Abstract
Oxidative stress is an exclusive biochemical complication affecting reproduction; hence, dietary antioxidant supplementation for its attenuation is a required nutrition – reproduction improvement strategy. On this background, Chlorella vulgaris (a natural antioxidant) was supplemented to grower female rabbits to maturity. The rabbits were thirty-five in number randomly distributed into five experimental groups in a completely randomized design. Control group was fed only basal feed while treatment groups were fed diets containing 40 %, 60 %, 80 % and 100 % Chlorella vulgaris biomass as T1, T2, T3 and T4 respectively at 500 mg per animal body weight (kg) along with the basal feed daily. Performance records were obtained, blood was collected, and at the end uterus, ovaries and liver were removed from sacrificed animals for analysis. Serum, uterus and liver oxidative stress status were determined while RNA isolated from liver and ovaries samples were used for antioxidant genes expression analysis. Oxidative stress status and antioxidant enzymes activities were determined using chemical assays while antioxidant gene expression levels were determined using real-time quantitative PCR system. There was significant difference in feed intake (p < 0.014), final body weights (p < 0.008), empty carcass weights (p < 0.001) and commercial carcass weights (p < 0.001) of the rabbits as results of the microalgae supplementation. There was also significant difference in malondialdehyde (MDA) concentrations (p < 0.050), total antioxidant capacity (TAC) (p < 0.050) and protein carbonyl (PCO) concentrations (p < 0.050) due to the supplementation of the microalgae; in addition, supplementation of the microalgae significantly improved activities of superoxide dismutase (SOD) (p < 0.050), catalase (CAT) (p < 0.050) and reduced glutathione (GSH) concentration (p < 0.050). Furthermore, there was significant difference in relative expression of primary antioxidant genes sod1 (p < 0.050) and gpx1 (p < 0.050); however, there was no significant difference in relative expression of bre (p > 0.050) and ucp1 (p > 0.050). The study concluded from the outcomes stated above that supplementation of microalgae Chlorella vulgaris improved performances of rabbits through attenuation of oxidative stress, enhancement of antioxidant enzymes activities as well as up-regulation of primary antioxidant genes. Hence, it was recommended as dietary supplement for protection against oxidative stress and improved productivity in rabbits and other food producing mammalian species. In addition, further studies into assessment of its effects on expression of transcripts and immune modulation genes in rabbits and other animals is warranted as future studies in order to established its potential as beneficial nutraceutical for animals and human.
Keywords: Agriculture, Biotechnology, Cancer research, Genetics, Physiology, Toxicology, Molecular biology, Zoology, Oxidative stress, Chlorella vulgaris, Antioxidant enzymes, sod1, gpx1
1. Introduction
Oxidative stress negatively affects reproductive system and reproductive outputs starting from oocytes production, conception, and embryo development to parturition. Oxidative stress complexity could be trace to mitochondria leakages of electrons during stimulation of NADPH via oxidative phosphorylation causing cascades of cellular and tissue damage as results of compromised internal antioxidant defence against prooxidants [1, 2, 3]. Dietary supplementation of antioxidants is one of the most valid strategies for its management because oxidative stress is almost inevitable especially in animals due to increased energy requirement associated with growth and economic activities such as reproduction, milk and meat yield [4]; additional antioxidants supplied exogenously complement innate antioxidant apparatus of the body together for protection against activities of free radical generations and halting cascades of oxidative stress damages [5].
Oxidative stress as an imbalance between prooxidants and antioxidants compromise animal performance and production, reduce animal productivity and negatively impact animals’ health; therefore, because antioxidants are capable of attenuating the menace, antioxidant supplementation is not only a nutritional strategy for improving productivity but also a palliative health promotion strategy for increasing animal production outputs [6]. However, commonly used synthetic antioxidants including butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tertiary butyl hydroquinone (TBHQ) for management of oxidative stress in food producing animals are becoming source of growing concerns because of their carcinogenic tendencies which remains a major threat to health; therefore, exploration of natural antioxidants for oxidative stress attenuation is a required animal production and health research [7].
Microalgae are examples of natural sources of antioxidants for potential exploration because they contained modified metabolites of functional benefits suitable for improving quality of human and animal lives [8]. Some of these secondary metabolites in microalgae are biological resources with industrial applications such as thickeners and or emulsifiers in food processing, complementary drugs in nutraceutical and pharmaceuticals industries; and biomedical materials in alternative medicine practices as well as feed additives in animals and fisheries production [9]. Their mechanisms of actions are diverse from direct scavenging on free radicals or prooxidant to inhibiting cascades of biochemical processes leading to prooxidants generation of oxidative stress products.
Chlorella vulgaris is a popular microalga commercially cultivated across the world and rich in bioactive compounds including carotenes, astaxanthin, lutein and fucoxanthin; in addition to these compounds, it was also reported to contain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which are all bioactive compounds of functional values [10]. The microalga is also identified as direct scavenger of free radicals causing oxidative stress; and according to Rodriguez-Garcia et al. [11] the microalga has higher antioxidants power compared with synthetic antioxidants commonly used in foods and animal production; it is a natural growth promoter, immune booster, and tissues rebuilder [12, 13]. Therefore, this study because of antioxidant potential of Chlorella vulgaris investigated effects of its supplementation on performance and oxidative stress in rabbits through assessment of oxidative stress products generation, antioxidant enzymes activities and expression of primary antioxidant genes.
2. Materials and methods
2.1. Location and duration of the study
The experiment duration was 120 days; animal experimentation took place at the Laboratory Animal Section of Experimental Livestock Unit (ELU) of ICAR – National Institute of Animal Nutrition and Physiology (NIANP), Bangalore, India. The institute is located at Adugodi a suburb of Bangalore city; Karnataka, India. It is a Tropical Savannah area located on coordinates longitude 12.97o N and latitude 77.56o E; average monthly temperature of the area is 23.9 °C while warmest temperature is 27.6 °C, annual rainfall is 970 mm usually spread clearly in a season different from dry season. Approval of the animal experimentation protocol was given from a joint consideration of Institutional Animal Ethics Committee (IAEC) of ICAR – NIANP, Bangalore; India and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
2.2. Animals and their management
Thirty-five female growing New Zealand White rabbits were used for the study. The animals were divided into 5 experimental groups of 7 animals per group in a complete randomized design experiment. The animals were sourced from Biogen Biotechnology (P) Ltd., Laboratory Animal Facility, Attibele; Anekal - Taluk, Bangalore - 562107, India. Biogen is a laboratory animal breeding company in India with registration number 971/PO/RcBiBt/S/2006/CPCSEA. Upon delivery of the rabbits to the animal house, they were randomly distributed into the experimental groups and acclimatized for a period of 14 days before commencement of the trial.
The rabbits were housed individually in aluminium coated wire mesh cages; animals in control group were fed only basal feed without Chlorella vulgaris supplements while animals in treatment groups were supplemented with 200, 300, 400, and 500 mg Chlorella vulgaris biomass as T1, T2, T3 and T4 respectively along with the basal feed daily throughout the experimental period while water and basal feed (Krishna Valley Agrotech, India) were provided ad-libitum via automatic nipple drinkers and feeding trough. Nutri-functional properties of the supplement diets were determined through subjected to proximate and in-vitro free radical attenuation analysis (Table 1).
Table 1.
Chemical and nutri-functional composition of the Chlorella vulgaris supplement diets.
| Parameters | T1 | T2 | T3 | T4 | Units |
|---|---|---|---|---|---|
| Dry matter | 93.95 | 94.38 | 96.54 | 92.33 | % dry matter |
| Crude protein | 30.46 | 44.36 | 53.86 | 61.15 | % of dry matter |
| Total ash | 2.23 | 3.59 | 4.30 | 5.28 | % of dry matter |
| Metabolizable energy | 3433 | 3025 | 3183 | 3648 | Kcal/Kg |
| Crude fibre | 0.92 | 1.75 | 1.71 | 2.90 | % of dry matter |
| Ether extract | 1.22 | 1.13 | 1.18 | 0.80 | % of dry matter |
| Chlorella vulgaris | 200 | 300 | 400 | 500 | mg/500 mg |
| Total phenolic content | 39.38 | 36.28 | 41.16 | 43.93 | mg/g ellagic acid equivalent |
| Total antioxidant concentration | 670.30 | 601.21 | 760.00 | 902.54 | μmol/g ascorbic equivalent |
| H2O2 Scavenging Power | 47.44 | 23.26 | 59.58 | 41.14 | % |
| FRAP | 1.34 | 1.20 | 1.52 | 2.48 | -n/a- |
Source: Laboratory analysis. Ferric Reducing Antioxidant Power (FRAP).
Chlorella vulgaris used in this study was an organically produced commercial microalga rich in antioxidant and other bioactive beneficial compounds procured from Seagrass Tech (Pvt.) Ltd, 600087 – India. The microalgae was manufactured organically without use of synthetic chemical in closed batch production system, it was delivered as a fine green powder biomass screened for its safety of use in animal experimental consumption by evaluation of its heavy metal and microbial compositions. These assessments indicated that the microalga in its biomass form used in this study is safe for animals’ consumption (Table 2).
Table 2.
Sample information of Chlorella vulgaris biomass used in this study.
| Parameters | Methods | Specification | Analysis |
|---|---|---|---|
| Physical appearance | Visual examination | Fine green powder | Fine green powder |
| Particle sizes | AOAC 973.03 | 100 % pass through 100 mesh | Complies |
| Arsenic as As | AOAC 993.14 | <0.5 ppm | Below detection limit |
| Lead as Pb | AOAC 993.14 | <1 ppm | Below detection limit |
| Aerobic plate count | AOAC 966.23 | <200,000 cfu/g | 300 cfu/g |
| E. Coli | AOAC 991.14 | Absent | Absent |
| Salmonella sp | AOAC 998.09 | Absent | Absent |
| Staphylococcus aureus | AOAC 975.55 | Absent | Absent |
| Coliforms | AOAC 966.24 | <10 cfu/g | <10 cfu/g |
| Yeast/mold | AOAC 997.02 | <500 cfu/g | <10 cfu/g |
| Chlorophyll | AOAC 942.04 | 0.25 % | 2.67 % |
| Beta carotene | AOAC 955.10 | Nil | 0.1 mg/g |
| Packaging | Bagging | Dry, cool away from light | 1 kg/bag |
Source: Seagrass Tech Lab (2018).
2.3. Observation and recording of animal performances
Performance parameters were recorded daily, weekly and at the end of the study. Feed intake was recorded daily from evaluation of differences in feed served and remnants by each animal in gram (g). On weekly basis, body weight change was determined from difference in body weight of the current week and body weight of the previous week for each animal using small digital scale with accuracy of 0.2 g (Atom Scale, India).
2.4. Determination of serum and tissues oxidative stress status
2.4.1. Sample collection and storage
Blood was collected from each animal into vacutainers kept at room temperature for approximately 15 min allowing clotting then centrifuged at 2000 rpm for 10 min using bench top centrifuge (Remi Centrifuge, India). Supernatant layers were collected into test bottles and kept at – 80 °C for downstream analysis. For tissue oxidative stress markers, at the end of the experiment the animals were randomly selected from each group and sacrificed by sharp cutting through jugular vein after being fasted overnight. Liver and uterus of the sacrificed animals were removed into phosphate buffer (pH 7.4), snap frozen in liquid nitrogen then stored at – 80 °C for downstream enzymes analysis.
2.4.2. Preparation of tissue homogenates
Tissues homogenates were prepared as 10 % in Tris HCl buffer (1 mM ethylene diamine tetra acetic acid, 1 mM dithiothreitol and 50 mM Tris HCl) used for antioxidant enzymes analysis. The tissues homogenates were prepared with 100 mg of each tissue sampled in triplicates then lysed under cold liquid nitrogen and added with 1 mL Tris HCl buffer as homogenizer followed by centrifuge at 3500 rpm for 15 min at 4 °C. Collected supernatants were stored at – 80 °C for downstream antioxidant enzymes activities.
2.4.3. Protein estimation
Amount of protein in tissue homogenates was determined using Bicinchoninic Acid (BCA) Protein Assay Kit (G-Biosciences, USA). According to the manufacturers’ protocol; briefly, it involves preparation of protein standards of known concentrations using bovine serum albumin (BSA) through serial dilution of 300 μL of BSA from stock into 150 μL Tris HCl buffer; then to 25 μL of each homogenate sample and standards, 200 μL BCA working solution was added and incubated at 37 °C (Labnet Inc., USA). After the incubation, absorbance of the samples and the standards were determined at 562 nM using Thermo Multiskan GO micro plate reader (Thermo Fisher Scientific, Finland).
2.4.4. Serum and tissues superoxide dismutase enzymes activities
Enzymatic activities of superoxide dismutase were determined using chemical assay developed on the background of pyrogallol autooxidation in the presence of diethylenetriaminepentaacetic acid (DEPTA) at pH 8.5 by 50 %; this principle was established and verified by Marklund and Marklund [14] and its modification according to Guvala et al [15] was adopted for use in this study. The chemical assay comprised of 50mM Tris cacodylate buffer, 1mM DEPTA and 20mM Pyrogallol solution prepared in 10 mM HCl. The assay was used to produce reaction mixtures of each sample comprising Tris buffer, Pyrogallol and respective serum and tissues samples. The reaction mixtures were individually set for absorbance reading at wavelength 420 nm in UV/Vis spectrophotometer (Biochrom Libra S32, UK). The reaction time was 180 s and at the end of spectrometer absorbance readings, inhibition of pyrogallol autooxidation and activity of the superoxide dismutase enzymes in the samples were calculated.
2.4.5. Serum and tissues catalase enzymes activity
Serum and tissues samples prepared as described above were used for determination of catalase activities based on their ability to decompose hydrogen peroxide (H2O2) into H2O and O2 (EC 1.11.1.6). The catalase enzyme assay composition includes 50 mM of potassium phosphate buffer (pH-7.0) prepared from di-potassium hydrogen phosphate, potassium dihydrogen phosphate; and 100 μM hydrogen peroxide. Blank (reaction mixture without sample) and test reaction mixture comprising the assay and samples were subjected to spectrophotometer reading at 240 nm; absorbance changes were noted at every 30 s throughout 180 s reaction time for each sample. Catalase enzyme activities were calculated from changes in absorbance at 240 nm per minutes using milli molar extinction coefficient of H2O2 at 240nm.
2.4.6. Serum and tissues reduced glutathione
Reduced glutathione was determined following protocol of Moron et al [16] on the principles that the enzyme is capable of reacting with Ellman's reagent 5,5′- dithiobis (2-nitrobenzoic acid) to form 5′-thio-2-nitrobenzoic acid (TNB) which absorb UV – light at 412 nm. The assay involved precipitation of the samples in 20 % trichloro acetic acid then centrifuge the mixture at 2000 rpm for 2 min at 4 °C (Eppendorf Centrifuge 5424 R, Germany). Supernatant of 50 μL were obtained after the centrifuge; for each reaction mixture, the supernatant was added with 100 μL of phosphate buffer (200 mM, pH 8.0) and 50 μL of 5mM 5,5′- diothiobis (2-nitrobenzoic acid) then incubated for 10 min at room temperature. Reduced glutathione standards were prepared and alongside with the test samples were put up for absorbance reading at 412 nm in micro plate reader (Thermo Fisher Scientific, Finland); activities of reduced glutathione in each reaction was calculated μmol of GSH per g of the sample for tissue and μmol per mL for serum samples.
2.4.7. Serum and tissue lipid peroxidation
The levels of lipid peroxidation in the serum and tissues were determined according to procedures of Ohkawa et al [17]. Serum samples were used undiluted while 10 % tissue homogenates prepared using potassium chloride was used for tissues lipid peroxidation analysis. The assay reaction mixture comprised 0.5 mL samples, 0.2 mL 8 % sodium n-dodecyl sulphate, 1 mL 20 % acetic acid and 1 mL 0.8 % 2-thiobarbituric acid (TBA). These reaction mixtures were boiled for 1 h at 95 °C in water bath (Brookefield-Ametek, India); the reactions were terminated by cooling on ice for 10 min each. After cooling a mixture of n-butanol and pyridine (15:1) was added to each reaction vortex briefly then centrifuge at 4000 rpm for 10 min. Organic supernatant layers were taken from the samples and set for absorbance readings in micro plate reader at 532 nm; molar extinction coefficient of malondialdehyde (MDA) was used for estimation of the lipid peroxidation products quantity expressed in nanomole (nmol) per mg for tissue and per mL for serum samples.
2.4.8. Serum and tissues protein carbonylation
Protein carbonyl was determined based on protocol of Colombo et al [18] a procedure involving labelling of protein carbonyl with 2,4-dinitrophenylhydrazine (DNPH) to form its conjugate dinitrophenylhydrazones (DNP). For serum 100 μL of serum was used while for tissue 1 mg protein sample per tissue was used; both serum and tissue sample were individually incubated with 100 μL of DNPH (20 mM prepared in 2N HCl) for 60 min in dark room. After the incubation, 1 mL 20 % acetic acid was added and incubated on ice for 15 min then centrifuge at 10000 rpm for 5 min at 4 °C; the supernatant was discarded while the protein pellets were washed in ethyl acetate and ethanol mixture (1:1) then vortex mixed followed by centrifuge at 10000 rpm for 5 min at 4 °C.
The procedure of washing and pellet collection was repeated twice for each sample after which the pellets were resuspended in 1 mL guanidine hydrochloride (6 M prepared in 50 mM phosphate buffer pH 2.3). The process of re-suspension involved incubation of the pellets in the guanidine solution at 37 °C for period of 30 min. After complete dissolution of the pellets in the guanidine solution, they were put up for absorbance reading at 366 nm in microplate reader Thermo Multiskan GO micro plate reader (Thermo Fisher Scientific, Finland). Protein carbonyl concentration (μM/mL) was calculated using molar absorption coefficient of 22,000 M−1cm−1.
2.4.9. Serum and tissues total antioxidant capacity
Overall antioxidant capacities of the serum and tissues samples were determined using protocol described by Benzie and Strain [19]; the principle of the assay is based on redox reduction of iron (III) to iron (II) at low pH. In this study, a chemical assay containing 25 mL sodium acetate buffer (300 mM, pH 3.6), 2.5 mL of 10 mM 2,4,6-Tri-(2 pyridyl)-s-trizine prepared in 40 mM HCl and 2.5 mL Ferric chloride (20 mM prepared in distilled water) was prepared and used working assay. Reaction mixture comprising50 μL of samples and 200 μL of the FRAP working solution were incubated for 30 min at 37 °C (Labnet Inc., USA) after which absorbance was measured at 493 nm using microplate reader. Ascorbic acid was used as standard for calculating antioxidant power associated with colour changes and reduction of Fe3+ to Fe2+; total antioxidant concentration was expressed as μmol per mL for serum and as μmol per gram for tissues.
2.5. Quantification of antioxidant genes expression in liver and ovaries of the rabbits
2.5.1. Sample collection and RNA isolation
200 mg liver and ovary samples were collected in triplicate from each sacrificed animal into 200 μL of RNAlater (Invitrogen Bioservices, USA) and stored at – 80 °C ahead of downstream analysis. For RNA isolation, 50 mg of collected samples each in triplicates were crushed under liquid nitrogen after being washed in phosphate buffer (pH 7.4); after proper lysis they were homogenized TRIzol reagent (Life Technologies, USA).
After homogenization of the samples, they were subjected to mechanical cell disruption for separation of membranes from the cells using a mechanical disruptor (Scientific Industries, USA). The disruption was followed by 5 min incubation at room temperature and phases separations by addition of 1 mL chloroform followed by rigorous mixing for 15 s each. Then each of the samples was centrifuge at 12000 rcf for 15 min at 4 °C. After the centrifuge 500 μL RNA clear aliquot were obtained from each sample into 500 μL isopropanol. The mixture of RNA and isopropanol were then gently mix for 15 s each and incubated for 10 min for RNA precipitation; then centrifuge again for 10 min at 12000 x g at 4 °C. After this centrifuge, the RNA pellets were collected while the isopropanol was discarded then the pellets were washed with 1 mL ethanol and centrifuge for 5 min at 12000 x g at 4 °C and allowed to evaporate. After evaporation the RNA pellets were dissolved by adding 50 μL nuclease free water, then subjected to purity and concentration evaluation using Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., USA).
2.5.2. cDNA synthesis
Revertaid cDNA synthesis kit was used for synthesizing of cDNA from isolated RNA samples according to the manufacturers’ protocol (Thermo Fisher Scientific, USA); after synthesizing, from respective cDNA templates, 50 ng/uL of each sample in triplicates were used for real time quantification using StepOne Real-Time PCR system (Applied Biosystem, USA).
2.5.3. Real-time PCR quantification of relative expression of the target genes
Takara SYBR® premix (Takara Bio Inc., Japan) was used for the comparative quantification of relative expression of the target genes in the animals. The quantification reaction followed Takara SYBR® premix protocol for Applied Biosystems’ 7300/7500/7500 Fast Real-Time PCR System and StepOne Real-Time PCR System. The first step of the reaction ran for 30 s at 95 °C; the second step (denaturation) ran first at 95 °C which for 5 s while annealing of the reaction mixture at 60 °C which ran for a period of 60 s in a continuous cycle of 40 runs followed by melt curve stage.
2.5.4. Primers and primer designing
Primers used for the real-time quantifications were designed using NCBI database given below (Table 2); while glyceraldehyde-3-phosphate dehydrogenase (gapdh) was used as internal reference control gene. The gene product yield was confirmed in 1.8 % agarose gel electrophoresis (Tarsons Product Pvt. Ltd., India).
2.6. Statistical analysis of data
Performance records, oxidative stress biomarkers, antioxidant enzymes activities and fold changes of relative expression of the genes were subjected to one – way analysis of variance (ANOVA); significant means were determined at p < 0.05 while means separation was done with Duncan test procedure of SPSS version 20.0. In addition, there was comparative analysis of relative expression of primary antioxidant genes in liver and ovaries using Mann-Whitney U test for comparative ranking of the genes expression patterns in both tissues.
3. Results
3.1. Animals’ performances characteristics
There was no significant difference in initial weights of the rabbits; mean initial weight was 1060 ± 29.42 g (p < 0.17). However, there was significant difference in final weights of the rabbits, the finishing weight was 2383 ± 40.99 g (p < 0.008). There was also significant difference in feed intakes (p < 0.014); mean feed intake was 100.56 ± 1.22 g while minimum and maximum feed intakes were 90.60 g and 110.26 g respectively. Furthermore, there was significant difference in commercial carcass weight (p < 0.001); similarly, significant difference was observed in the empty carcass weight (p < 0.001) as well as the rabbits’ skin weight (p < 0.001). Weight after slaughtering was 2325 ± 39.07 g (p < 0.004), blood weight was 58.94 ± 5.54 g (p < 0.006), empty carcass weight 1801.74 ± 52.78 g (p < 0.001), commercial carcass weight was 1419.72 ± 34.36 g (p < 0.001), skin weight was 236.96 ± 7.2 g and feed conversion ratio was 10.32 ± 0.45 (p > 0.05) respectively (Table 3).
Table 3.
List of target genes and their primers evaluated in the rabbits.
| S/N | Gene Name | Primers type | Primer Sequence (5′ to 3′) | Primers Length (bp) | Product size (bp) | Accession No |
|---|---|---|---|---|---|---|
| 1 | Oviductal glycoprotein 1 (OVGP1) | F | TTCCTTGCCTGAGACCAGTT | 20 | 121 | NM_001105687.1 |
| R | CTCTCTCTCGGGGTGGTCATC | 21 | ||||
| 2 | Uncoupling protein 1 (UCP1) | F | CCTAACAACTGGAGGCGTGG | 20 | 177 | NM_001171077.1 |
| R | GGAGTTGTCCCTTTCCACAGA | 21 | ||||
| 3 | Oxidative stress responsive 1 (OXSR1) | F | GTTTGCGTAAAGGTGGGTGG | 20 | 120 | XM_008252717.2 |
| R | AGCAGGCACACACATCACTG | 20 | ||||
| 4 | Brain and reproductive organ-expressed (TNFRSF1A modulator) (BRE) | F | TCAGTCACTTTGGCACAGGT | 20 | 120 | XM_002709945.3 |
| R | GGAAAAACAGGGGCAGGTCA | 20 | ||||
| 5 | Oryctolagus cuniculus superoxide dismutase 1 (SOD1) | F | GGTGGTCAAGGGACGCATAA | 20 | 205 | NM_001082627.2 |
| R | CACATCAGCCACACCATTGC | 20 | ||||
| 6 | Oryctolagus cuniculus glutathione peroxidase 1 (GPX1) | F | TTTGGGCATCAGGAGAACGC | 20 | 211 | NM_001085444.1 |
| R | TGATGAACTTGGGGTCGGTC | 20 | ||||
| 7 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F | TCGGAGTGAACGGATTTGGC | 20 | 181 | NM_001082253.1 |
| R | TTCCCGTTCTCAGCCTTGAC | 20 |
3.2. Oxidative stress status and antioxidant enzymes activities
There was significant difference in serum oxidative stress status of the rabbits; mean malondialdehyde (MDA) concentration was 18.40 ± 0.91 nmol/mL (p < 0.010) while minimum and maximum concentrations were 11.51 and 27.60 nmol/mL respectively. Total antioxidant concentration (TAC) was 65.00 ± 8.60 μmol/mL (p < 0.005) while minimum and maximum total antioxidant concentrations were 14.10 and 170.57 μmol/mL respectively. Protein carbonyl (PCO) concentration was 7.28 ± 0.34 μmol/mL (p < 0.001) while minimum and maximum concentrations were 5.45 and 10.23 μmol/mL respectively. Superoxide dismutase (SOD) activities was 172.40 ± 7.8 U/mL (p < 0.042) while minimum and maximum activities were 123.10 and 261.50 U/mL respectively. Catalase (CAT) enzyme activities was 4.06 ± 0.26 U/mL (p > 0.05) while minimum and maximum activities were 1.72 and 6.19 U/mL respectively. Reduced glutathione (GSH) concentration was 10.11 ± 0.62 μmol/mL (p < 0.017) while minimum and maximum concentrations were 6.11 and 17.39 μmol/mL respectively (Table 4).
Table 4.
Effect of Chlorella vulgaris supplementation on performances of the rabbits at finishing stage.
| Parameters | Control | T1 | T2 | T3 | T4 | SEM | p-value |
|---|---|---|---|---|---|---|---|
| Feed intake (g) | 100.32b | 105.92b | 101.81b | 101.11b | 93.67a | 1.22 | 0.014 |
| Initial body weight (g) | 1031.50 | 987.55 | 1199.25 | 1065.25 | 1019.25 | 29.42 | 0.170 |
| Final body weight (g) | 2152.30a | 2322.70ab | 2545.30bc | 2428.20bc | 2471.30bc | 40.99 | 0.008 |
| Weight after slaughter (g) | 2103.10a | 2268.30ab | 2503.50bc | 2374.00bc | 2376.20bc | 39.07 | 0.004 |
| Blood weight (g) | 49.20a | 54.40a | 41.80a | 54.20a | 95.10b | 5.54 | 0.006 |
| Commercial carcass weight (g) | 1269.10a | 1332.70ab | 1666.10b | 1428.50b | 1402.20b | 34.36 | 0.001 |
| Skin weight (g) | 203.20a | 240.40b | 285.50bc | 234.20b | 221.50ab | 7.20 | 0.001 |
| Feed conversion ratio | 10.12 | 10.95 | 10.84 | 10.42 | 8.21 | 0.45 | 0.324 |
a,b,cMeans with different superscript in the same row are different (p < 0.05) for the parameters measured.
Control - group without supplement diet.
T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass.
T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass.
T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass.
T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
Similarly, there was significant difference in malondialdehyde (MDA) concentration of the rabbits’ liver 6.61 ± 1.54 nmol/mg (p < 0.001) while minimum and maximum concentrations were 0.15 and 19.65 nmol/mg respectively. Superoxide dismutase (SOD) enzyme activities was 169.70 ± 18.3 U/mg (p < 0.002) while minimum and maximum activities were 73.40 and 376.10 U/mg respectively. Catalase (CAT) enzyme activities in the liver was 8.70 ± 0.30 U/mg (p < 0.010) while minimum and maximum activities were 7.14 and 13.37 U/mg respectively. Reduced glutathione (GSH) concentration was 9.71 ± 0.49 μmol/g (p < 0.001) while minimum and maximum glutathione reduced were 6.09 and 15.37 μmol/g respectively (Table 5).
Table 5.
Effect of Chlorella vulgaris supplements on serum oxidative stress biomarkers in the rabbits.
| Parameters | Control | T1 | T2 | T3 | T4 | p - values |
|---|---|---|---|---|---|---|
| MDA (nmol/mL) | 23.90 ± 1.66a | 15.37 ± 2.24b | 16.88 ± 0.94b | 19.09 ± 0.92b | 16.78 ± 1.32b | 0.01 |
| PCO (μmol/mL) | 7.74 ± 0.55b | 5.88 ± 0.22a | 6.99 ± 0.49ab | 9.61 ± 0.23c | 6.23 ± 0.30a | 0.001 |
| TAC (μmol/mL) | 37.9 ± 4.50a | 112.5 ± 21.3c | 62.80 ± 20.10ab | 63.4 ± 20.05ab | 80.3 ± 5.75bc | 0.005 |
| SOD (U/mL) | 158.5 ± 9.50ab | 203.8 ± 22.97b | 146.2 ± 14.70a | 157.7 ± 13.10ab | 196.2 ± 3.80b | 0.04 |
| CAT (U/mL) | 3.80 ± 0.35 | 4.64 ± 0.57 | 5.13 ± 0.53 | 3.33 ± 0.58 | 3.41 ± 0.50 | 0.10 |
| GSH (μmol/mL) | 7.18 ± 0.54a | 9.05 ± 0.40ab | 10.21 ± 0.22abc | 10.92 ± 0.47bc | 13.19 ± 2.27c | 0.02 |
a,b,c Means with different superscript in the same row are different (p < 0.05) for each of the parameters.
Control - group without supplement diet.
T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass.
T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass.
T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass.
T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
There was also significant difference in malondialdehyde (MDA) concentration of the rabbits’ uterus, mean concentration was 1.70 ± 0.46 nmol/mg (p < 0.001) while minimum and maximum concentrations were 0.03 and 5.48 nmol/mg respectively. Total antioxidant concentration (TAC) was 244.00 ± 18.00 μmol/mg (p < 0.012) while minimum and maximum antioxidant capacity were 138.00 and 510.00 μmol/mg respectively. Superoxide dismutase (SOD) enzyme activities in the uterus was 144.40 ± 18.4 U/mg (p < 0.002) while minimum and maximum activities were 48.10 and 350.90 U/mg respectively. Catalase (CAT) enzyme activities in the uterus was 3.87 ± 0.02 U/mg (p < 0.001) while minimum and maximum activities were 3.73 and 3.97 U/mg respectively. Reduced glutathione (GSH) concentration was 9.52 ± 0.48 μmol/g (p < 0.001) while minimum and maximum glutathione reduced were 6.88 and 14.36 μmol/g respectively (Tables 6 and 7).
Table 6.
Effect of Chlorella vulgaris supplements on liver oxidative stress biomarkers of the rabbits.
| Parameters | Control | T1 | T2 | T3 | T4 | p - values |
|---|---|---|---|---|---|---|
| MDA (nmol/mg) | 18.91 ± 0.38a | 0.54 ± 0.15b | 2.58 ± 1.00bc | 5.05 ± 1.31d | 5.99 ± 1.89d | 0.001 |
| PCO (nmol/100mg) | 7.00 ± 0.60 | 5.14 ± 0.32 | 8.08 ± 0.52 | 6.11 ± 1.53 | 7.00 ± 1.00 | 0.23 |
| TAC (μmol/mg) | 50.47 ± 1.08 | 59.10 ± 3.70 | 65.90 ± 4.92 | 60.3 ± 4.38 | 60.4 ± 1.06 | 0.28 |
| SOD (U/mg) | 110.10 ± 22.4a | 190.40 ± 18.10b | 135.3 ± 4.40bc | 126.213.18bc | 286.70 ± 52.4c | 0.002 |
| CAT (U/mg) | 9.30 ± 0.11bc | 8.33 ± 0.15ab | 8.05 ± 0.37ab | 8.00 ± 0.14ab | 10.35 ± 1.03c | 0.01 |
| GSH (μmol/g) | 6.30 ± 0.10b | 6.58 ± 0.38b | 5.30 ± 0.35ab | 5.00 ± 0.13ab | 8.85 ± 1.12c | 0.001 |
a,b,c Means with different superscript in the same row are different (p < 0.05) for the parameters measured.
Control - group without supplement diet.
T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass.
T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass.
T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass.
T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
Table 7.
Effect of Chlorella vulgaris supplements on uterus oxidative stress biomarkers in the rabbits.
| Parameters | Control | T1 | T2 | T3 | T4 | p-value |
|---|---|---|---|---|---|---|
| MDA (nmol/mg) | 5.25 ± 0.10c | 2.57 ± 0.24b | 0.22 ± 0.10a | 0.38 ± 0.20a | 0.08 ± 0.01a | 0.001 |
| PCO (nmol/100mg) | 25.49 ± 3.66 | 22.96 ± 4.15 | 14.53 ± 1.08 | 24.57 ± 6.90 | 23.00 ± 2.88 | 0.40 |
| TAC (μmol/mg) | 149.50 ± 6.00a | 332.20 ± 64.15b | 255.50 ± 13.14b | 248.10 ± 9.80b | 235.00ab | 0.01 |
| SOD (U/mg) | 84.80 ± 13.2a | 165.10 ± 10.5a | 110.10 ± 9.6a | 100.90 ± 5.8a | 261.50 ± 5.8b | 0.002 |
| CAT (U/mg) | 3.85 ± 0.01b | 3.89 ± 0.02c | 3.93 ± 0.14bc | 3.94 ± 0.12d | 3.75 ± 0.01a | 0.001 |
| GSH (μmol/mg) | 8.36 ± 0.01ab | 7.59 ± 0.32a | 10.94 ± 0.39bc | 11.58 ± 1.02c | 9.17 ± 1.14abc | 0.02 |
a,b,c Means with different superscript in the same row are different (p < 0.05) for the parameters measured.
Control - group without supplement diet.
T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass.
T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass.
T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass.
T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
3.3. Patterns of primary antioxidant genes expression levels
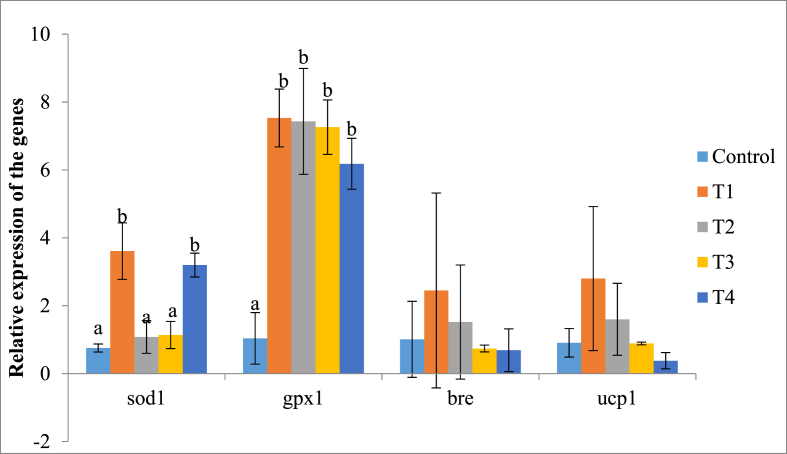
In the liver, there was significant difference in relative expression of the primary antioxidant genes sod1 (p < 0.009) and gpx1 (p < 0.004) but there were no significant differences in relative expression of bre (p < 0.65) and ucp1 (p < 0.58). Mean relative expression of sod1 was 2.02 ± 0.35 fold changes while minimum and maximum expressions were 0.34 and 5.18 fold changes respectively. Antioxidant gene gpx1 was up-regulated in all the treatment groups with mean fold change value of 5.89 ± 0.76 while minimum and maximum fold changes were 0.22 and 9.88 respectively. All treatment groups had significantly higher relative expression of gpx1 compared with control group (Fig. 1). Relative expression of bre was not significantly different among the groups; mean fold change for the gene was 1.28 ± 0.39 (p < 0.65); there were also no significant differences expression of ucp1 1.32 ± 0.46 (p < 0.58) minimum and maximum fold changes of the gene were 0.06 and 7.06 respectively.
Fig. 1.
Relative expression of primary antioxidant genes in liver of the rabbits. Bars with different labels are significantly different in expression of the genes determined (p < 0.05). Control - group without supplement diet. T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass. T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass. T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass. T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
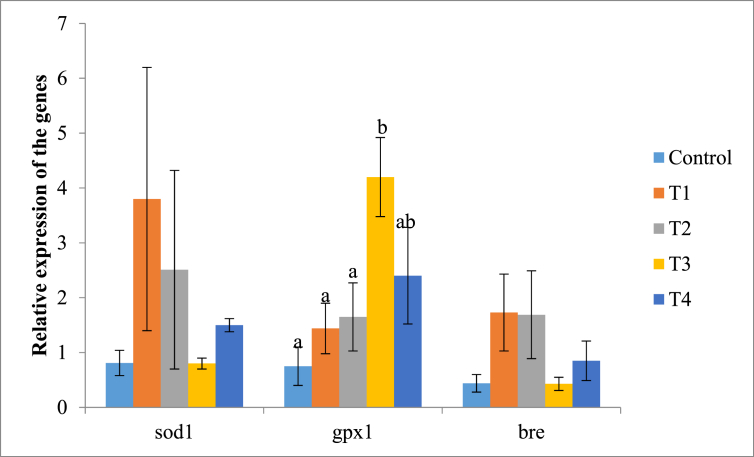
Pattern of relative expression of primary antioxidant genes in the ovaries is also comparable with relative expression levels in the liver because antioxidant gene gpx1 was significantly up-regulated although there were no significant differences in relative expression of sod1 and bre; mean fold changes of gpx1 was 2.07 ± 0.39 (p < 0.030) while minimum and maximum fold changes were 0.05 and 5.47 respectively. Mean relative expression of sod1 gene was 1.76 ± 0.61 fold changes (p < 0.42) while minimum and maximum fold changes were 0.11 and 8.60 respectively. Relative expression of bre was 1.03 ± 0.24 fold changes (p < 0.23) while minimum and maximum fold changes were 0.12 and 2.86 respectively. The relative level of antioxidant genes expression in the ovaries indicated sod1 and gpx1 were higher in the treatment groups compared with control group (Fig. 2).
Fig. 2.
Relative expression of primary antioxidant genes in ovaries of the rabbits. Bars with different labels are significantly different in expression of the genes determined (p < 0.05). Control - group without supplement diet. T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass. T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass. T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass. T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
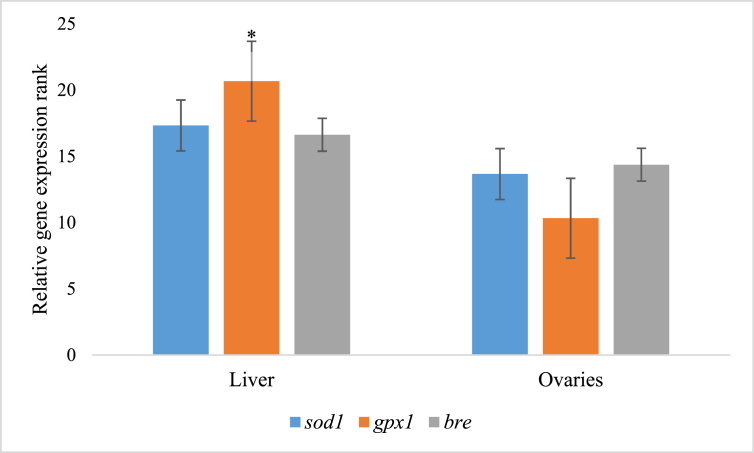
Comparative analysis of variance between relative expression of sod1, gpx1 and bre using Mann-Whitney U statistics indicated that there was significant difference in level of gpx1 expression between liver and ovaries (p < 0.001) but there were no significant differences in expression of sod1 (p < 0.25) and bre (p < 0.48); however relative expression of the two genes ranked higher in liver than ovaries (Figs. 3 and 4).
Fig. 3.
Comparative expression of primary antioxidant genes in the rabbits' liver and ovaries (*p < 0.05). Control - group without supplement diet. T1 - group supplemented with 200 mg/kg BW Chlorella vulgaris biomass. T2 - group supplemented with 300 mg/kg BW Chlorella vulgaris biomass. T3 - group supplemented with 400 mg/kg BW Chlorella vulgaris biomass. T4 - group supplemented with 500 mg/kg BW Chlorella vulgaris biomass.
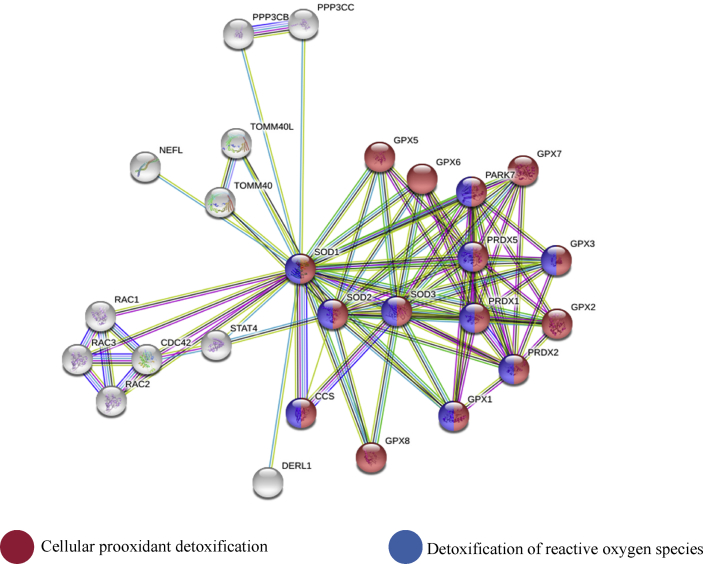
Fig. 4.
Functional enrichment analysis of superoxide dismutase gene expression biological impacts in the rabbits.
4. Discussion
4.1. Effects of Chlorella vulgaris supplementations on performances of the rabbits
Chlorella vulgaris is a microalgae commonly cultivated on commercial scale in different parts of the world; its applications cut-across food industries, pharmaceuticals and animal production [20]. In animal production, it is being used as animal and fish feeds additives because of its nutritional profile [21]. In this present study, supplementation of the microalgae to rabbits showed that it is a potential for improving production performances and efficiencies of feeds utilization because despite reduction in feed intake as results of the supplementation final body weight of the rabbits in the treatment groups were higher compared with control group (Table 4). The reduction in feed intake was not because of non-palatability of the microalgae or as results of toxicity but due to improved efficiency of feed utilization of the animals which was evident with their higher final body weights compared with control; furthermore, feed conversion ratio of the control was higher compared with the treatment groups. All these are indications that supplementation of the microalgae has significant economic benefits since it reduced feed intake, improved feed conversion ratio and increased productivity.
Chlorella vulgaris supplementation in this study due to reduction in feed intakes however cannot be interpreted to be that the rabbits rejected feed due to the supplement because productivity was not compromised in fact it increased. This justification agreed with submission of Halle et al [22] who observed that supplementation of Chlorella vulgaris in laying birds led to reduction in feed intake without reduction in productivity of the birds. In fact, all the birds’ productivity indicators increased in the supplemented groups; according to the report production performances were significantly higher compared with control group because the supplemented groups had higher egg weight, higher laying percentages, higher hen day production and lower feed conversion ratio.
Similarly, supplementation of Chlorella vulgaris in fish was also reported to improved productivity, dietary lipid utilization and muscle pigmentation which are all identified as products quality improvement in fish [23]. Chlorella vulgaris supplementation and reduced feed intake in this study could be linked with improved biodiversity of intestinal microbes of the rabbit thereby contributing to better gut health. Supplementation of the microalga since it is a green alga with enormous amount of starch and cellulose led to increased population of lactic acid bacteria and ability of the gut microflora to degrade algal polysaccharides and other complex plant polymers in the basal feed increased and in overall led to better digestive efficiency of the animals which was the reason why despite the reduced feed intake, there was higher product yields [23].
This is possible in rabbits because they are hindgut fermenters capable of degrading complex biomolecules in their caecum as result of the increased microflora population and diversity. Again, as a way of improving gut health, the diversity is capable of reducing population of non-beneficial microbes in the guts of the rabbit and hence contributed to improvement of the animals’ health and productivity [24]. It can be deduced from this study thereof that supplementation of Chlorella vulgaris is a strategy for reducing rabbits feed intake, improving gut health and productivity. Higher finishing weights of the supplemented groups, higher commercial carcass weight as well as higher skin weight are indications attestation to improved nutritional efficiencies as result of the supplementation. These observations are in agreements with previous studies on Chlorella vulgaris feeding in other animal species where it was regarded as microalga with potential nutritive values capable of enhancing some biochemical and physiological functions for improved immunity and growth [25].
4.2. Effects of Chlorella vulgaris supplements on oxidative stress status of the rabbits
Oxidative stress products generation is a major patho-physiological mechanism of oxidative stress damages; malondialdehyde and protein carbonyls are leading among these products and their increasing concentration corresponds with poor performance [26]. In this study, supplementation of Chlorella vulgaris significantly reduced generation of these products on one hand and on the other hand it led to increased total antioxidant concentration in the serum, uterus and liver of the rabbits. Significant reduction of these products in all these tissues indicated that the supplement diets contributed to overall antioxidant defense system of the rabbits across different tissues of the animals’ body. Specifically, the supplementation led to inhibition of malondialdehyde production which is a cellular mechanism protecting cell membrane from lipid peroxidation damage; and a cellular mechanism for promoting animal production and performance. Therefore, it could be derived that protection against oxidative stress due to the supplementation is a strategy for promoting animal production performances [27].
Chlorella vulgaris is a rich source of polyphenolic compounds [28]; hence, reduction in oxidative stress products concentration in this study can be linked with antioxidant capabilities of the polyphenolic contents of the microalgae. Polyphenols are bioactive compounds capable of reducing oxidative stress products if they are biologically available in animals’ gut for absorption. Since rabbits are hind-gut fermenters, there is possibility of maximum bioavailability of the polyphenols because both enzymatic and microbial degradation of bounded polyphenols takes place within the gastro-intestinal tracts [29]. The higher bioavailability of polyphenols as results of these multiple absorption was responsible for the increased total antioxidant concentration in the supplemented animals in this study. The absorbed polyphenols from the microalgae at cellular level hence inhibited oxidative stress and its associated compromise while several repeats of this process could lead to non-disturbance of cellular activities and therefore reduced oxidative stress products generation [30]. In this present study, significantly decreased generation of oxidative stress products across different body tissues and systems implied that at the minimal and maximal levels of supplementation used in this study, oxidative stress can be attenuated using microalgae Chlorella vulgaris because of bioactive compounds it contained. Some of these bioactive compounds including carotenes, zeaxanthin, astaxanthin, fucoxanthin, vitamins and minerals were reported in studies where similar microalgae fed to rabbits led to decrease in production of oxidative stress products including malondialdehyde [31, 32].
Lipid peroxidation is the chief oxidative stress effect and most prevalent form of oxidative stress compromise at cellular level; it is a toxic process to the cells causing cell loss of membrane integrity, cell death and activation of genetic programme cell death [33]. Compromised reproductive inefficiencies in male such as spermatozoa dysfunctions, low epididymis sperm concentrations, poor sperm motility and abnormal sperm morphology are also linked with increasing lipid peroxidation [34]. In female, infertility triggered by endometriosis is also reportedly linked with increased production of oxidative stress products specifically increasing levels of malondialdehyde; furthermore, lipid peroxidation is also mechanism of oxidative stress associated withdecline oocytes quality [35, 36]. Therefore, for improving reproduction and promotion of reproductive health according to this present study, Chlorella vulgaris supplementation is an option due to its capacity to inhibit lipid peroxidation. In agreement with this, earlier studies also reported inhibition of lipid peroxidation and improved performances using varied derivatives of Chlorella vulgaris in different models [37, 38, 39, 40].
4.3. Effects of Chlorella vulgaris supplementations on antioxidant enzymes activities and reduced glutathione concentrations in the rabbits
In this study, supplementation of microalgae Chlorella vulgaris led to enhanced antioxidant enzymes activities as well as increased concentration of reduced glutathione; these are indicators that the alga has strong antioxidant capabilities. In animal production, this is beneficial because it demonstrated that at minimal supplementation levels the microalgae is an additive whose supplementation is capable of improving productivity. In a similar submission Lee et al [41] stated that short-term Chlorella vulgaris supplementation improved activities of antioxidant enzymes. The enhancement of antioxidant status by supplementation of Chlorella vulgaris can be biologically explained to be as results of complementary roles of antioxidants in the microalga which contributed to free radical scavenging capacities of the internal antioxidant system serving as second line of defence and thereby increase activities of primary antioxidants enzymes activities. This underlying biochemical mechanism led to conservation of primary antioxidant enzymes depletion hence higher activities [42].
Modulation of antioxidant enzymes by Chlorella vulgaris in this study is in agreement with previous reports that supplementation of Chlorella vulgaris in human or animal models [43]. Chlorella vulgaris increases total antioxidant capability of body systems and tissues, and kept degradation of cellular membrane at bay due to inhibition of lipid peroxidation. These are the reasons for increased activities of the antioxidant enzymes [44, 45, 46]. Higher concentrations of reduced glutathione in animals of the treatment groups compared with control also signified that supplementation of Chlorella vulgaris led to adequate elimination of hydrogen peroxide because glutathione as a non-enzyme antioxidant has complex metabolic role and capable of decomposing hydrogen peroxides.
Biochemical activities of glutathione apart from decomposition of hydrogen peroxides also involve enhancement of the primary antioxidant enzymes activities, and participation in third line of defence such as cellular protection against toxic agents resulting from lipid peroxidation and restoration of cellular balance of reduced and oxidized glutathione [47]. These mechanisms of reduced glutathione in oxidative stress protection is complex antioxidant defence process that can be associated with the supplementation of Chlorella vulgaris in this study because apart from the increase concentration of glutathione, there was also significant higher activities of catalase antioxidant enzymes in the treatment groups compared with control which indicated that the treatment groups had better hydrogen peroxide decomposition potential compared with the control group [48].
4.4. Effects of Chlorella vulgaris supplementations on relative expression of primary antioxidant genes in liver and ovaries of the rabbits
This study outcomes of gene expression showed that supplementation of Chlorella vulgaris to the rabbit exhibited protection against oxidative stress across all lines of oxidative stress defence systems because significant up-regulation of the primary antioxidant genes sod1 and gpx1 indicated that at immediate cellular defence stage; there is adequate protective actions of antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase in suppression, detoxification and activation of free radicals dismutation as well as capabilities for decomposition of hydrogen peroxides and overall prevention of free radicals formation by inhibition of mechanisms leading to their production [49].
It is also evident that the up-regulations of these genes in the supplemented rabbits confirmed molecular relevance of Chlorella vulgaris as a source of antioxidants capable of preventing metabolic and reproductive oxidative stress damages. This could be attributed to complementary actions of the microalga antioxidant components including carotenoids, astaxanthin, fucoxanthin, vitamins and minerals which are bioactive compounds capable of chain breaking, scavenging and neutralizing free radicals. These are also factors leading to up-regulation of these genes [50].
Sod1 a protein-coding gene with capacity of biding copper and zinc ions is one of the enzymes primarily responsible for protection against superoxide radical destruction. It is also active against superoxide radical and hydrogen peroxide in both the cytoplasm and mitochondria therefore its up-regulation as result of Chlorella vulgaris supplementation indicated complete cellular protection. Sod1 is also involved in pharmacokinetic and reactive oxygen species detoxification pathways hence the microalga is a drug candidate in development of therapeutic solution for oxidative stress attenuation [51, 52].
In this study, it is also evident that as the rabbit matures there are increasing metabolic activities due to growth and development of reproductive organs leading to cellular generation of reactive oxygen species and superoxide radicals [53]. However, in the supplemented group the superoxide radicals were converted to hydrogen peroxide (H2O2) by superoxide dismutase activities. This is because in the supplemented group there was higher adequate cyclical reaction of neutralizing H2O2 into water and molecular oxygen; this was why the supplemented rabbit had lowered levels of oxidative stress status via reduced malondialdehyde concentration, reduced protein carbonyl concentration and higher total antioxidant capacity [54]. Molecular basis of these protective mechanisms was the up-regulation of the antioxidant genes.
Furthermore, up-regulation of sod1 due to supplementation of Chlorella vulgaris in this study indicated that the microalga is an excellent source of antioxidant for oxidative stress attenuation because sod1 gene is associated with several other genes working together for cellular prooxidants detoxification, response to toxic compounds in the cells, and detoxification of reactive oxygen species. This is in agreement with Ismail et al [55] who reported that supplementation of Spirulina which is also a microalga contributed to oxidative stress protection in a network of gene relationships linked with up-regulation of sod1. These genes along with sod1 jointly contribute to cellular prooxidant detoxification and specifically detoxification of reactive oxygen species (Fig. 3).
Glutathione peroxidase (gpx1) is another antioxidant gene up-regulated as result of the supplementation of Chlorella vulgaris in this study; the gene is also a protein coding gene and it belongs to peroxidase family whose responsibilities include protection against oxidative stress. The gene when expressed is responsible for reduction of organic hydroperoxides and detoxification of hydrogen peroxide. Up-regulation of these genes is associated with hydrogen peroxide detoxification and catalytic activation of drugs against oxidative stress and selenoius acid related therapeutic agents [56, 57, 58]. Molecular advantage of this is that the microalgae Chlorella vulgaris can be exploited as feed additive and or drug agent with bioactive compounds that can contribute to favourable cellular transcriptions.
The gene is expressed in both liver and ovaries indicating that metabolically and reproductively there was adequate oxidative stress protections in the rabbits. This is ultimate benefits from Chlorella vulgaris supplementation because up-regulation of gpx1 is a promoter of oxidoreductase catalytic activities at cellular levels. This is a molecular protective catalysis of an oxidation-reduction (redox) reaction; it is a reversible chemical reaction in which oxidation state of an atom or atoms within a molecule is altered; two substrates are involved and one of the substrate acts as electron donor then becomes oxidized, while the other acts as electron acceptor and becomes reduced [59]; this cellular mechanism is responsible for interactive relationship between gpx1 and sod1for protection against oxidative stress. Molecular function of gpx1 expression is also related to enhancement of glutathione-transferase activities; biological cellular mechanism promoting total antioxidant protection. The implication of this is that up-regulation of gpx1 could enhance activities of glutathione-S-transferase which again signified that the microalga supplementation contributed to total antioxidant protection [60].
Specific benefits of this gene in enhancement of glutathione-S-transferase activities is that the enzyme has capacity to protect against neuron oxidative stress damages and by extension it could be deduced that supplementation of microalga Chlorella vulgaris can be exploited as supplement nutraceutical for protection of oxidative stress associated with nervous systems [61]. Deficiency of glutathione peroxidase expression is related to occurrence of many diseases including haemolytic anaemia, diabetes mellitus, ischemia and retina vascular diseases among others; what is common to all these diseases however is oxidative stress complexities. Therefore, it up-regulation in the treatment groups could explain the reason for reduced lipid peroxidation products, higher antioxidant potential and enhanced antioxidant enzymes activities since its deficiency is related to higher oxidative stress damages [62].
Co-expression of glutathione peroxidase and sod1 can be regarded as another important biological benefit of Chlorella vulgaris supplementation effects in oxidative stress protection in this study because this interaction inhibits cellular modification of amino acids metabolism and can be used to adjudge Chlorella vulgaris as source of antioxidants with wide range of biological protective activities against oxidative stress. The interactive co-expression identified between sod1 and gpx1 in this present study agreed with submissions efficacy of Chlorella vulgaris as source of antioxidant; the co-expression was responsible for effective protection against oxidative stress [63, 64].
5. Conclusions
Oxidative stress is an inevitable biochemical process compromising animal performances and the implications transcend beyond physiological dysfunction to economic loss since it manifested as relevant mediator of reduced animal productivity in form of lower finishing weights as discovered in this study. Dietary supplementation of Chlorella vulgaris could serve as nutritional strategy for attenuation of oxidative stress for improving animal performance and reproduction because its supplementation led to higher finishing weights, higher commercial carcass yields as well as uteri-ovarian oxidative stress protector which could contribute to better reproduction. Supplementation of Chlorella vulgaris also manifested its antioxidant capabilities in multiple folds; from inhibition of oxidative stress products generation, to enhancement of antioxidant enzymes activities and up-regulation of primary antioxidant genes in liver and ovaries as important organs responsible in coordination of metabolic and reproductive activities. Therefore, the microalgae Chlorella vulgaris was recommended as supplement for protection against oxidative stress and improved productivity in rabbits and other food producing mammalian species. In addition, further studies into assessment of its effects on expression of transcripts and immune modulation genes in animal models is warranted in order to established its potential as nutraceutical.
Declarations
Author contribution statement
Sikiru A.B.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Arangasamy A.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alemede I.C., Egena S.S.A.: Conceived and designed the experiments; Wrote the paper.
Guvvala P.R.: Analyzed and interpreted the data. Ippala J.R., Bhatta R.: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Department of Biotechnology, Government of India; The World Academy of Sciences, Italy; and ICAR-National Institute of Animal Nutrition and Physiology, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are greatful to The World Academy of Sciences (TWAS), Trieste, Italy; Department of Biotechnology (DBT), Government of India for joint award of PhD Fellowship to Sikiru A.B. at ICAR-NIANP, Bengaluru, India under which this study was carried out. We acknowledge and thank authorities of Federal University of Technology, Minna, Nigeria for permitting Sikiru A.B. to take up the fellowship. Special thanks to The Director of ICAR-National Institute of Animal Nutrition and Physiology (NIANP), Bengaluru, India for providing enabling facilities and supporting the study. We are also greatful to Dr. Selvaraju S. and Dr. Sejian V. all of ICAR-NIANP for respectively allowed use of real-time PCR machine and microplate reader in their laboratories for this research.
References
- 1.Bryant D.M. Energy expenditure in wild birds. Proc. Nutr. Soc. 1997 Nov;56(3):1025–1039. doi: 10.1079/pns19970107. [DOI] [PubMed] [Google Scholar]
- 2.Speakman J.R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2007 Aug 8;363(1490):375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkling T., Blanchard P., Chastel O., Glauser G., Vallat-Michel A., Hatch S.A., Danchin E., Helfenstein F. Reproductive effort and oxidative stress: effects of offspring sex and number on the physiological state of a long-lived bird. Funct. Ecol. 2017 Jun;31(6):1201–1209. [Google Scholar]
- 4.Descalzo A.M., Sancho A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008 Jul 1;79(3):423–436. doi: 10.1016/j.meatsci.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Castillo C., Pereira V., Abuelo Á., Hernández J. Effect of supplementation with antioxidants on the quality of bovine milk and meat production. Sci. World J. 2013;2013 doi: 10.1155/2013/616098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karami M., Alimon A.R., Sazili A.Q., Goh Y.M. Meat quality and lipid oxidation of infraspinatus muscle and blood plasma of goats under dietary supplementation of herbal antioxidants. J. Anim. Vet. Adv. 2010;9(22):2839–2847. [Google Scholar]
- 7.Qin Y. Bioactive Seaweeds for Food Applications. Academic Press; 2018 Jan 1. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products; pp. 135–152. [Google Scholar]
- 8.Porse H., Rudolph B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phys. 2017 Oct 1;29(5):2187–2200. [Google Scholar]
- 9.Ötleş S., Pire R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001 Nov 1;84(6):1708–1714. [PubMed] [Google Scholar]
- 10.Aizzat O., Yap S.W., Sopiah H., Madiha M.M., Hazreen M., Shailah A., Junizam W.W., Syaidah A.N., Srijit D., Musalmah M., Anum M.Y. Modulation of oxidative stress by Chlorella vulgaris in streptozotocin (STZ) induced diabetic Sprague-Dawley rats. Adv. Med. Sci. 2010 Jan 1;55(2):281–288. doi: 10.2478/v10039-010-0046-z. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Garcia I., Guil-Guerrero J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008 Jun 1;108(3):1023–1026. doi: 10.1016/j.foodchem.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Guzman S., Gato A., Calleja J.M. Anti-inflammatory, analgesic and free radical scavenging activities of the marine microalgaeChlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001 May;15(3):224–230. doi: 10.1002/ptr.715. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud A.E., Naguib M.M., Higazy A.M., Sultan Y.Y., Marrez D.A. Effect of substitution soybean by blue green alga Spirulina platensis on performance and meat quality of growing rabbits. Am. J. Food Technol. 2017;12:51–59. [Google Scholar]
- 14.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974 Sep;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 15.Guvvala P.R., Ravindra J.P., Rajani C.V., Sivaram M., Selvaraju S. Protective role of epigallocatechin-3-gallate on arsenic induced testicular toxicity in Swiss albino mice. Biomed. Pharmacother. 2017 Dec 1;96:685–694. doi: 10.1016/j.biopha.2017.09.151. [DOI] [PubMed] [Google Scholar]
- 16.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979 Jan 4;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 17.Okhawa H., Ohishi N., Yogi K. Assay for lipid peroxidation in animal tissue by TBA reaction. Anal. Biochem. 1979;95:35–38. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Colombo G., Clerici M., Garavaglia M.E., Giustarini D., Rossi R., Milzani A., Dalle- Donne I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr., B. 2016 Apr 15;1019:178–190. doi: 10.1016/j.jchromb.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996 Jul 15;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Celi P., Gabai G. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front. Vet. Sci. 2015 Oct 26;2:48. doi: 10.3389/fvets.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lum K.K., Kim J., Lei X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotech. 2013 Dec;4(1):53. doi: 10.1186/2049-1891-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halle I., Janczyk P., Freyer G., Souffrant W.B. Effect of microalgae Chlorella vulgarison laying hen performance. Arch. Zootec. 2009;12(2):5–13. [Google Scholar]
- 23.Gouveia L., Choubert G., Gomes E., Rema P., Empis J. Use of Chlorella vulgaris as a carotenoid source for rainbow trout: effect of dietary lipid content on pigmentation, digestibility and retention in the muscle tissue. Aquat. Int. 1998 Aug 1;6(4):269–279. [Google Scholar]
- 24.Zheng L., Oh S.T., Jeon J.Y., Moon B.H., Kwon H.S., Lim S.U., An B.K., Kang C.W. The dietary effects of fermented Chlorella vulgaris (CBT®) on production performance, liver lipids and intestinal microflora in laying hens. AJAS (Asian-Australas. J. Anim. Sci.) 2012 Feb;25(2):261. doi: 10.5713/ajas.2011.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang H.K., Salim H.M., Akter N., Kim D.W., Kim J.H., Bang H.T., Kim M.J., Na J.C., Hwangbo J., Choi H.C., Suh O.S. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013 Mar 1;22(1):100–108. [Google Scholar]
- 26.Agarwal A., Allamaneni S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online. 2004 Jan 1;9(3):338–347. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- 27.Fulia F., Gitto E., Cuzzocrea S., Reiter R.J., Dugo L., Gitto P., Barberi S., Cordaro S., Barberi I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J. Pineal Res. 2001 Nov;31(4):343–349. doi: 10.1034/j.1600-079x.2001.310409.x. [DOI] [PubMed] [Google Scholar]
- 28.Li H.B., Cheng K.W., Wong C.C., Fan K.W., Chen F., Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007 Jan 1;102(3):771–776. [Google Scholar]
- 29.Dangles O., Dufour C., Manach C., Morand C., Remesy C. Methods in enzymology. Vol. 335. Academic Press; 2001 Jan 1. Binding of flavonoids to plasma proteins; pp. 319–333. [DOI] [PubMed] [Google Scholar]
- 30.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015 Apr 16;73(6):348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 31.Kim M.Y., Cheong S.H., Lee J.H., Kim M.J., Sok D.E., Kim M.R. Spirulina improves antioxidant status by reducing oxidative stress in rabbits fed a high-cholesterol diet. J. Med. Foods. 2010 Apr 1;13(2):420–426. doi: 10.1089/jmf.2009.1215. [DOI] [PubMed] [Google Scholar]
- 32.Mazo V.K., Gmoshinskiĭ I.V., Zilova I.S. Microalgae Spirulina in human nutrition. Voprosy pitaniia. 2004;73(1):45–53. [PubMed] [Google Scholar]
- 33.Riss T.L., Moravec R.A. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2004 Feb 1;2(1):51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- 34.Türk G., Çeribaşı S., Sönmez M., Çiftçi M., Yüce A., Güvenç M., Kaya Ş.Ö., Çay M., Aksakal M. Ameliorating effect of pomegranate juice consumption on carbon tetrachloride-induced sperm damages, lipid peroxidation, and testicular apoptosis. Toxicol. Ind. Health. 2016 Jan;32(1):126–137. doi: 10.1177/0748233713499600. [DOI] [PubMed] [Google Scholar]
- 35.De Ziegler D., Borghese B., Chapron C. Endometriosis and infertility: pathophysiology and management. The Lancet. 2010 Aug 28;376(9742):730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 36.Mihalas B.P., De Iuliis G.N., Redgrove K.A., McLaughlin E.A., Nixon B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Scientific Rep. 2017 Jul 24;7(1):6247. doi: 10.1038/s41598-017-06372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kouba A., Velíšek J., Stará A., Masojídek J., Kozák P. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus) BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/408270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janczyk P., Langhammer M., Renne U., Guiard V., Souffrant W.B. Effect of feed supplementation with Chlorella vulgaris powder on mice reproduction. Arch. Zootech. 2006;9:122–134. [Google Scholar]
- 39.Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006 Mar 1;46(2):185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H., Wang X., Zhou Y., Yao H., Ahmad F. Evaluation of the toxicity of ZnO nanoparticles to Chlorella vulgaris by use of the chiral perturbation approach. Anal. Bioanal. Chem. 2014 Jun 1;406(15):3689–3695. doi: 10.1007/s00216-014-7773-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee S.H., Kang H.J., Lee H.J., Kang M.H., Park Y.K. Six-week supplementation with Chlorella has favorable impact on antioxidant status in Korean male smokers. Nutrition. 2010 Feb 1;26(2):175–183. doi: 10.1016/j.nut.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Lee H.S., Choi C.Y., Cho C., Song Y. Attenuating effect of chlorella supplementation on oxidative stress and NFκB activation in peritoneal macrophages and liver of C57BL/6 mice fed on an atherogenic diet. Biosci. Biotechnol. Biochem. 2003;67(10):2083–2090. doi: 10.1271/bbb.67.2083. [DOI] [PubMed] [Google Scholar]
- 43.Panahi Y., Mostafazadeh B., Abrishami A., Saadat A., Beiraghdar F., Tavana S., Pishgoo B., Parvin S.H., Sahebkar A. Investigation of the effects of Chlorella vulgaris supplementation on the modulation of oxidative stress in apparently healthy smokers. Clin. Lab. 2013 Jan 1;59(5-6):579–587. doi: 10.7754/clin.lab.2012.120110. [DOI] [PubMed] [Google Scholar]
- 44.Shimada M., Hasegawa T., Nishimura C., Kan H., Kanno T., Nakamura T., Matsubayashi T. Anti-hypertensive effect of γ-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double-blind study. Clin. Exp. Hypertens. 2009 Jan 1;31(4):342–354. doi: 10.1080/10641960902977908. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T. Effect of gamma-aminobutyric acid-rich chlorella on blood pressure in mildlyhypertensive subjects. Jpn. Pharmacol. Ther. 2000;28(6):529–534. [Google Scholar]
- 46.Sansawa H., Inoue K., Shirai T. Effect of Chlorella tablet ingestion on mild hypercholesterolemic patients. J. Jpn. Soc. Food Sci. Tech. (Japan) 2002 [Google Scholar]
- 47.Emri T., Pócsi I., Szentirmai A. Glutathione metabolism and protection against oxidative stress caused by peroxides in Penicillium chrysogenum. Free Radical Biol. Med. 1997 Jan 1;23(5):809–814. doi: 10.1016/s0891-5849(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 48.Verduyn C., Giuseppin M.L., Scheffers W.A., van Dijken J.P. Hydrogen peroxide metabolism in yeasts. Appl. Environ. Microbiol. 1988 Aug 1;54(8):2086–2090. doi: 10.1128/aem.54.8.2086-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018 Dec 1;54(4):287–293. [Google Scholar]
- 50.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003 Oct;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M., Saw H.P., Cui F.F., Lin S.Y., Chang H.T., Chiu C.D. pLG72 induces superoxide radicals via interaction and aggregation with SOD1. Free Radic. Res. 2018 Sep 2;52(9):970–976. doi: 10.1080/10715762.2018.1504293. [DOI] [PubMed] [Google Scholar]
- 52.Abbasi-Oshaghi E., Mirzaei F., Pourjafar M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study. Int. J. Nanomed. 2019;14:1919. doi: 10.2147/IJN.S192382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization Glucose-6-phosphate dehydrogenase deficiency. WHO working group. Bull. World Health Organ. 1989;67(6):601–611. [PMC free article] [PubMed] [Google Scholar]
- 54.McDonagh E.M., Bautista J.M., Youngster I., Altman R.B., Klein T.E. PharmGKB summary: methylene blue pathway. Pharmacogenetics Genom. 2013 Sep;23(9):498. doi: 10.1097/FPC.0b013e32836498f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ismail M., Hossain M., Tanu A.R., Shekhar H.U. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. BioMed. Res. Int. 2015;2015 doi: 10.1155/2015/486120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treitinger A., Spada C., Verdi J.C., Miranda A.F., Oliveira O.V., Silveira M.V., Moriel P., Abdalla D.S. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur. J. Clin. Invest. 2000 May;30(5):454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 57.Burk R.F., Hill K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015 Jul 17;35:109–134. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- 58.López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007 Jul 8;252(1):1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Harris M.A. Gene ontology: tool for the unification of biology. Nat. Genet. 2000 May 1;25(1):25. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forgione M.A., Weiss N., Heydrick S., Cap A., Klings E.S., Bierl C., Eberhardt R.T., Farber H.W., Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002 Apr 1;282(4):H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 61.Steinberg M., Brauer M.J., Necheles T.F. Acute hemolytic anemia associated with erythrocyte glutathione-peroxidase deficiency. Arch. Int. Med. 1970 Feb 1;125(2):302–303. [PubMed] [Google Scholar]
- 62.de Haan J.B., Bladier C., Griffiths P., Kelner M., O’Shea R.D., Cheung N.S., Bronson R.T., Silvestro M.J., Wild S., Zheng S.S., Beart P.M. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J. Biol. Chem. 1998 Aug 28;273(35):22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 63.Yu B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994 Jan 1;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 64.Qian H., Chen W., Sun L., Jin Y., Liu W., Fu Z. Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology. 2009 Jul 1;18(5):537–543. doi: 10.1007/s10646-009-0311-8. [DOI] [PubMed] [Google Scholar]