Abstract
Introduction
We investigated the effects of zerumbone (1 and 10 mg/kg) against hyperactivity, anxiety and memory impairment in scopolamine-induced dementia in Sprague-Dawley rats.
Methods
Open field tests, elevated plus maze and Morris water maze were performed to assess general locomotor activity, anxiety-like behaviours and learning and memory processes respectively in rats pretreated with scopolamine.
Results
Scopolamine-treated rats showed high total activity, stereotype, and total distance travelled in the open field arena, reduced number of entries to open arms, decreased the percentage of time spent in open arms and higher escape latency time in the Morris water maze test. Interestingly, single administration of zerumbone (1 and 10 mg/kg) reversed the hyperactivity, anxiety-like behaviours, and learning impairment effects of scopolamine in the three experimental model studied respectively.
Discussion
Our findings demonstrated that the scopolamine-induced impairment of learning and memory was reversed by the administration of zerumbone. As a conclusion, our findings presented the positive effects of zerumbone on dementia-like behaviours in the animal model used and could possibly contribute for future research to manage hyperactivity, anxiety, and learning disabilities.
Keywords: Dementia, Alzheimer's disease, Scopolamine, Learning and memory deficit, Anxiety, Hyperactivity, Zerumbone
1. Background
Dementia is a term generally used to describe a decline in mental ability associated with cognition, memory, or other thinking skills. This deterioration eventually interferes with occupational functioning and social activities. Alzheimer's disease (AD), among the common type of dementia, is a progressive neurodegenerative disease. It is characterized by the loss of memory accompanied by behavioural disturbances thereby negatively impacting daily activities. AD accounts for nearly 70% of dementia cases worldwide [1]. Among the pathophysiological changes that occur leading to AD are alteration of cholinergic neurons, tau proteins, and accumulation of the β-amyloid peptide. Studies have shown that the decrease in acetylcholine (ACh) in the brain may be linked to cognitive impairment. In addition, an increase in acetylcholinesterase (AChE), an enzyme responsible for degrading ACh, has been observed in patients with AD. Common AD symptoms are memory loss; difficulties in planning, solving problems, speaking, writing, and completing familiar tasks; disorientation; and changes in behaviours such as anxiety and depression [2]. As the disease progresses, the symptoms will worsen. Diagnosis of AD can be carried out in several days or weeks by obtaining a medical and family history of the patient, observing the changes in behaviour and thinking skills, cognitive tests, examination on physical and neurological conditions, blood tests, and brain imaging [3]. Early stages of memory impairment can be associated with cholinergic deficits in the hippocampus and cerebral cortex regions [4]. Neuropsychiatric symptoms such as depression, apathy, aggression, and psychosis share similar pathogenic processes with AD beside their unique pathogenic processes [5].
Various animal models have been developed to evaluate dementia based on their diverse pathophysiological basis. Scopolamine acts as a competitive antagonist at muscarinic receptors. It damages central cholinergic functions, leading to learning and memory impairment in rodents. This mimics the loss of cortical cholinergic neurons and deficient central cholinergic functions seen in AD brains. As scopolamine is an anticholinergic agent, it blocks ACh binding sites resulting in high concentrations of ACh. This results in damaging the hippocampus nerves, eventually leading to memory loss and learning problems [6].
At present, there is no permanent cure for dementia-related disorders and existing medications do not provide satisfactory improvements. There are also various adverse effects associated with existing treatment. Plant-derived compounds have played an important part as a source in formulating synthetic drugs. Zerumbone (2,6,9,9-tetramethyl-[2E,6E, 10E]-cycloundeca-2,6,10-trien-1-one), a sesquiterpenoid, is abundant in rhizomes of Zingiber zerumbet (L) Smith, a subtropical ginger plant. Zerumbone was reported to inhibit the proliferation of colon [7] and breast cancers [8], suppress skin tumours in mice [9], and block tumor necrosis factor-induced nuclear factor-κB activation in various cancer cell lines [10]. The in vitro studies of zerumbone showed that it has the potential to inhibit acetylcholinesterase enzyme and could be a great source as an anti-AD agent [11].
In the present study, we investigated the therapeutic effects of zerumbone on locomotor activities, anxiety-like behaviours and learning and memory processes in the scopolamine-induced dementia rats.
2. Methods
2.1. Animals
Twenty four male Sprague-Dawley rats (weighing 200–250 g) were housed at the animal house of Center for Drug Research, Universiti Sains Malaysia, with room temperature maintained at 24 ± 1°C, 12 hours light/dark cycle. Animals were acclimatized for 1 week before experiments with free access to standard laboratory food pellets and water. The behavioural experiments were conducted in accordance with current guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Universiti Putra Malaysia (UPM) (Ref: UPM/IACUC/AUP-R032/2017).
2.2. Drugs
Tween 20 and scopolamine hydrobromide were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). Scopolamine was dissolved in normal saline (0.9% NaCl). Zerumbone was dissolved in Tween 20 and normal saline at a ratio of 5:95. Treatments were administered intraperitoneally in a volume of 10 mL/kg.
2.3. Experimental design
Animals were randomly selected into four groups (n = 6). Group 1 received 5% Tween 20 dissolved in normal saline (control group) and group 2 received scopolamine 20 mg/kg (negative control); group 3 and 4 received zerumbone 1 and 10 mg/kg, respectively (treatment groups). Scopolamine 20 mg/kg was injected to all groups except the control group. After 30 min, zerumbone with the respective doses were administered to groups 3 and 4. Behavioural tests were performed after 1 hour of the last treatments.
2.4. Open field test
The open field test (OFT) is performed to measure locomotor activities and general performance effects of administered drugs in rodents, which could affect the performance of learning and memory. The automated open field consists of a 40 cm × 40 cm × 35 cm arenas, which are divided into equal squares. The arena was virtually divided by software (ActiTrack v2.7.13, Panlab) into central and peripheral zones. After treatment, each animal was placed individually at the centre of the open field arena, and locomotion was recorded for 20 min. During this period, the total activity, stereotypes, the mean velocity (cm/s), total distance travelled (cm), number of rearing, number of entry to central zone, and time spent in central zone were automatically recorded [12].
2.5. Elevated plus maze
The elevated plus maze (EPM) is used to evaluate anxiety level in rodents, following the procedure described previously [13]. The apparatus consists of two open arms (50 cm × 10 cm) and two covered arms (50 cm × 10 cm × 40 cm). With the arms extended from a central platform (10 cm × 10 cm). The apparatus was made of stainless steel and elevated to a height of 50 cm above the floor. After treatment, each animal was placed individually at the central square of the maze facing on open arms and allowed to explore the maze freely. The behaviour of animals was recorded for 5 minutes and a number of parameters were measured including total arms entry, number of entry to close and open arms, percentage of time spends in open arms, and percentage of entry to open arms.
2.6. Morris water maze
The Morris water maze (MWM) was used to evaluate learning and memory functions in the rat. The apparatus consists of a circular water pool (120 cm in diameter with a depth of 60 cm) which is divided to four equal quadrants (north, south, east, and west) with some external cues. The pool was filled with water (25 ± 1˚C), and nontoxic white colour was added into the water to make it opaque. A circular platform (10 cm in diameter) was placed in the centre of the quadrant 1 cm below the water surface. The MWM test consists of five days training sessions with four consecutive trials per day followed by intertrial interval of 30s. For each acquisition trial sessions (days 1–5) rats were placed individually in the water facing the pool wall and allowed to swim freely to find the hidden platform within 60s. They were gently guided to the platform if they could not find it and allowed to stay on it for 20s before being removed. The platform was placed at constant position throughout training while the starting points were randomly selected. Mean escape latency time (ELT) of rat to find the hidden platform is recorded as an index of acquisition or learning. On the 6th day of treatment, the platform was removed from the pool for probe trial test of 60s. The average time spent in the target quadrant, the number of entries to the target quadrant, and the first latency entrance to the target quadrant were recorded as an index of spatial memory [14].
3. Statistical analysis
All the results were expressed as mean ± standard error of mean and behavioural scores within OFT, EPM, and MWM (except ELT) were statistically analysed using one-way analysis of variance followed by Tukey's post hoc test using GraphPad Prism 5 software. Two-way analysis of variance followed by Bonferroni's post hoc test was used to analyse ELT results. P < .05 was considered statistically significant.
4. Results
4.1. Effect of zerumbone on locomotors activity
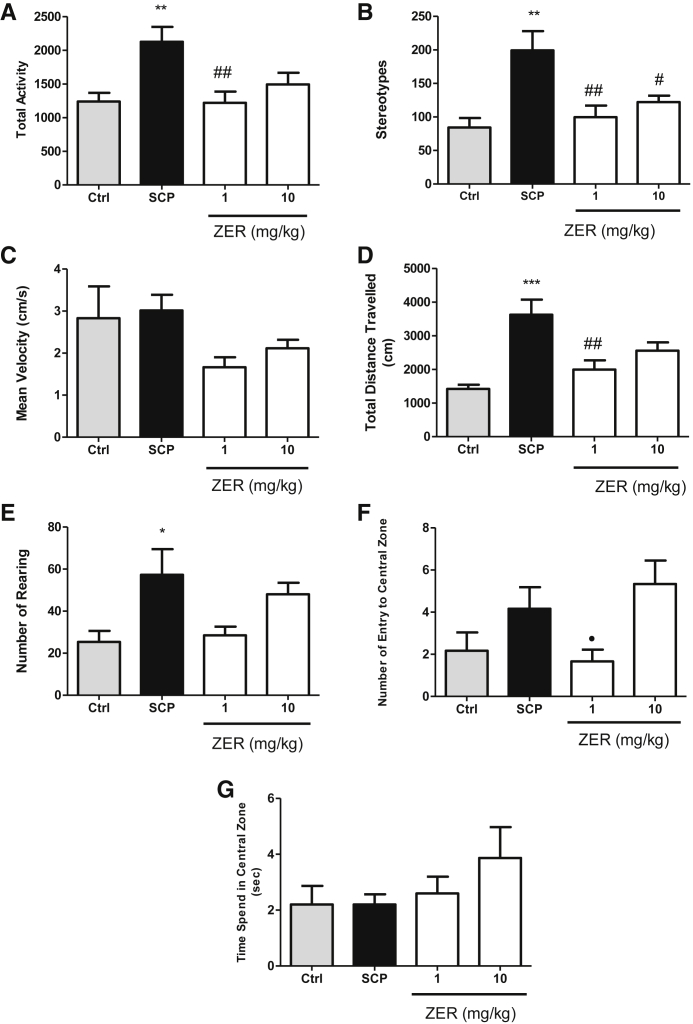
In the OFT, scopolamine-treated rats showed significantly higher stereotype. Both doses of zerumbone (1 and 10 mg/kg) groups decreased the hyperlocomotion effect of scopolamine (Fig. 1). In addition, lower dose of zerumbone (1 mg/kg) significantly decreased the total activity and total distance travelled by the rats compared with the scopolamine alone group. The time spent in the central zone and the mean velocities of all treated groups are at the same level as control group which demonstrated exploration and motor performance in rats, respectively. The lower dose of zerumbone (1 mg/kg) significantly reduced number of entry to central zone compared with the higher dose of zerumbone (10 mg/kg). Besides, no significant differences were observed between the two doses of zerumbone (1 and 10 mg/kg) groups pretreated with scopolamine and control groups among all parameters such as total activity, stereotypes, total distance travelled, number of rearing, number of entry to central zone, and time spent in central zone.
Fig. 1.
Effect of zerumbone (1 and 10 mg/kg) in the open field test on the total activity (A), stereotypes (B), the mean velocity (cm/s) (C), total distance travelled (cm) (D), number of rearing (E), number of entry to central zone (F), and time spent in central zone (%) (G) on the SCP pretreated rats. Data expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey's post hoc test. ***P < .001 and **P < .005 and *P < .01 compared with the control group. ##P < .005 and #P < .001 compared with the SCP group. •P < .05 as compared to zerumbone 10 mg/kg pretreated with SCP group. SEM, standard error of mean; ANOVA, analysis of variance; SCP, scopolamine.
4.2. Effect of zerumbone on anxiety-like behaviour
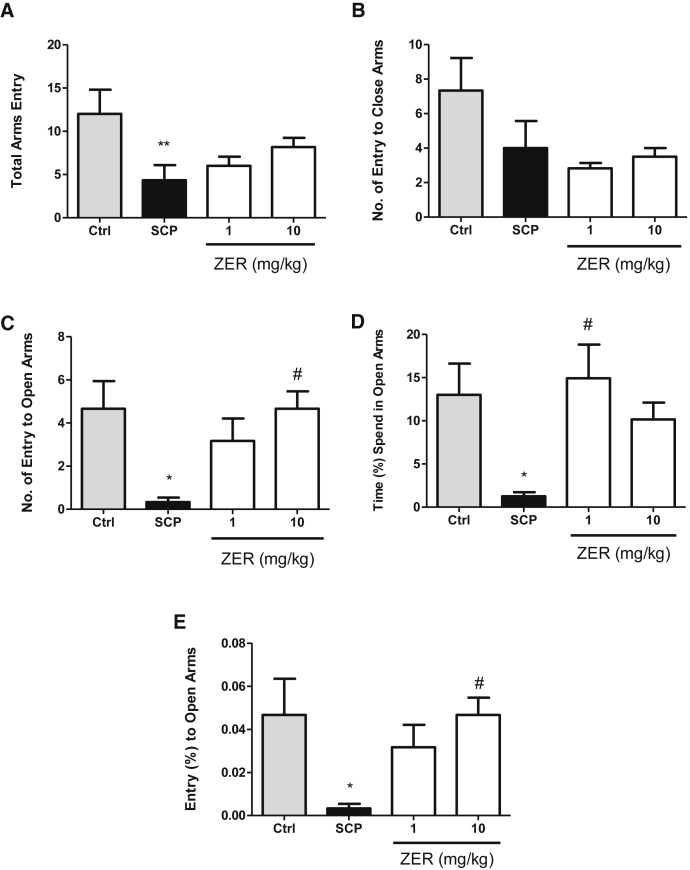
In the EPM task as shown in Fig. 2, lower number of entry, a lower percentage of entry to open arms, and lower percentage of time spent in open arms during a 5-minute session are indicated as higher anxiety-like behaviours in rats. Based on the results obtained, scopolamine group showed significantly lower percentage and number of entry to open arms compared with all other groups. The higher dose of zerumbone (10 mg/kg) group pretreated with scopolamine significantly increased the percentage and number of entry to open arms. In addition, the lower dose of zerumbone (1 mg/kg) group pretreated with scopolamine also significantly increased the percentage of time spent in open arms compared with scopolamine-treated group. Besides, there is no significant difference between both doses zerumbone (1 and 10 mg/kg) groups.
Fig. 2.
Effect of zerumbone (1 and 10 mg/kg) in the elevated plus maze on the total arms entry (A), number of entry to close arms (B), number of entry to open arms (C), percentage of time spent in open arms (D), and percentage of entry to open arms (E) on the scopolamine pretreated rats. Data expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey's post hoc test. *P < .05 compared with the control group. #P < .05 compared with the SCP group. ANOVA, analysis of variance; SEM, standard error of mean.
4.3. Effect of zerumbone on learning and memory
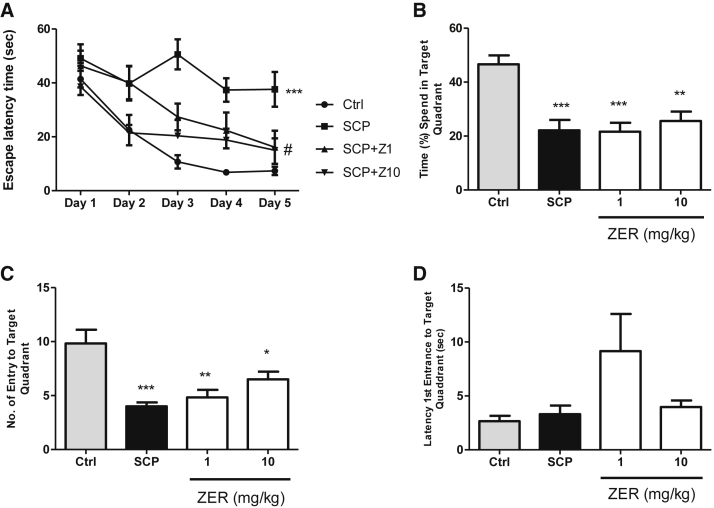
In the MWM as shown in Fig. 3, reduction in the mean of ELT indicated as improvement in the learning process. While, higher percentage of time spent in target quadrant, higher number of entry to target quadrant, and lower latency first entrance to target quadrant are indicated as improvement in reference memory. Scopolamine-treated rats showed significantly higher ELT compared with all other groups on the last day of training. Both doses of zerumbone (1 and 10 mg/kg) significantly decreased ELT compared with the scopolamine only group on day 3 and 5 of training. Therefore, both doses of zerumbone improved learning process in rats pretreated with scopolamine. However, zerumbone groups pretreated with scopolamine did not show any statistically significant difference compared with scopolamine-treated group in number of entry and latency of the first entrance to target quadrant as well as percentage of time spent in target quadrant.
Fig. 3.
Effect of zerumbone (1 and 10 mg/kg) in the Morris water maze on the escape latency time (A), percentage of time spent in target quadrant (B), number of entry to target quadrant (C), and latency first entrance to target quadrant (D) on the scopolamine pretreated rats. Data expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey's post hoc test. ***P < .001 compared with the control group. Two-way ANOVA followed by Bonferroni post hoc test was used to analyze escape latency time and ***P < .001 compared with the control group. #P < .05 compared with the SCP group. ANOVA, analysis of variance; SEM, standard error of mean.
5. Discussion
Scopolamine-induced dementia is widely used to evaluate the potential of therapeutic agents to treat AD. Based on studies on the rat brain, scopolamine causes impairment to learning and memory through degeneration and dysfunction of cortical cholinergic neurons which are the result of several genes expression related to muscarinic receptor signalling pathways, apoptosis, and cell differentiation. It has been reported that memory impairment effects of scopolamine could be related to high level of lipid peroxidation and low amount of antioxidant in brain [15].
In the present study, we performed three well-characterized behavioural tests (OFT, EPM, and MWM) to investigate whether acute administration of zerumbone with two dosages (1 and 10 mg/kg) causes reduction in hyperactivity, anxiety-like behaviour, and memory deficit in Sprague-Dawley rats pretreated with scopolamine. Scopolamine, which is a muscarinic antagonist drug, blocks presynaptic muscarinic acetylcholine receptors turning to activate dopamine neurons resulting in hyperactivity [16]. In acute overdosage, scopolamine shows toxic properties on central nervous system and leads to injury in hippocampal circuits that may result in cognitive and memory impairment [13].
In this study, the OFT was performed to evaluate general locomotors activity of rats. Based on the results, scopolamine-treated rats exhibited hyperactivity/locomotion behaviour, as indicated by significantly increased total activity, stereotype, and total distance travelled in the arena of the OFT. This hyperactivity effect of scopolamine reversed to normal condition with single administration of zerumbone. The higher dose of zerumbone (10 mg/kg) also reversed the scopolamine effect only by reducing stereotype. Moreover, scopolamine-treated rats showed cognitive impairment and memory deficit as indicated by increased escape latency time, lower number of entry to target quadrant, and reduction in percentage of time spent in target quadrant in MWM test. Both doses of zerumbone (1 and 10 mg/kg) in scopolamine pretreated rats significantly showed improvement in acquisition trials as evidenced by reduction in the escape latency time as compared with scopolamine group. This result suggests that even the low dose of zerumbone has the potential to improve learning process. However, no differences were observed between zerumbone and scopolamine groups on reference memory during probe trial in the MWM test.
This study also demonstrated that memory impairment effect of scopolamine is related to anxiety as assessed in an EPM behavioural test. The EPM task is widely used to predict the anxiety responses of drugs in rodents. It has been reported that single or repeated administration (i.p.) of scopolamine caused anxiety and depression-like behaviour in rats [13,17]. These findings are in agreement with our results that indicated significant reduction of number of entry to open arms, decreased percentage of time spent in open arms, and decreased percentage of entry to the open arms of the EPM test. However, this reduction was reversed by single administration of either dose of zerumbone (1 or 10 mg/kg). A previous study suggested that the anxiety indicators parameters in the EPM such as the percentage of time spent in open arms and the number of entry to open arms are related to GABAA receptor complex [18]. Various terpenes are reported to mediate anxiolytic mechanisms involving GABAA receptors [13]. Based on these reports, we propose that the bioactive compound, zerumbone used in this study, which is a sesquiterpene of Zingiber zerumbet Smith, decreased anxiety-like behaviours in scopolamine pretreated rats.
Collectively, these data suggest that improvement in learning within MWM tests along with an increase of anxiolytic-like behaviour within EPM and reduction in hyperactivity within OFT could be related to the administration and effects of zerumbone against scopolamine-induced hyperactivity, memory deficit, and anxiety in laboratory Sprague-Dawley rats.
6. Conclusion
Our findings demonstrated that zerumbone treatment caused a reduction in hyperactivity, anxiety, and facilitated learning improvement on scopolamine-induced dementia rats. In conclusion, we suggest that zerumbone could be a new lead compound for the development of anxiolytic drugs to prevent or treat some conditions close to AD.
Research in Context.
-
1.
Systematic review: Dementia is a term generally used to describe a decline in mental ability associated with cognition, memory, or other thinking skills. This deterioration eventually interferes with occupational functioning and social activities. Alzheimer's disease, among the common type of dementia, is a progressive neurodegenerative disease.
-
2.
Interpretation: In this study, we investigated the effect of zerumbone against memory impairment, anxiety, and hyperactivity effects in Sprague-Dawley rats. Scopolamine-treated rats showed high total activity, stereotype, and total distance travelled in the open field arena. Interestingly, single administration of zerumbone at doses of 1 and 10 mg/kg reversed the hyperactivity, anxiety-like behaviour, and learning impairment effects of scopolamine to normal condition.
-
3.
Future directions: The study concludes the positive effect of zerumbone on dementia-like behaviour and might be useful for the treatment of hyperactivity, anxiety, and learning disabilities.
Acknowledgments
The author wishes to express sincere appreciation to the Center for Drug Research, Universiti Sains Malaysia for their facilities and support for this research.
This study was supported by the Putra Grant – Geran Putra Berimpak (Grant No: 9659000) from Universiti Putra Malaysia and the e-Science Fund Scheme (Grant No: 5450778) from the Ministry of Science, Technology & Innovation (MOSTI), Malaysia.
Footnotes
The authors declare no conflict of interests.
References
- 1.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association, Alzheimer Association 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Association, Alzheimer Association 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 4.Behl P., Edwards J.D., Kiss A., Lanctot K.L., Streiner D.L., Black S.E. Treatment effects in multiple cognitive domains in Alzheimer's disease: a two-year cohort study. Alzheimers Res Ther. 2014;6:48. doi: 10.1186/alzrt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X.-L., Hu N., Tan M.-S., Yu J.-T., Tan L. Behavioral and psychological symptoms in Alzheimer's disease. Biomed Res Int. 2014;2014:927804. doi: 10.1155/2014/927804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sodhi R.K., Jaggi A.S., Singh N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014;109:73–86. doi: 10.1016/j.lfs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Murakami A., Takahashi D., Kinoshita T., Koshimizu K., Kim H.W., Yoshihiro A., Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α, β-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23:795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 8.Kirana C., McIntosh G.H., Record I.R., Jones G.P. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003;45:218–225. doi: 10.1207/S15327914NC4502_12. [DOI] [PubMed] [Google Scholar]
- 9.Murakami A., Tanaka T., Lee J.Y., Surh Y.J., Kim H.W., Kawabata K., Ohigashi H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int J Cancer. 2004;110:481–490. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]
- 10.Takada Y., Murakami A., Aggarwal B.B. Zerumbone abolishes NF-κB and IκBα kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 11.Bustamam A., Ibrahim S., Al-Zubairi A., Met M., Syam M. Zerumbone: a natural compound with anti-cholinesterase activity. Am J Pharmacol Toxicol. 2008;3:209–211. [Google Scholar]
- 12.Seibenhener M.L., Wooten M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydin E., Hritcu L., Dogan G., Hayta S., Bagci E. The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol. 2016;53:6557–6567. doi: 10.1007/s12035-016-9693-9. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher M., Burwell R., Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 2015;129:540–548. doi: 10.1037/bne0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaki H.F., Abd-El-Fattah M.A., Attia A.S. Naringenin protects against scopolamine-induced dementia in rats. Bull Fac Pharm Cairo Univ. 2014;52:15–25. [Google Scholar]
- 16.Roffman J.L., Tanner A.S., Eryilmaz H., Rodriguez-Thompson A., Silverstein N.J., Ho N.F., Abi-Dargham A. Dopamine D1 signaling organizes network dynamics underlying working memory. Sci Adv. 2016;2:e1501672. doi: 10.1126/sciadv.1501672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmati B., Kiasalari Z., Roghani M., Khalili M., Ansari F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm Biol. 2017;55:958–965. doi: 10.1080/13880209.2017.1285320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Snafi A.E. A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR J Pharm. 2016;6:17–42. [Google Scholar]