Abstract
Histiocytic Sarcoma is a rare malignant hematopoietic neoplasm that can present in extranodal sites including lymph nodes, skin, gastrointestinal tract, and the central nervous system. Only 10% of cases manifest as skin lesions and very few are reported in the head and neck. The authors report a case of histiocytic sarcoma of the eyelid in a 72-year-old male that was clinically diagnosed as a chalazion. Initial excision was not sent for routine histopathological assessment and the patient was subsequently lost to follow up. Recurrence occurred at the eyelid site and additional lesions were found on the forearms, abdomen, and right knee. Histopathological assessment of one of these other sites confirmed the diagnosis of histiocytic sarcoma. To our knowledge, this is the first reported case of disseminated histiocytic sarcoma that originally presented in the ocular adnexa (eyelid). And, as the initial lesion was not sent to Pathology and therefore potentially missed, this case highlights the importance of submitting tissue, including chalazia, for pathologic evaluation.
Keywords: Histiocytic sarcoma, Chalazion, Immunohistochemistry, Diagnosis
Introduction
Histiocytoses are rare disorders characterized by the accumulation of cells thought to be derived from macrophages or dendritic cells and known for protean manifestations in the ocular adnexa, orbit, and even within the eye. More than 100 subtypes of histiocytoses have been described with a wide range of clinical and histologic presentations, proving very difficult to diagnose for clinicians and pathologists.1 In this report, we discuss a patient with histiocytic sarcoma (HS) of the upper eyelid that was previously diagnosed and treated as a chalazion.
Case report
A 72-year-old male patient was referred to the Oculoplastics service for a right upper eyelid lesion. The patient’s ocular history was significant for a previous excision of a “chalazion” at the same site one year prior. The patient was subsequently lost to follow up. Histopathology was not performed on the original excised tissue.
At the time of presentation, the patient described the lesion as intermittently painful with recurrent bleeding cycles and an increase in size over the past year. On examination, there was a 5 × 15 × 17 mm firm and mildly tender nodule of the right upper eyelid. The remainder of the ocular examination was unremarkable. An incisional biopsy was performed. Histopathology showed a complete effacement of the tissue by diffuse sheets of discohesive, atypical histiocytic-appearing cells within fibrous connective tissue and infiltration in a small portion of skeletal muscle (Fig. 1). Mitotic figures were numerous and perineural invasion and necrosis were prominent features (Fig. 1). An extensive panel of immunostains revealed the following phenotype for the cellular population: CD68, muramidase, CD4 and CD33 positive; Ki-67 positive in approximately 20% of cell; and negativity for CD3, CD8, CD20, CD21, CD34, S100 and pan-cytokeratin (Fig. 2). This phenotype is consistent with a histiocytic lineage. The increased Ki-67 is indicative of neoplastic process.
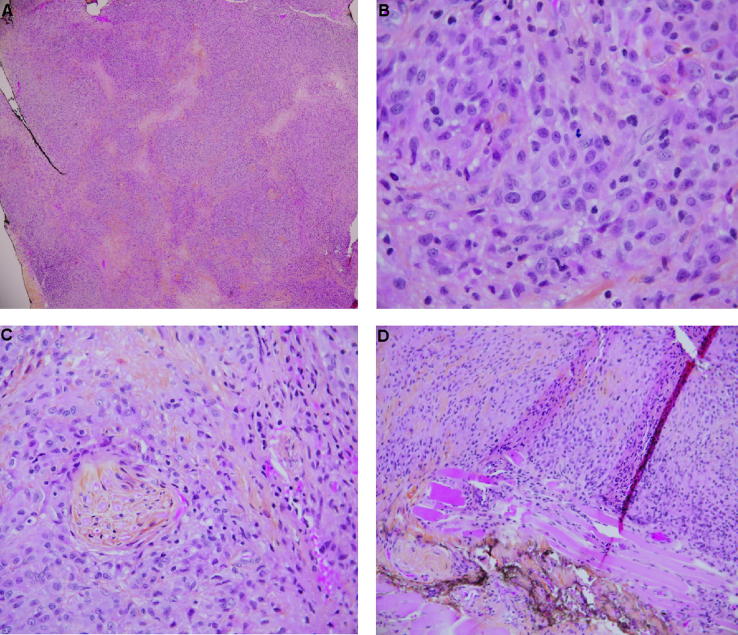
Fig. 1.
Right upper eyelid excisional biopsy stained with hematoxylin-phloxine and saffron. (A) Low powered magnification (original magnification 10×) image illustrating a predominant homogenous population of pale amphophilic cells with interspersed fibrous connective tissue and areas of pale necrosis consistent with tissue destruction. Complete effacement of the normal eyelid tissue is evident, features not-suggestive of a chalazion. (B) High powered magnification (original magnification 40×) image illustrating the homogenous population of the eyelid lesion consisting of discohesive, large nuclear cells with ample eosinophilic cytoplasmic and very prominent nucleoli. Nuclear atypica is evident throughout the image. Mitotic figures and nuclear groves can be seen. Lipogranulomas were absent. (C) Higher powered magnification (original magnification 20×) image of the eyelid lesion illustrating perineural invasion. (D) Low powered magnification (original magnification 10×) image of the eyelid lesion illustrating skeletal muscle invasion.
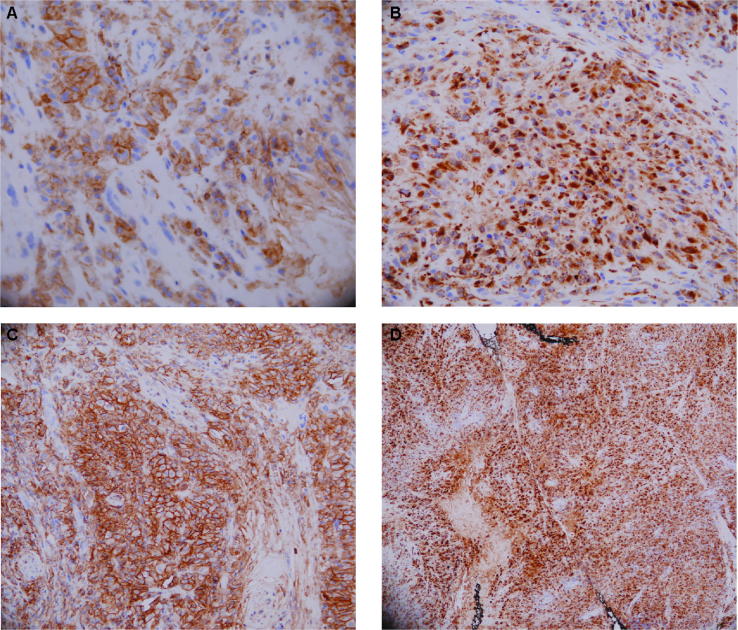
Fig. 2.
Immunophenotyping of the right upper eyelid lesion. Immunohistochemical staining of the large cell neoplastic lesion using antibodies against (A) CD4, (B) Muramidase, (C) CD33 and (D) CD68. (A) Image illustrating the CD4 positive nature of the cellular population. (B) Image illustrating the muramidase positive phenotype of the cellular population. (C) Image illustrating the CD33 positive phenotype of the cellular population. (D) Image highlighting the CD68 positive nature a representative section of the eyelid lesion.
The patient was referred to oncology for further assessment and treatment. Physical examination at this time showed small nodules of the right upper eyelid and violaceous nodules on the right and left forearm, abdomen and right knee. Biopsy confirmed an identical histopathology and immunophenotype as the eyelid. During his work-up, the patient was also discovered to have a secondary squamous cell carcinoma of the hypopharynx. Treatment focused on the hypopharyngeal carcinoma and the patient underwent a total laryngectomy, bilateral neck dissection and right subtotal thyroidectomy. He was subsequently referred for adjuvant radiation.
Discussion
Histiocytic sarcoma (HS) is a rare malignant neoplasm of lymphoid and hematopoietic tissue characterized by the World Health Organization in 2008 as a malignant proliferation of cells with morphological and phenotypical features of mature tissue histiocytes.2 Exceedingly rare, HS represents less than 1% of all non-Hodgkin’s lymphomas. It commonly presents in lymph nodes, however is also found in the gastrointestinal tract, spleen, soft tissue, central nervous system and skin.3 To date, there are no reports of a HS presenting in the ocular adnexa.
HS can present in all ages, with a bimodal distribution in ages 0–29 years and 50–69 years. It often presents with non-specific clinical findings, and occurs typically with (less commonly without) constitutional symptoms such as fever, fatigue, weight loss, night sweats, or weakness.4 Due to the wide range of presentations in a variety of extranodal sites, HS is notoriously difficult to diagnose. It is a diagnosis of exclusion with broad differential diagnoses including other histiocytic-dendritic cell disorders such as reactive histiocytic proliferations and dendritic cell neoplasms; large cell non- Hodgkin lymphoma, especially anaplastic large cell lymphoma and diffuse large B-cell lymphoma; malignant melanoma; undifferentiated large cell carcinoma; and monocytic leukemia.4
The tumour consists of a discohesive population of large rounded to polygonal cells with histiocytic features: cytoplasm that varies from being abundant and eosinophilic to sometimes foamy or clear and nuclei that are large, round to oval, indented, grooved, convoluted or irregularly folded, and often eccentrically placed with atypia varying from mild to severe. Mitotic activity is consistently seen but can vary depending on the case. Necrosis is often a feature. There is often an accompanying inflammatory infiltrate of lymphocytes, plasma cells, neutrophils, benign histiocytes, and eosinophils.4
Immunohistochemistry is essential to the diagnosis; HS must have expression of one or more of the histiocytic markers CD163 (hemoglobin scavenger receptor, the most specific immuno-marker for histiocytes), CD68 (less specific), and lysozyme (or muramidase, of weakest specificity), and absence of immuno-markers for B- and T- lymphocytes, Langerhans cells (CD1a, langerin/CD207), follicular dendritic cells (CD21, CD23, CD35), epithelial cells (pancytokeratin, EMA), melanocytes (HMB 45, Melan A), and myeloid cells (CD 13, myeloperoxidase, CD 33). Some tumours can express a variety of markers that stain histiocytes such as CD4, CD43, CD45, among others; some can be positive for S100 but are usually patchy and weak.4 However, as none of the above immuno-markers for histiocytes (CD163, CD68, and lysozyme) are 100% specific, the utilization of a panel of markers is recommended.
The management of HS consists primarily of wide surgical excision and/or chemotherapy and/or radiotherapy. Chemotherapeutic regimes include cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and prednisone, methotrexate, doxorubicine, cyclophosphamide, etosposide, mechlorethamine, vincristine, and procarbazine (Pro-MACE-CHOP).5 Most cases of HS present at an advanced clinical stage with limited response to chemotherapy and high mortality; most patients die of progressive disease within two years. However, there is a small subset of patients with localized disease that can have a favourable long-term outcome.5
We report the first case of HS of the ocular adnexa that presented as a recurrent chalazion-like lesion on the eyelid. The location of the HS delayed the original diagnosis given the common presentation of chalazions for excision. When biopsied one year after the initial excision, dissemination was noted to the forearms, abdomen, and right knee and a secondary right hypopharyngeal squamous cell carcinoma was diagnosed.
HS is notoriously difficult to diagnose for ophthalmologists and pathologists alike, due to its broad differential diagnoses. HS is associated with poor prognoses despite appropriate treatment. Our case illustrates the unique presentation of HS to the eyelid and the importance of considering HS in the differential diagnosis of a recurrent chalazion. This case also highlights the importance of submitting chalazion specimens for histopathologic evaluation, which in this case, would have likely led to an earlier diagnosis and possibly prevented dissemination of the disease.
Conflict of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors would like to acknowledge the assistance of Drs. D. Lebrun and D. Good from the division of Hematopathology, Queen’s University of Pathology and Molecular Medicine in the diagnosis of this case.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Emile J.-F., Abla O., Fraitag S. Revised classification of histiocytoses and the macrophage – dendritic cell lineages. Blood. 2016;127(22):2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos J.A., Abbondanzo S.L., Barkman C.L. Histiocytic sarcoma: a study of five cases including the histiocytic marker CD163. Mod Pathol. 2005;18:693–704. doi: 10.1038/modpathol.3800346. [DOI] [PubMed] [Google Scholar]
- 3.Clifton W., Akinduro O., Lopez-Chiriboga S. Infection or glioma? The false dilemma of primary central nervous system histiocytic sarcoma. World Neurosurg. 2017 doi: 10.1016/j.wneu.2017.07.001. Jul 12 S1878-8750 (17)3001-2. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi E., Nakamura S. Histiocytic sarcoma: An updated literature review based on the 2008 WHO classification. J Clin Exp Hematop. 2013;53(1):1–8. doi: 10.3960/jslrt.53.1. [DOI] [PubMed] [Google Scholar]
- 5.Hornick J.L., Jaffe E., Fletcher C.D.M. Extranodal histiocytic sarcoma Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J. Surg Pathol. 2004;28:1133–1144. doi: 10.1097/01.pas.0000131541.95394.23. [DOI] [PubMed] [Google Scholar]