Abstract
Trochlear dysplasia is the most commonly encountered pathoanatomy in patients who present with patellar instability. Outcomes of trochleoplasty procedures have shown low rates of recurrent instability and high patient-reported outcome scores. This article describes a “thin-flap” groove-deepening trochleoplasty combined with medial patellofemoral ligament reconstruction with a gracilis allograft and lateral retinacular lengthening to treat recurrent patellar instability due to high-grade trochlear dysplasia. This technique can obviate tibial tubercle osteotomy by normalizing the position of the trochlear groove and, subsequently, decreasing the tibial tubercle–to–trochlear groove distance.
Patellar instability (PI) is a complex problem that can cause significant disability and is a risk factor for osteoarthritis.1 Several risk factors for PI have been identified, including age, activity level, female sex, trochlear dysplasia (TD), patella alta, genu valgum, femoral anteversion, and external tibial torsion.2, 3, 4 Of these risk factors, TD is the most commonly encountered pathoanatomy. DeJour et al.5 reported that up to 90% of patients who underwent surgical stabilization for PI had radiographic signs of TD. Several “groove-deepening” trochleoplasty procedures have been developed that have yielded consistent results with low rates of recurrent instability and high patient-reported outcome scores.6, 7, 8
This article describes a “thin-flap” groove-deepening trochleoplasty combined with medial patellofemoral ligament (MPFL) reconstruction with a gracilis allograft and lateral retinacular lengthening to treat recurrent PI due to high-grade TD. We also detail how this procedure can normalize an abnormal tibial tubercle–to–trochlear groove (TT-TG) distance without the need for a tibial tubercle osteotomy (TTO).
Diagnostic Evaluation and Surgical Indications for Trochleoplasty
Physical examination involves a comprehensive evaluation of the lower extremity to include assessment of coronal-plane alignment, the rotational profile, and ligamentous sufficiency. Directed evaluation of the patellofemoral joint includes an assessment of the apprehension sign and lateral translation of the patella up to 90° of knee flexion. If the patient has apprehension and/or continued lateral translation beyond 45° to 60° of knee flexion, high-grade dysplasia is suspected. The J sign is evaluated with the patient sitting and performing an active knee extension. If the patella begins to translate laterally prior to the last 30° of terminal knee extension, this is consistent with a flat or convex trochlea either pushing or allowing the patella to track laterally.
An imaging workup including anteroposterior, 45° posteroanterior notch, lateral, and 15° to 20° axial Merchant radiographs is performed. A lateral view allows assessment of the DeJour classification.9 An axial assessment of dysplasia is made from axial magnetic resonance imaging cuts. From the axial magnetic resonance imaging cuts, the lateral trochlear inclination angle is measured. A measurement of 0° or a negative measurement on the first slice showing articular cartilage confirms a flat or convex trochlea that is indicated for a groove-deepening trochleoplasty.
Surgical Technique
The patient receives general anesthesia in the supine position, and a thigh-high tourniquet is placed. The affected extremity is prepared and draped in the usual sterile fashion. Diagnostic arthroscopy is not routinely performed unless there is a known loose body or if the health of the patellofemoral cartilage is in question.
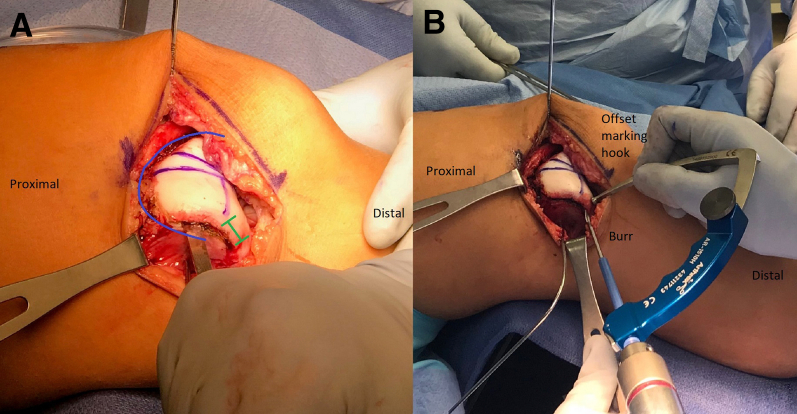
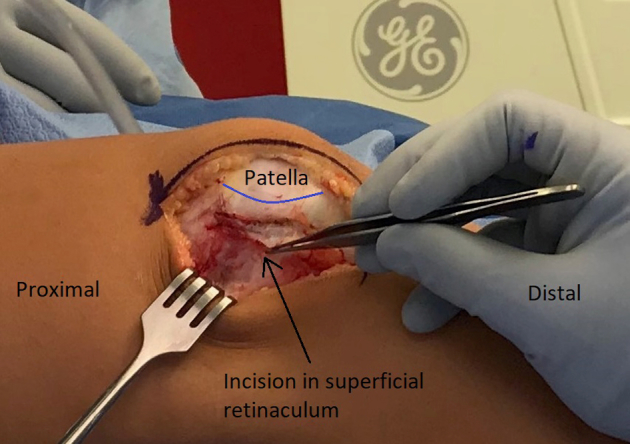
The leg is exsanguinated with an Esmarch bandage, and the tourniquet is inflated. The leg is placed over a tibial triangle to keep the knee flexed approximately 45°. An 8- to 10-cm incision is made on the lateral border of the patella, and dissection is carried down to the lateral retinaculum (Fig 1). The retinaculum is opened in a Z fashion with the superficial layer incised off the patella for a distance of 2 to 3 cm posteriorly. The deep layer is incised along with the synovium to expose the joint (Fig 2). The lateral patellotibial ligament and remaining lateral structures are released down to the level of the tibial tubercle directly off the lateral edge of the patellar tendon. The quadriceps tendon is split proximally until the patella is easily dislocated medially (Fig 3). The ligamentum mucosum and the lateral fat pad are also routinely cut to aid in exposure. A 2- or 2.4-mm K-wire is placed in the medial distal femur to hold the patella medially. The trochlear morphology is assessed visually and via palpation. The patient's native trochlear groove is highlighted with a surgical marking pen (Video 1). By use of the roof of the intercondylar notch as a reference, the planned new groove is marked to end just lateral to the roof of the notch. The distance between the planned new groove and the proximal aspect of the native groove is measured (Fig 4). This distance represents how much the trochlear groove will be lateralized and how much the TT-TG will be decreased. The distal transition between the normal and dysplastic aspects of the trochlea is determined next. At this junction, a third mark is made that denotes the distal aspect of the new groove (Fig 4).
Fig 1.
The patient is supine for the procedure. The lateral aspect of a right leg, positioned in approximately 45° of flexion on a tibial triangle, is shown. An 8- to 10-cm incision is made on the lateral border of the patella (blue line), and dissection is carried down to the lateral retinaculum. The superficial layer of fibers is incised.
Fig 2.
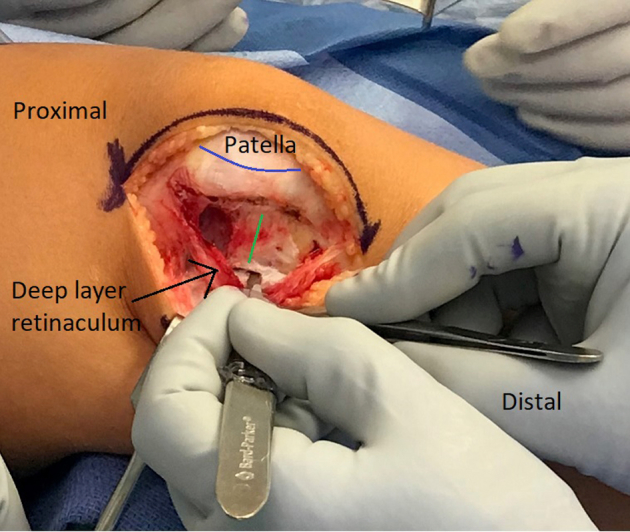
Right leg. The retinaculum is opened in a Z fashion with the superficial layer incised off the patella (blue line), and the deep layer is incised along with the synovium to expose the joint. The distance between the incision of the superficial and deep layers is marked (green line).
Fig 3.
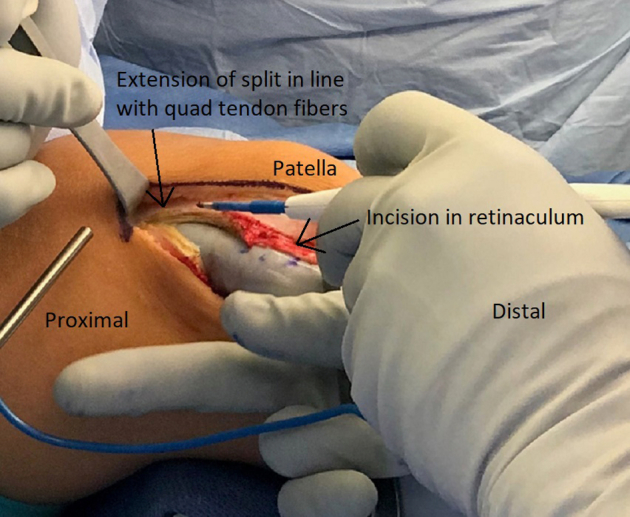
Right leg. The quadriceps tendon is split proximally until the patella is able to be easily dislocated medially. The split is an extension of the incision in the retinaculum and should ideally be in line with the quadriceps (quad) tendon fibers.
Fig 4.
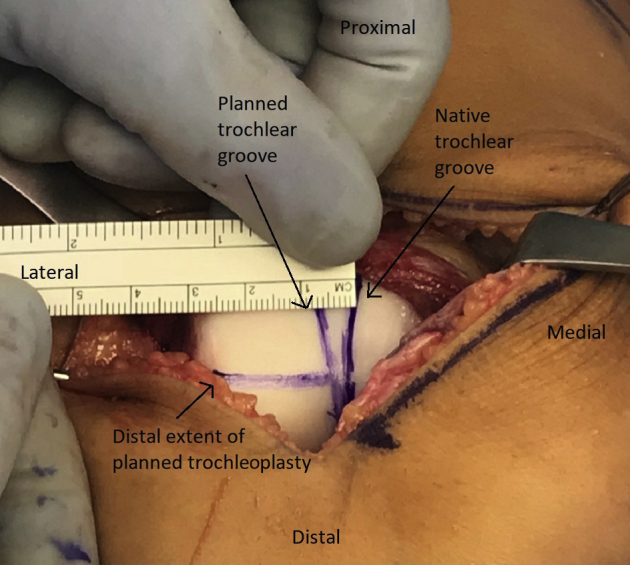
Right leg. The patient's native trochlear groove (medial vertical marker line) is highlighted with a surgical marking pen. By use of the roof of the intercondylar notch as a reference, the planned new groove (lateral vertical marker line) is marked to end just lateral to the roof of the notch. The distance between the planned new groove and the proximal aspect of the native groove is measured. The distal transition between the normal and dysplastic aspects of the trochlea is determined as well, and a third mark is made that denotes the distal aspect of the new groove (horizontal marker line).
Prior to flap elevation, a mark is made approximately 5 mm below the chondral surface on the lateral trochlea to denote where the osteochondral flap will be elevated. An osteotome is used at this level to penetrate the subchondral bone 2-3 mm. This initial cut is started approximately 1 to 1.5 cm distal to the location of the distal aspect of the planned new groove and is continued all the way around to the distal aspect of the medial trochlea (Fig 5A). Curved and straight osteotomes are used interchangeably to elevate the osteochondral flap. Next, a specialized offset marking hook and burr (Arthrex, Naples, FL) are loaded on an anterior cruciate ligament guide (Arthrex). The burr is advanced into the subchondral bone and is used in a pivoting motion with the tip of the burr and marking hook held stationary while the hand holding the drill moves back and forth to resect bone (Fig 5B, Video 1). The burr and osteotomes are used interchangeably to mobilize the osteochondral flap. The flap is then elevated to expose the subchondral bone of the trochlea.
Fig 5.
Right leg. (A) To elevated the cartilage flap, an osteotome is used approximately 5 mm below the chondral surface on the lateral trochlea to penetrate the subchondral bone approximately 5 to 10 mm. This initial cut is started approximately 1 to 1.5 cm distal (green line, with hash ends marking this distance) to the location of the distal aspect of the planned new groove and is continued all the way around to the distal aspect of the medial trochlea (blue line). (B) A specialized offset marking hook and burr (Arthrex) are loaded on an anterior cruciate ligament guide (Arthrex). The burr is advanced into the subchondral bone and is used in a pivoting motion with the tip of the burr and marking hook held stationary while the hand holding the drill moves back and forth to resect bone.
Prior to bone removal, a narrow, straight osteotome is passed from proximal to distal in the subchondral bone underneath the flap where the base of the new trochlear groove is planned. Next, with care taken to preserve the height of the lateral condyle, an osteotome is used to remove wedges of bone. In general, 8 to 10 mm of bone is removed to re-create a lateral trochlear inclination angle between 15° and 20° and a trochlear depth between 5 and 10 mm. The process is repeated on the medial side, and the groove is contoured to create a smooth transition distally. Any extra-articular spur or prominence is removed with a curved osteotome such that the proximal trochlea is flush with the anterior cortex of the femur. The burr used to detach the osteochondral flap can be used to smooth any irregularities or rough edges in the subchondral bone. Once an appropriate groove has been created, the osteochondral flap is molded into position manually. If the flap is not easily moldable, the burr can be used to remove excess bone off the undersurface of the flap until it is moldable.
Fixation of the flap is achieved using 3.5-mm PushLock knotless suture anchors (Arthrex) and 3 strands of No. 1 Vicryl (Ethicon, Somerville, NJ). The Vicryl sutures are cut in half to produce 6 strands of suture. These sutures are loaded through the eyelet of the suture anchor and evened out so that the midpoint of the sutures is through the eyelet with equal lengths of suture on either side, thus creating 12 effective free ends of suture. The first anchor is placed in the distal aspect of the new groove. Next, 4 strands of suture are placed through a second anchor, and this is placed at the proximal aspect of the new groove. Manual reduction of the flap is performed during anchor placement to minimize the chance of suture cut-through and to maximize the depth of the new groove. If the flap is long, an anchor can be placed halfway up the new groove. Four strands of suture are similarly placed with suture anchors proximally both medially and laterally to secure the medial and lateral facets of the trochlea (Fig 6). The K-wire holding the patella medially is removed, and the patella is located on the new trochlear groove.
Fig 6.
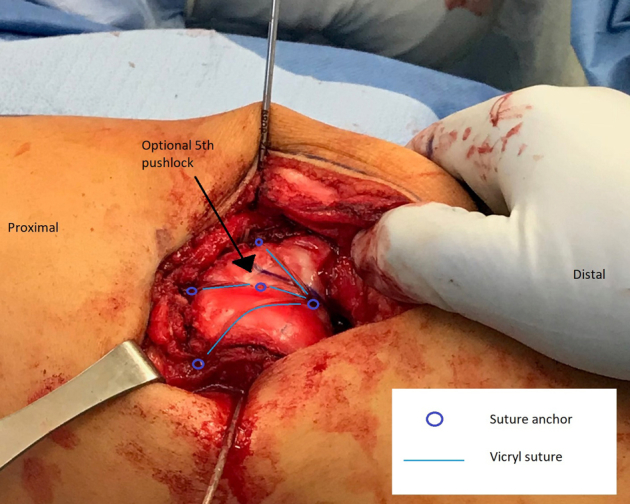
Right leg. After the cartilage flap is adequately prepared, it is secured in place in the new trochlear groove distally, at the midpoint of the flap centrally, and proximally with medial, central, and lateral anchors (blue circles). The Vicryl suture (blue lines) between anchors compresses the cartilage flap into place.
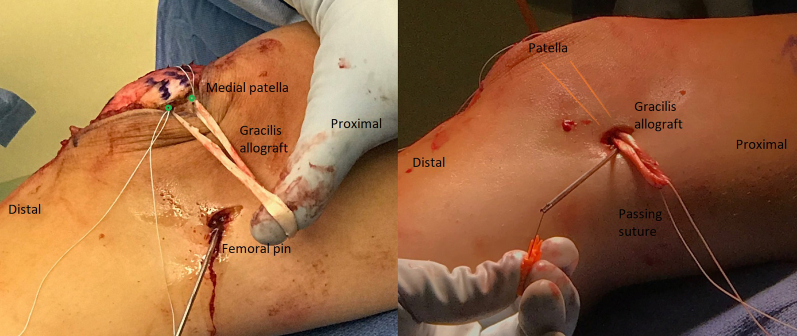
MPFL reconstruction is commenced by dissecting through the lateral incision over the anterior surface to the medial border of the patella. Guide pins are placed in the upper half of the patella. Fluoroscopy is used to confirm appropriate pin placement in the patella as well as appropriate Beath pin placement in the femur (Fig 7). Femoral tunnel placement is performed as described by Schöttle et al.10 through a 2-cm incision on the medial femur. The Beath pin is advanced through the lateral femoral cortex and out the skin of the lateral thigh. Patellar sockets are drilled to accommodate 3.5-mm SwiveLock suture anchors (Arthrex), and the femoral tunnel is drilled with a 7-mm reamer. The femoral tunnel is typically drilled 40 to 50 mm to ensure that all the graft will fit in the tunnel. The layer between the medial retinacular structures and the capsule is dissected from the patella to the femoral tunnel, and a passing suture is placed. A nitinol wire is placed in the femoral tunnel alongside the Beath pin.
Fig 7.
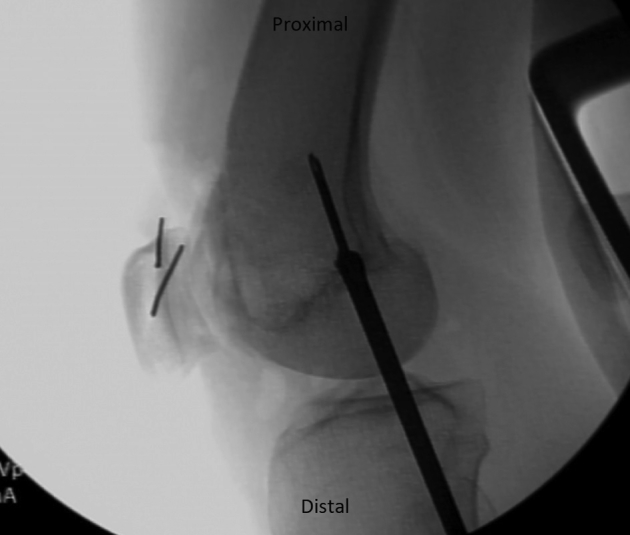
Right leg. Lateral fluoroscopy is used during medial patellofemoral ligament reconstruction. Confirmation of pin placement both in the patella and in the femur at the Schöttle point is shown. A reamer can be placed at the medial femoral cortex to confirm the location of the aperture along the length of the pin.
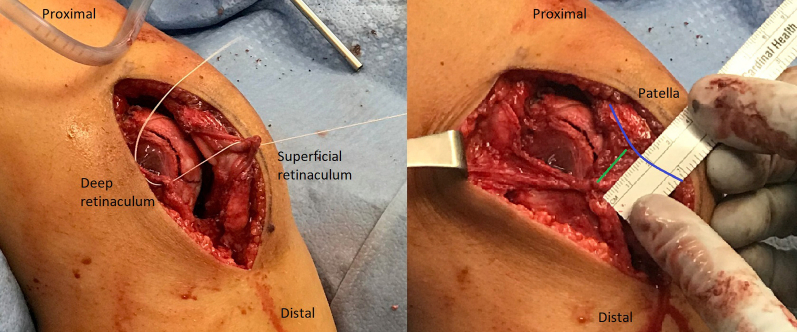
A gracilis allograft, reinforced on each free end with No. 2-0 FiberWire (Arthrex), is first placed in the patella and then shuttled down to the femur with the preplaced passing suture, loaded onto the eyelet of the Beath pin, and placed into the femur (Fig 8). With the knee maintained in 45° of flexion, a 6 × 23–mm BioComposite interference screw (Arthrex) is placed in the femoral tunnel. After screw placement, the patella is checked to ensure that 1 quadrant of lateral translation is present without medial tilt and that the knee can be fully flexed. If placement is not satisfactory, the screw is removed and replaced until appropriate patellar stability is achieved. The wound and joint are washed with saline solution, and the knee is flexed 70°. The lateral retinaculum is closed by suturing the superficial layer of the retinaculum to the deep layer at whatever point the superficial layer comfortably lies. This completes the lateral retinacular lengthening. We have observed that 1 to 2 cm of length is typically achieved (Fig 9).
Fig 8.
Right leg. For medial patellofemoral ligament reconstruction, a gracilis allograft is reinforced on each free end with No. 2-0 FiberWire. It is first placed in the patella (A) and then shuttled down to the femur with a preplaced passing suture (B, the gracilis allograft is deep to the skin and its position is marked with orange lines). A passing suture is loaded onto the eyelet of the Beath pin, and the graft is placed into the femur for final fixation (not shown).
Fig 9.
Right leg. (A) The lateral retinaculum is closed by suturing the superficial layer of the retinaculum to the deep layer at whatever point the superficial layer comfortably lies. (B) Typically, 1 to 2 cm of length is achieved (distance marked by green line), as illustrated. Blue line indicates the patella.
The quadriceps tendon is closed with No. 0 Vicryl suture, and the wound is closed in layered fashion. A sterile dressing is applied.
Postoperative Management
The patient is discharged home as an outpatient. He or she is encouraged to bear weight as tolerated and range the knee as tolerated right away. Physical therapy is commenced within a week of surgery to re-establish motion and work on early muscle activation and swelling reduction. A hinged knee brace is optional and should not be worn for more than 2 to 3 weeks, and crutches are used until gait normalizes. If rehabilitation proceeds appropriately, the patient may begin to return to impact activities at 3 months after surgery. Full clearance for sports typically occurs between 4 and 6 months after surgery. Table 1 describes pearls and pitfalls of the described technique, and Table 2 lists advantages and disadvantages.
Table 1.
Pearls and Pitfalls of Trochleoplasty, MPFL Reconstruction, and Open Lateral Lengthening for Patellar Instability in Setting of High-Grade Trochlear Dysplasia
| Pearls |
| The surgeon should ensure adequate exposure to ensure lateral and medial exposure of the patella prior to lateral arthrotomy; the soft-tissue planes should be undermined to create an adequate mobile window. This allows medial patellar access for graft placement for MPFL reconstruction. |
| The surgeon should plan to undermine the cartilage flap 1.0-1.5 cm distal to the new planned trochlear groove. This optimizes the contour of the trochlea from distal to proximal to prevent any acute angles. |
| Fluoroscopy should be used to ensure proper placement of the MPFL reconstruction tunnels in the femur and patella. |
| The MPFL reconstruction femoral tunnel should be drilled to an adequate length (40-50 mm) to avoid graft-tunnel mismatch. |
| The surgeon should fix the MPFL reconstruction at 45° of flexion and check range of motion and patellar stability after fixation. |
| Pitfalls |
| Excision of too much lateral-column bone during flap creation can compromise trochlear stability—removed bone should be saved because it can be used to build up the lateral column if needed. |
| Molding the cartilage flap can be difficult if it is too thick—additional subchondral bone should be carefully removed with a burr. |
| Suture cut-through on the cartilage can occur if the flap is too thin or long—holding manual reduction of the flap during anchor insertion can help minimize this and occasionally an additional anchor at the midpoint of the flap can help mitigate the stress on the suture. |
| Over- and under-tensioning of the lateral retinaculum should be avoided by closing it where the superficial layer lies comfortably with the knee at 70° of flexion—typically, 1-2 cm of length is achieved. |
MPFL, medial patellofemoral ligament.
Table 2.
Advantages and Disadvantages of Trochleoplasty, MPFL Reconstruction, and Open Lateral Lengthening for Patellar Instability in Setting of High-Grade Trochlear Dysplasia
| Advantages |
| No split in the articular cartilage is required to create the new trochlear groove. |
| The entire procedure can be performed with a single lateral parapatellar incision (except the femoral pin for MPFL reconstruction). |
| The procedure uses absorbable suture and no hardware that requires routine removal. |
| Use of an offset burr minimizes the risk of creating a cartilage flap that is too thin. |
| Disadvantages |
| Preparation of the cartilage flap to achieve the proper thickness can be time-consuming and requires meticulous work—when too thick, it is difficult to mold, and when too thin, it may crack, buckle, or get cut through by suture. |
| The central distal push lock required violation of a small area of articular cartilage. |
| A large flap area is required to heal back to the metaphyseal bone. |
| There is a risk of patellar fracture with fixation of MPFL reconstruction. |
MPFL, medial patellofemoral ligament.
Discussion
Although historical reports of trochleoplasty procedures have been mixed, recent studies detailing the results of a groove-deepening trochleoplasty with or without an associated MPFL reconstruction have shown consistently good results with extremely low rates of repeated instability, high rates of patient satisfaction, and little to no evidence of progression to osteoarthritis.6, 8, 11, 12, 13, 14, 15 Whereas there are reports that have detailed good outcomes of isolated MPFL reconstruction in the setting of high-grade TD,16 a recent study by Hiemstra et al.17 reported that disease-specific outcomes of isolated soft-tissue procedures in the setting of high-grade TD were worse and rates of recurrent instability were higher than in patients with low-grade TD.
Although some of the aforementioned reports contained some patients in whom a TTO was performed to either medialize, anteriorize, and/or distalize the patella, most of these patients did not undergo distal realignment surgery. The lack of a routine need for a TTO in the setting of high-grade TD is supported by Fucentese et al.,18 who showed that a groove-deepening trochleoplasty reduced the TT-TG measurement, on average, by 6 mm. More recently, Brady et al.19 showed that as the degree of TD increased, so did the average TT-TG measurement, thus suggesting a correlation between them. Given the aforementioned findings and our experience, the trochlear groove is pathologically medialized in the setting of high-grade TD and a groove-deepening trochleoplasty will place the trochlear groove in a more appropriate, lateral position. This will lower the TT-TG measurement by decreasing the “TG” portion as opposed to the traditional manipulation of the “TT” portion. Thus, it is our preference to consider a TTO only in cases of extreme instability when excessive rotational pathology is present (e.g., a TT-TG measurement of >30 mm and a fixed patellar dislocation through a range of motion). Otherwise, a trochleoplasty not only will correct the underlying pathology but also can normalize the TT-TG measurement.
A few different types of groove-deepening trochleoplasty procedures have been described. The technique described in our article is a thin-flap trochleoplasty. Other authors have described a thick-flap trochleoplasty in which more subchondral bone is left on the undersurface of the trochlea.7, 8, 20 It is our preference to perform the thin-flap trochleoplasty for the following reasons: (1) a lateral approach can allow lateral retinacular lengthening and thus more complete soft-tissue rebalancing, (2) the scar from a lateral approach is smaller and is less likely to cause kneeling pain than a midline scar, and (3) a thin-flap trochleoplasty allows for more thorough molding of the cartilage. In contrast to other described techniques,21 our technique does not require a formal split in the articular cartilage while still allowing for precise cartilage molding.
In summary, the benefits of a thin-flap trochleoplasty procedure combined with MPFL reconstruction and lateral lengthening include correction of TD with consistently good results, with extremely low rates of repeated instability, high rates of patient satisfaction, and little to no evidence of progression to osteoarthritis.6, 8, 11, 12, 13, 14, 15 The procedure does not require any permanent hardware that routinely needs to be removed or is likely to lead to hardware-related complications. The risks and long-term effects of thin-flap trochleoplasty on chondrocytes and underlying subchondral bone are not fully known at this point, and further study should be performed to confirm that no deleterious effects occur. Future studies are also needed to determine any difference between thick- and thin-flap trochleoplasty procedures combined with MPFL reconstruction and lateral lengthening. However, the groove-deepening trochleoplasty without the need for a routine TTO is supported in the literature.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: J.L.P. is a consultant for Arthrex, Ceterix, and Grand Rounds. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Steps for performing open lateral lengthening, trochleoplasty, and medial patellofemoral ligament reconstruction in the setting of high-grade trochlear dysplasia. The procedure is performed on a right leg in the supine position through a lateral parapatellar incision. A thorough discussion of planning the new trochlear groove and how this corrects aspects of trochlear dysplasia pathoanatomy is highlighted. Pearls for a safe technique regarding osteochondral flap elevation are also emphasized.
References
- 1.Sanders T.L., Pareek A., Johnson N.R., Stuart M.J., Dahm D.L., Krych A.J. Patellofemoral arthritis after lateral patellar dislocation: A matched population-based analysis. Am J Sports Med. 2017;45:1012–1017. doi: 10.1177/0363546516680604. [DOI] [PubMed] [Google Scholar]
- 2.Sanders T.L., Pareek A., Hewett T.E., Stuart M.J., Dahm D.L., Krych A.J. High rate of recurrent patellar dislocation in skeletally immature patients: A long-term population-based study. Knee Surg Sports Traumatol Arthrosc. 2018;26:1037–1043. doi: 10.1007/s00167-017-4505-y. [DOI] [PubMed] [Google Scholar]
- 3.Lewallen L.W., McIntosh A.L., Dahm D.L. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41:575–581. doi: 10.1177/0363546512472873. [DOI] [PubMed] [Google Scholar]
- 4.Fithian D.C., Paxton E.W., Stone M.L. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32:1114–1121. doi: 10.1177/0363546503260788. [DOI] [PubMed] [Google Scholar]
- 5.Dejour H., Walch G., Nove-Josserand L., Guier C. Factors of patellar instability: An anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2:19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 6.Longo U.G., Vincenzo C., Mannering N. Trochleoplasty techniques provide good clinical results in patients with trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2018;26:2640–2658. doi: 10.1007/s00167-017-4584-9. [DOI] [PubMed] [Google Scholar]
- 7.Dean C.S., Chahla J., Serra Cruz R., Cram T.R., LaPrade R.F. Patellofemoral joint reconstruction for patellar instability: Medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5:e169–e175. doi: 10.1016/j.eats.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara I., Bua N., Smith T.O., Ali K., Donell S.T. Deepening trochleoplasty with a thick osteochondral flap for patellar instability: Clinical and functional outcomes at a mean 6-year follow-up. Am J Sports Med. 2015;43:2706–2713. doi: 10.1177/0363546515597679. [DOI] [PubMed] [Google Scholar]
- 9.Dejour D., Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15:39–46. doi: 10.1097/JSA.0b013e31803035ae. [DOI] [PubMed] [Google Scholar]
- 10.Schöttle P.B., Schmeling A., Rosenstiel N., Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 11.Testa E.A., Camathias C., Amsler F., Henle P., Friederich N.F., Hirschmann M.T. Surgical treatment of patellofemoral instability using trochleoplasty or MPFL reconstruction: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25:2309–2320. doi: 10.1007/s00167-015-3698-1. [DOI] [PubMed] [Google Scholar]
- 12.Camathias C., Studer K., Kiapour A., Rutz E., Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44:2855–2863. doi: 10.1177/0363546516652894. [DOI] [PubMed] [Google Scholar]
- 13.Balcarek P., Rehn S., Howells N.R. Results of medial patellofemoral ligament reconstruction compared with trochleoplasty plus individual extensor apparatus balancing in patellar instability caused by severe trochlear dysplasia: A systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25:3869–3877. doi: 10.1007/s00167-016-4365-x. [DOI] [PubMed] [Google Scholar]
- 14.Banke I.J., Kohn L.M., Meidinger G. Combined trochleoplasty and MPFL reconstruction for treatment of chronic patellofemoral instability: A prospective minimum 2-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2014;22:2591–2598. doi: 10.1007/s00167-013-2603-z. [DOI] [PubMed] [Google Scholar]
- 15.Ntagiopoulos P.G., Byn P., Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41:998–1004. doi: 10.1177/0363546513482302. [DOI] [PubMed] [Google Scholar]
- 16.Steiner T.M., Torga-Spak R., Teitge R.A. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34:1254–1261. doi: 10.1177/0363546505285584. [DOI] [PubMed] [Google Scholar]
- 17.Hiemstra L.A., Kerslake S., Loewen M., Lafave M. Effect of trochlear dysplasia on outcomes after isolated soft tissue stabilization for patellar instability. Am J Sports Med. 2016;44:1515–1523. doi: 10.1177/0363546516635626. [DOI] [PubMed] [Google Scholar]
- 18.Fucentese S.F., Schottle P.B., Pfirrmann C.W., Romero J. CT changes after trochleoplasty for symptomatic trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2007;15:168–174. doi: 10.1007/s00167-006-0140-8. [DOI] [PubMed] [Google Scholar]
- 19.Brady J.M., Sullivan J.P., Nguyen J. The tibial tubercle-to-trochlear groove distance is reliable in the setting of trochlear dysplasia, and superior to the tibial tubercle-to-posterior cruciate ligament distance when evaluating coronal malalignment in patellofemoral instability. Arthroscopy. 2017;33:2026–2034. doi: 10.1016/j.arthro.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Dejour D., Byn P., Ntagiopoulos P.G. The Lyon's sulcus-deepening trochleoplasty in previous unsuccessful patellofemoral surgery. Int Orthop. 2013;37:433–439. doi: 10.1007/s00264-012-1746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laidlaw M.S., Feeley S.M., Ruland J.R., Diduch D.R. Sulcus-deepening trochleoplasty and medial patellofemoral ligament reconstruction for recurrent patellar instability. Arthrosc Tech. 2018;7:e113–e123. doi: 10.1016/j.eats.2017.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Steps for performing open lateral lengthening, trochleoplasty, and medial patellofemoral ligament reconstruction in the setting of high-grade trochlear dysplasia. The procedure is performed on a right leg in the supine position through a lateral parapatellar incision. A thorough discussion of planning the new trochlear groove and how this corrects aspects of trochlear dysplasia pathoanatomy is highlighted. Pearls for a safe technique regarding osteochondral flap elevation are also emphasized.