Abstract
Peters’ anomaly accounts for the highest type of Anterior Segment Dysgenesis (ASD). The main features of Peters’ anomaly are: congenital corneal opacity centrally, defect in the posterior stroma and absence of Descemet’s membrane and the endothelium. However, this condition has wide clinical and histopathological variations in appearance, associations and severity. In this case series, we summarize 6 corneas in 5 Saudi cases of Peters’ anomaly (and describe 2 in detail) with unique histopathological findings that are additional to the typical known ones, shedding some light on the nomenclature of these variants according to the reported cases in the English-written literature. This will widen the spectrum of findings known to ophthalmic pathologists and ophthalmologists about this anomaly. This is also of importance in the assessment of the congenital glaucoma cases commonly seen in Saudi Arabia that often happens in association with ASD.
Keywords: Peters’ anomaly, Corneal opacity, Posterior keratoconus, Myxoma, Anterior Segment Dysgenesis, Glaucoma
Introduction
The essential features of Peters’ anomaly are: congenital corneal opacity centrally, defect in the posterior stroma and absence of Descemet’s membrane and the endothelium.1 However, other unusual findings are often encountered in the so called: “Peters’ anomaly variants”. Such cases have been described and reported with variable clinical and histopathological features. Within the long period of our experience- exceeding 25 years of practice- in an eye tertiary care center we have encountered 6 corneas in 5 Saudi patients with unusual features. In this paper, we are summarizing these cases with a review of the most relevant literature, providing detailed description of 2 cases that were not previously published.
Case reports
All cases with the tissue diagnosis of Peters’ anomaly variant diagnosed at our institute over the last 25 years were isolated and reviewed by a single pathologist. We have collected 6 corneas from 5 Saudi patients with unusual Peters’ anomaly where the main characteristics were found in addition to other interesting findings leading to the diagnosis of “Peters’ anomaly variant”. Examples of the typical histopathological findings of classic Peters’ anomaly are demonstrated in Fig. 1 for better understanding.
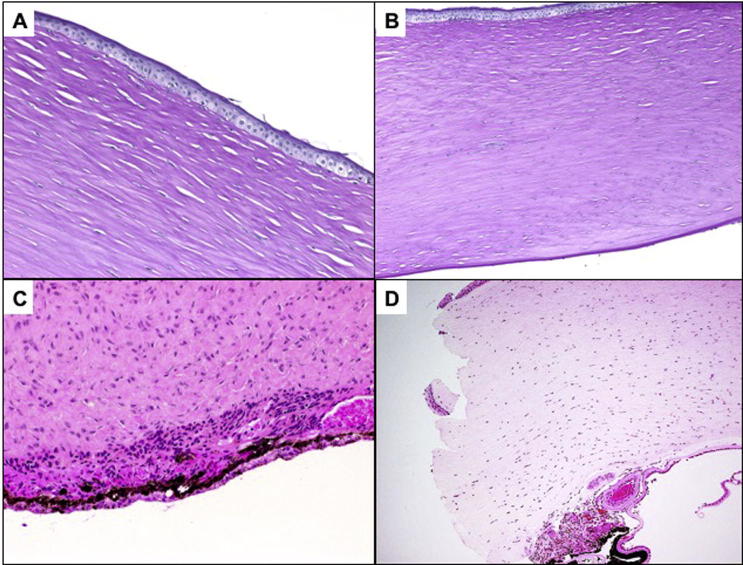
Fig. 1.
(A) An example of Peters’ anomaly with anterior bullous changes, absent Bowman’s layer and alteration of the normal stromal lamellar architecture. (Original magnification ×200 Periodic Acid Schiff). (B) Lower power of the same cornea. Note the thickened corneal stroma with edema and intact peripheral portion of Descemet’s membrane (Original magnification ×100 Periodic Acid Schiff). (C) The classic absence of Descemet’s membrane centrally with adherent iris tissue in the area of defective Descemet’s membrane (Original magnification ×200. Hematoxylin & Eosin). (D) Another cornea in Peters’ anomaly with cataract and lenticular adhesion (Type 2) showing thick corneal stroma, total absence of Descemet’s membrane, and adherent iris tissue as well as a residual lens capsule following cataract surgery (Original magnification ×100 Hematoxylin & Eosin).
The commonest unique observation in these cases was posterior concavity of the cornea or the so-called “keratoconus posticus circumscriptus” in 5 corneas of 4 patients with bilateral involvement. One patient had bilateral penetrating keratoplasty (PKP) in our institution (Fig. 2A and B). The second observation was a thickened multilaminated Descemet’s membrane in 2/6 (Cases 4 and 6) (Fig. 2C). The third unusual finding was a central corneal stromal myxoma in Cases 3 and 5 (Fig. 3). Finally, one case showed the presence of unilateral corneal opacity and stromal keratitis (Fig. 4).
Fig. 2.
(A)The histopathological appearance of the posterior concavity in one of the cases of posterior keratoconus. (Original magnification ×100. Hematoxylin & Eosin). (B) Higher power of the same cornea showing thick interrupted Bowman’s layer and absent Descemet’s membrane centrally. (Original magnification ×200, Periodic Acid Schiff). (C) One of the cases of Peters’ variant with absent Descemet’s membrane centrally and thick multilaminated Descemet’s membrane (Black arrow) at the periphery. (Original magnification ×100, Periodic Acid Schiff).
Fig. 3.
(A) The clinical appearance of the bilateral corneal opacity in Case 5. (B) The elevated central part of the right eyelid skin because of the underlying dome-shaped corneal mass. (C) The histopathology of the corneal opacity in the right eye showing neovascularization and adherent iris tissue. (Original magnification ×50. Hematoxylin & Eosin). (D) Higher magnification of the anterior part of the cornea showing keratinized thick epithelium. (Original magnification ×100. Hematoxylin & Eosin). (E) The histopathological appearance of the dome-shaped area of the cornea showing sub-epithelial myxoma composed of stellate and spindle cells. (Original magnification ×200. Hematoxylin & Eosin).
Fig. 4.
(A) Clinical appearance of the right cornea with unilateral central opacity in case 6. (B) The clinical appearance of the clear left eye cornea in the same patient. (C) The histopathological appearance of the right cornea showing central stromal scarring and infiltration by chronic inflammatory cells in addition to the absence of Descemet’s membrane. (Original magnification ×100. Hematoxylin & Eosin). (D) Higher magnification of the inflammatory cells, which are positive with CD68 stain. (Original magnification ×400).
Cases 1 to 4: These cases are not described in detail. The summary of the demographic, clinical and histopathological key features of these 4 cases is presented in Table 1.
Table 1.
Demographics, clinical features and unusual histopathological finding(s) in 6 eyes of 5 patients with Peters’ variant.
| Case # eye | Agea | Gender | Clinical Features | Unusual histopathological finding(s) | FU |
|---|---|---|---|---|---|
| Case 1 OD | 1 Month | F | Diffuse blue-gray corneal opacity except for central cornea, OU Secondary Angle Closure Glaucoma, OU Clinically: intra-corneal cyst confirmed by Ultrasound bio-microscopy, OU |
Stromal thinning of the central portion of the cornea, broad concave defect of the posterior surface of the cornea = Keratoconus posticus circumscriptus. | 2 years |
| Case 2 OS⁎ | 1 Month | F | Stromal thinning centrally with broad concave defect and PAS-faintly positive membrane posteriorly and Inflammatory cells within the cavity of the posterior defect= Keratoconus posticus circumscriptus. No Endothelial cells |
||
| Case 3 OD | 4 months | M | Dense central corneal scar OU Stromal Corneal vascularization OD Glaucoma OU |
Stromal marked thickening, loss of normal lamellar architecture, neovascularization and variable scarring. Centrally the corneal stroma replaced by loose myxoid stroma with proliferating spindle – shaped cells overlying an area of posterior corneal concavity= Keratoconus posticus circumscriptus. Central Myxoma |
3 years |
| Case 4 OD | 30 years | M | Central corneal scar OD | Stromal posterior concavity= Keratoconus posticus circumscriptus. Descemet’s membrane (at periphery): thickened, multilaminated and irregular with few central and peripheral guttata and moderately attenuated endothelium. Endothelial cells extend over the guttata and some show pigment phagocytosis |
5 years |
| Case 5 OS | 15 days | F | Thick, dense and dome-shaped corneal opacity, peripheral clear corneal zone of 1–2 mm, OD Central corneal opacity measuring 7 mm, OS |
Keratinized epithelium with intact portions of Bowman’s layer, peripherally. Stromal central myxomatous area with stellate- shaped cells, loose stroma and deep stromal neovascularization= Central Myxoma |
1 year |
| Case 6 OD | 5 months | F | Central corneal opacity sparing the peripheral 1–2 mm, OD Clear cornea, OS |
Stromal central defect with absent Descemet’s membrane= Keratoconus posticus circumscriptus Stromal infiltration with chronic inflammatory cells (Positive for CD3 and CD68). ? Von Hippel. Descemet’s membrane: intact at both ends, thickened and multilaminated |
11 years |
Age at presentation; F: Female; M: Male; OD: Right eye; OS: Left eye; OU: Both eyes; FU: Follow up.
Cases 1 & 2 represent 2 corneas from the same patient.
The last 2 cases in the same Table: one with a myxomatous lesion (Case 5) and one with unilateral Peters’ anomaly rare variant (Case 6) are further described with more details as follows:
Case 5: A 15 days- old female newborn was referred with whitish corneal opacities in both eyes since birth. Prenatal history was unremarkable however her family history was positive for paternal bilateral corneal opacity suspicious of Peters’ and treated by PKP in the left eye. Examination under general anesthesia showed corneal diameter of 12.5 mm in both eyes, intraocular pressure (IOP) of 15.5 and 9.5 mmHg in the right and left eyes respectively. The slit lamp examination showed thick, dense and dome-shaped right corneal opacity with very limited peripheral clear zone of 1–2 mm, inferior intra-stromal corneal blood vessels embedded within the elevated part of the cornea. The anterior and posterior segments could not be visualized in that eye. The left eye showed central corneal opacity measuring 7 mm in diameter. The anterior segment (iris/lens) as well as the posterior segment was within normal limits (Fig. 3A and B). PKP was performed in the right eye (OD) with donor and recipient trephination size of 8 mm. Histopathological examination showed keratinized epithelium with intact portions of Bowman’s layer and stroma peripherally. Centrally, there was a thick myxomatous area with stellate-shaped cells, loose stroma and deep stromal neovascularization (Fig. 3C, D and E). Thin Descemet’s membrane was seen at one end of the corneal tissue but absent along the remaining cornea with iridocorneal adhesions. The patient was followed up until the age of 11 months with stable central corneal scar, infero-temporal iridectomy and controlled IOP on the left. The right eye showed hypotony (IOP of 5 mmHg) in addition to a failed graft with iridocorneal peripheral adhesions.
Case 6: A 5 months-old female who was a product of full term, uncomplicated pregnancy presented with large corneas in both eyes and central corneal opacity, OD since birth. She was diagnosed elsewhere to have congenital glaucoma at the age of 1 month and had glaucoma filtering surgery in the form of trabeculotomy in both eyes followed by a glaucoma drainage device (Ahmad valve), OD because of uncontrolled IOP. Her visual examination showed poor fixation of the right eye while she could fix and follow objects with her left eye. The IOP measured 29 mmHg, OD and 19 mmHg, OS. Corneal diameters measured 12 mm and 13 mm in the right and left eyes respectively. The cornea of the right eye showed central corneal opacity sparing the peripheral 1–2 mm with a clear lens. Both the cornea and lens were clear in the left eye (Fig. 4A and B). PKP on the right was performed at the age of 11 years with guarded prognosis followed by acute graft rejection after 3 weeks. There was no further plan for any surgical management. The histopathological examination of the right cornea showed characteristic findings of Peters’ anomaly with an absent Bowman’s layer, central stromal defect, and absence of Descemet’s membrane centrally. Descemet’s membrane at both ends was intact with focal areas where it was thickened and multilaminated. There was stromal infiltration by chronic inflammatory cells. The cells were CD3 and CD68 positive (Fig. 4C and D).
Discussion
Peters’ anomaly accounts for the highest type of abnormality in the so-called anterior segment dysgenesis (ASD).1 The incidence is estimated to be 44–60 cases per year in the USA.2
Peters’ was first reported in the year 1906 in patients who presented with central corneal opacity and iridocorneal adhesions. One of the largest retrospective series on ASD-related congenital corneal opacities was conducted in Japan and clearly demonstrated that Peters’ anomaly was the commonest clinical diagnosis.3 In that study involving 220 eyes of 139 patients, Peters’ anomaly was found in about 73% of the eyes.3 It is now considered to be a genetic disease, known to be sporadic but can be sometimes inherited.4 The inheritance pattern can be either autosomal dominant or recessive.5
The commonest ocular associations are glaucoma 20%, microphthalmos 18% and coloboma in 6%.6 Peters’ anomaly and persistent hyperplastic primary vitreous (PHPV) have been usually found as an isolated ocular disease and have been reported as an uncommon clinical complex with poorly explained developmental mechanism that can link the pathogenesis embryologically.7 Peters’ anomaly is divided into 2 types: type I shows the typical central corneal opacity and the iridocorneal adhesions with or without lens changes and type II, which shows cataract or lenticular adhesions. The later type can be related to faulty separation of the lens vesicle from the surface ectoderm. Other advocated developmental mechanisms for Peters’ anomaly include abnormal migration of neural crest cells and intrauterine corneal inflammation.8, 9 Peters’ Plus Syndrome (PPS) on the other hand demonstrates the mentioned anterior chamber defect and other systemic abnormalities.
Histopathologically, the characteristic findings are mainly the central deficiency of the posterior stroma; Descemet’s membrane and the endothelium giving rise to the central corneal opacity seen clinically.1, 7, 10 The lenticular changes can be also observed as well as the iridocorneal adhesions such as demonstrated in Fig. 1D.10 Changes in Bowman’s layer have been also described as being absent (as demonstrated in Fig. 1A) or thickened and hyperplastic.10, 11, 12
Nischal and his coauthors have studied 22 eyes in 13 patients with ultrasound bio microscopy (UBM) as an aid for proper clinical diagnosis, which was further confirmed histopathologically.10 The commonest diagnosis in their series was Peters’ anomaly in 9 cases (70%). 2 of their patients with final histological diagnosis of Peters’ anomaly had bilateral corneal involvement, were misdiagnosed clinically as corneal ectasia and sclerocornea. The UBM in these cases was found to be consistent with Peters’ anomaly.10
One of the cases reported by Nischal (case 5 in his series) had a very similar histopathological appearance to our 5 corneas with a prominent central defect in the posterior cornea or the central concavity but they did not use the terminology: “keratoconus posticus circumscriptus” or (posterior keratoconus). Case 1 in our table has been diagnosed histopathologically (in both eyes) as posterior keratoconus, however this concavity was noted as an intrastromal cyst and has been reported.13 The thickened Descemet’s membrane has been recently reported in association with Peters’ anomaly and described as a multi-layered structure.1 We had previously reported one of our cases (case 3) of corneal myxoma in association with Peters’ anomaly.14 This has been also described in relation to birth injuries and as a primary myxoma in Down’s syndrome with keratoconus.15, 16, 17
Our last case (Case 6) with the clinical impression of glaucoma showed typical findings of Peters’ anomaly with an absent Bowman’s layer, central stromal defect and absence of Descemet’s membrane.18 However, Descemet’s membrane at both ends was thickened and multilaminated. This case was unusual as the patient presented with bilateral congenital glaucoma but unilateral right eye corneal opacity, which was found later to be consistent with Peters’ anomaly. The presence of stromal chronic inflammation in that case might be due to the previous surgeries especially with the defect in Descemet’s membrane centrally where inflammatory cells might have infiltrated the posterior stroma, therefore the diagnosis of Von Hippel variant in our case was not certain. Stromal keratitis -as a congenital finding- has been originally described by Von Hippel in 1897 then reported in the English-written literature by Jepson in 1963. In his case the corneal tissue showed a wedge-shaped keratitis starting from the limbus and involving the central cornea. In his case, there was an associated PHPV and microphthalmos.9 However, our case remains unique because of the unilaterality in the occurrence of the ASD. Unilateral Peters’ anomaly is often reported, sometimes with other types of congenital abnormalities in the other eye.19, 20 In the study of the ASD in Japan, they had 160 eyes in 109 patients with Peters’ anomaly, out of which bilateral involvement was evident in almost half the patients while 30 patients only had normal fellow eye such as our last case.3 The treatment in such cases can be challenging.
Conclusion
In conclusion, Peters’ anomaly has diverse clinical features and histopathological variants as part of faulty development in the anterior segment development. In our case series of 6 corneas, we have encountered posterior concavity in 5, thick multilaminated Descemet’s membrane at the peripheral ends of the cornea in 2, associated myxoma in 2 corneas (one of which has been previously reported by one of the authors) and finally a single case with unilateral Peters’ anomaly and stromal keratitis, which is similar in appearance to the rare Von Hippel entity. Further histopathological multi centric studies in our region are needed to better identify the wide variations in Peters’ anomaly, the associated glaucoma, the genetic background and the prevalence of unilateral cases in our population.
Acknowledgments
Acknowledgement
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declared that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Ni W., Wang W., Hong J., Zhang P., Liu C. A novel histopathologic finding in the Descemet’s membrane of a patient with Peters’ Anomaly: a case report and literature review. BMC Ophthalmol. 2015;15:139. doi: 10.1186/s12886-015-0131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurilec J.M., Zaidman G.W. Incidence of Peters’ anomaly and congenital corneal opacities interfering with vision in the United States. Cornea. 2014;33:848–850. doi: 10.1097/ICO.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 3.Shigeyasu C., Yamada M., Mizuno Y., Yokoi T., Nishina S., Azuma N. Clinical features of anterior segment dysgenesis associated with congenital corneal opacities. Cornea. 2012 Mar;31(3):293–298. doi: 10.1097/ICO.0b013e31820cd2ab. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari R., Ferri S., Whittaker B., Liu M., Lazzaro D.R. Peters’ anomaly: review of the literature. Cornea. 2011;30:939–944. doi: 10.1097/ICO.0b013e31820156a9. [DOI] [PubMed] [Google Scholar]
- 5.Iseri S.U., Osborne R.J., Farrall M., Wyatt A.W., Mirza G., Nürnberg G. Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat. 2009;30:1378–1386. doi: 10.1002/humu.21079. [DOI] [PubMed] [Google Scholar]
- 6.Najjar D.M., Christiansen S.P., Bothun E.D., Summers C.G. Strabismus and amblyopia in bilateral Peters’ anomaly. JAAPOS. 2006;10:193–197. doi: 10.1016/j.jaapos.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara A., Ozeki H., Matsunaga N., Nozaki M., Ashikari M., Shirai S. Histopathological examination of two cases of anterior staphyloma associated with Peters’ anomaly and persistent hyperplastic primary vitreous. Br J Ophthalmol. 2001;85:1421–1425. doi: 10.1136/bjo.85.12.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traboulsi E.I., Maumenee I.H. Peters’ anomaly and associated congenital malformations. Arch Ophthalmol. 1992;110:1739–1742. doi: 10.1001/archopht.1992.01080240079035. [DOI] [PubMed] [Google Scholar]
- 9.Jepson C.N. The von Hippel anomaly of the cornea associated with hyperplasia of the primary vitreous. Surv Ophthalmol. 1963;8:207–209. [PubMed] [Google Scholar]
- 10.Nischal K.K., Naor J., Jay V., MacKeen L.D., Rootman D.S. Clinicopathological correlation of congenital corneal opacification using ultrasound biomicroscopy. Br J Ophthalmol. 2002;86:62–69. doi: 10.1136/bjo.86.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend W.M., Font R.L., Zimmerman L.E. Congenital corneal leukomas.2. Histopathologic findings in 19 eyes with central defect in Descemet’s membrane. Am J Ophthalmol. 1974;77:192–206. [PubMed] [Google Scholar]
- 12.Stone D.L., Kenyon K.R., Green W.R., Rayan S.J. Congenital central corneal leukoma (Peters’ anomaly) Am J Ophthalmol. 1976;81:173–193. doi: 10.1016/0002-9394(76)90729-7. [DOI] [PubMed] [Google Scholar]
- 13.Khan A.O., Alkatan H., Al-Gehedan S., Al-Rashed W. Bilateral congenital stromal cyst of the cornea. JAAPOS. 2007;11(4):400–401. doi: 10.1016/j.jaapos.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.O., Al-katan H., Al-Gehedan S. Infantile corneal myxoma. JAAPOS. 2008;12(2):207–209. doi: 10.1016/j.jaapos.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Robinson J.W., Brownstein S., Mintsioulis G. Corneal myxoma arising in a patient with repeated phototherapeutic keratectomies. Cornea. 2006;25(9):1111–1114. doi: 10.1097/01.ico.0000225710.54888.a1. [DOI] [PubMed] [Google Scholar]
- 16.Alkatan H., Al-Arfaj K. Myxoma of the cornea. Ann Saudi Med. 2008;28(4):297–299. doi: 10.5144/0256-4947.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leger F., Sawan B., Mortemousque B., Williamson W., Vital C. Corneal myxoma associated with keratoconus and Down’s syndrome. Cornea. 2000;19(4):561–563. doi: 10.1097/00003226-200007000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Al Shamrani M., Al Hati K., Alkatan H., Alharby M., Jastaneiah S., Song J. Pathological and immunohistochemical alterations of the cornea in congenital corneal opacification secondary to primary congenital glaucoma and peters anomaly. Cornea. 2016 Feb;35(2):226–233. doi: 10.1097/ICO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 19.Cho D., Choi D., Nam W. Unilateral Peters’ anomaly with chorioretinal coloboma in the other eye. Korean J Ophthalmol. 2011;25(5):352–354. doi: 10.3341/kjo.2011.25.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basdekidou C1., Dureau P., Edelson C., De Laage De Meux P., Caputo G. Should unilateral congenital corneal opacities in Peters' anomaly be grafted? Eur J Ophthalmol. 2011;21(6):695–699. doi: 10.5301/EJO.2011.6317. [DOI] [PubMed] [Google Scholar]