Abstract
Biopolymer-based composition with adding of conductive polymer poly-(3,4-ethylenedioxythiophene) polystyrenesulfonate (PEDOT PSS) was made by mixing of iota-carrageenan (CRG), polyvinyl alcohol (PVA) and PEDOT PSS followed by freezing/thawing cycles. The method is environmentally friendly and based on the formation of polymer matrix upon of mixing CRG, PVA and PEDOT PSS and formation of porous physical gel due to freezing/thawing cycles. It is necessary to mention that all components are well-known as biocompatible materials. The resulting material is stable in water and also has swelling capability both in distilled water and physiological solutions. Structure of material was characterized by means of X-ray diffraction, optical and electron microscopy. Electrophysical investigations also were performed. The conductivity of the gel immersed in distilled water is comparable with the dry gel value and close to 0.01 [S/cm].
Keywords: Biomedical engineering, Nanotechnology, Nanotechnology fabrication, Biomedical devices, Polymers, Biomedical materials, Materials synthesis, Hydrogels, Biopolymers, Iota-carrageenan, Polyvinyl alcohol, PEDOT PSS, Conductive polymers
1. Introduction
Electroconductive hydrogels are three-dimensional water-swollen networks of cross-linked polymer chains that possess a combination of unique properties: high electrical conductivity due to comprising of a conductive electroactive polymer [1, 2, 3, 4]; transmittance, [5, 6]; biocompatibility [7, 8]; swelling capacity [9]; a high degree of hydration [10].
The resistivity of electroconductive gels is comparable to observable one in some metals and inorganic semiconductors. Even though these hybrid materials have sufficiently high conductivity they simultaneously retain the mechanical properties of the polymer matrix such as flexibility and strength.
To prepare electroconductive hydrogels the family of highly conjugated polymers as polypyrrole, polyaniline and polythiophene are often used [11, 12, 15]. Several research works are focused on polyaniline hydrogels development [13, 14]. The combination of polyaniline with nanocellulose gives a possibility to develop elastomer material with promising electrochemical capabilities [13]. Polypyrrole also can be a part of very interesting hydrogels that are very promising for future applications [15]. For example, the possibility to make self-healing electroconductive nanocellulose-mediated materials with polypyrrole is already demonstrated [16]. The derivative of polythiophene – poly-(3,4-ethylenedioxythiophene) (PEDOT) – appears to be attractive because of its high conductivity and also its exceptional electrochemical stability in its oxidized state [12, 17]. Moreover, this polymer demonstrates good biocompatibility [18, 19]. PEDOT is known to be an water-insoluble polymer, however, supplementation of polyelectrolyte, polystyrenesulfonate (PSS), to PEDOT makes the complex of PEDOT:PSS water-soluble and significantly enhances its stability [15, 17].
PEDOT:PSS based hydrogels can be utilized as electrodes in biofuel cells [20] and biosensors [21, 22], also in optoelectronics where PEDOT:PSS has established itself as the most efficient electroconductive polymer [23, 24], in optogenetics in view of the good cytocompatibility with the neurons [25], in neuroprosthetics where e.g. authors [18] presented the possibility of controlling the behavior of cells bioelectrochemically on the implantable electrodes.
Herein we elaborate a method of producing a three-component hydrogel based on electrically conductive polymer PEDOT:PSS, the polysaccharide iota-carrageenan (CRG) and polyvinyl alcohol (PVA). We selected CRG due to its high swelling capacity and easy gel-forming [26, 27, 28, 32, 36]. Besides, CRG with its significant number of functional groups is capable to application-driven tailoring of the microstructure of hydrogels [27]. PVA gives the possibility to impart mechanical strength and stability to the hydrogel [29, 30, 31, 32, 33, 35]. It is necessary to mention that, today PVA is one of most actively used components for hydrogels [37, 38]. The different variants of PVA-based hydrogels with CRG [35, 36], cellulose [39, 40] and other component including conductive polymers are described [15, 37].
PEDOT:PSS, CRG and PVA are considered as non-toxic and biocompatible components [29, 30, 31, 34]. In the process of synthesis, we carried out a physical method, namely "freezing-thawing", that allows avoiding the use of cross-linking agents and other toxic substances [15]. This method is widely using for environmentally friendly and in some cases biocompatible hydrogels synthesis [15, 36]. "Freezing-thawing" was already successfully applied for synthesis of CRG-PVA hydrogels in our previous work [35] and in the study of Hosseinzadeh et al. [36]. In paper [35] was demonstrated the possibility of iota-CRG-PVA hydrogel to carry photocatalytic nanoparticles thus this system can be promising as a basis for new functional hydrogels.
2. Methods
2.1. Materials
Next materials were used: PEDOT:PSS (1.3wt % dispersion in H2O, Sigma-Aldrich), iota-CRG from Special Ingredients (Great Britain) and PVA from Avilon-Kompani Khim (122000 g/mol, Russia) without further purification.
2.2. Preparation of hydrogel
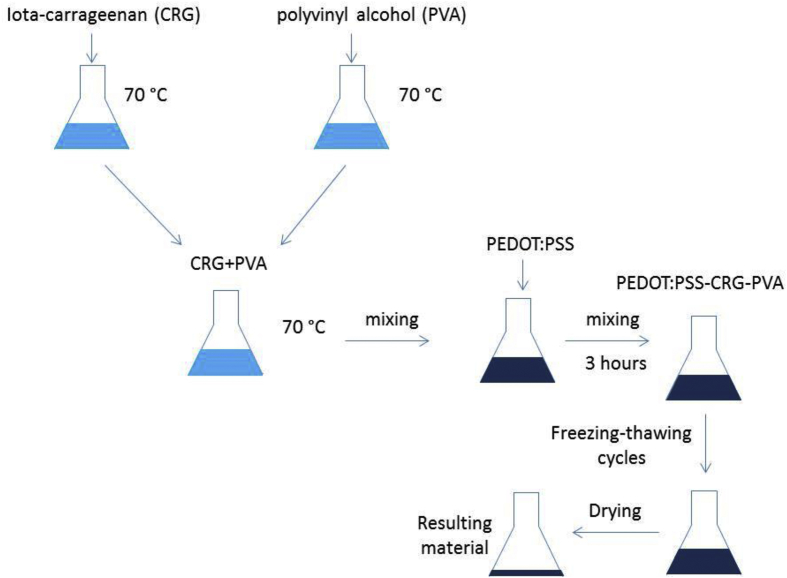
Hydrogel preparation starts from CRG and PVA dissolving in distilled water in equal volume in the separate flasks at the temperature of 70°С. The concentrations of both reagents were the same and equal to 1.3 wt%. After dissolving processes CRG and PVA solutions are mixed with each over. Next, the chosen amount of PEDOT:PSS (1.3 wt%) was added in the mixture. Resulting solution contains PEDOT:PSS, CRG and PVA was steered for 3 h with rotation frequency equal to 500–550 rpm. Biosan MSH-300i magnetic stirrer was used for this process. During steering, temperature stays constant and equal to 70°С. After this process, the reaction flask is cooling down to the room temperature. Next four freezing/thawing cycles were provided. The length of each cycle was 24 h. The freezing temperature was equal to -12°С and the thawing temperature was equal to +25°С. After four cycles hydrogels were dried at temperature equal to 40°С until constant weight.
A schematic view of hydrogel preparation is presented in Fig. 1. Received material can be swelled in different water solutions to receive hydrogels. The preparation method was designed based on the research [35].
Fig. 1.
A schematic view of hydrogel preparation. CRG – iota-carrageenan; PVA – polyvinyl alcohol; PEDOT:PSS - poly-(3,4-ethylenedioxythiophene) polystyrenesulfonate.
There are five hydrogels was prepared with ratio of PEDOT:PSS, CRG, and PVA 1:1:1; 2:1:1; 3:1:1; 4:1:1 and 5:1:1.
2.3. X-ray diffraction
Diffraction patterns of PEDOT:PSS, CRG, PVA and received hydrogels in the dried forms were obtained at the BELOK beamline of the Kurchatov Synchrotron Radiation Source (NRC “Kurchatov Institute”, Moscow). The storage ring “Siberia-2” operated with the next conditions: electron energy of 2.5 GeV and electron current stored of about 100 mA. Monochromated by using a Si(111) double-crystal monochromator the photon beam was doubly focused on the sample position by the sagittally bent second crystal of the monochromator and total-reflection multi-segmented mirror. Diffraction data were acquired using a Rayonix SX-165 2D CCD-detector (2048х2048 pixels, 79 μm pixel size) at λ = 0.09699 nm, sample-to-detector distance of 80 mm and exposure time of 300 s per frame with two-pass Multi-Read option to reduce random background. Dried samples were measured as self-supporting films (PEDOT:PSS-CRG-PVA, PEDOT:PSS, CRG, PVA samples) under the ambient conditions. The 2D images were integrated into standard I(2θ) format using the Fit2D code [41].
2.4. Swelling study
For determination of swelling capacity in dependence on time, the weighted amount of dry sample of material was placed into 10 ml of aqueous solution (distilled water or 0.15M NaCl solution, depending on experiment type). Each 5 min sample removing from aqueous solution and blotted with filter paper for surface water removing. Next sample weighed by using analytical weighing machine. Such the swelling/weighting steps were repeated with a constant time interval of 5 min. Experiments stopped then three consecutive measurements would remain equal to each over with the accuracy of 0.003 g.
The degree of swelling was calculated by the next equation [35]:
| (1) |
Where – is the mass of the initial (dry) sample in g; - degree of swelling %; - every given moment of time; – mass of wet sample on the moment of time in g.
2.5. Thermogravimetric analysis (TGA)
Thermal analysis was performed by using TGA (PerkinElmer, Pyris 1 Thermogravimetric Analyzer). The apparatus was continually flushed with nitrogen (100 ml/min). Measurements were implemented in the temperature range from 30 °C to 700 °C, at a heating rate of 10 °C/min.
2.6. Electrophysical and electrochemical analysis
A Keithley 4200 SCM station was used to characterize the resistivity of the gel samples both in dry state. Sheet resistance measurements were carried out in a standard aligned equidistance four-probe and van der Pauw setup. The gel samples with 20 × 20 mm2 film areas were prepared for each ratio of PEDOT:PSS-CRG-PVA. Also, this system was used for gel behavior observation during swelling/drying.
The swelled hydrogels electrochemical characterization was carried out based on the three-electrode system with Ag/AgCl as a reference electrode. Measurements were provided by using potentiostat-galvanostat Autolab PGSTAT 302N. Current-voltage characteristics (CV) were performed in a potential range of -0,3–0,8 V vs. Ag/AgCl reference electrode under a sweep rate of 20–100 mV/s. For conductivity testing of swelled hydrogel – tested material was placed on two gold electrodes.
2.7. Microscopy of samples
The porous structure of hydrogels was observed with an optical microscope Carl Zeiss Axio Imager M2m in transmission mode.
Hydrogel morphology was studied in a scanning electron microscope (SEM) Versa 3D DualBeam (FEI, USA) in environmental SEM (ESEM) mode. The images were obtained with the gaseous secondary electron detector (GSED) with accelerating voltage of 10 kV and a current of 60 pA. The samples were placed on the Peltier stage, which was precooled to 3 °C. During the experiment, the humidity in the vacuum chamber was consistently decreased from 100% to 60% to reveal the microstructure of the samples.
3. Results and discussion
The presented in Fig. 1 general scheme showing a method of preparation of material by mixing iota-carrageenan, polyvinyl alcohol and PEDOT:PSS solutions followed by alternating freezing and thawing cycles. The received material next can be swelled in water to receive hydrogels, thus it makes possible to prepare those hydrogels in water solutions with different ionic strength for different possible applications.
Enhancement of PEDOT:PSS content was focused on the maximization of the electrical conductivity of hydrogel. But the hydrogel with highest PEDOT:PSS content (ratio of PEDOT:PSS, CRG, and PVA - 5:1:1) became highly unstable during swelling. This gel degraded after 5 min both in neat solution and in physiological solution. This effect caused by a low concentration of PEDOT:PSS in the initial solution that leads to an increase of water content in the mixing stage of hydrogel preparation (see Fig. 1) that in its turn leads to the decreasing of resulting stability of hydrogel. This observation is in correlation with results, presented in [38] concerning PVA hydrogels. Thus, in further research we do not consider hydrogel with a ratio of PEDOT:PSS, CRG, and PVA - 5:1:1.
3.1. X-ray diffraction
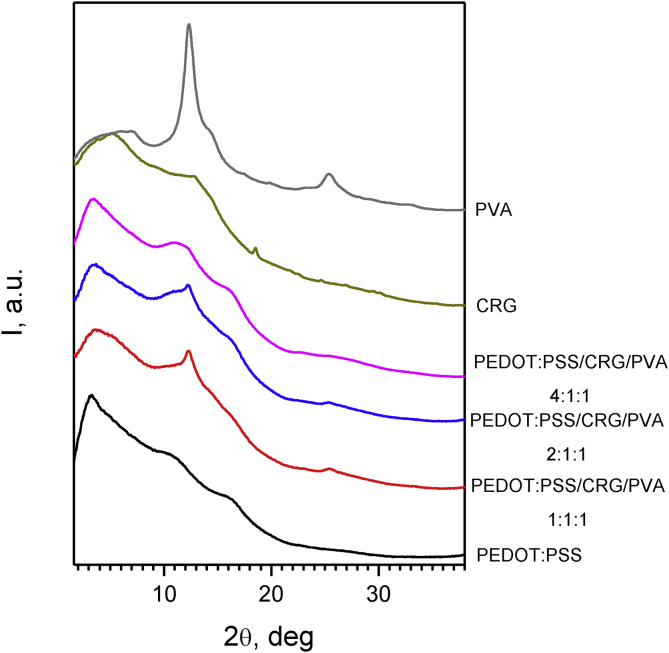
All hydrogels under study are non-crystalline exhibiting only strongly broadened halos in their diffraction patterns. Nevertheless, the different components are characterized by diffraction features located at different scattering angles, which are preserved in multi-component composites. More specifically, PEDOT:PSS reveals a triangular-shaped maximum at ca. 3.3 deg clearly distinguishable for all composites. PVA is most ordered among polymers under investigation in comparison with CRG and PEDOT:PSS. PVA diffraction pattern reveals three broad peaks at ca. 12.3, 14.0, and 25.4 deg. These peaks progressively diminish in intensity as the PVA fraction in the all PEDOT:PSS CRG PVA composites decrease (see Fig. 2). The diminishing of PVA peaks correlated with increasing of PEDOT:PSS content in the gels. Also increasing of PEDOT:PSS peak at ca. 3.3 deg is observed in correlation with increasing of its content in gels. This means that composite gel formed via PVA crystalline regions.
Fig. 2.
Powder X-ray diffraction patterns of the hydrogels (λ = 0.9699 Å).
3.2. Swelling study
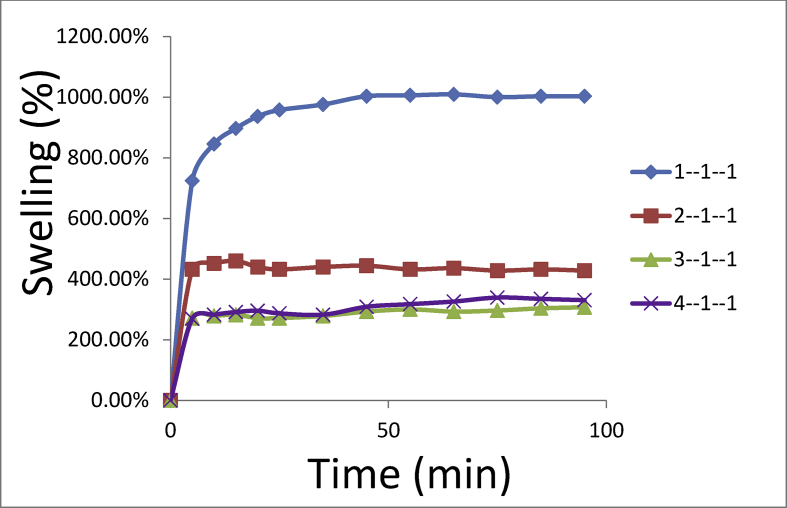
The swelling curves for the PEDOT:PSS-CRG-PVA hydrogels with different concentrations of PEDOT:PSS for a physiological solution (0,15M NaCl) are demonstrated in Fig. 3. We found that all the hydrogels are stable regardless of the concentration of the PEDOT:PSS.
Fig. 3.
Swelling behavior of PEDOT:PSS-CRG-PVA hydrogels with different concentrations of PEDOT:PSS in physiological solution (0.15 M NaCl solution).
The degrees of swelling for the hydrogels are: PEDOT:PSS-CRG-PVA (with ratio of reagents 1:1:1) – 1000 ± 7%; PEDOT:PSS-CRG-PVA (with ratio of reagents 2:1:1) – 430 ± 3%; PEDOT:PSS-CRG-PVA (with ratio of reagents 3:1:1) – 305 ± 8%; PEDOT:PSS-CRG-PVA (with ratio of reagents 4:1:1) – 330 ± 7%. Those degrees were observed at the time longer than 100 min for 0.15 M NaCl solution.
The data shows the increase of PEDOT:PSS concentration decreases of swelling degree of samples in physiological solution because of the complex formation of i-CRG sulphate groups and PEDOT. One can notice that further increase of PEDOT:PSS concentration leads to a slight increase of swelling degree because of lower concentration of PVA (the main gel-forming polymer in this system) after initial mixing (see Fig. 1).
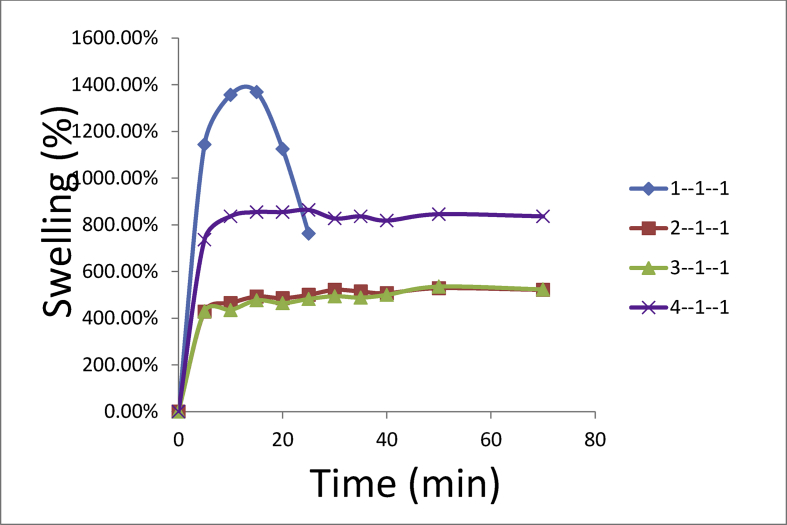
The swelling curves for the PEDOT:PSS-CRG-PVA hydrogels with different concentrations of PEDOT:PSS for a neat water solution are demonstrated in Fig. 4. The first sample with low concentration of PEDOT:PSS is unstable and begins to degrade after 10 min because of the scarcity of sodium ions concentration for CRG molecules complex formation. This sample is starting to degrade in water and after 25 min lounging into smaller pieces. On the contrary hydrogels with higher PEDOT content are quite stable due to CRG- PEDOT:PSS complex formation. The degrees of swelling for the hydrogels are: PEDOT:PSS-CRG-PVA (with ratio of reagents 2:1:1) – 510 ± 10%; PEDOT:PSS-CRG-PVA (with ratio of reagents 3:1:1) – 510 ± 10%; PEDOT:PSS-CRG-PVA (with ratio of reagents 4:1:1) – 835 ± 5%. Those degrees were observed at the time longer than 80 min. Because of aggregation of carrageenan macromolecules in the presence of sodium ions [42] the swelling degree of PEDOT:PSS-CRG-PVA gels in physiological solution lower than in water. And again as in physiological solution increase of PEDOT:PSS concentration from PEDOT:PSS-CRG-PVA (with ratio of reagents 3:1:1) to PEDOT:PSS-CRG-PVA (with ratio of reagents 4:1:1) leads to increase of swelling degree because of lower concentration of PVA. In this case one can observe a stronger effect because the gels are quite week in water media. Further increase of PEDOT:PSS ratio (PEDOT:PSS, CRG, and PVA - 5:1:1) causes instability of the gel as it was mentioned above.
Fig. 4.
Swelling behavior of the PEDOT:PSS-CRG-PVA hydrogels with different concentrations of PEDOT:PSS in a neat water solution.
We are interested in the experiment which carried out in a physiological solution (0.15 M NaCl solution) because further research in the future will be focused on applications related to applications in biological systems.
3.3. Microscopy of samples
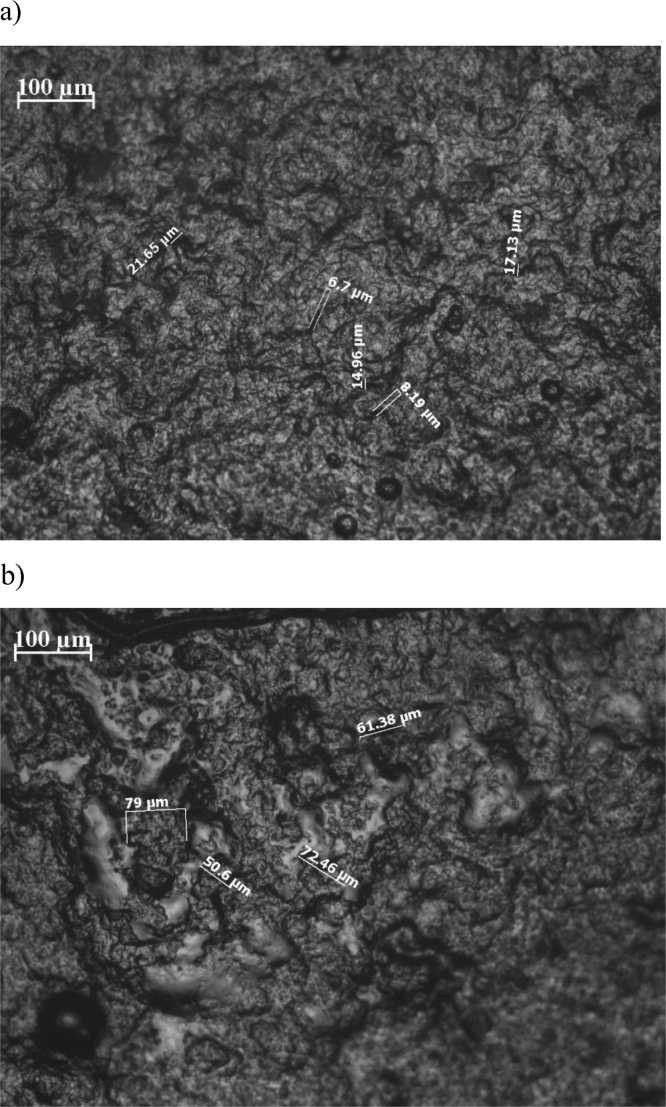
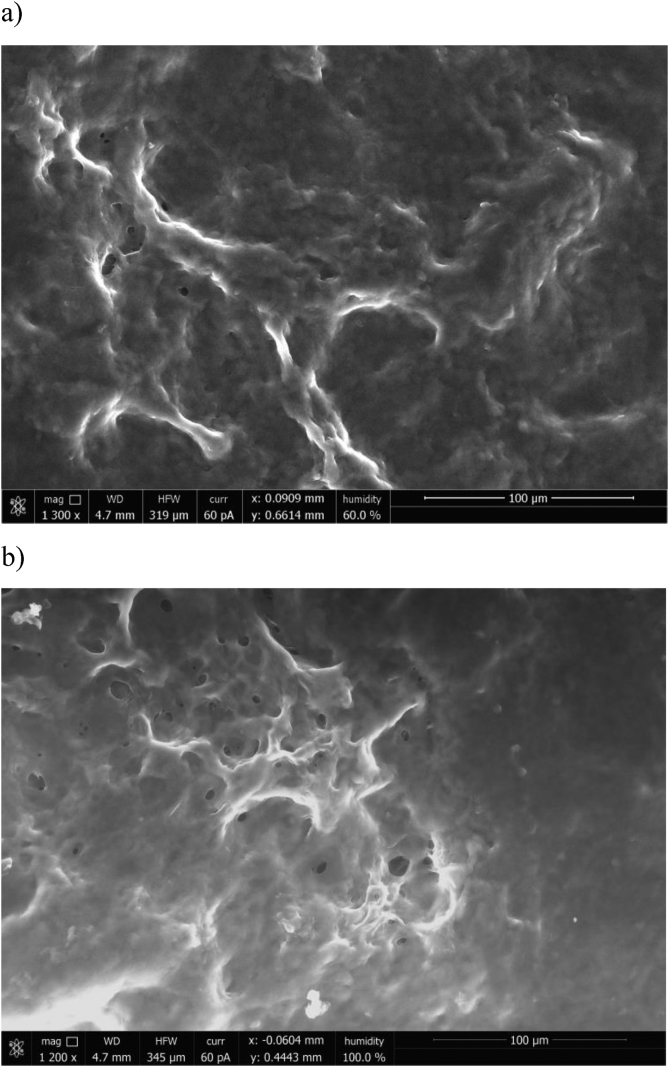
The main driving force of gel formation in PEDOT:PSS-CRG-PVA solutions is aggregation of PVA molecules upon separation of polymer phase during crystallization of water. So after thawing PVA will form a gel with a filled by water pores. The structure of hydrogels was observed using optical and electron microscopy before (see Fig. 5a) and after (see Figs. 5b, 6) equilibrium swelling in a neat water solution. Optical microscopy images show a porous structure of the hydrogel. Characteristic pore diameter can be estimated from 5 to 20 μm for hydrogels before swelling due to pore shrinkage under water evaporation and from 50 to 70 μm after swelling of dried gel. Results of ESEM study presented in Fig. 6 shows the absence of microphase separation of PEDOT:PSS, CRG, and PVA.
Fig. 5.
The microscopic image (transmission mode) of the PEDOT:PSS-CRG-PVA hydrogel (with ratio of reagents 4:1:1) before swelling (a) and after swelling (b).
Fig. 6.
ESEM image of PEDOT:PSS-CRG-PVA hydrogel (with ratio of reagents 4:1:1) with different humidity – a) 60% and b) equal to 100%.
3.4. Thermogravimetric analysis (TGA)
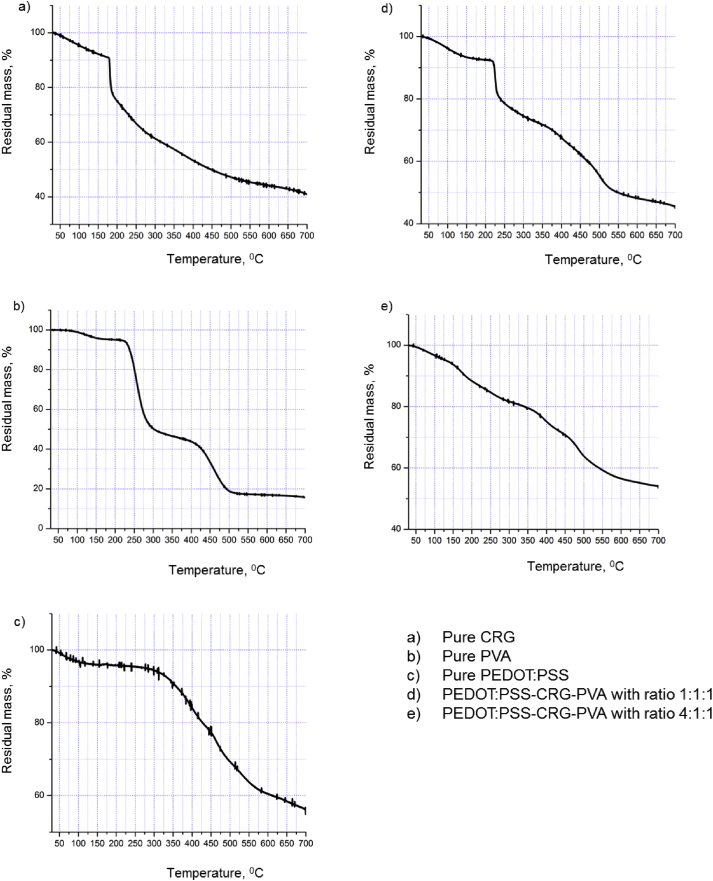
TGA was used to understand the thermal stability of the hydrogels. Reducing the mass of all the samples in the first stage (5–10 %) can be explained by the presence of the remaining moisture. Then, primarily CRG starts to decompose abruptly at 175 °C, and the increase of temperature up to 700 °C leads to lose the mass to 40%. PVA and PEDOT:PSS begins to decompose at 230 °C and 300 °C, respectively. The most thermally stable sample among the hydrogels is the first with the ratio of reagents 1:1:1, which has a decomposition temperature of 230 °C and mass loss of 55% at the 700 °C. Hydrogels PEDOT:PSS-CRG-PVA with ratios 2:1:1 3:1:1 and 4:1:1 have a lower decomposition temperature (180 °C, 177 °C and 170 °C), mass losses of 50%, 52% and 55%, respectively, at the ultimate temperature. The curves for initial reagents CRG, PVA, PEDOT:PSS and their based hydrogels PEDOT:PSS-CRG-PVA with different ratios of reagents 1:1:1, and 4:1:1, are presented in Fig. 7.
Fig. 7.
TGA curves: a) pure CRG; b) pure PVA; c) pure PEDOT:PSS; d) and e) PEDOT:PSS-CRG-PVA hydrogels with the different ratios of reagents - 1:1:1, and 4:1:1, respectively.
It can be concluded that with the increment of electroconductive PEDOT:PSS concentration in hydrogels thermal stability of three-based hydrogels PEDOT:PSS-CRG-PVA decreases (1:1:1–230 °C, 2:1:1–180 °C, 3:1:1–177 °C, 4:1:1–170 °C). However, if we consider these hydrogels as implantable materials in the future, all three gels with different ratios are undoubtedly suitable for experiments.
3.5. Electrophysical and electrochemical analysis
Film resistivity was examined at several points on a 20 × 20 mm2 film areas for four samples with different PEDOT:PSS content. Table 1 is showing the results of dry gels resistivity. The differences in resistivity number for each sample is most probably caused by the film thickness variation. The lesser resistivity was found for gel with a ratio of component 4:1:1 and equal to 15–30 [kOhm/sq] for dry sample. Taking into account the dry film thickness ∼40 mkm the measured sheet conductivity about 0.01 [S/cm] of the dry gel is two orders less than clean PEDOT:PSS one ∼1 [S/cm].
Table 1.
Resistivity of dry samples.
| Material | Specific resistivity kOhm/sq |
|---|---|
| PEDOT:PSS-CRG-PVA 1:1:1 | 57–102 |
| PEDOT:PSS-CRG-PVA 2:1:1 | 31–58 |
| PEDOT:PSS-CRG-PVA 3:1:1 | 22–46 |
| PEDOT:PSS-CRG-PVA 4:1:1 | 15–30 |
The resistance of the swelled hydrogel was higher in comparison with the same samples in dry state (Table 2). Absence of data for PEDOT:PSS-CRG-PVA 1:1:1 is explained its low stability in neat water (see Fig. 4). As in the case of dry gel, the differences in resistivity number for each sample are most probably caused by the film thickness variation. The lower PEDOT:PSS ration leads to higher resistance, but in the case of swelled hydrogels influence of porosity can take place [43, 44]. Growth of porosity during hydrogel swelling may cause the change of electric current percolation net which leads to resistance enlargement [44]. This phenomenon in those hydrogels requires further investigation in future research. The measured sheet conductivity of PEDOT:PSS-CRG-PVA with reagent ratios 4:1:1 was about 0.005 [S/cm] in physiological solution and 0.002 [S/cm] in neat solution.
Table 2.
Resistivity of swelled hydrogel.
| Material | Specific resistivity in neat water kOhm/sq | Specific resistivity in physiological solution kOhm/sq |
|---|---|---|
| PEDOT:PSS-CRG-PVA 1:1:1 | - | 252–348 |
| PEDOT:PSS-CRG-PVA 2:1:1 | 117–202 | 59–88 |
| PEDOT:PSS-CRG-PVA 3:1:1 | 96–174 | 47–71 |
| PEDOT:PSS-CRG-PVA 4:1:1 | 83–152 | 32–64 |
The interesting phenomenon was observed during hydrogel swelling. Current-Voltage characteristic (CVC) of hydrogel with a 4:1:1 ratio of PEDOT:PSS-CRG-PVA during swelling is presented in Fig. 8. It is shown that hydrogel change its properties during swelling and restore it during drying. The film conductivity of the gel immersed in distilled water is comparable with the dry gel value. The hysteresis of CVC is observed (see Fig. 8). The CVC does not cross the axis zero point confirming the EMF sources existence in the hydrogel system under study. It should be noted that the voltage in the experiment does not exceed 0.9 V to prevent water electrolysis. The question concerning the origin of the EMF sources and their localization is needed the additional investigation.
Fig. 8.
a) Current-Voltage characteristic of hydrogel during swelling in standard measurement geometry with aligned equidistance placed four probes. Voltage U1 on the first probe varied in the range from -0.9 to +0.9V, the 4th probe was grounded. b) Current-Voltage characteristic of Voltage U23 = U2–U3 between probes 2 and 3 of hydrogel during swelling.
4. Conclusions
Our work shows the possibility of obtaining of biopolymer-based material with conductivity without potentially hazardous linking agents. The method is environmentally friendly and based on the formation of polymer matrix upon of mixing CRG, PVA and PEDOT PSS and formation of porous physical gel in this matrix due to freezing/thawing cycles. It is necessary to mention that all components are well-known as biocompatible materials [5, 12, 15, 37, 42]. This means that received hydrogels is expected also can be biocompatible, further research in this field will be provided.
The film conductivity of the gel immersed in distilled water is comparable with the dry gel value and close to 0.01 [S/cm] in dry state and close to 0.005 [S/cm] as a hydrogel. Further research of those materials will be focused on the understanding of effects with EMF sources, porosity influence, on increasing of material conductivity closer to PEDOT PSS one and providing biocompatibility tests.
Declarations
Author contribution statement
Pavel Gotovtsev: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gulfiya Badranova: Conceived and designed the experiments; Performed the experiments.
Yan Zubavichus & Nikolay Chumakov: Performed the experiments; Analyzed and interpreted the data.
Christina Antipova, Mikhail Presniakov & Roman Kamyshinsky: Performed the experiments.
Timofei Grigoriev: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Kazbek Tokaev: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by RFBR according to the research project No18-29-23024mk.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. V.G.Valeev (NRC “Kurchanov Institute”) for fruitful discussions. We acknowledge Resource Centers of organic and hybrid materials, optical microscopy and spectroscopy, probe and electron microscopy and electro physical methods of National Research Centre « Kurchatov institute».
References
- 1.Chikar Jennifer A., Hendricks Jeffrey L., Richardson-Burns Sarah M., Raphael Yehoash, Pfingst Bryan E., Martin David C. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials. 2012;33:1982–1990. doi: 10.1016/j.biomaterials.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hira Steven M., Payne Christine K. Protein-mediated synthesis of the conducting polymer PEDOT:PSS. Synth. Met. 2013;176:104–107. [Google Scholar]
- 3.Zhou Hui, Yao Wei, Geng Li, Wang Jing, Lu Yun. Graphene/poly(3,4-ethylenedioxythiophene) hydrogel with excellent mechanical performance and high conductivity. Carbon. 2013;59:495–502. [Google Scholar]
- 4.Pana Lijia, Yu Guihua, Zhai Dongyuan, Lee Hye Ryoung, Zhao Wenting, Liu Nian, Wang Huiliang, Tee Benjamin C.-K., Shi Yi, Cui Yi, Bao Zhenan. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. 2012;109(24):9287–9292. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiseppi-Elie Anthony. Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials. 2010;31:2701–2716. doi: 10.1016/j.biomaterials.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 6.Devaki Sudha Janardhanan, Narayanan Rohini Kuttiplavil, Sarojam Sivakala. Electrically conducting silver nanoparticle–polyacrylic acid hydrogel by in situ reduction and polymerization approach. Mater. Lett. 2014;116:135–138. [Google Scholar]
- 7.Cheong G.L. Mario, Lim Khoon S., Jakubowicz Anais, Martens Penny J., Laura A., Poole-Warren, Green Rylie A. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater. 2014;10:1216–1226. doi: 10.1016/j.actbio.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Karagkiozaki V., Karagiannidis P.G., Gioti M., Kavatzikidou P., Georgiou D., Georgaraki E., Logothetidis S. Bioelectronics meets nanomedicine for cardiovascular implants: PEDOT-based nanocoatings for tissue regeneration. Biochim. Biophys. Acta. 2013;1830:4294–4304. doi: 10.1016/j.bbagen.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Rivero Rebeca E., Molina Maria A., Rivarola Claudia R., Barbero Cesar A. Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sens. Actuators B. 2014;190:270–278. [Google Scholar]
- 10.Kotanen Christian N., Nolan Wilson A., Dong Chenbo, Dinu Cerasela-Zoica, Justin Gusphyl A., Guiseppi-Elie Anthony. The effect of the physicochemical properties of bioactive electroconductive hydrogels on the growth and proliferation of attachment dependent cells. Biomaterials. 2013;34:6318–6327. doi: 10.1016/j.biomaterials.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Balint Richard, Cassidy Nigel J., Cartmell Sarah H. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Yang C., Suo Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018;3:125–142. [Google Scholar]
- 13.Han J., Lu K., Yue Y., Mei C., Huang C., Wu Q., Xu X. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crops Prod. 2019;128:94–107. [Google Scholar]
- 14.Pan L., Yu G., Zhai D., Lee H.R., Zhao W., Liu N., Wang H., Tee B.C.-K., Shi Y., Cui Y., Bao Z. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9287–9292. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuk H., Lu B., Zhao X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019;48:1642–1667. doi: 10.1039/c8cs00595h. [DOI] [PubMed] [Google Scholar]
- 16.Ding Q., Xu X., Yue Y., Mei C., Huang C., Jiang S., Wu Q., Han J. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater. Interfaces. 2018;10:27987–28002. doi: 10.1021/acsami.8b09656. [DOI] [PubMed] [Google Scholar]
- 17.Groenendaal L., Jonas Friedrich, Freitag Dieter, Pielartzik Harald, Reynolds John R. Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv. Mater. 2000;12(7):481–494. [Google Scholar]
- 18.Collazos-Castro Jorge E., Polo José L., Hernández-Labrado Gabriel R., Padial-Cañete Vanesa, García-Rama Concepción. Bioelectrochemical control of neural cell development on conducting polymers. Biomaterials. 2010;31:9244–9255. doi: 10.1016/j.biomaterials.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 19.Richardson-Burns Sarah M., Hendricks Jeffrey L., Foster Brian, Povlich Laura K., Kim Dong-Hwan, Martin David C. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT)around living neural cells. Biomaterials. 2007;28:1539–1552. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Chao, Zhang Libin, Ding Lili, Ren Hongqiang, Cui Hao. Effect of conductive polymers coated anode on the performance of microbial fuel cells (MFCs) and its biodiversity analysis. Biosens. Bioelectron. 2011;26:4169–4176. doi: 10.1016/j.bios.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Li Lanlan, Shi Ye, Pan Lijia, Yi Shia, Yu Guihua. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J. Mater. Chem. B. 2015 doi: 10.1039/c5tb00090d. [DOI] [PubMed] [Google Scholar]
- 22.Ismail Yahya A., Martinez Jose G., Al Harrasi Ahmad S., Kim Seon J., Otero Toribio F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens. Actuators B. 2011;160:1180–1190. [Google Scholar]
- 23.Rutledge Steven A., Helmy Amr S. Etch-free patterning of poly(3,4-ethylenedioxythiophene)−poly(styrenesulfonate) for optoelectronics. ACS Appl. Mater. Interfaces. 2015 doi: 10.1021/am507981d. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang Jianyong, Chu Chi-Wei, Chen Fang-Chung, Xu Qianfei, Yang Yang. High-conductivity poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005;15(2):203–208. [Google Scholar]
- 25.Gerwig Ramona, Fuchsberger Kai, Schroeppel Birgit, Link Gordon Steve, Heusel Gerhard, Kraushaar Udo, Schuhmann Wolfgang, Stett Alfred, Stelzle Martin. PEDOT–CNT composite microelectrodes for recording and electrostimulation applications: fabrication, morphology, and electrical properties. Front. Neuroeng. 2012 doi: 10.3389/fneng.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prajapati V.D., Maheriya P.M., Jani G.K., Solanki H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014;105:97–112. doi: 10.1016/j.carbpol.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Zhan X., Wan J., Wang Y., Wang C. Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015;121:27–36. doi: 10.1016/j.carbpol.2014.11.063. [DOI] [PubMed] [Google Scholar]
- 28.Campo V.L., Kawano D.F., da Silva D.B., Jr., Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis – a review. Carbohydr. Polym. 2009;77:167–180. [Google Scholar]
- 29.Kenawy E.-R., Kamoun E.A., Mohy Eldin M.S., El-Meligy M.A. Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: synthesis and characterization for biomedical applications. Arabian J. Chem. 2014;7:372–380. [Google Scholar]
- 30.Mishra S.K., Ferreira J.M.F., Kannan S. Mechanically stable antimicrobial chitosan-PVA-silver nanocomposite coatings deposited on titanium implants. Carbohydr. Polym. 2015;121:37–48. doi: 10.1016/j.carbpol.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Qi X., Hu X., Wei W., Yu H., Li J., Zhang J., Dong W. Investigation of Salecan/poly(vinyl alcohol) hydrogelsprepared by freeze/thaw method. Carbohydr. Polym. 2015;118:60–69. doi: 10.1016/j.carbpol.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Dafader N.C., Manir M.S., Alam M.F., Swapna S.P., Akter T., Huq D. Effect of kappa-carrageenan on the properties of poly(vinyl alcohol) hydrogel prepared by application of gammaradiation. SOP Trans. Appl. Chem. 2015;2:1–12. [Google Scholar]
- 33.Varshney L. Role of natural polysaccharides in radiation formation of PVA–hydrogel wound dressing. Nucl. Instrum. Methods Phys. Res., Sect. B. 2007;255:343–349. [Google Scholar]
- 34.Li L., Ni R., Shao Y., Mao S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014;103:1–11. doi: 10.1016/j.carbpol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Badranova G.U., Gotovtsev P.M., Zubavichus Y.V., Staroselskiy I.A., Vasiliev A.L., Trunkin I.N., Fedorov M.V. Biopolymer-based hydrogels for encapsulation of photocatalytic TiO2 nanoparticles prepared by the freezing/thawing method. J. Mol. Liq. 2016;223:16–20. [Google Scholar]
- 36.Hosseinzadeh H., Zoroufi S., Mahdavinia G.R. Study on adsorption of cationic dye on novel kappa-carrageenan/poly(vinyl alcohol)/montmorillonite nanocomposite hydrogels. Polym. Bull. 2015;72:1339–1363. [Google Scholar]
- 37.Jiang S., Liu S., Feng W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011;4:1228–1233. doi: 10.1016/j.jmbbm.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Hassan C.M., Peppas N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules. 2000;33:2472–2479. [Google Scholar]
- 39.Han J., Yue Y., Wu Q., Huang C., Pan H., Zhan X., Mei C., Xu X. Effects of nanocellulose on the structure and properties of poly(vinyl alcohol)-borax hybrid foams. Cellulose. 2017;24:4433–4448. [Google Scholar]
- 40.Han J., Wang H., Yue Y., Mei C., Chen J., Huang C., Wu Q., Xu X. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon. 2019;149:1–18. N. Y. [Google Scholar]
- 41.Hammersley A.P. FIT2D: a multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016;49:646–652. [Google Scholar]
- 42.Piculell L. Effects of ions on the disorder—order transitions of gel-forming polysaccharides. Food Hydrocolloids. 1991;5(Issues 1–2):57–69. [Google Scholar]
- 43.Costa C.M., Rodrigues L.C., Sencadas V., Silva M.M., Rocha J.G., Lanceros-Méndez S. Effect of degree of porosity on the properties of poly(vinylidene fluoride-trifluorethylene) for Li-ion battery separators. J. Membr. Sci. 2012;407–408:193–201. [Google Scholar]
- 44.Hunt A., Ewing R., Ghanbarian B. 2014. Pecrolation Theory for Flow in Porous Media. [Google Scholar]