Studies of colorectal cancer (CRC) traditionally focused on the role of epithelial genetic mutations and signaling pathway dysregulation with an underappreciation of faulty signaling impact from the stromal microenvironment in this context.1 The colonic stroma consists of professional and nonprofessional cells with fibroblasts, myofibroblasts, and telocytes found in close contact with the epithelia.2, 3 These latter cells are responsible for the contribution of the microenvironment surrounding the epithelium. The central role played by nonmyofibroblastic Foxl1+ telocytes as the source of indispensable intestinal stem cell niche factors was shown recently.3, 4 Although mutation of PTEN in epithelium of various organs leads to cancer development, we and others showed that specific intestinal epithelial cell ablation of Pten is insufficient to initiate neoplasia.5, 6 Such observations on epithelial phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling suggest a greater role for extra-epithelial signaling in gut tumor development.
Here, using the Foxl1Cre recombinase, we generated a mouse model featuring a specific deletion of Pten in gut telocytes (PtenΔFoxl1+) (Supplementary Figure 1), allowing dissection of the specific contribution of PTEN signaling in colonic telocytes. As previously shown with abrogation of Bmpr1aΔFoxl1+,7, 8 and herein with Pten, the deregulation of important cell signaling in gut telocytes strongly impacted colon epithelial homeostasis (Figure 1). By 75 days, PtenΔFoxl1+ colon spontaneously developed an average of 28 polyps as opposed to none in controls. Although normal colonic mucosal architecture was observed in both control and PtenΔFoxl1+ mice, mutants showed regions developing into lesions characterized by gut-associated lymphoid tissues, epithelial hyperplasia developing into benign lymphoid aggregates, and hamartomatous polyps composed of a mixture of epithelial and mesenchymal cells. These non-neoplastic lesions did not progress to carcinoma with age and were histologically similar to human hamartomatous polyps described in Cowden’s disease. Thus, telocyte PTEN signaling is a key singular protective pathway against colonic polyposis initiation.
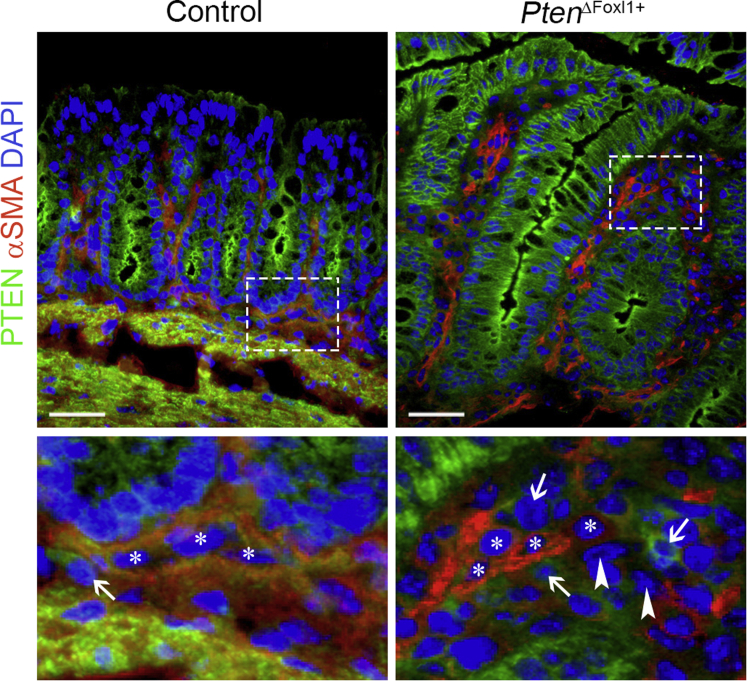
Supplementary Figure 1.
Validation of the loss of PTEN in colonic telocytes of PtenΔFoxl1+mice. PTEN immunostaining (signal in green) is observed in the epithelium of both control and PtenΔFoxl1+ mice. Co-staining of PTEN and α-smooth muscle actin (αSMA) (signal in red) showed that immunoreactivity to PTEN was specifically lost in some αSMA- mesenchymal cells (white arrowheads) as opposed to αSMA+ mesenchymal cells (white asterisks) and other αSMA- mesenchymal cells (white arrows) corresponding to fibroblasts, which retain PTEN expression in PtenΔFoxl1+ mice. Control mice showed PTEN immunoreactivity in αSMA- (white arrow) and αSMA+ (white asterisks) mesenchymal cells. Scale bars: 25 μm. DAPI, 4′,6-diamidino-2-phenylindole.
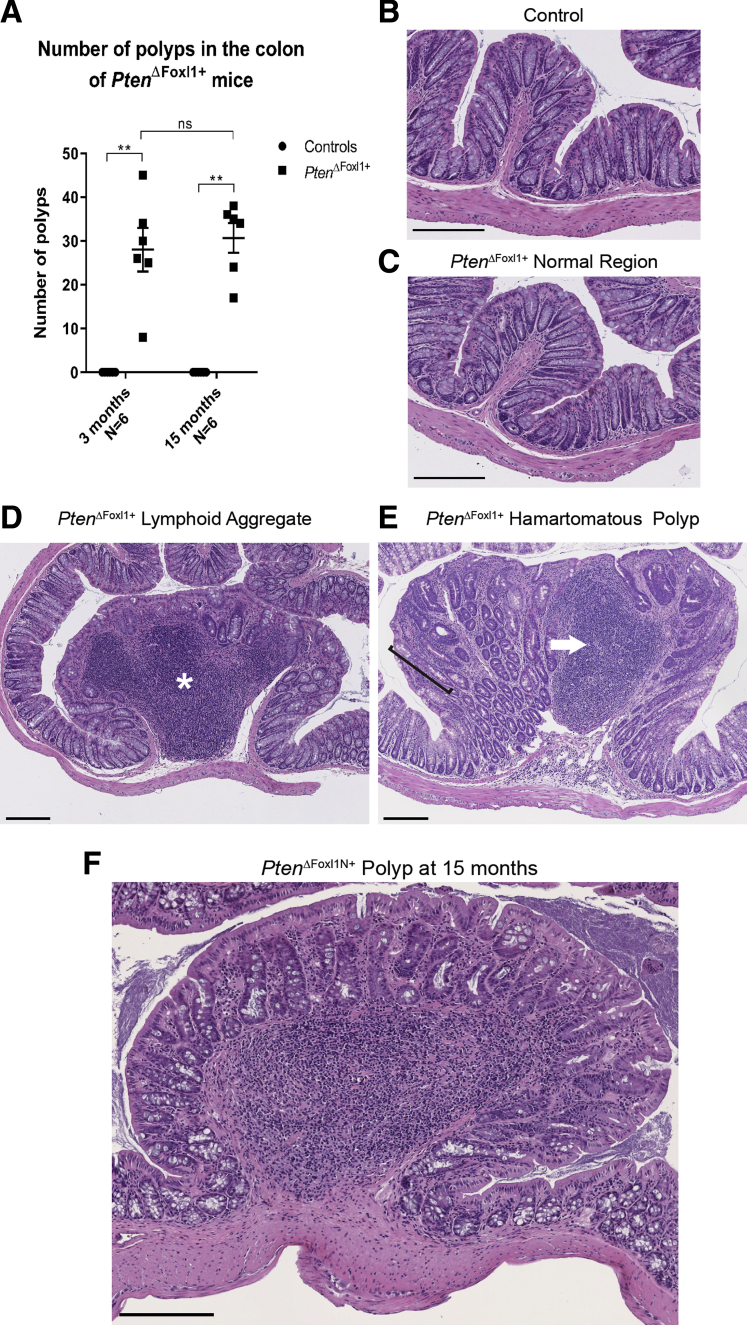
Figure 1.
PtenΔFoxl1+mice develop spontaneous polyps in their colon by 75 days. (A) Polyps were counted from 3- and 15-month control and PtenΔFoxl1+ colons. H&E staining of (B) control and (C) PtenΔFoxl1+ normal colonic mucosa. (D) Lymphoid aggregates in PtenΔFoxl1 colon featured large gut-associated lymphoid tissues (white asterisk) and epithelial hyperplasia. (E) Hamartomatous polyps showed a mixture of epithelial and mesenchymal cells, longer crypts (black bracket), and immune cell infiltrates (white arrow). (F) Polyps in 15-month-old mutants did not progress into carcinoma. Data are expressed as means ± SEM. Mann–Whitney U test: **P ≤ .01. Scale bars: 200 μm.
Studies showed that telocytes play a crucial role in secreting epithelial-interacting factors.3, 4 Disruption of PTEN signaling in telocytes altered the subepithelial milieu (Supplementary Figure 2). Proliferating cell nuclear antigen immunostaining showed increases in the number of proliferating cells in the epithelium (3.18-fold) and surrounding mesenchyme (1.45-fold) in mutants when compared with controls. Co-immunostaining of vimentin and α-smooth muscle actin showed a remodeling of nonprofessional cell populations in mutants compared with controls. Nonprofessional cells are strongly bioactive through secretion of soluble factors impacting the extracellular milieu supporting epithelial cells. To investigate for precocious altering events, mucosa of 30-day-old control and PtenΔFoxl1+ mice were analyzed for deregulated production of secreted factors. Altered expression of growth factors, extracellular matrix remodeling factors, cytokines, and chemokines was observed in PtenΔFoxl1+ mice (Supplementary Tables 1 and 2). By deleting PTEN signaling in telocytes, we show how these cells play a key role in regulating the secretion of epithelial, stromal, and immune cell interacting soluble factors, promoting a potent tumor microenvironment without pre-existing epithelial hyperplasia.
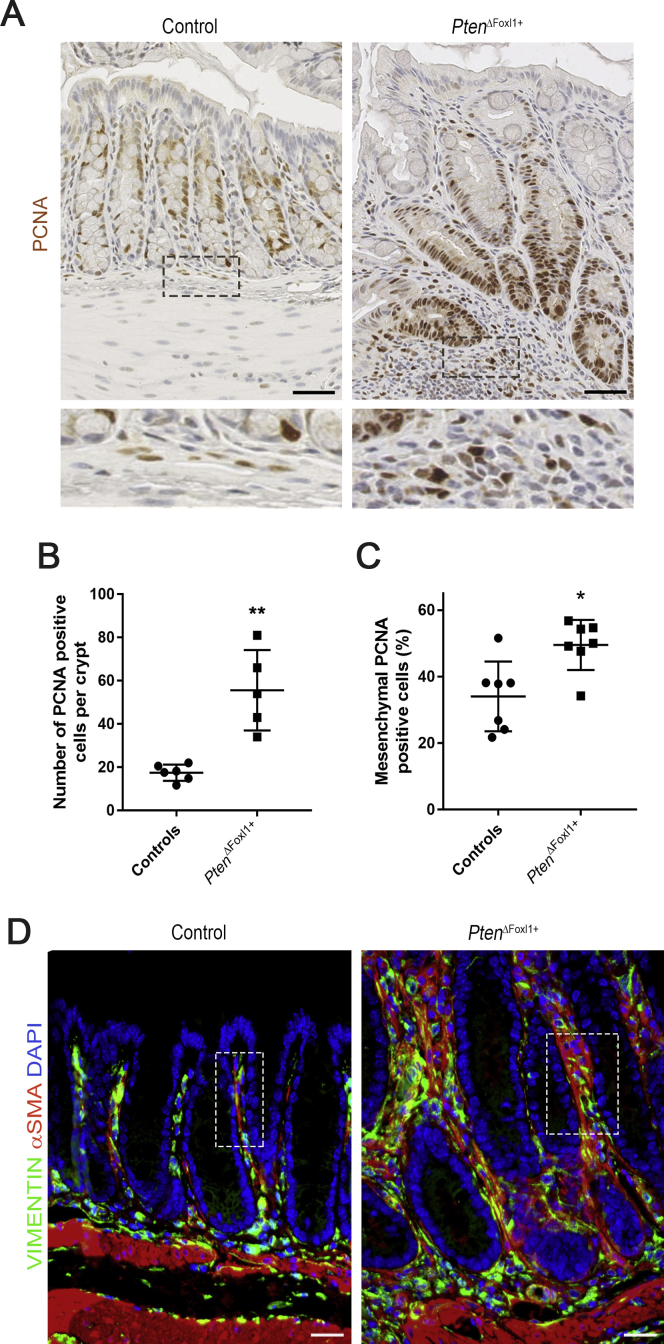
Supplementary Figure 2.
Increased mucosal proliferation and mesenchymal cellular remodeling in PtenΔFoxl1+mice. (A) Proliferation was analyzed by immunohistochemistry using a proliferating cell nuclear antigen (PCNA) antibody in 75-day-old control and PtenΔFoxl1+ mice. Proliferative cells (brown signal) were found in a very limited number of pericryptal mesenchymal cells in control mice. Cell counts of PCNA-stained cells showed a significant increase in the number of proliferating cells in the (B) epithelium of hyperplastic crypts (3.18-fold) (N = 5) and in the (C) mesenchyme (1.45-fold) of PtenΔFoxl1+ mice (N = 7) compared with controls (N = 6 and N = 7, respectively). (D) Co-immunostaining against α-smooth muscle actin (αSMA) (red staining, muscle cells, and myofibroblasts) and vimentin (green staining, fibroblasts, Foxl1+ cells, and myofibroblasts) was performed on colonic sections of control and PtenΔFoxl1+ mice. Myofibroblasts (yellow), fibroblasts, and Foxl1+ cells (green) and muscle cells (red) were present in the mesenchyme of both control and mutant mice, with a robust increase in all cell types in polyp pericryptal mesenchyme of PtenΔFoxl1+ mice. For example, in the delimitated areas, epithelial cells represent 71%, fibroblasts and Foxl1+ cells represent 14%, myofibroblasts represent 5%, and muscle cells represent 10% of all cells in the control mouse compared with 50% of epithelial cells, 18% of fibroblasts and Foxl1+ cells, 20% of myofibroblasts, and 12% of muscle cells in the mutant mouse. Error bars represent SEM (Mann–Whitney U test: *P < .05; **P < .01). Five to 10 crypts per mouse were counted and each dot represents the average for 1 mouse. Scale bars: (A) 50 μm and (D) 25 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Because PtenΔFoxl1+ mice only develop benign polyps without cancerous progression, we hypothesized that the absence of Pten in telocytes in concert with a mutated epithelium could enable the evolution of the cancer cascade. The generation of compound mutant mice with PtenΔFoxl1+ and an ubiquitous ApcMin/+ mutation uncovered key synergistic cross-talk between defective subepithelial mesenchyme and a primed oncogenic epithelium, impacting epithelial tumor multiplicity (Figure 2). Our results show that at 75 days (compound mice died at approximately 85 days), this double mutation (PtenΔFoxl1+;ApcMin/+) leads to accelerated polyposis initiation compared with ApcMin/+ animals (average, 18 and 3 adenomas, respectively), although no polyps were found in controls. This particular genetic combination is not sufficient to enable malignant transformation of a preneoplastic epithelium at 75 days of age. This could be explained by the limited life expectancy of the compound mice. This short latency is indubitably detrimental to the acquisition of key mutations allowing tumor progression. Second, the use of a different genetic epithelial mutation in combination with our dysfunctional mesenchyme should be explored. The serrated mouse models of CRC feature tumors that progress into adenocarcinomas with metastasis potential.9, 10 Serrated CRC involves initial mutations such as KRAS or BRAF, but not APC. Because the loss of PTEN signaling in telocytes lead to an epithelial hyperplasia within 85 days, its association with epithelial mutations of the serrated route potentially could lead to the formation of more invasive lesions.
Figure 2.
Pten-deficient telocytes and ApcMin/+mutation increases adenoma multiplicity but not epithelial transformation in mice. Total (A) colonic polyps and (B) adenomas were counted from 75-day-old ApcMin/+, PtenΔFoxl1+, and PtenΔFoxl1+;ApcMin/+ mice. (C) Histologic assessment showed an increase of adenoma multiplicity in PtenΔFoxl1+;ApcMin/+ when compared with ApcMin/+ colons (N = 6 and 5, respectively). Asterisks indicate adenomatous polyps. (D) All ApcMin/+ polyps were assessed histologically as adenomas. (D) Adenomas, (E) lymphoid aggregates, and (F) a mixed adenoma were found in PtenΔFoxl1+;ApcMin/+ colons. Data are expressed as means ± SEM. Mann–Whitney U test: **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001. Scale bars: (A) 1 mm, (D–F) 500 μm.
The present results indicate that loss of Pten in telocytes is sufficient to trigger the development of spontaneous colonic polyposis. Our findings further support that telocytes abrogated for PTEN signaling feature an altered stroma cell ratio and secretory profile leading to the establishment of a toxic microenvironment impacting colonic epithelial homeostasis. The current observations suggest that synergy between Pten-deficient telocytes and an oncogenically primed epithelium toward cancer progression may require a mutation from pathways such as the serrated route, as opposed to the traditional pathway involving APC mutation.
Footnotes
Author contributions Marie-Josée Langlois, Nathalie Rivard, Francois Boudreau, Julie C. Carrier, and Nathalie Perreault conceived and designed the experiments; Marie- Josée Langlois contributed to results shown in Figures 1 and 2, Supplementary Figures 1 and 2, and Table 1; Raphaëlle Servant contributed to results shown in Supplementary Figures 1 and 2; Vilcy Reyes-Nicolas and Christine Jones contributed to results shown in Supplementary Table 2; Sébastien A. B. Roy contributed to results shown in Figures 1 and 2; Marie-Josée Langlois, Vilcy Reyes-Nicolas, Christine Jones, Marilène Paquet, Nathalie Rivard, Julie C. Carrier, Francois Boudreau, and Nathalie Perreault analyzed the data; Nathalie Rivard, Francois Boudreau, and Nathalie Perreault contributed to reagents/materials/analysis tools; Raphaëlle Servant contributed to figure preparation; and Nathalie Perreault prepared and wrote the manuscript. All authors reviewed the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This research was supported by the Cancer Research Society (N.P., F.B., J.C.C., and N.R.), and the Canadian Institutes of Health ResearchMOP-136917 (N.P.).
Supplementary Materials and Methods
Animals
C57BL/6-ApcMin/+ (002020) and BALB/c-Ptenfx/fx (004597) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The C57BL/6J Foxl1Cre transgenic line was provided by Dr K. H. Kaestner.1 BALB/c-Ptenfx/fx mice were backcrossed with C57BL/6J mice for more than 18 generations. All mutations were genotyped according to a previously published protocol1 or as directed by The Jackson Laboratory. All experiments were approved by the Animal Research Committee of the Faculty of Medicine and Health Sciences of the Université de Sherbrooke (Animal welfare committee approval number FMSS-308-17). The study followed the standards and policies of the Canadian Council on Animal Care in sciences.
Polyp Counts
Methylene blue–stained polyps were visualized under a stereomicroscope. Polyp sizes were measured with a digital caliper (Fisher Scientific, Waltham, MA) and polyp numbers were counted from the duodenum to the rectum as previously described.2, 3
Histologic Staining, Immunochemistry, and Immunofluorescence
Tissues were fixed, paraffin-embedded, sectioned, and stained as described previously.2, 4, 5, 6, 7, 8, 9 Immunohistochemistry staining of proliferating cell nuclear antigen (18197; Abcam, Cambridge, MA), anti–α-smooth muscle actin (A2547; Sigma-Aldrich, Oakville, ON, Canada), and antivimentin (5741; Cell Signaling Technology, Danvers, MA) were performed as previously described.2, 4, 5, 6, 7, 8, 9, 10 Immunofluorescence against PTEN (9559; Cell Signaling) and α-smooth muscle actin (A2547; Sigma) was performed on optimal cutting temperature–embedded tissues as previously described.4 Alexa Fluor–conjugated antibodies for all immunofluorescence studies were obtained from Invitrogen (Carlsbad, CA). Immunofluorescence images were acquired with a Leica (Leica Microsystems Inc, Concord, ON, Canada) DLMB2 microscope equipped with a DFC300FX camera and Leica FireCAM 3.4.1 software. Otherwise, slides were visualized with a NanoZoomer slide scanner and NDP.view2 software (Hamamatsu Corporation, Bridgewater, NJ).
Cytokine and Chemokine Assays
Cytokine and chemokine production was determined with antibody arrays from RayBiotech (Peachtree Corners, GA) according to the manufacturer’s protocols. The Mouse Cytokine Array G1000 was used on total protein extracts from the colon of 30-day-old mutant and control mice. The arrays were scanned with the Cy3 channel using a ScanArray Express dual-color confocal laser scanner and ScanArray Express software (PerkinElmer, Waltham, MA).
RNA Extraction and Gene Expression Analysis
Total RNA was isolated and processed using the Totally RNA extraction kit (Ambion, Carlsbad, CA). Reverse-transcription polymerase chain reaction and quantitative real-time polymerase chain reaction were performed as described previously.2, 4, 5, 6, 7, 8, 9 For every quantitative polymerase chain reaction run, a no-template control was performed for each primer pair, each of which was consistently negative. Primer sequences are available upon request.
Data Analysis
All epithelial cell counts were performed on well-oriented crypts. To determine the ratio of proliferative mesenchymal cells shown in supplementary Figure 2, the number of proliferating cell nuclear antigen–positive cells relative to the total number of mesenchymal cells in a defined area was counted. Statistical tests used are described in figure legends. Data are expressed as means ± SEM. Graphs and statistics were generated with GraphPad Prism (GraphPad Software Inc, San Diego, CA). All authors had access to the study and reviewed and approved the final manuscript.
Supplementary Table 1.
Modulated Cytokines/Chemokines in Colons of 30-Day-Old PtenΔFoxl1+ Mice
| Factors | Fold change | P value |
|---|---|---|
| Growth factors and growth factor regulators | ||
| Insulin like growth factor binding protein 5 | 3.22 | .001 |
| Insulin like growth factor binding protein 6 | -5.26 | .005 |
| Osteoprotegerin | 1.74 | .034 |
| Stromal cell-derived factor 1/chemokine (C-X-C motif) ligand 12 | 6.14 | .012 |
| Soluble tumor necrosis factor receptor II | 1.47 | .026 |
| TROY | 1.86 | .004 |
| Thymic stromal lymphopoietin | 2.09 | .001 |
| Vascular endothelial growth factor A | 1.98 | .042 |
| Vascular endothelial growth factor receptor 2 | 3.87 | .011 |
| Extracellular matrix remodeling factors | ||
| Matrix metalloproteinase 3 | 11.71 | .003 |
| Pro-matrix metalloproteinase 9 | 14.14 | 1.45E-05 |
| Tissue inhibitors of metalloproteinases-1 | 4.42 | .014 |
| Tissue inhibitors of metalloproteinases-2 | -2.59 | .062 |
| Inflammatory cytokines and chemokines | ||
| BLC | 28.39 | .050 |
| CD30 ligand | 8.63 | .005 |
| CD40 | 2.06 | .004 |
| EOTAXIN/chemokine (C-C motif) ligand 11 | 2.93 | .004 |
| Glucocorticoid-induced TNFR family related gene | 2.15 | 2.0E-04 |
| Granulocyte-macrophage colony stimulating factor | 4.65 | .001 |
| Interleukin 4 | 17.13 | .049 |
| Interleukin 6 | 17.19 | .050 |
| Interleukin 9 | 6.43 | .002 |
| Interleukin 12 | 1.74 | .012 |
| Interleukin 13 | 6.67 | .129 |
| Interleukin 15 | 1.82 | .001 |
| Interferon-inducible T cell alpha chemoattractant/chemokine (C-X-C motif) ligand 11 | -2.51 | .039 |
| L-selectin | 5.92 | .008 |
| Lymphotactin | 19.80 | .039 |
| Monocyte chemoattractant protein 1/ chemokine (C-C motif) ligand 2 | 22.77 | .001 |
| Monocyte Chemoattractant Protein 5/ Chemokine (C-C motif) Ligand 12 | 7.53 | .00022 |
| Macrophage-derived chemokine/ chemokine (C-C motif) ligand 22 | 11.65 | .005 |
| Monokine indiced by Gamma/Chemokine (C-X-C motif) ligand 9 | 6.30 | .026 |
| Regulated on activation, normal T cell expressed and secreted | 2.42 | .004 |
NOTE. Fold change represents the ratio of mean value (mutant/control) of cytokine/chemokine expression analyzed using the RayBiotech Mouse Cytokine Antibody Array G series 1000 on total colonic protein extracts from 30-day-old mice (N = 4). Negative values indicate a reduction in PtenΔFoxl1+ mice compared with controls. Statistical probability was determined using the Student t test.
Supplementary Table 2.
Gene Expression Levels of Secreted Factors in Colons of 30- to 45-Day-Old PtenΔFoxl1+ Mice
| Factors | Fold change | P value |
|---|---|---|
| Growth factors and growth factors regulators | ||
| Insulin like growth factor binding protein 5 | 20.70 | .008 |
| Insulin like growth factor binding protein 6 | -0.74 | .206 |
| Stromal cell-derived factor 1/ chemokine (C-X-C motif) ligand 12 | 1.05 | .841 |
| TROY | 1.50 | .691 |
| Vascular endothelial growth factor A | 1.05 | .042 |
| Extracellular matrix remodeling factors | ||
| Pro-matrix metalloproteinase 9 | 41.70 | .008 |
| Tissue inhibitors of metalloproteinases-1 | 7.00 | .008 |
| Tissue inhibitors of metalloproteinases-2 | 1.72 | .056 |
| Inflammatory cytokines and chemokines | ||
| Interleukin 13 | 40.05 | .036 |
| Interleukin 15 | 1.18 | .548 |
| Monocyte chemoattractant protein 5/chemokine (C-C motif) ligand 12 | 6.03 | .016 |
| Monokine indiced by Gamma/ chemokine (C-X-C motif) ligand 9 | 23.05 | .079 |
| EOTAXIN/chemokine (C-C motif) ligand 11 | 2.47 | .064 |
NOTE. Fold change represents the ratio of mean value (mutant/control) of total colonic RNA extracts from 30- to 45-day-old mice (N = 5). Negative values indicate a reduction in PtenΔFoxl1+ mice compared with controls. Statistical probability was determined using the Student t test.
References
- 1.Taddei M.L. Cancer Lett. 2013;341:80–96. doi: 10.1016/j.canlet.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 2.McLin V.A. Gastroenterology. 2009;136:2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Shoshkes-Carmel M. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki R. Cell Mol Gastroenterol Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langlois M.J. FASEB J. 2009;23:1835–1844. doi: 10.1096/fj.08-123125. [DOI] [PubMed] [Google Scholar]
- 6.Marsh V. Nat Genet. 2008;40:1436–1444. doi: 10.1038/ng.256. [DOI] [PubMed] [Google Scholar]
- 7.Allaire J.M. Int J Cancer. 2016;138:2700–2712. doi: 10.1002/ijc.30001. [DOI] [PubMed] [Google Scholar]
- 8.Roy S.A. Sci Rep. 2016;6:32759. doi: 10.1038/srep32759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies E.J. J Pathol. 2014;233:27–38. doi: 10.1002/path.4312. [DOI] [PubMed] [Google Scholar]
- 10.Jackstadt R. J Pathol. 2016;238:141–151. doi: 10.1002/path.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Sackett S.D. Genesis. 2007;45:518–522. doi: 10.1002/dvg.20315. [DOI] [PubMed] [Google Scholar]
- 2.Langlois M.J. FASEB J. 2009;23:1835–1844. doi: 10.1096/fj.08-123125. [DOI] [PubMed] [Google Scholar]
- 3.Perreault N. Genes Dev. 2005;19:311–315. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allaire J.M. Am J Physiol Gastrointest Liver Physiol. 2011;300:G586–G597. doi: 10.1152/ajpgi.00041.2010. [DOI] [PubMed] [Google Scholar]
- 5.Allaire J.M. Int J Cancer. 2016;138:2700–2712. doi: 10.1002/ijc.30001. [DOI] [PubMed] [Google Scholar]
- 6.Auclair B.A. Gastroenterology. 2007;133:887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Gagne-Sansfacon J. PLoS One. 2014;9:e98751. doi: 10.1371/journal.pone.0098751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloum F. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1065–G1079. doi: 10.1152/ajpgi.00176.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S.A. Sci Rep. 2016;6:32759. doi: 10.1038/srep32759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulombe G. Mol Cell Biol. 2013;33:2275–2284. doi: 10.1128/MCB.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]