dentistry: a review of basic concepts and applications in dentistry. Acta Odontol Scand. 2014;72:1–12. doi: 10.3109/00016357.2013.795660. [DOI] [PubMed] [Google Scholar]
- 11.Lackmann JW, Bandow JE. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl Microbiol Biotechnol. 2014;98:6205–6213. doi: 10.1007/s00253-014-5781-9. [DOI] [PubMed] [Google Scholar]
- 12.Jablonowski L, Koban I, Berg MH, Kindel E, Duske K, Schröder K, et al. Elimination of E. Faecalis by a new non-thermal atmospheric pressure plasma handheld device for endodontic treatment. A preliminary investigation. Plasma Process Polym. 2013;10:499–505. [Google Scholar]
- 13.Yang B, Chen J, Yu Q, Li H, Lin M, Mustapha A, et al. Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J Dent. 2011;39:48–56. doi: 10.1016/j.jdent.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho BH, Han GJ, Oh KH, Chung SN, Chun BH. The effect of plasma polymer coating using atmospheric-pressure glow discharge on the shear bond strength of composite resin to ceramic. J Mater Sci. 2010;46:2755–2763. [Google Scholar]
- 15.Nam SH, Lee HJ, Hong JW, Kim GC. Efficacy of nonthermal atmospheric pressure plasma for tooth bleaching. Sci World J. 2015;2015:581731. doi: 10.1155/2015/581731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canullo L, Peñarrocha D, Clementini M, Iannello G, Micarelli C. Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: radiographic results at 24-month follow-up of a RCT. Clin Oral Implants Res. 2015;26:8–14. doi: 10.1111/clr.12290. [DOI] [PubMed] [Google Scholar]
- 17.Koban I, Holtfreter B, Hübner NO, Matthes R, Sietmann R, Kindel E, et al. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro - proof of principle experiment. J Clin Periodontol. 2011;38:956–965. doi: 10.1111/j.1600-051X.2011.01740.x. [DOI] [PubMed] [Google Scholar]
- 18.Carreiro AF, Delben JA, Guedes S, Silveira EJ, Janal MN, Vergani CE, et al. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J Periodontol. 2019;90:507–515. doi: 10.1002/JPER.18-0366. [DOI] [PubMed] [Google Scholar]
- 19.Yoon SY, Kim KH, Seol YJ, Kim SJ, Bae B, Huh SR, et al. Effects of metastable species in helium and argon atmospheric pressure plasma jets (APPJs) on inactivation of periodontopathogenic bacteria. J Korean Phys Soc. 2016;68:1176–1191. [Google Scholar]
- 20.Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: an experimental study in dogs. Clin Oral Implants Res. 2007;18:655–661. doi: 10.1111/j.1600-0501.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 21.Ehlbeck J, Schnabel U, Polak M, Winter J, von Woedtke T, Brandenburg R, et al. Low temperature atmospheric pressure plasma sources for microbial decontamination. J Phys D Appl Phys. 2010;44:013002 [Google Scholar]
- 22.Laroussi M, Mendis DA, Rosenberg M. Plasma interaction with microbes. New J Phys. 2003;5:41.1–41.10. [Google Scholar]
- 23.Venezia RA, Orrico M, Houston E, Yin SM, Naumova YY. Lethal activity of nonthermal plasma sterilization against microorganisms. Infect Control Hosp Epidemiol. 2008;29:430–436. doi: 10.1086/588003. [DOI] [PubMed] [Google Scholar]
- 24.Laroussi M, Sayler GS, Glascock BB, McCurdy B, Pearce ME, Bright NG, et al. Images of biological samples undergoing sterilization by a glow discharge at atmospheric pressure. IEEE Trans Plasma Sci. 1999;27:34–35. [Google Scholar]
- 25.Lee K, Paek KH, Ju WT, Lee Y. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. J Microbiol. 2006;44:269–275. [PubMed] [Google Scholar]
- 26.Montie TC, Kelly-Wintenberg K, Roth JR. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans Plasma Sci. 2000;28:41–50. [Google Scholar]
- 27.Kim SJ, Yoon SY, Kim GH. Bullet velocity distribution of a helium atmospheric-pressure plasma jet in various N2/O2 mixed ambient conditions. IEEE Trans Plasma Sci. 2015;43:2054–2063. [Google Scholar]
- 28.Miura T, Egawa M, Ito T, Eguro T, Tanabe K, Yoshinari M. Debridement effect on periodontal pathogen Porphyromonas gingivalis cultured on titanium by application of atmospheric-pressure plasma. J Biomed Sci Eng. 2017;10:51–59. [Google Scholar]
- 29.Kim MS, Lee JS, Shin HK, Kim JS, Yun JH, Cho KS. Prospective randomized, controlled trial of sinus grafting using Escherichia-coli-produced rhBMP-2 with a biphasic calcium phosphate carrier compared to deproteinized bovine bone. Clin Oral Implants Res. 2015;26:1361–1368. doi: 10.1111/clr.12471. [DOI] [PubMed] [Google Scholar]
- 30.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis . Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahasneh A, Darby M, Tolle SL, Hynes W, Laroussi M, Karakas E. Inactivation of Porphyromonas gingivalis by low-temperature atmospheric pressure plasma. Plasma Med. 2011;1:191–204. [Google Scholar]
- 32.Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 33.Preissner S, Wirtz HC, Tietz AK, Abu-Sirhan S, Herbst SR, Hartwig S, et al. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: An in-vitro-study. J Biophotonics. 2016;9:637–644. doi: 10.1002/jbio.201500189. [DOI] [PubMed] [Google Scholar]
- 34.Sladek RE, Filoche SK, Sissons CH, Stoffels E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett Appl Microbiol. 2007;45:318–323. doi: 10.1111/j.1472-765X.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 35.Berney M, Hammes F, Bosshard F, Weilenmann HU, Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl Environ Microbiol. 2007;73:3283–3290. doi: 10.1128/AEM.02750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shashurin A, Keidar M, Bronnikov S, Jurjus RA, Stepp MA. Living tissue under treatment of cold plasma atmospheric jet. Appl Phys Lett. 2008;93:181501 [Google Scholar]
- 37.Vleugels M, Shama G, Deng XT, Greenacre E, Brocklehurst T, Kong MG. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans Plasma Sci. 2005;33:824–828. [Google Scholar]
- 38.Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, et al. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym. 2007;4:370–375. [Google Scholar]