Abstract
Background
The investigation and knowledge of calcium handling mechanisms in the plasmodium has been considered as a potential biological target against malaria.
Objective
This study deals with the evaluation of inhibitory activity of secondary metabolites of ethylacetate partitioned-fraction of Adansonia digitata stem bark extract on malaria-associated protein using in silico docking studies.
Materials and methods
Molecular docking and virtual screening was performed to understand the mechanism of ligand binding and to identify potent calcium transporter inhibitors. The stem bark extracts of A. digitata contains rich sources of phytochemicals. The secondary metabolites were determined by HPLC-DAD and HRGC-MS analysis. The major chemical constituent present in the ethylacetate partitioned-fraction of A. digitata stem bark extract were examined for their antiplasmodial activity and were also involved in docking study.
Results
The secondary metabolites, quercetin and apigenin inhibited the formation of β-hematin. The results showed that all the selected compounds in the A. digitata showed binding energy ranging between -6.5 kcal/mol and -7.1 kcal/mol. Among the two chemical constituents, apigenin has the highest docking score along with the highest number of hydrogen bonds formed when compared to quercetin. Analysis of the results suggests that apigenin and quercetin could act as an anti-malaria agent.
Conclusion
Molecular docking analysis could lead to further development of potent calcium transporter inhibitors for the prevention and treatment of malaria and related conditions.
Keywords: Bioinformatics, Biocomputational method, Computational biology, Computer-aided drug design, Pharmaceutical science, Biochemistry, AutoDock tool, HPLC-DAD analysis, HRGC-MS analysis, Malaria, Secondary metabolites
1. Introduction
Protozoan malaria parasites of the genus Plasmodium go through repetitive cycles of asexual multiplication in suitable vertebrate hosts. After multiplication, the parasites develop inside the host's erythrocyte followed by the destruction of the host cell and invasion of new erythrocytes. Parasitism of Plasmodium presents a fascinating issue with respect to transport of ions and metabolites, because the parasite must adjust itself to both the intracellular and extracellular ionic environment.
Differences in the concentrations of Na+, K+ and Ca2+ in the plasma and erythrocyte cytosol of the host have been reported. In addition, the host-cell's cytosolic levels of K+ decrease significantly and the levels of Na+ also increases as the parasite develops within the cell, while a high K+ and low Na+ level is maintained in the parasite's cytosol [1]. This propose that the parasite has a way of maintaining constant cytosolic levels of the alkali cations even with respective to fluctuation of the cation levels in the host erythrocyte cytosol.
Within the red blood cell, the growth of human malaria parasite P. falciparum has been shown to require external calcium ion (Ca2+) and this is linked with obviously elevated calcium ion concentration intracellularly [2, 3]. The elevated Ca2+ concentration in the red blood cell is raised due to increase in plasma membrane permeability to the cation and a concurrent inhibition of its Ca2+-ATPase [4]. The significance of calcium ion signaling and its indirect consequence on malaria parasite cells have been reported to include regulation of transcription factors and cell cycle arrest [5].
The importance of the regulation of Ca2+ for malaria parasites have been indicated by the fact that disturbance in culture of Ca2+ transport by Ca2+ ionophores arrests the growth of P. falciparum, the human malaria parasite [6]. Regulation of the cytoplasmic concentration of Ca2+ is basically accomplished by the operation of Ca2+ pumps which transfer Ca2+ across cellular membranes against a concentration gradient [7]. Intracellular sequestration and extrusion of excess calcium is achieved through Ca2+-ATPase pump and a Na+-Ca2+ exchanger in the plasma membrane [8, 9]. Interference in intracellular Ca2+ homeostasis results to parasite death, making Ca2+ regulation an important target for antimalarial drugs.
Phytochemicals are biologically active, naturally occurring chemical compounds found in plants, which provide health benefits for humans in addition to those attributed to macronutrients and micronutrients. The major classes of phytochemicals with disease-preventing functions are antioxidant, anticancer, detoxifying, immunity-potentiating and neuropharmacological agents. Each class of these functional agents consists of a wide range of chemicals with different potency; examples of these plant chemicals are: flavonoids, saponins, alkaloids, tannins, glycosides, anthraquinones, sterols and triterpenes [10, 11]. A number of modern drugs have been isolated from natural sources and many of these isolations were based on the uses of the agents in traditional medicine [12]. Almost all medicinal plants have active ingredients which are responsible for most of the biological activities they exhibit (Table 2).
Table 2.
Compounds identified from Ethylacetate fraction of A. digitata extract using gas chromatography-mass spectrophotometry (GC-MS).
| Peak | RT (min) | % of Total Area | Compound |
|---|---|---|---|
| 1. | 37.36 | 1.99 | Bicyclo [3.1.1] Heptane |
| 2. | 38.58 | 7.19 | n-Hexadecanoic acid |
| 3. | 38.73 | 9.90 | n-Hexadecanoic acid |
| 4. | 39.68 | 1.84 | Phytol |
| 5. | 39.76 | 14.25 | Cis-13,16-Docosadienoic acid, methyl ester |
| 6. | 39.90 | 45.69 | Cis-vaccenic acid |
| 7. | 40.01 | 11.04 | Octadecanoic acid |
| 8. | 40.16 | 5.59 | 2-Propanone, 1-(3,5,5-trimethyl-2-cyclohexen-1-ylidene |
| 9. | 42.01 | 2.51 | Phthalic acid, hept-4-yl-Isohexeyl ester |
Adansonia digitata is a perennial plant, commonly called baobab, in English and “Ose” in Yoruba language. It is the most widespread of the Adansonia species on the African continent, found in the hot, dry savannahs of sub-Saharan Africa. A. digitata is recognized as an effective treatment for many diseases. It is indigenous in many African countries [13].
The plant parts, especially bark fibers, fruit pulp, seeds and leaves, have been used traditionally for medicinal and nutritional purposes [13, 14]. Studies by Ajaiyeoba [15], Musila et al. [16] and Adeoye and Bewaji [17], reported that A. digitata has significant antimalarial properties. Its medicinal applications include treatment of intestinal and skin disorders and various uses as anti-inflammatory, anti-pyretic and analgesic agents [18, 19].
Computational (in silico) methods have been developed and widely applied to pharmacology hypothesis development and testing. These in silico methods include database searching, quantitative structure-activity relationships, similarity searching, pharmacophore identification, computational modeling and docking. Such methods have seen frequent use in the discovery and optimization of novel molecules with affinity to a target, the clarification of absorption, distribution, metabolism, excretion and toxicity properties as well as physicochemical characterization [20]. This study investigates the inhibitory effects of the compounds identified from ethylacetate partitioned fraction of Adansonia digitata stem bark extract by high performance liquid chromatography-diode array detector (HPLC-DAD) and gas chromatography-mass spectrometry (HRGC-MS) with target protein; calcium transporters (Ca2+-ATPase and CAX) associated with malaria.
2. Materials and methods
2.1. Chemicals and reagents
Hematin, oleic acid, chloroquine diphosphate, sodium dodecyl sulfate (SDS), apigenin, quercertin, rutin, luteolin, gallic acid, chlorogenic acid and caffeic acid were obtained from Sigma-Aldrich, USA. Ethylacetate, Chloroform, dimethylsolfoxide (DMSO), hydrochloric acid (HCl), sodium acetate, sodium bicarbonate and sodium hydroxide pellet were obtained from BDH Chemicals Ltd, Poole, England. Others are of analytical grade.
2.2. Solvent partitioning
About 500mL of distilled water was added to 35g of the dried crude methanol extract of A. digitata stem bark to form a slury, this was poured inside a separating funnel and washed repeatedly with chloroform until near exhaustion. The marc remaining was finally washed with ethylacetate until near exhaustion. The aqueous fraction was filtered with a filter paper to remove plant fibers. The obtained fractions: chloroform fraction of A. digitata (CFAD) and ethylacetate fraction of A. digitata (EFAD) were concentrated under pressure using rotary evaporator at 40 °C. The concentrate was heated over a water bath to obtain a solvent free extract, which was stored in a refrigerator at 4 °C.
2.3. Analysis of secondary metabolites by high performance liquid chromatography-doide array detector (HPLC-DAD)
Analysis of secondary metabolites in EFAD was carried out using a reverse phase HPLC-DAD. A. digitata extracts (crude and partitioned fractions) at a concentration of 15 mg/mL was injected by means of a model SIL-20A Shimadzu Auto sampler. Separations were carried out using Phenomenex C18 column (4.6 mm × 250 mm x 5 μm particle size) according to the method described by Colpo et al. [21] with slight modifications. Quantifications were carried out by integration of the peaks using the external standard method. The chromatography peaks were established by comparing its retention time with those of reference standards and by DAD spectra (wavelength from 200 nm to 500 nm). All chromatography operations were carried out at ambient temperature and in triplicate as described by Boligon et al. [22].
2.4. Identification of chemicals using gas chromatography-mass spectrophotometry
Identification of constituents of ethylacetate partitioned fraction of A. digitata using HRGC-MS. The GC-MS analysis was performed using Agilent Technologies GC-MS (Model 7890A) equipped with Agilent 19091 S–433HP-5MS 5% phenyl methyl polysiloxane column (30 m × 250 μm x 0.25μm f.t). The analysis works on the principle that a mixture will separate into individual substances when heated. Pure helium gas was used as a carrier gas at a flow rate of 1.5 mL/min. GC-MS analysis resulting in chromatogram was compared to complete library using data base of National Institute of Standard and Technology (NIST).
2.5. In vitro antiplasmodial effects of apigenin and quercetin
Antiplasmodial activity of apigenin and quercetin from ethylacetate partitioned fraction of A. digitata stem bark extract was evaluated by the method explained by Afshar et al. [23] with some modifications. Different concentrations (0.02–0.32 mg/mL in DMSO) of the extracts and pure compounds (apigenin and quercetin) were prepared. 100μL of the sample was added to the reaction mixture which contains 250 μL each of 3 mM of hematin, 10 mM oleic acid, and 1 M HCl. The volume of the resulting mixture was adjusted to 1.0 mL using sodium acetate buffer, pH 5, and incubated for about 12 h over night at 37 °C. Chloroquine diphosphate and artesunate were used as a positive control. After incubation, samples were centrifuged (using refrigerated Sigma 3–30K, Germany) at 14000 rpm for 10 min. The hemozoin pellets were washed repeatedly and incubated at 37 °C for 15 min with constant shaking in 2.5% (w/v) SDS in phosphate buffered saline. Then, the final wash was done using 0.1 M sodium bicarbonate until a clear supernatant is observed which is usually achieved in three washes. The supernatant was discarded after the final wash and the pellets were dissolved in 1.0 mL of 0.1 M NaOH. Hemozoin content was determined by measuring the absorbance with a UV-visible spectrophotometer at 400 nm. The results were expressed as % inhibition (I%) of heme crystallization using the following equation:
| I% = [(AN−AS)/AN]×100 |
where, AN = absorbance of control and AS = absorbance of samples.
2.6. Target identification
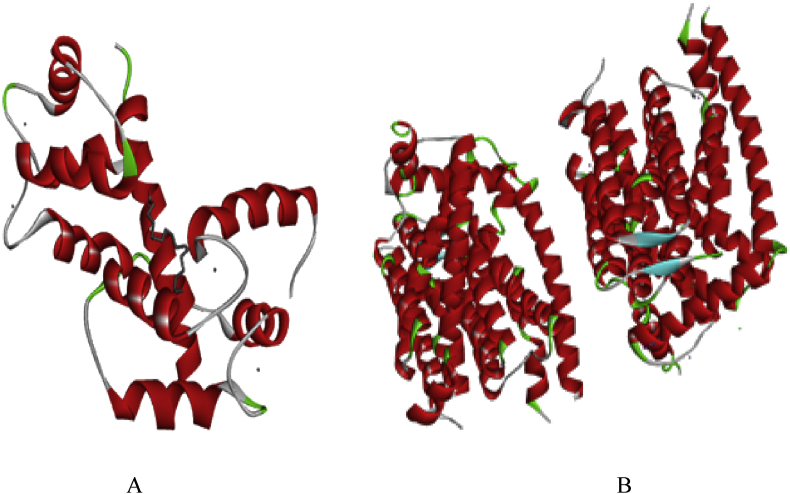
To study the nature of interactions, binding mode and selectivity of calcium transporters with secondary metabolites of A. digitata, docking was carried out with AutoDock 4.2. AutoDock Tool was used for creating PDBQT files from traditional PDB files [24]. The three dimensional structure of calcium ATPase Serca (PDB ID: 2KNE) [25] and calcium transporters (CAX) (PDB ID: 4K1C) [26] was obtained from Protein Data Bank (PDB) database. This structure was determined using X-ray diffraction (Fig. 1).
Fig. 1.
3D structure of malaria-associated calcium transporter proteins. Calcium ATPase (2KNE) (A) and Calcium transporter CAX (4K1C) (B).
2.7. Ligand identification
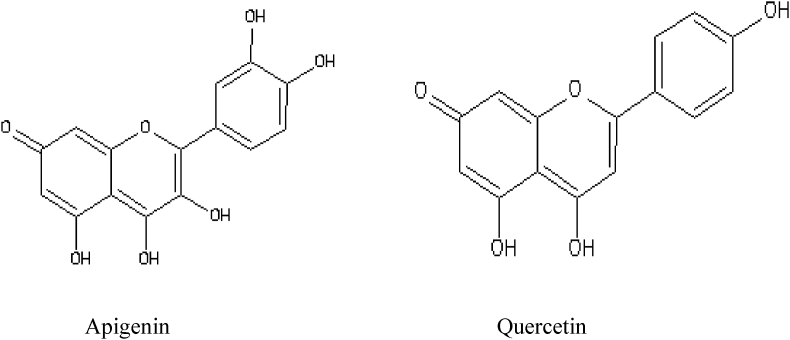
The major secondary metabolites present in the ethylacetate fraction of A. digitata stem bark extract (apigenin and quercetin) quantified and identified by HPLC-DAD were used as the ligand. The ligands were retrieved from www.zinc.docking.org. The structure was downloaded in Mole 2 file format and was then converted to PDB format using OPEN BABEL 2.2.1 [27] and further used for docking studies (Fig. 2).
Fig. 2.
2D structure of the ligands.
2.8. Molecular docking studies
Calcium ATPase (2KNE) and Ca transporter CAX (4K1C) were downloaded from protein databank ( http://www.rcsb.org/). Apigenin, quercetin were used as the ligand and they were downloaded from http://www.zinc.docking.org. The various ligands and water molecules were deleted from the original protein. The ligands were re-docked into the active site of each protein as a method of revalidation. A flexible cis-vaccenic acid, apigenin and quercetin structures were prepared in a PDBQT file with appropriate torsion numbers. Molecular docking was carried out using AutoDock vina [28]. The root mean square deviation (RMSD) and affinity energy [28] were used in selecting the best interaction proses. The protein-ligand interactions were identified using discovery studio visualizer (version 16).
2.9. AutoDock calculation
Docking can be performed using different methods. The most effective method is Lamarckian genetic algorithm. AutoDock was run several times to obtain various docked conformations, and used to analyze the predicted docking energy. The binding sites for these molecules were selected based on the ligand-binding pocket of the templates [29]. Auto dock tools provide a choice of methods to analyze the results of docking simulations such as, conformational similarity, visualizing the binding site and its energy and other parameters like intermolecular energy and inhibition constant [30].
2.10. Statistical analysis
Results calculated from replicate data were expressed as means ± standard error of means (SEM). Results were analyzed using one-way ANOVA followed by Tukey post-hoc test for comparison of means between control and test groups. The level of significance was set at p < 0.05. Statistical analysis and inhibitory concentration at 50% (IC50) were performed using Graph Pad Prism (ver.5.0a).
3. Results
3.1. Secondary metabolites of partitioned fraction of crude extract of A. digitata stem bark
Natural foods and food-derived antioxidants such as phenolic phytochemicals have interesting biological properties. The secondary metabolite profiles of compounds and vegetables matrices change on the base of season and cultivar, as reported by Blasi et al. [31] and on the base of extraction methods, as reported by Rocchetti et al. [32].
Table 1 shows the phenolics and flavonoids composition of partitioned fraction of crude extract of A. digitata stem bark. HPLC finger printing of partitioned fractions of A. digitata revealed the presence of gallic acid (tR = 11.87 min, peak 1), chlorogenic acid (tR = 21.09 min, peak 2), caffeic acid (tR = 23.49 min, peak 3), rutin (tR = 33.71 min, peak 4), quercetin (tR = 41.93 min, peak 5), luteolin (tR = 51.78 min, peak 6) and apigenin (tR = 62.34 min, peak 7).
Table 1.
Phenolic acids and flavonoid composition of partitioned fraction of crude extract of A. digitata stem bark using HPLC-DAD.
| Compounds | EFAD (mg/g) | CFAD (mg/g) |
|---|---|---|
| Gallic acid | 2.35 ± 0.02a | 0.06 ± 0.01a |
| Chlorogenic acid | 4.86 ± 0.03b | 0.09 ± 0.04a |
| Caffeic acid | 2.29 ± 0.01a | 0.17 ± 0.02b |
| Rutin | 1.07 ± 0.01c | - |
| Quercetin | 6.19 ± 0.02d | - |
| Luteolin | 4.54 ± 0.01b | - |
| Apigenin | 7.60 ± 0.01e | - |
EFAD = Ethyl acetate partitioned fraction of A. digitata and CFAD = Chloroform partitioned fraction of A. digitata; the mean of: – (not detected).
Results are expressed as mean ± standard error of three determinations. Averages followed by different letters differ by Tukey test at p < 0.05.
3.2. Effects of ethylacetate partitioned fraction of A. digitata and some phenolics on β-hematin (synthetic hemozoin) formation
The results from the cell free β-hematin formation assay of ethylacetate partitioned fraction of A. digitata stem bark extract and some secondary metabolites are shown in Table 3. The inhibition of β-hematin formation is presented as percentage (% I). Artesunate and chloroquine were used as positive control with the most potent antiplasmodial activity and the extracts solvent (DMSO) was used as negative control without any antiplasmodial activity. The secondary metabolites and the extract fraction had considerable antiplasmodial activity especially apigenin which showed the most potent activity when compared to the reference antimalarial drugs. The IC50 values of ethylacetate extract fraction, quercetin, and apigenin are 0.065, 0.049, and 0.107 respectively while that of artesunate and chloroquine are 0.005 and 0.002 respectively (Table 4).
Table 3.
Percentage inhibition (%) of β-hematin formation.
| Conc. mg/mL | EFAD | Chloroquine | Artesunate | Quercetin | Apigenin |
|---|---|---|---|---|---|
| 0.02 | 4.88 ± 0.00c | 6.27 ± 0.01c | 28.57 ± 0.06a | 5.23 ± 0.00c | 12.54 ± 0.01b |
| 0.04 | 5.92 ± 0.00c | 40.77 ± 0.01a | 53.31 ± 0.02a | 9.06 ± 0.00c | 22.65 ± 0.04b |
| 0.08 | 17.77 ± 0.05b | 46.69 ± 0.01a | 54.01 ± 0.01a | 25.09 ± 0.05b | 23.34 ± 0.03b |
| 0.16 | 21.25 ± 0.04c | 47.74 ± 0.01a | 54.70 ± 0.01a | 25.44 ± 0.05c | 38.33 ± 0.01b |
| 0.32 | 22.30 ± 0.04c | 48.08 ± 0.01a | 55.75 ± 0.01a | 25.78 ± 0.05c | 42.50 ± 0.01b |
The results are mean ± SEM of three determinations.
Table 4.
The IC50 values of ethylacetate extract fraction and the phenolics.
| Compound/Extract | IC50 (mg/mL) |
|---|---|
| EFAD | 0.065 |
| Chloroquine | 0.002 |
| Artesunate | 0.005 |
| Quercetin | 0.049 |
| Apigenin | 0.107 |
3.3. Molecular docking
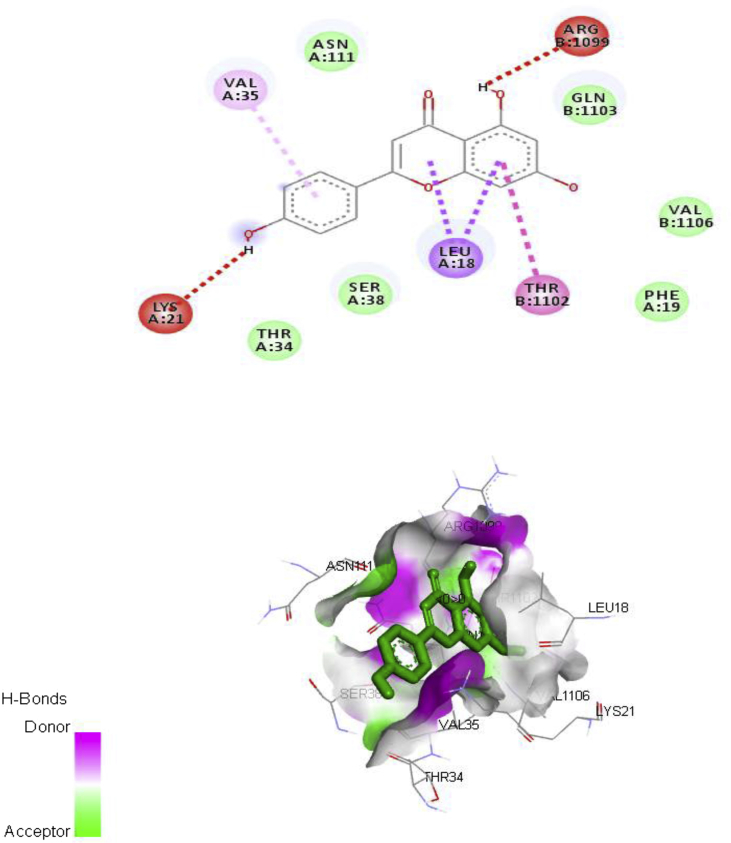
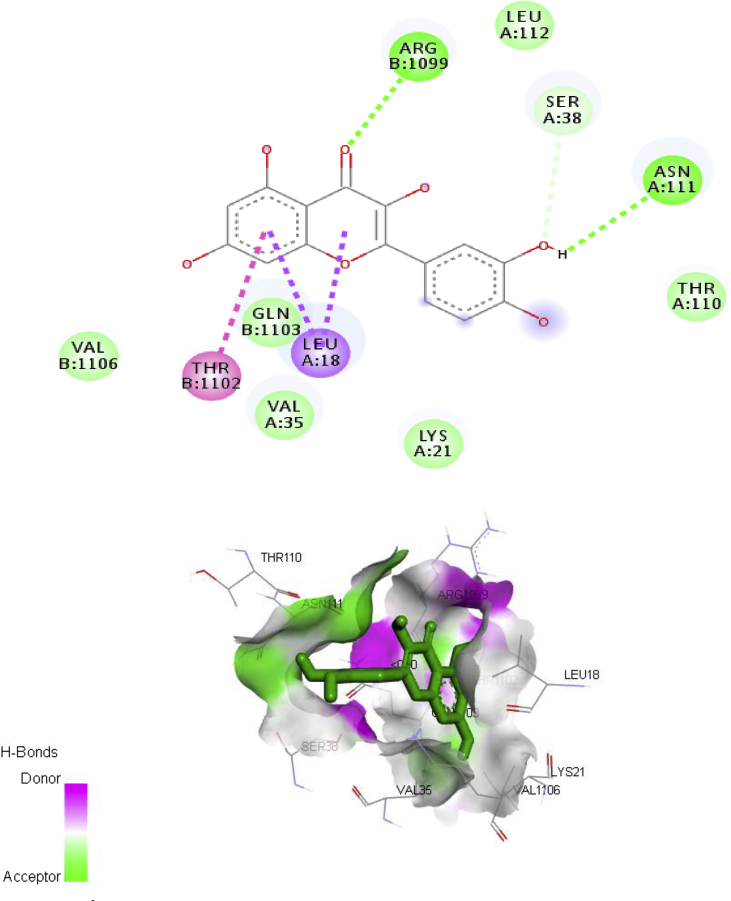
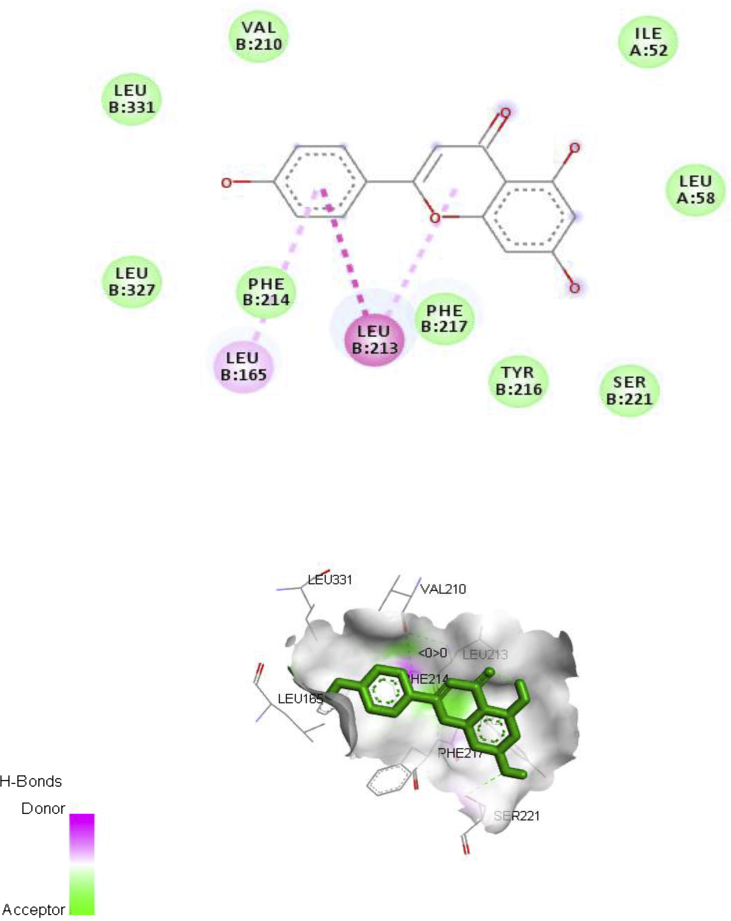
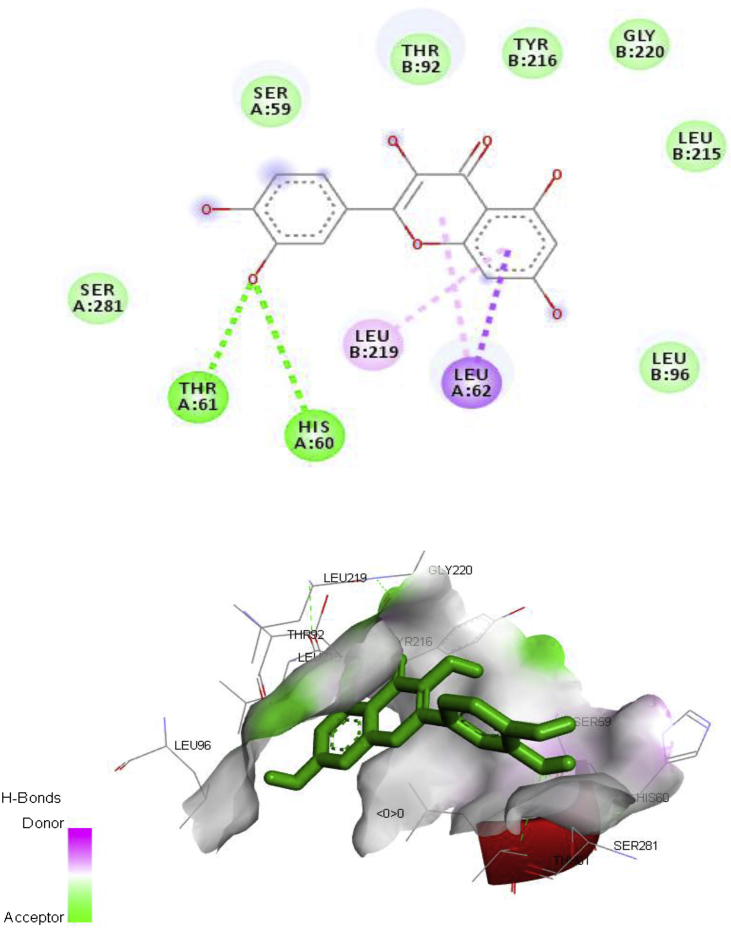
The major chemical constituent present in the ethylacetate partitioned-fraction of A. digitata stem bark extracts were involved in docking study (Figs. 3, 4, 5, and 6) using AutoDock 4.2, based on the Lamarckian principle. The results revealed that all the selected compounds in the A. digitata showed binding energy ranging from -6.5 kcal/mol to -7.1 kcal/mol. Among two chemical constituents, apigenin has the highest docking score along with the highest number of hydrogen bonds when compared to quercetin. The interaction of these constituents with the Ca2+ATPase (2KNE) and Ca2+ transporter CAX (4K1C) is presented in Table 5.
Fig. 3.
Ca2+ATPase (2KNE) – Apigenin interaction.
Fig. 4.
Ca2+ATPase (2KNE) – Quercetin interaction.
Fig. 5.
Ca2+ transporter CAX (4K1C) – Apigenin interaction.
Fig. 6.
Ca2+ transporter CAX (4K1C) – Quercetin interaction.
Table 5.
Summary of binding affinities and ligand-amino acid interactions.
| S/N | Protein | Compound | Binding affinities (Kcal/mol) | Ligand-Amino acid interactions |
|---|---|---|---|---|
| 1. | Calcium ATPase SERCA | Apigenin | -6.6 | Lys21, Leu18, Thr1102, Arg1099, Val35 |
| Quercetin | -6.5 | Thr1102, Leu18, Asn111, Ser38, Arg1099 | ||
| 2. | CAX | Apigenin | -7.1 | Leu213, Leu165 |
| Quercetin | -6.8 | Thr61, His60, Leu219, Leu62 |
4. Discussion
Many of the antioxidants and therapeutic actions of phytochemicals are thought to be associated with their biologically active polyphenol components, such as flavonoids and phenolic acids, which has powerful antioxidant activities [33].
High performance liquid chromatography (HPLC) finger printing is a tool for estimating the phytochemicals in plant samples. It is a technique employed to identify, separate, quantify and purify individual components of a mixture. It gives both quantitative and qualitative information and makes possible the screening of samples for the presence of new compounds. The HPLC fingerprint of the ethylacetate partitioned fraction of crude extract of A. digitata stem bark (EFAD) showed the presence of phenolics (gallic, chlorogenic and caffeic acids) and flavonoids (rutin, quercetin, luteolin and apigenin). In the EFAD, chlorogenic acid, quercetin, apigenin and luteolin constituted the bulk of phenolics with apigenin being the most abundant. The amount of apigenin is statistically higher when compared to other flavonoids present in the EFAD. The observations from this study however supports the claim that quercetin and apigenin are well known as flavonoid with strong antioxidant properties capable of efficiently scavenging lipid free radicals and hydroxyl radicals and also reported to reduce oxidative stress.
During proteolysis of haemoglobin in the parasite food vacuole where hemoglobin is enzymatically digested, large quantities of toxic heme are released which are rapidly converted to highly insoluble and relatively unreactive microcrystalline dimer called hemozoin. Chloroquine and related drugs have been demonstrated to inhibit synthetic hemozoin (β-hematin) formation.
In the in vitro studies, EFAD exhibited a considerable activity by inhibiting the formation of β-hematin. The secondary metabolites, quercetin and apigenin also had considerable antiplasmodial activity. The percentage inhibition of β-hematin formation by apigenin was comparable to the standard drug chloroquine and artesunate. Artesunate was observed to have higher percentage inhibition of β-hematin formation but its IC50 was higher than chloroquine. This could be as a result of the mechanism of action of artesunate which has been attributed to the generation of free radicals [34] through the cleavage of its endoperoxide bridge leading to free radicals and oxidative stress [35, 36] which may not be through inhibition of hemozoin polymerization. The mechanism of action of chloroquine against Plasmodium parasite has been reported and established to be through inhibition of hemozoin polymerization. The result of this study is in line with other previous work reported. Chloroquine had the highest antiplasmodial activity with the lowest IC50 value when compared to artesunate, other phenolics and the extract.
Protein-ligand docking is an essential tool for computational drug design and it is widely used in pharmaceutical companies [37]. Protein–ligand docking is used to predict the position and orientation of a ligand when it is bound to a protein receptor or enzyme. Pharmaceutical research employs docking techniques for a variety of purposes, mostly in the virtual screening of large databases of available chemicals in order to select likely drug candidates. In order to understand the mechanism of ligand binding and to identify potent calcium transporter inhibitors, a study involving molecular docking and virtual screening was performed. Out of three chemical constituents, apigenin and quercetin has the highest docking score along with the highest number of hydrogen bonds formed while the cis-vaccenic acid has the least docking score with the least hydrogen bonds. Analysis of the results of the autodock software shows that apigenin, quercetin and cis-vaccenic acid have a considerable binding affinity with the calcium transporters.
5. Conclusion
From this study, apigenin and quercetin inhibit the formation of β-hematin. The in silico molecular docking analysis revealed that apigenin has the highest binding affinity with the highest number of hydrogen bond. This study suggests that apigenin and quercetin could serve as inhibitors of calcium transport proteins associated with malaria. However, further, work can be extended to experimental animals to study the effect of apigenin and quercetin on malaria.
Declarations
Author contribution statement
Akinwunmi O. Adeoye: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
John O. Olanlokun, Habib Tijani: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Segun O. Lawal, Cecilia O. Babarinde, Mobolaji T. Akinwole: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Clement O. Bewaji: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Pillai A.D., Addo R., Sharma P., Nguitragool W., Srinivasan P., Desai S.A. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodeling. Mol. Mircobiol. 2013;88:20–34. doi: 10.1111/mmi.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Strehler E.E., Caride A.J., Filoteo A.G., Xiong Y., Penniston J.T., Enyedi A. Plasma membrane Ca2+ATPases as dynamic regulators of cellular calcium handling. Ann. N. Y. Acad. Sci. 2007;1099:226–236. doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagamune K., Moreno S.N., Chini E.N., Sibley L.D. Calcium regulation and signaling in the apicomplexan parasites. Subcell. Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 6.Alleva L.M., Kirk K. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 2001;117:121–128. doi: 10.1016/s0166-6851(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 7.Hajnoczky G., Csordas G., Madesh M., Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J. Physiol. 2000;529(1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia C.R.S. Calcium homeostasis and signaling in the blood-stage malaria parasite. Parasitol. Today. 1999;15(12):488–491. doi: 10.1016/s0169-4758(99)01571-9. [DOI] [PubMed] [Google Scholar]
- 9.Hajnoczky G., Csordas G., Yi M. Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/s0143416002001872. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi Nat. Prod. Commun. 2015;10(8):1403–1408. [PubMed] [Google Scholar]
- 11.Cossignani L., Blasi F., Simonetti M.S., Montesano D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food analytical methods. 2018;11(4):1180–1188. [Google Scholar]
- 12.Rizvi M.M.A., Irshad M., Hassadi G.E., Younis S.B. Bioefficacies of Cassia fistula: an Indian labrum (review) Afr. J. Pharm. Pharmacol. 2009;3(6):287–292. [Google Scholar]
- 13.Sidibe M., Williams J.T. 4th International Centre for Underutilised Crops, Southampton, UK. 2002. Baobab – Adansonia digitata. Fruits for the future; pp. 96–105. [Google Scholar]
- 14.Chadare F.J., Linnemann A.R., Hounhouigan J.D., Nout M.J.R., Van Boekel M.A.J. Baobab food products: a review on their composition and nutritional value. Crit. Rev. Food Sci. Nutr. 2009;49:254–274. doi: 10.1080/10408390701856330. [DOI] [PubMed] [Google Scholar]
- 15.Ajaiyeoba E.O. Vol. 167. 2005. In vivo antimalarial activity of methanolic extract of A. digitata stem bark in mice model. New Strategy against Ancient Scourge. (Proceedings of the 4th Multilateral Initiative on Malaria Pan-Africa Malaria Conference Yaounde). [Google Scholar]
- 16.Musila M.F., Dossaji S.F., Nguta J.M., Lukhoba C.W., Munyao J.M. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J. Ethnopharmacol. 2013;146:557–561. doi: 10.1016/j.jep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Adeoye A.O., Bewaji C.O. Therapeutic potentials of Adansonia digitata (Bombacaceae) stem bark in Plasmodium berghei-infected mice. J. Biol. Sci. 2015;15:78–84. [Google Scholar]
- 18.Ramadan A., Harraz F.M., El-Mougy S.A. Anti-inflammatory, analgesic and antipyretic effects of the fruit pulp of Adansonia digitata. Fitoterapia. 1994;65:418–422. [Google Scholar]
- 19.Karumi Y., Augustine A.I., Umar I.A. Gastroprotective effects of aqueous extract of Adansonia digitata leaf on ethanol-induced ulceration in rats. J. Biol. Sci. 2008;8:225–228. [Google Scholar]
- 20.Ekins S., Mestres J., Testa B. In silico pharmacology for drug discovery: applications to targets and beyond. Br. J. Pharmacol. 2007;152(1):21–37. doi: 10.1038/sj.bjp.0707306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colpo E., Vilanova C.D., Reetz L.G., Duarte M.M., Farias I.L., Meinerz D.F. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition. 2014;30:459–465. doi: 10.1016/j.nut.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Boligon A.A., Piana M., Schawnz T.G., Pereira R.P., Rocha R.B.T., Athayde M.L. Chromatographic analysis and antioxidant capacity of Tabernaemontana catharinensis. Nat. Prod. Commun. 2014;9(1):61–64. [PubMed] [Google Scholar]
- 23.Afshar F.H., Delazar A., Janneh O., Nazemiyeh H., Pasdaran A., Nahar L. Evaluation of antimalarial, free-radical scavenging and insecticidal activities of Artemisia scoparia and A. spicigera, Asteraceae. Braz. J. Pharmacol. 2011;21:986–990. [Google Scholar]
- 24.Khodade P., Prabhu R., Chandra N. Parallel implementation of autodock. J. Appl. Crystallogr. 2007;40:598–599. [Google Scholar]
- 25.Juranic N., Atanasova E., Filoteo A.G., Macura S., Prendergast F.G., Penniston J.T., Strehler E.E. Calmodulin wraps around its binding domain in the plasma membrane Ca2+ pump anchored by a novel 18-1 motif. J. Biol. Chem. 2010;285:4015–4024. doi: 10.1074/jbc.M109.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waight A.B., Pederson B.P., Schlessinger A., Bonomi M., Chau B.H., Roe-Zuru Z., Risenmay A.J., Sali A., Stroud R.M. Structural basis for altering access of eukaryotic calcium/proton exchanger. Nature. 2013;499:107–110. doi: 10.1038/nature12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner J.G., Kevin E.G., Mark W., David D.A., Cornelis J.V. Optimizing the use of opensource software applications in drug discovery. Drug Discov. Today. 2006;11(3):127–132. doi: 10.1016/S1359-6446(05)03692-5. [DOI] [PubMed] [Google Scholar]
- 28.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multi-threading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang M.W., Ayeni C., Breuer S. Virtual screening for HIV protease inhibitors: a comparison of AutoDock 4 and vina. PLoS One. 2010;5:11955. doi: 10.1371/journal.pone.0011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H., Lee J., Lee S. Critical assessment of the automated AutoDock as a new docking tool for virtual screening. Proteins. 2006;65:549–554. doi: 10.1002/prot.21183. [DOI] [PubMed] [Google Scholar]
- 31.Blasi F., Urbani E., Simonetti M.S., Chiesi C., Cossignani L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016;89:202–207. [Google Scholar]
- 32.Rocchetti G., Blasi F., Montesano D., Ghisoni S., Marcotullio M.C., Sabatini S., Lucini L. Edible nuts deliver polyphenols and their transformation products to the large intestine: impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019;115:319–327. doi: 10.1016/j.foodres.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 33.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efferth T., Sauerbrey A., Olbrich A., Gebhart E., Rauch P., Weber H.O. Molecular models of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 35.Meshnick S.R., Yang Y.Z., Lima V., Kuypers F., Kamchonwongpaisan S., Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu) Antimicrob. Agents Chemother. 1993;37:1108–1114. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner G.H., Oh C.H., Wang D., Gerena L., Milhous W.K., Meshnick S.R. Mechanism-based design, synthesis and in vitro antimalarial testing of new 4-methylated trioxanes structurally related to artemisinin: the importance of a carbon-centered radical for antimalarial activity. J. Med. Chem. 1993;37:1256–1258. doi: 10.1021/jm00035a003. [DOI] [PubMed] [Google Scholar]
- 37.Kolb P., Ferreira R.S., Irwin J.J., Shoichet B.K. Docking and chemoinformatic screens for new ligands and targets. Curr. Opin. Biotechnol. 2009;20:429–436. doi: 10.1016/j.copbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]